Abstract
The mechanistic target of rapamycin complex 1 (mTORC1) kinase is a sensor of different environmental conditions and regulator of cell growth, metabolism and autophagy. mTORC1 is activated by Rag GTPases, working as RagA:B and RagC:D heterodimers. Rags control mTORC1 activity by tethering mTORC1 to the lysosomes where it is activated by Rheb GTPase. RagA:B, active in its GTP-bound form, is inhibited by GATOR1 complex, a GTPase activating protein (GAP) and GATOR1 in turn is negatively regulated by GATOR2 complex. Sestrins are stress-responsive proteins that inhibit mTORC1 via activation of AMP-activated protein kinase (AMPK) and tuberous sclerosis complex. Here we report an AMPK-independent mechanism of mTORC1 inhibition by Sestrins mediated by their interaction with GATOR2. As a result of this interaction the Sestrins suppress lysosomal mTOR localization in a Rag-dependent manner. This mechanism is potentially involved in mTORC1 regulation by amino acids, rotenone and tunicamycin, connecting stress response with mTORC1 inhibition.
Keywords: Sestrin, mTORC1, GATOR1, GATOR2, RagA:B
INTRODUCTION
The evolutionary conserved mechanistic target of rapamycin (mTOR) protein kinase is a critical regulator of cell growth and metabolism. It exists as two separate protein complexes called mTORC1, composed of mTOR, Raptor, GβL/mLST8, PRAS40 and DEPTOR, and mTORC2, containing mTOR, Rictor, GβL/mLST8 and mSIN1, which have different substrate specificities and control distinct but overlapping processes (Laplante and Sabatini, 2012). mTORC1 stimulates protein and lipid biosynthesis through several well characterized substrates including p70S6K, an upstream kinase for ribosomal protein S6, and 4EBP1, an inhibitor of the translational initiation factor eIF-4E (Dann et al., 2007; Hay and Sonenberg, 2004; Wullschleger et al., 2006). mTORC1 also activates the major lipogenic regulator – transcription factor SREBP1(Laplante and Sabatini, 2012). Another critical function of mTORC1 is inhibition of autophagy via direct phosphorylation of ULK1 and ATG13 (Laplante and Sabatini, 2012). By contrast, mTORC2 regulates glucose metabolism via direct phosphorylation of AKT, a critical regulator of glucose transport and glycolysis (Laplante and Sabatini, 2012). mTORC1 activity is regulated by two types of small GTPases: Rheb and members of the Rag family that form RagA:B and RagC:D heterodimers (Bar-Peled and Sabatini, 2014). GTP-loaded Rheb binds and activates mTORC1 and Rheb activity itself is controlled by the tuberous sclerosis protein complex (TSC), composed of TSC1, TSC2 and TBC1D7 subunits, which works as a GTPase-activating protein (GAP) for Rheb (Dibble and Manning, 2013). TSC activity is suppressed by insulin and growth factors, which stimulate AKT-dependent phosphorylation of TSC2 and displace the TSC complex from the lysosomes, the site at which Rheb is located and functions to activate mTORC1 (Menon et al., 2014). In contrast, many stress conditions including energy shortage, reactive oxygen species or DNA damage inhibit mTORC1 via phosphorylation of TSC2 by the AMP-activated kinase (AMPK) (Mihaylova and Shaw, 2011). AMPK is activated by AMP as well as ADP, which accumulate during energy shortage, and is inhibited by ATP (Hardie et al., 2012).
Another critical branch of mTORC1 regulation is controlled by amino acids (AA) via the RagA:B and RagC:D heterodimers (Sancak et al., 2008). While RagA:B is active in its GTP-bound form, RagC:D needs to be GDP bound. The RagA:B and RagC:D complexes tether mTORC1 to the lysosomes where it is activated by Rheb. RagA:B activity is controlled by the Ragulator complex, which is composed of MP1, p14, p18, HBXIP and C7orf59 and functions as a guanine-nucleotide exchange factor (GEF) (Bar-Peled and Sabatini, 2014). RagC:D is controlled by the tumor suppressor folliculin, which functions as a RagC:D GAP (Bar-Peled and Sabatini, 2014). The Rag heterodimers sense amino-acids via v-ATPase, a lysosomal protein which detects AA availability (Bar-Peled and Sabatini, 2014). Attempts to find GAP for RagA:B led to identification of the GATOR super-complex, which is composed of two complexes: GATOR1 and GATOR2. GATOR1, containing DEPDC5, Nprl2 and Nprl3 functions as a GAP for RagA:B. GATOR2, composed of Mios, WDR59, WDR24, Seh1L and Sec13, is a negative regulator of GATOR1, however its mechanism of action remains obscure (Bar-Peled et al., 2013).
We have recently identified Sestrins, encoded by the Sesn1, Sesn2 and Sesn3 genes in mammals and the dSesn gene in Drosophila, as stress-inducible regulators of AMPK-mTORC1 signaling (Budanov et al., 2010). Expression of the Sestrins is induced by various stress insults via stress-responsive transcriptional factors such as p53 and FoxO and in turn the Sestrins control major cellular processes including cell viability, antioxidant defense, cell growth and metabolism (Lee et al., 2013; Lee et al., 2012). Sestrin induction results in inhibition of mTORC1 activity through AMPK stimulation (Budanov et al., 2010). Here we describe AMPK-independent mechanism of mTORC1 regulation by the Sestrins, in which the Sestrins inhibit mTORC1 localization to the lysosomes in a Rag-dependent manner through an interaction with GATOR2.
RESULTS
Sestrins Interact with GATOR2
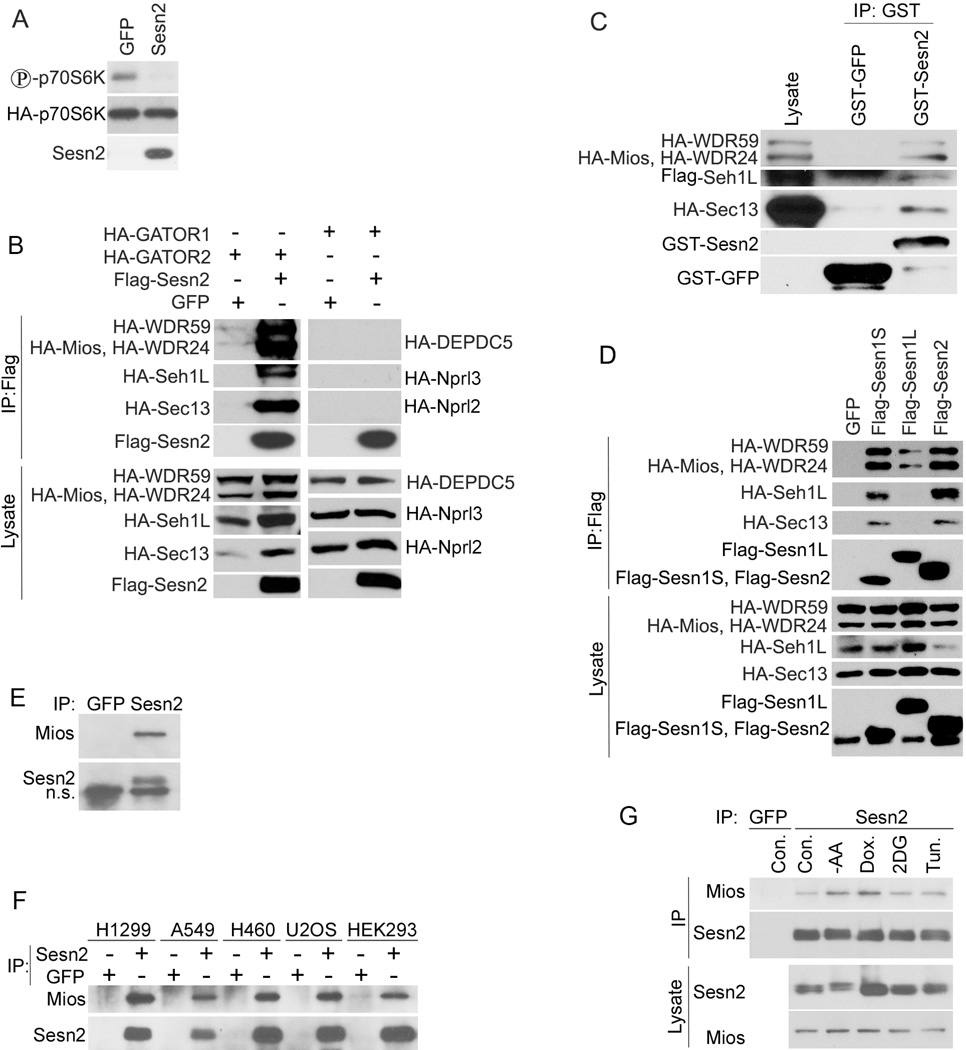
We have shown that the Sestrins inhibit mTORC1 in human cancer cell lines and in Drosophila in the AMPK- and TSC-dependent manner (Budanov and Karin, 2008; Lee et al., 2009). To further analyze the AMPK dependence of mTORC1 inhibition by the Sestrins, we co-transfected immortalized AMPK α−/− mouse embryonic fibroblasts (MEF) with HA-p70S6K- and either Sesn2- or GFP-expressing constructs. Surprisingly, we observed strong inhibition of p70S6K phosphorylation upon Sesn2 expression (Figure 1A), suggesting the operation of an AMPK-independent mechanism of mTORC1 inhibition by the Sestrins. To better examine the role of AMPK in immortalized MEF we co-transfected AMPK α−/− cells with AMPKα1- and HA-p70S6K- together with either GFP- or Sesn2-expressing constructs and measured phosphorylation of AMPK and its targets as well as mTORC1 targets in the presence or absence of Sesn2 by immunoblotting. While we observed that Sesn2 had similar effects on p70S6K and 4EBP1 phosphorylation in AMPK α−/− and AMPKα1-reconstituted cells, it stimulates AMPK, Acetyl-CoA carboxylase (ACC) and ULK1 phosphorylation only in the AMPKα1-reconstituted cells (Figure S1A), indicating that inhibition of p70S6K phosphorylation by Sesn2 can be AMPK-independent. To study the possible impact of AMPK on mTORC1 inhibition by the Sestrins in another cell line where we previously observed strong AMPK activation by Sesn2 (Budanov and Karin, 2008), we co-transfected HEK293T cells with HA-p70S6K- together with either GFP- or Sesn2-expressing constructs and treated them with AMPK inhibitor compound C or vehicle control. While p70S6K phosphorylation was strongly inhibited in control cells, it was partially relieved by compound C indicating that two parallel mechanisms of mTORC1 inhibition by Sesn2 operate in these cells (Figure S1B). To identify Sestrin-interacting proteins that could be involved in this process we performed tandem affinity purification using human mammary epithelial MCF10A cells infected with SBP (Streptavidin-Binding Peptide)-Flag-Sesn2 retroviral expressing construct. Sesn2-containing protein complexes were purified and analyzed by mass spectrometry (MS), which identified GATOR2 proteins Mios, WDR59, WDR24, SehlL and Sec13 as putative Sesn2-interacting partners (data not shown). To test whether the interaction between Sesn2 and GATOR2 is specific, we co-transfected Flag-Sesn2 together with HA-tagged Mios, WDR59, WDR24, Seh1L and Sec13 expressing constructs, or in parallel with constructs expressing HA-tagged GATOR1 proteins: DEPDC5, Nprl2 and Nprl3. Immunoprecipitation of Flag-Sesn2 with anti-Flag beads followed by immunoblot analysis revealed that all GATOR2 proteins were co-precipitated with Flag-Sesn2, but not GFP or Flag-GFP (Figures 1B and S1C). However we did not observe any interaction between Sesn2 and GATOR1 (Figure 1B). To determine whether Sesn2 can bind GATOR2 in vitro indicating its avidity to this complex, we isolated GATOR2 from HEK293T cells and performed in vitro binding assay with bacterially-purified either GST-Sesn2 or GST-GFP proteins bound to GST-beads. GST-Sesn2 but not control GST-GFP efficiently bound GATOR2 as demonstrated by immunoblot analysis of the GST-Sesn2 complexes after incubation with GATOR2 (Figure 1C). We demonstrated earlier that the intact Sesn2 molecule was required for mTORC1 inhibition and deletion mutants lacking the N-terminal (ΔN), C-terminal (ΔC) or middle (ΔM) part of the protein lost their inhibitory effect on mTORC1 (Budanov and Karin, 2008). To analyze whether these mutants are able to interact with GATOR2 we co-expressed Sesn2 deletion mutants with GATOR2 and analyzed the interactions by immunoprecipitation and immunoblotting. While the intact Sesn2 strongly interacted with GATOR2, the ΔN- and ΔC- Sesn2 truncated mutants showed almost no interaction with GATOR2, although the ΔM mutant showed some residual activity, indicating that intact C-and N-termini can be involved in the interaction with GATOR2 (Figure S1D). Other Sestrin family members, Sesn1 and Sesn3, also negatively regulate mTORC1 activation and may have identical functions to Sesn2 (Budanov et al., 2010). Sesn1 is expressed as a short form Sesn1S (55kDa, the most similar to Sesn2), and a long-form Sesn1L, with an extended N-terminus. We co-transfected Flag-tagged Sesn1S- and Sesn1L- with HA-tagged GATOR2-expressing constructs into HEK293T cells and incubated the lysates with anti-Flag beads. While Sesn1S showed strong interaction, Sesn1L interacted poorly with GATOR2 (Figure 1D). To study whether endogenous Sesn2 and GATOR2 interact, we conducted immunoprecipitation with anti-Sesn2 antibody from MCF10A cells and analyzed GATOR2 components by immunoblotting. After trying different commercially-available antibodies we were able to detect only Mios in the Sesn2 immunoprecipitates (Figure 1E). To examine whether Sesn2 can co-precipitate Mios in various cell lines of different origins we immunoprecipitated Sesn2 from cells with intact as well as inactivated p53 or Lkb1 (WT p53: A549, H460, U2OS; p53-deficient: H1299, HEK293; WT Lkb1: H1299, 293, U2OS; Lkb1-deficient: A549, H460). We found strong co-precipitation of Sesn2 and Mios in all these cell lines (Figure 2F) regardless of their relative expression level (Figure S1E). Presuming that stress factors can affect the interaction between Sesn2 and Mios, we incubated MCF10A cells with AA-free medium, DNA-damaging drug doxorubicin, glycolytic inhibitor 2-deoxyglucose (2DG) or ER stress inducer tunicamycin. We observed that AA starvation and doxorubicin enhanced the interaction between Sesn2 and Mios, while 2DG and tunicamycin had only marginal effects, although either 2DG or tunicamycin induced Sesn2 expression in these cells (Figure 2G).
Figure 1. Sestrins Inhibit mTORC1 in an AMPK-Independent Manner and Interact with GATOR2.
(A) Immortalized AMPK α−/− MEF cells were co-transfected with HA-p70S6K together with either GFP-or Sesn2-expressing constructs. 48 hrs later cells were lysed, HA-p70S6K was immunoprecipitated with anti-HA beads and phosphorylation and expression of the corresponding proteins were analyzed by immunoblotting. (B) Sesn2 directly interacts with GATOR2 but not GATOR1. Flag-Sesn2 was co-transfected with HA-tagged GATOR2- or GATOR1- expressing constructs to HEK293T cells, immunoprecipitated with anti-Flag beads and the proteins were analyzed by immunoblotting with anti-HA or anti-Flag antibodies. (C) Sesn2 binds GATOR2 in vitro. GATOR2 was purified from HEK293T cells using anti-Flag-beads, eluted with Flag peptide and incubated with bacterially purified GST-Sesn2 or control GST-GFP protein overnight followed by immunoblot analysis with HA or GST antibodies. (D) Sesn1 binds GATOR2. Flag-Sesn1S-, Flag-Sesn1L- or Flag-Sesn2- were co-transfected together with the GATOR2-expressing constructs and analyzed by immunoblotting as in (B). (E) Endogenous Sesn2 interacts with the GATOR2 Mios protein in non-transformed MCF10A mammary epithelial cells. Endogenous Sesn2 was immunoprecipitated with anti-Sesn2 antibodies (anti-GFP antibodies of the same type were used as a control) and immunoblotted with anti-Mios and anti-Sesn2 antibodies. (F) Endogenous Sesn2 interacts with Mios in different human cancer cell lines. Experiment was performed as in (E). (G) Different stress conditions can affect Sesn2-Mios interaction. MCF10A cells were treated with AA-free medium, doxorubicin, 2DG and tunicamycin and Sesn2-Mios interactions were examined as in (E).
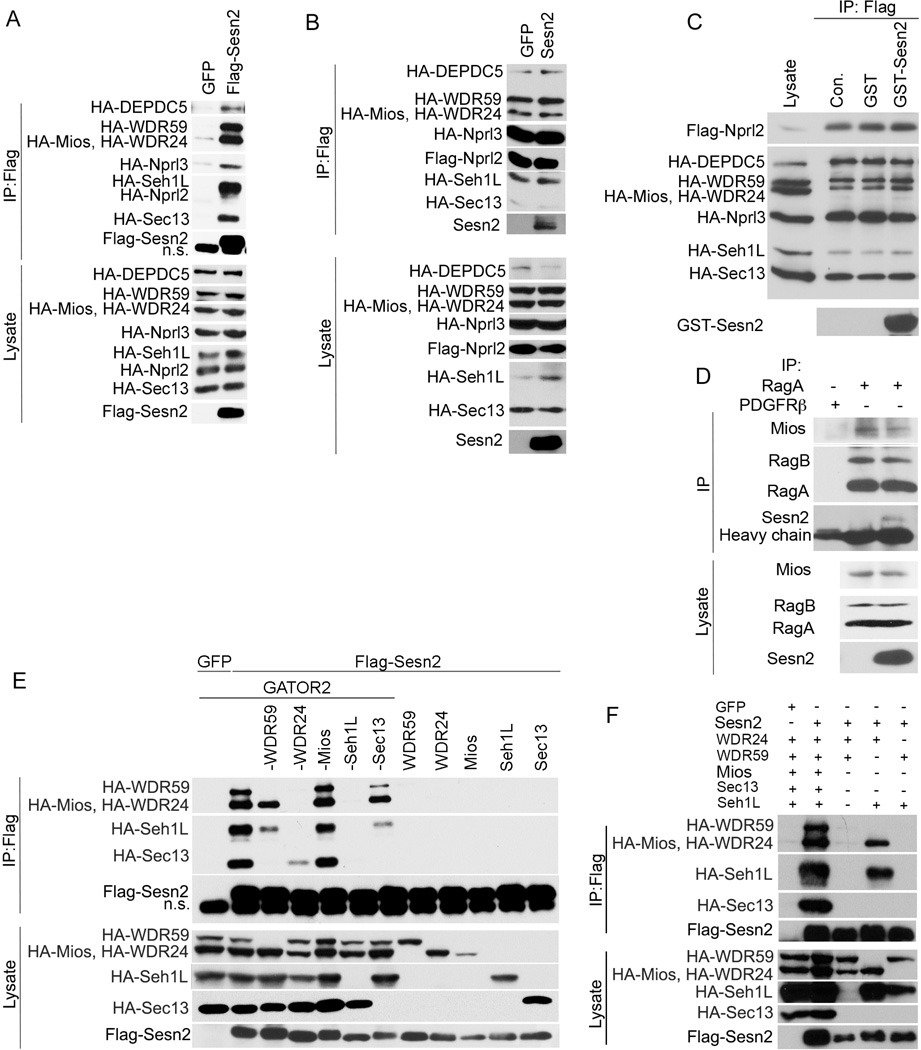
Figure 2. Sesn2 Does not Disrupt GATOR1–GATOR2 Interactions and Does not Interact with the Separate GATOR2 Components.
(A) Sesn2 interacts with GATOR1 in the presence of GATOR2. HEK293T cells were co-transfected with either Flag-Sesn2- or GFP-expressing constructs with all HA-tagged GATOR-expressing plasmids. Flag-Sesn2 was immunoprecipitated with anti-Flag beads and the proteins within Sesn2 complex and in the cell lysates were analyzed by immunoblotting with anti-Flag or anti-HA antibodies. (B) Overexpression of Sesn2 does not affect GATOR1–GATOR2 interactions. All GATOR proteins were ectopically expressed in the presence of either Sesn2 or GFP, immunoprecipitated via Flag-Nprl2 with anti-Flag beads and analyzed by immunoblotting as in (A). (C) Sesn2 does not affect interaction between GATOR1 and GATOR2 in vitro. All GATOR proteins were ectopically expressed in HEK293T cells. Immunocomplexes containing Flag-Nprl2 and other HA-tagged GATOR proteins were isolated with anti-Flag beads, incubated overnight with bacterially purified GST-Sesn2 and examined by immunoblotting as in (A). (D) Overexpression of Sesn2 does not have a strong impact on the GATOR-RagA:B interactions. HEK293T cells were infected with either Sesn2- or control GFP-expressing constructs and Rag complexes were immunoprecipitated with anti-RagA or control anti-PDGFRβ antibody and analyzed by immunoblotting with anti-RagA and anti-Mios antibodies. (E) Sesn2 interacts with whole GATOR2 but not its separate components. Flag-Sesn2 expressing construct was co-transfected with different combinations of the components of GATOR2-expressing constructs (HA-WDR59, HA-WDR24, HA-Mios, HA-Seh1L and HA-Sec13) into HEK293T cells. Flag-Sesn2 complexes were pulled down with anti-Flag beads and analyzed by immunoblotting as in (A). (F) Sesn2 interacts with a combination of WDR24 and Seh1L proteins. Flag-Sesn2 expressing plasmid was co-transfected with different combinations of WDR24-, Seh1L- and WDR59-expressing constructs. The Flag-Sesn2 complexes were immunoprecipitated with anti-Flag beads and analyzed by immunoblotting as in (A).
Sesn2 Does not Inhibit Complex formation Between Ectopically-Expressed GATOR1 and GATOR2 and Does not Interact with Individual GATOR2 Proteins
As previously described, Sesn2 does not directly interact with GATOR1 (Figure 1B). To study whether Sesn2 can interact with GATOR1 through GATOR2 we co-transfected Flag-Sesn2 together with all HA-tagged GATOR-expressing constructs and immunoprecipitated Sesn2 with anti-Flag beads followed by immunoblot analysis. We found that in the presence of GATOR2, GATOR1 co-precipitated with Sesn2, indicating that the interactions between GATOR1 and GATOR2, and Sesn2 and GATOR2 are not mutually exclusive (Figure 2A). To study whether Sesn2 can affect the interaction between GATOR1 and GATOR2, which could explain how the Sestrins inhibit mTORC1 activity, we co-transfected all the GATOR components together with Sesn2-expressing plasmid. We pulled down GATOR proteins using anti-Flag beads which immunoprecipitated Flag-Nprl2 protein together with all other members of GATOR1 and GATOR2. Comparison of the amounts of GATOR2 proteins co-purified with GATOR1 showed no difference whether Sesn2 was present or absent from the complex, indicating that direct inhibition of the GATOR1–GATOR2 interaction might not be the primary mechanism by which Sestrins modulate GATOR activity (Figure 2B). To confirm this, we pulled down the GATOR1–GATOR2 complex with anti-Flag beads and incubated the beads overnight with a high excess of bacterially-purified GST-Sesn2 followed by immunoblot analysis of the GATOR proteins. Again, we did not observe any effect of Sesn2 on the interaction between GATOR1 and GATOR2 proteins indicating that Sesn2 does not affect the GATOR1–GATOR2 interactions (Figure 2C). GATOR interacts with Rag proteins via the GATOR1 sub-complex (Bar-Peled et al., 2013). To study whether ectopically-expressed Sesn2 can regulate interaction between Rags and GATOR we immunoprecipitated endogenous RagA:B proteins with anti-RagA antibodies and analyzed the complex with anti-Mios antibodies. We observed that Sesn2 does not significantly affect Rag-GATOR interactions. Moreover Sesn2 was found in the complex suggesting that Sesn2 can directly modulate GATOR activities toward Rags (Figure 2D).
To determine which GATOR2 proteins directly interact with Sesn2, we co-transfected Flag-Sesn2- with individual HA-tagged GATOR2-expressing constructs and immunoprecipitated Flag-Sesn2 with anti-Flag beads. In parallel we co-transfected Flag-Sesn2- with different combinations of GATOR2-expressing constructs. As indicated in Figure 2E, most GATOR2 proteins when transfected individually were expressed at much lower levels than when they all were co-expressed. Surprisingly, binding of Sesn2 to any individual GATOR2 component was barely detectable (Figure 2E). Furthermore, the removal of individual GATOR2 components other than Mios weakened the GATOR2 association with Sesn2 (Figure 2E). Elimination of WDR59 had a partial effect on the interaction between Sesn2 and the other GATOR2 proteins, and exclusion of either WDR24 or Seh1L almost completely blocked the assembly of Sesn2-GATOR2 complexes, suggesting that WDR24 and Seh1L could constitute the binding site for Sesn2 (Figure 2E). Because we found that WDR24 and Seh1L are required for interaction between Sesn2 and GATOR2, while WDR59 omission had a partial effect, we analyzed which of these 3 proteins might be responsible for the interaction with Sesn2. We co-transfected HEK293T cells with Flag-Sesn2 and a combinations of 2 of 3 constructs co-expressing WDR24, Seh1L and WDR59 and immunoprecipiated Flag-Sesn2 with anti-Flag beads. We observed that Flag-Sesn2 efficiently co-precipitated with the pair of WDR24 and Seh1L, but not the other protein combinations indicating that the WDR24-Seh1L pair provides a binding site for Sesn2 (Figure 2F).
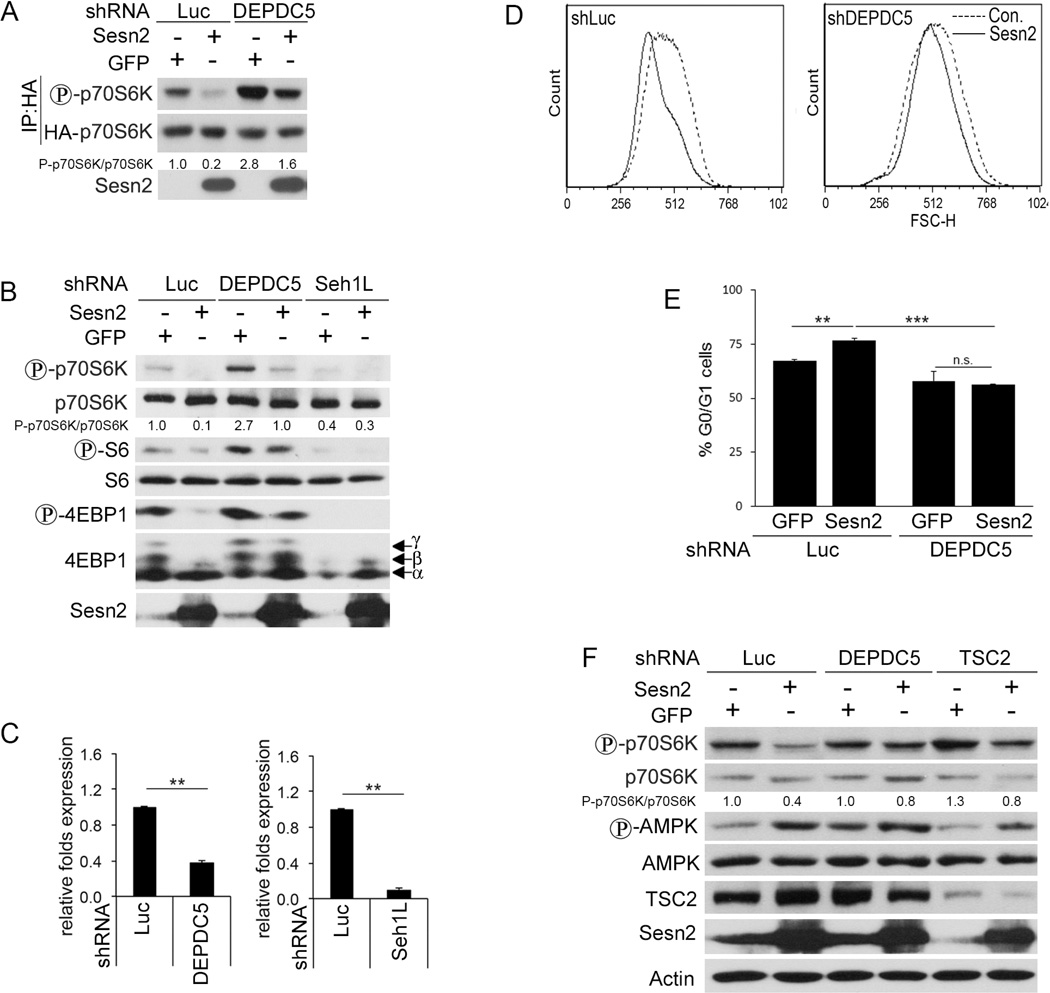
Sesn2 Inhibits mTORC1 in a GATOR-Dependent Manner
As previously reported, GATOR2 activates mTORC1 by inhibiting GATOR1, which is GAP for RagA:B (Bar-Peled et al., 2013). To study whether mTORC1 inhibition by Sesn2 depends on GATOR1, we co-transfected HEK293T cells with HA-p70S6K and either control shLuciferase (shLuc) or shDEPDC5 shRNA constructs together with GFP- or Sesn2-expression vectors and monitored mTORC1 activity by p70S6K phosphorylation (Budanov and Karin, 2008). DEPDC5 silencing strongly compromised the inhibition of p70S6K phosphorylation by Sesn2 (Figure 3A). To determine whether Sesn2 inhibits phosphorylation of endogenous p70S6K and 4EBP1 in a GATOR-dependent manner, we silenced either DEPDC5 or Seh1L in MCF10A cells and confirmed the downregulation of DEPDC5 or Seh1L expression by quantitative real time PCR (qPCR) (Figure 3B and C), followed by infection of the cells with either Sesn2- or GFP- expression vectors. DEPDC5 knockdown compromised the suppression of mTORC1 activity by Sesn2 as indicated by higher levels of phosphorylation of p70S6K and 4EBP1 in the Sesn2-infected cells (Figure 3B). Knockdown of Seh1L itself inhibited mTORC1 activity and Sesn2 expression did not have an additional effect on phosphorylation of p70S6K and 4EBP1 (Figure 3B). The major mTORC1 function is regulation of cell growth, which is also associated with cell proliferation (Wullschleger et al., 2006). To study whether Sesn2 regulates cell growth and proliferation in a GATOR-dependent manner we measured cell size in DEPDC5-silenced or control cells infected with either GFP- or Sesn2-expressing lentiviruses. While we observed inhibition of cell growth by Sesn2 in control cells, no effect of Sesn2 on cell size was observed in the DEPDC5-silenced cells (Figure 3D). Ectopic expression of Sesn2 also increased the number of cells in G0/G1 phase indicating an inhibitory effect of Sesn2 on cell cycle and this effect was lost in the DEPDC5-silenced cells (Figure 3E). As we described earlier, Sesn2 inhibits p70S6K phosphorylation in H1299 cells, and these effects were compromised when TSC2 and AMPK were silenced (Budanov and Karin, 2008). To compare the inhibitory effect of Sesn2 on mTORC1 in the cells with inhibited AMPK-TSC2 axis and in the cells where GATOR1 activity is diminished, we ectopically expressed Sesn2 in either DEPDC5- or TSC2-silenced cells. While Sesn2 inhibited p70S6K phosphorylation in control cells, this effect was reduced in either TSC2- or DEPDC5-silenced cells, indicating that both pathways can contribute to mTORC1 inhibition by Sesn2 (Figure 3F). Although we observed an activation of AMPK phosphorylation in response to Sesn2 expression, it was higher in the DEPDC5-silenced cells, indicating that DEPDC5 silencing did not compromise AMPK activation by Sesn2, in accordance with the existence of an AMPK-independent mechanism of mTORC1 inhibition by the Sestrins.
Figure 3. Sesn2 Inhibits mTORC1 in a GATOR-Dependent Manner.
Suppression of mTORC1 by Sesn2 is compromised in DEPDC5-silenced HEK293T cells. HEK293T cells were co-transfected with HA-p70S6K together with either shLuc- or shDEPDC5-expressing constructs combined with either Sesn2- or GFP-expressing constructs and HA-p70S6K was immunoprecipitated with anti-HA beads followed by immunoblot analysis with the indicated antibodies. (B) Knockdown of DEPDC5 or Seh1L compromises an inhibitory effect of Sesn2 on mTORC1 in MCF10A cells. DEPDC5- or Seh1L-silenced cells were infected with Sesn2- or GFP-expressing lentiviruses. The protein phosphorylation and expression was detected by immunoblotting. (C) The inhibition of DEPDC5 or Seh1L in (B) is determined by qPCR. (D, E) Sesn2 inhibit cell growth (D) and cell cycle (E) in a DEPDC5-dependent manner. Cell size and cell cycle of DEPDC5-silenced or control MCF10A cells expressing Sesn2 or GFP were determined by flow cytometry. (F) Comparison of the effects of DEPDC5 and TSC2 silencing on inhibition of mTORC1 by Sesn2 in H1299 cells. The experiment was performed as in (B). Results in (E) and (C) are averages ± SD. ** indicates p< 0.01 and *** p< 0.001by Student’s t test.
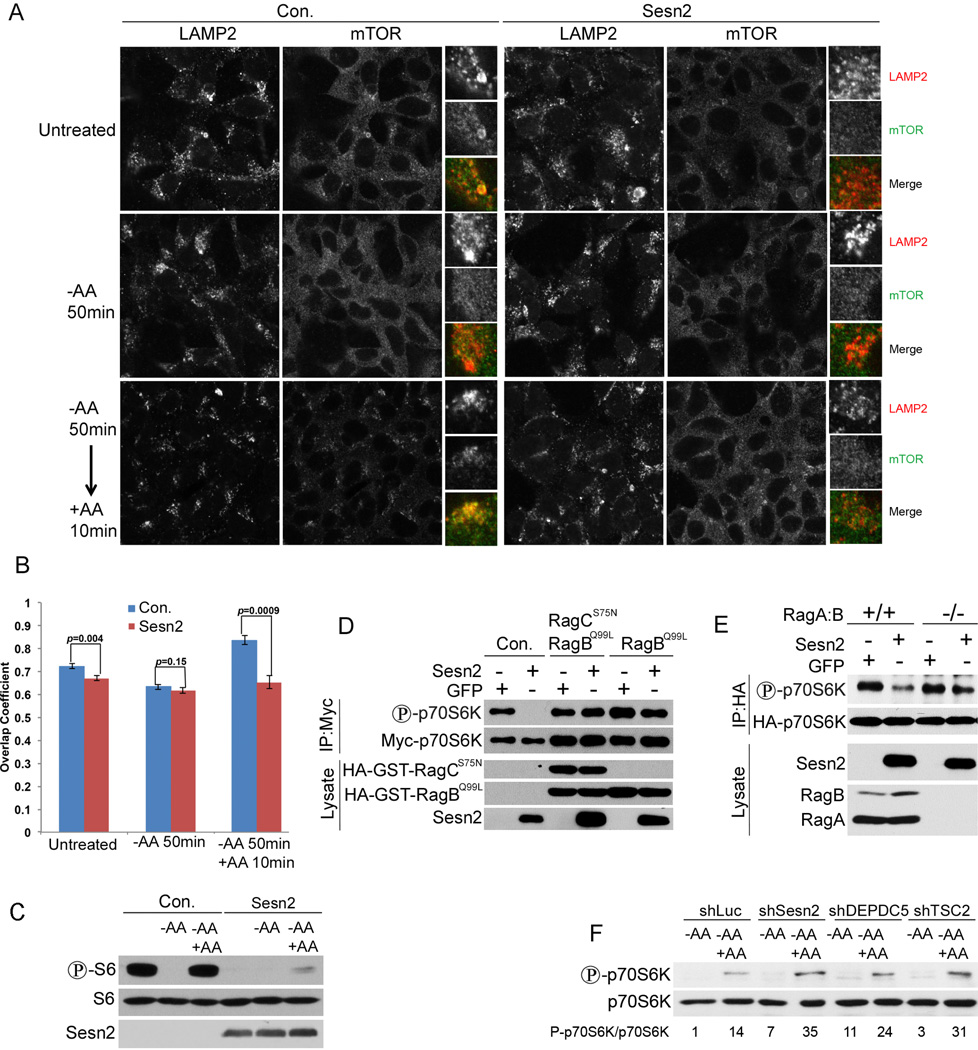
Sesn2 Regulates mTORC1 via Rag Proteins and Participates in AA Signaling
GATOR2 stimulates mTORC1 activity by suppressing the inhibition of RagA:B by GATOR1 (Bar-Peled et al., 2013). Rags tether mTORC1 to the lysosomes, where mTORC1 can interact with its activator Rheb. Incubation of cells with AA-free medium causes re-distribution of mTORC1 from the lysosomes to the cytoplasm (Sancak et al., 2008). To study the impact of Sesn2 on localization of mTORC1 in control, AA-deprived and AA-stimulated conditions we infected HEK293T cell with either Sesn2-expressing or control pLU construct and were able to reach almost 100% of infection (Figure S2A). Ectopic expression of Sesn2 inhibited the localization of mTORC1 to the lysosomes, similar to effects of AA starvation (Figures 4A, B and S2B). AA withdrawal in the presence of Sesn2 expression had no additive effects on mTORC1 localization and re-feeding with AA strongly stimulated mTOR re-distribution to the lysosomal compartment in control cells, but not in the cells infected with Sesn2 expressing construct (Figures 4A,B, and S2B). These findings were supported by analysis of mTORC1 activity using anti-phospho-S6 antibodies, which showed inhibition of S6 phosphorylation in the Sesn2-expressing cells in normal and AA starved conditions (Figure 4C). AA re-feeding caused restoration of S6 phosphorylation in control, but not in the Sesn2-expressing HEK293T and H1299 cells (Figures 4C and S2C), supporting the idea that Sesn2 can interfere with AA-stimulated mTORC1 activation. Rags play a critical role in mTORC1activation by AA causing mTOR re-distribution from the cytoplasm to the lysosomes (Sancak et al., 2008). To determine whether Rags are important for regulation of mTORC1 by Sesn2, we co-transfected HEK293T cells with Myc-p70S6K-, and either Sesn2- or control GFP-expressing constructs in the presence or absence of constitutively active RagCS75-and/or RagBQ99 (Sancak et al., 2008). Myc-p70S6K was pulled down with anti-Myc antibodies and analyzed by immunoblotting. While Sesn2 inhibited p70S6K phosphorylation in control cells, this effect was suppressed in cells expressing RagBQ99 and RagCS75 or RagBQ99 alone (Figure 4D). In a parallel experiment, we co-transfected HA-p70S6K together with either GFP- or Sesn2-expressing constructs into RagA:B−/− or control RagA:B+/+ cells and analyzed p70S6K phosphorylation by immunoblotting. While we observed an inhibition of p70S6K phosphorylation by Sesn2 in control cells, this effect was compromised in the RagA:B−/− cells (Figure 4E) supporting the importance of Rag proteins for suppression of mTORC1 by Sesn2. RagA:B is active in its GTP-bound form, and GATOR1 can regulate RagA:B by stimulating its GTPase activity (Bar-Peled et al., 2013). To determine whether Sesn2 regulates Rags controlling GDP/GTP loading, we analyzed RagB charging with guanine nucleotides in the presence or absence of ectopically expressed Sesn2 in HEK293T cells. Surprisingly, we did not see any significant difference in GDP/GTP ratio between control and the Sesn2-expressing cells, indicating that Sesn2 does not control mTORC1 via the mechanism involved in GDP/GTP charging of Rags (Figure S2D). To examine the impact of Sesn2 on AA signaling, we silenced Sesn2 in H1299 cells and compared activation of p70S6K by AA re-feeding in control and Sesn2-silenced cells. We observed that mTORC1 activation by AA was significantly higher in the Sesn2-silenced cells as compared to control, indicating the important role of Sesn2 in the AA-regulated mTORC1 signaling (Figure 4F). We also compared the effects of Sesn2 knockdown with the effects of knockdowns of the established mTORC1 regulators DEPDC5 and TSC2. Silencing either DEPDC5 or TSC2 enhanced mTORC1 activation by AA and the effects were similar to the effects of Sesn2 silencing, demonstrating that Sesn2 can be involved in the mTORC1 regulation by AA via the mechanisms mediated by both DEPDC5 and TSC2 (Figure 4F).
Figure 4. Sesn2 Suppresses mTORC1 Activity via Inhibition of mTOR Lysosomal Localization in a Rag-Dependent Manner.
(A) Sesn2 suppresses lysosomal mTOR localization in control and in AA-stimulated cells. HEK293T cells were infected with either Sesn2 or control pLU lentiviral construct, kept in AA-free medium for 50 min and re-stimulated with AA for 10 min followed by immunostaining with anti-mTOR and anti-LAMP2 antibodies. (B) Overlapping between LAMP2 and mTOR1 in (A) was determined with ZEN Litr 2012 software. (C) Sesn2 inhibits mTORC1 activity in control and AA-stimulated cells. HEK293T cells were treated as in (A), lysed and immunoblotted with phospho-S6, S6 and Sesn2 antibodies. (D) Overexpression of constitutively active RagCS75N and/or RagBQ99L proteins compromises the inhibitory effects of Sesn2 on mTORC1. HEK293T cells were co-transfected with Myc-p70S6K- together with either Sesn2- or GFP-expressing constructs in the presence or absence of constitutively active RagCS75N and/or RagBQ99L. Myc-p70S6K was immunoprecipitated with anti-Myc antibodies and phosphorylation and expression of the corresponding proteins were analyzed by immunoblotting. (E) The suppressive effect of Sesn2 on mTORC1 is compromised in the RagA:B−/− cells. RagA:B−/− cells or WT counterparts were co-transfected with HA-p70S6K- and either Sesn2- or GFP-expressing constructs, lysed 48 hrs later. HA-p70S6K was immunoprecipitated with anti-HA beads and analyzed by immunoblotting. (F) Silencing of Sesn2 enhances re-stimulation of mTORC1 by AA. Sesn2 or control either DEPDC5 or TSC2 genes were silenced by shRNA lentiviruses, incubated in AA-free medium for 50 min and stimulated with AA for 10 min. HA-p70S6K phosphorylation and expression were determined by immunoblotting.
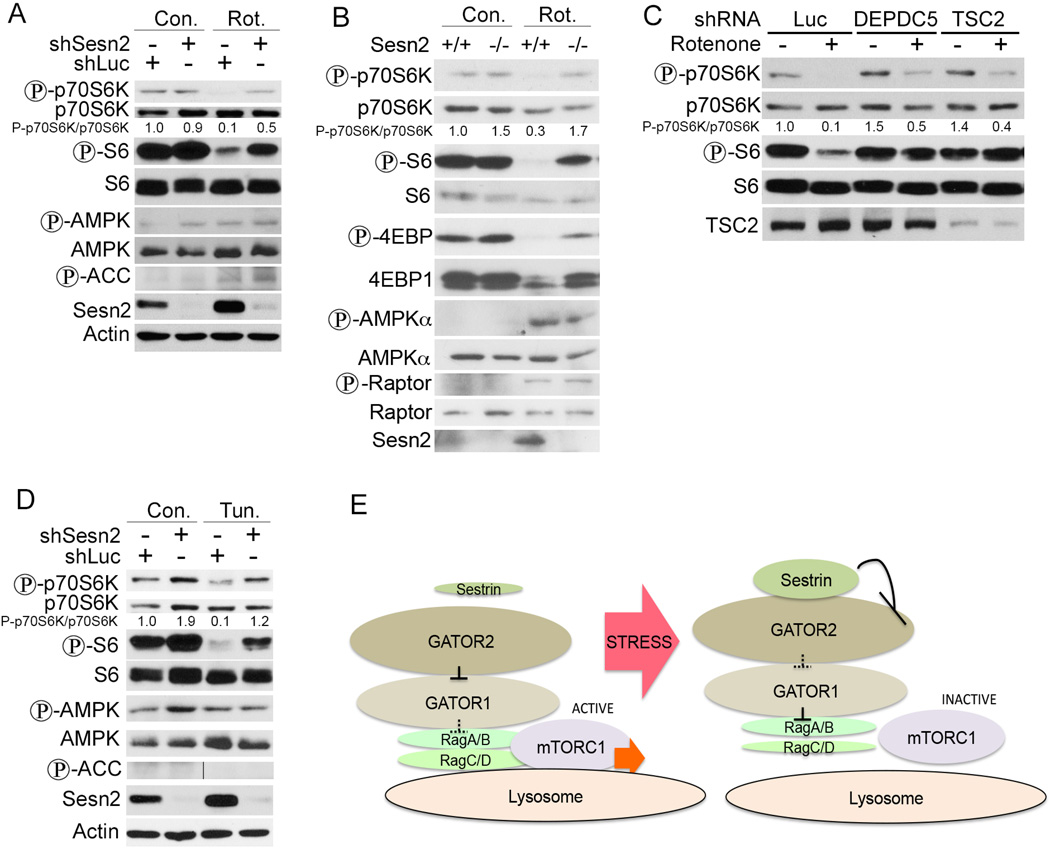
Sesn2 Inhibits p70S6K Phosphorylation in Response to Rotenone and Tunicamycin in an AMPK-Independent Manner
Sesn2 is induced during energy shortage as well as ER stress causing mTORC1 inhibition (Bae et al., 2013; Ben-Sahra et al., 2013). Previously we found that mTORC1 inhibition by Sesn2 depends in part on AMPK activation (Budanov and Karin, 2008). To determine whether inhibition of mitochondrial respiration can inhibit mTORC1 in a Sesn2-dependent manner, we treated Sesn2-silenced and control H1299 cells with rotenone, an inhibitor of the mitochondrial electron transport chain complex I. Analysis of p70S6K and S6 phosphorylation showed that rotenone inhibited mTORC1 activity in a Sesn2-dependent manner (Figure 5A). Rotenone treatment also enhanced phosphorylation of ACC at the AMPK sites, but Sesn2-silencing had little effect on these phosphorylation events (Figure 5A). To validate the role of Sesn2 in mTORC1 regulation by rotenone in an alternative system we treated Sesn2+/+ and Sesn2−/− fibroblasts with rotenone and found that Sesn2-deficiency strongly compromised mTORC1 inhibition by rotenone as determined by phosphorylation of the critical mTORC1 targets p70SK, S6 and 4EBP1, while phosphorylation of AMPK and its target Raptor were not affected by Sesn2-deficiency (Figure 5B). To study the impact of the downstream Sesn2 targets DEPDC5 and TSC on the regulation of mTORC1 by rotenone we treated DEPDC5- or TSC2- silenced cells with rotenone and observed that inhibition of p70S6K and S6 phosphorylation were DEPDC5- and TSC2-dependent (Figure 5C). We also studied the effects of ER stress on mTORC1 activity by treating Sesn2-silenced or control cells with tunicamycin. We observed that while tunicamycin decreased mTORC1 activity in a Sesn2-dependent manner, it had no effect on AMPK or ACC phosphorylation (Figure 5D).
Figure 5. Rotenone and Tunicamycin Regulate mTORC1 via Sesn2 Activation.
Rotenone (A, B and C) inhibits mTORC1 in a Sesn2-, DEPDC5- and TSC2- dependent and an AMPK-independent manner. (A) Sesn2-silenced or control H1299 cells were treated with rotenone for 10 hrs and phosphorylation and expression of the indicated proteins were determined by immunoblotting. (B) Sesn2+/+ and Sesn2−/− immortalized MEFs were treated with rotenone and analyzed by immunoblotting as in (A). (C) Rotenone regulates mTORC1 in DEPDC5- and TSC2-dependent manner. DEPDC5- or TSC2-silenced or control cells were treated with rotenone and analyzed as in (A). (D) Tunicamycin suppresses mTORC1 in a Sesn2-dependent manner. Sesn2-silenced or control H1299 cells were treated with tunicamycin for 10 hrs and phosphorylation and expression of the corresponding proteins were determined by immunoblotting. (E) A scheme indicating regulation of mTORC1 by Sestrins via a Rag-dependent mechanism. Sesn2 interacts with GATOR2 inhibiting its activity, leading to activation of GATOR1 within the GATOR supercomplex and inhibition of Rag-dependent recruitment of mTORC1 to the lysosomal membrane.
DISCUSSION
Previous studies have demonstrated that the Sestrins inhibit mTORC1 activity through activation of AMPK and TSC (Budanov et al., 2010). However, when studying the AMPK-dependence of mTORC1 inhibition by Sesn2, we observed strong inhibition of p70S6K phosphorylation even in an AMPK-null cells or in the cells where AMPK activity was suppressed by a specific inhibitor compound C (Figures 1A and S1A,B). To understand this phenomenon, we searched for new Sestrin-interacting proteins and found that Sesn2 interacts with the GATOR2, composed of Mios, WDR24, WDR59, Seh1L and Sec13. The function of GATOR2 is unknown, although it was demonstrated that GATOR2 suppresses inhibition of mTORC1 through GATOR1 within the GATOR super-complex (Bar-Peled et al., 2013). GATOR1 may function as a RagA:B GAP, and RagA:B is critical for regulation of TORC1 by GATOR (Bar-Peled et al., 2013). RagA:B regulates mTORC1 activity controlling its localization to the lysosomes, by placing it in close proximity of the major mTORC1 activator Rheb (Sancak et al., 2008). The mechanisms of the regulation of the Rag heterodimers themselves are not well known. Although it was previously reported that RagA:B activity is regulated via control of its GTPase activity (Sancak et al., 2008), it was later demonstrated that RagA:B GTP loading is not changed by amino-acid withdrawal, although lysosomal mTORC1 localization was still suppressed via the Rag-dependent mechanism (Oshiro et al., 2014). Interestingly, the vast majority of the RagA:B protein was detected in the cytoplasm, and only a small portion of it was associated with the lysosomes (Oshiro et al., 2014). Although GATOR1 works as a GAP for RagA:B, it is also not clear whether this is the sole mechanism of the RagA:B regulation by GATOR, how GATOR2 controls GATOR1 and what are the upstream mechanisms of GATOR regulation.
In the present work we examined the role of GATOR and Rags in the regulation of mTORC1 by the Sestrins and found that Sesn2 inhibits mTORC1 in a GATOR- and a Rag-dependent manner under normal cell culture conditions or upon AA re-feeding. Although a potential mechanism of regulation of GATOR by Sesn2 might involve dissociation of GATOR2:GATOR1 complexes, leading to GATOR1 activation and RagA:B inhibition, we did not see any inhibitory effect of Sesn2 on the GATOR1:GATOR2 interaction. Thus we conclude that the Sestrins affect the activity of entire GATOR, and suppress the inhibitory effects of GATOR2 on GATOR1 within GATOR compromising a stimulatory effect of RagA:B on mTORC1. Due to lack of information about the stoichiometry of the proteins in the complex, the structure and the function of the complex, at this point we cannot speculate on the precise mechanism through which the Sesn2 modulates GATOR activity. Notably, GATOR2 proteins contain WD40 domains involved in multiple protein-protein interactions (Xu and Min, 2011). The potential structure of the SEAC, the analog of GATOR complex in yeast, was reported recently where it was described that yeast WDR24 and Seh1L orthologs - Sea2 and Seh1 interact with each other on the tip of the SEAC complex forming a cleft (Algret et al., 2014). Interestingly, we demonstrated that Sesn2 interacts with GATOR2 via WDR24 and Seh1L, which together form a binding site for Sesn2. Sesn2, being located mostly in the cytoplasm (Budanov et al., 2004) (Figure S2A), could act by holding the GATOR-Rag complex in the cytoplasm and suppressing re-distribution of Rags to the lysosomal compartment where it can be activated by Ragulator and in turn activate mTORC1. In support of this theory, we did not see any co-localization of Sesn2 with the lysosomes (Figure S2A), indicating that Sesn2 plays a major role in mTORC1 regulation beyond the lysosomal compartment. Although it was demonstrated that one of the major functions of GATOR is to regulate RagA:B GTPse activity we did not observe any effect of Sesn2 on GDP/GTP loading, demonstrating that Sesn2 control GATOR and Rags not via regulation of GATOR1 GAP function but through other alternative mechanism such as retention of GATOR-Rag complexes in the cytoplasm preventing their activation.
We addressed the relative impact of the AMPK-TSC and GATOR-Rag axes in the regulation of mTORC1. According to our previous data, inhibitory effect of Sesn2 on mTORC1 was compromised but not completely suppressed in the TSC2-silenced cells (Budanov and Karin, 2008). The same partial effect was observed in the GATOR1-silenced cells (Figures 3A, B and F). Thus, both pathways can contribute to reach maximum inhibition of mTORC1 or under some conditions one branch might be pre-dominant over the other. For example, we have shown that rotenone inhibited mTORC1 in a Sesn2-dependent manner, although Sesn2 was not required for the AMPK activation under these conditions (Figure 5A, B). Thus rotenone can potentially suppress mTORC1 via the Sesn2-GATOR-Rag-dependent mechanism and we observed that silencing either DEPDC5 or TSC2 had a noticeable effect on mTORC1 inhibition by rotenone (Figure 5C). Alternatively, ER stress-induced mTORC1 inhibition operates via an AMPK-independent but a Sesn2-dependent mechanism (Figure 5D). Thus we conclude that in response to some stress insults Sesn2 inhibits mTORC1 via an AMPK-independent mechanism potentially operating via the GATOR-Rag axis. Interestingly, both the AMPK-TSC2- and the Rag-dependent pathways contribute to mTORC1 suppression under energy deficiency. For example, while in the early studies the major mechanism of the mTORC1 inhibition by glucose starvation and metformin was assigned solely to the AMPK-TSC2 regulation (Gwinn et al., 2008; Inoki et al., 2003), later it was revised and shown that both conditions suppress mTORC1 via the Rag-dependent mechanism (Efeyan et al., 2013; Kalender et al., 2010).
In conclusion, we demonstrate a novel route for inhibition of mTORC1 by the Sestrins through the regulation of GATOR and Rags (Figure 5E). Although the physiological function of GATOR is unknown, it was shown that GATOR1 works as a tumor suppressor and its components are mutated in several human cancers (Bar-Peled et al., 2013). Sesn1 and Sesn2 are targets of the major tumor suppressor p53, mutated in more than 50% of human cancers (Budanov, 2011; Budanov et al., 2002; Levine, 1997). Moreover we have shown that Sesn2 deficiency facilitates MEF transformation and accelerates growth of lung tumor xenografts (Budanov and Karin, 2008; Sablina et al., 2005). Thus the Sestrins might play an important role in the tumor suppressive network linking stress to mTORC1 activity in physiological as well as pathophysiological conditions.
Note added in proof: While our paper was under review two other groups reported the regulation of mTORC1 by Sestrins via the Rag-dependent mechanism (Chantranupong et al., 2014; Peng et al., 2014).
EXPERIMENTAL PROCEDURES
Cell Culture, Transfection, Infection and Treatment
Immortalized AMPK α−/− (a gift from Dr. B. Violett), RagA:B−/− (Kim et al., 2014), Sesn2−/− and WT MEF (Budanov and Karin, 2008), H1299, U2OS, A549, H460, and HEK293T cell were cultured in highglucose DMEM containing 10% FBS and penicillin/streptomycin. MCF10A cells were cultured in DMEM/F12 medium supplemented with 5% serum, 10µg/ml insulin, 20ng/ml EGF, 100ng/ml cholera toxin, 0.5 µg/ml hydrocortisone. All transfections were performed with Lipofectamine and Plus reagents (Life Technologies) and infections with lentiviral vectors were performed as described (Budanov and Karin, 2008). The treatments with 2DG, doxorubicin, tunicamycin and rotenone were performed for 10 hrs, and with AA free medium for 50 min.
Cell Lysis, Immunoprecipitation and Immunoblot Analyses
For immunoblot analysis cells were lysed in RIPA-SDS buffer and for immunoprecipitation cells were lysed in 0.3% NP40 or 0.3%CHAPS buffer as described in (Budanov and Karin, 2008). The lysates were incubated with the mix of indicated antibodies and protein A:G-Sepharose beads for 4 hrs (or alternatively with anti-Flag or anti-HA beads). After centrifugation the beads were washed 4 times with the lysis buffer. The proteins were resolved by SDS-PAGE, transferred onto PVDF membranes and probed with the relevant antibodies. The antibodies used for the experiments: anti-Flag from Sigma, anti- Sesn2 from Proteintech and Santa Cruz, anti-GFP, anti-GAPDH and anti-Actin from Santa Cruz, anti- LAMP2 from Abcam, anti-RagB from Novus Biological, all others from Cell Signaling Inc.
Constructs
HA-Tagged GATOR-expressing plasmids, Flag-Nprl2, HA-GST-RagBQ99L and HA-GST-RagCS75N, Mycp70S6K were from Addgene, HA-p70S6K, pLU, pLU-Flag-Sesn2, pLU-Flag-Sesn1S, pLU-Flag-Sesn1L, pLU-Flag-Sesn2-ΔN,-ΔC and ΔM, pLU-GFP, pLSLPw-shTSC2 were described in (Budanov and Karin, 2008) and shDEPDC5 was described in (Bar-Peled et al., 2013). The sequence for shSesn2 is 5’- GAAGACCCTACTTTCGGAT-3’ and for Seh1L is GAATCTATGAGGCACCAGATG. GST-Sesn2 and GST-GFP were obtained by inserting Sesn2 or GFP open reading frames into pGEX-2T plasmid.
Protein Purification from Bacteria
BL21 cells were transfected with GST-Sesn2 plasmid and bacterial culture was grown under 37°C till reached OD=0.4, induced with IPTG (1mM) and incubated 4hrs 27°C under extensive shaking. The bacteria were collected by centrifugation and lysed in the NETN buffer (20mM Tris pH=8, 100mM NaCl, 1mM EDTA, 5mM PMSF, protease/phosphatase inhibitors, 0.5% NP40). GST-Sesn2 was incubated with glutathione sepharose 4B (GE Healthcare) for 4hrs, eluted with 20mM reduced glutathione and dialyzed against PBS.
Immunocytochemistry
Cells were plated on coverslips, washed and fixed with 4% paraformaldehyde. Cells were permeabilized with 0.3% Triton X-100 and incubated with primary antibodies overnight. After 3 washes with PBS cells were incubated with Alexa Fluor-conjugated secondary antibodies (Invitrogen) and analyzed on Zeiss LSM700 confocal microscope.
Cell Size and Cell Cycle Examination
Cell size and cell cycle were determined by flow cytometry as described in (Budanov and Karin, 2008).
Statistical Analysis
Statistical analysis was performed with GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA), using Student's t test and one-way ANOVA. Statistical significance was defined as p < 0.05. Results are presented as mean ± standard deviation.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. B. Viollet for AMPK α−/− MEFs and L. F. Povirk for assistance with the GDT/GTP experiment. We thank R. Moran, N. Cruickshanks and A. Ivanov for reading and commenting on the manuscript and Nadushka Pryadilova for everyday support. This work was supported by NIH RO1 CA172660 to A.B and the superfund research program P42 ES010337 to M.K.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
AUTHOR CONTRIBUTIONS
A.P. analyzed mTOR regulation by Sestrins; A.N. studies Sestrin-GATOR interactions; B.D. examined mTOR localization; K.A. analyzed GDP/GTP loading; W.W. performed MS analysis; YC.K. generated RagA:B-deficient cells; KL.G. and M.K. contributed to MS analysis and manuscript preparation; A.V.B. designed the experiments and wrote the paper.
SUPPLEMENTAL INFORMATION
Supplemental Information contains Supplemental Experimental Procedures and two figures and can be found with this article online at
REFERENCES
- Algret R, Fernandez-Martinez J, Shi Y, Kim SJ, Pellarin R, Cimermancic P, Cochet E, Sali A, Chait BT, Rout MP, et al. Molecular architecture and function of the SEA complex, a modulator of the TORC1 pathway. Molecular & cellular proteomics: MCP. 2014 doi: 10.1074/mcp.M114.039388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SH, Sung SH, Oh SY, Lim JM, Lee SK, Park YN, Lee HE, Kang D, Rhee SG. Sestrins activate Nrf2 by promoting p62-dependent autophagic degradation of Keap1 and prevent oxidative liver damage. Cell metabolism. 2013;17:73–84. doi: 10.1016/j.cmet.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Bar-Peled L, Chantranupong L, Cherniack AD, Chen WW, Ottina KA, Grabiner BC, Spear ED, Carter SL, Meyerson M, Sabatini DM. A Tumor suppressor complex with GAP activity for the Rag GTPases that signal amino acid sufficiency to mTORC1. Science. 2013;340:1100–1106. doi: 10.1126/science.1232044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Peled L, Sabatini DM. Regulation of mTORC1 by amino acids. Trends in cell biology. 2014 doi: 10.1016/j.tcb.2014.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Sahra I, Dirat B, Laurent K, Puissant A, Auberger P, Budanov A, Tanti JF, Bost F. Sestrin2 integrates Akt and mTOR signaling to protect cells against energetic stress-induced death. Cell death and differentiation. 2013;20:611–619. doi: 10.1038/cdd.2012.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov AV. Stress-responsive sestrins link p53 with redox regulation and mammalian target of rapamycin signaling. Antioxidants & redox signaling. 2011;15:1679–1690. doi: 10.1089/ars.2010.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov AV, Karin M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell. 2008;134:451–460. doi: 10.1016/j.cell.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov AV, Lee JH, Karin M. Stressin' Sestrins take an aging fight. EMBO Mol Med. 2010;2:388–400. doi: 10.1002/emmm.201000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budanov AV, Sablina AA, Feinstein E, Koonin EV, Chumakov PM. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science. 2004;304:596–600. doi: 10.1126/science.1095569. [DOI] [PubMed] [Google Scholar]
- Budanov AV, Shoshani T, Faerman A, Zelin E, Kamer I, Kalinski H, Gorodin S, Fishman A, Chajut A, Einat P, et al. Identification of a novel stress-responsive gene Hi95 involved in regulation of cell viability. Oncogene. 2002;21:6017–6031. doi: 10.1038/sj.onc.1205877. [DOI] [PubMed] [Google Scholar]
- Chantranupong L, Wolfson RL, Orozco JM, Saxton RA, Scaria SM, Bar-Peled L, Spooner E, Isasa M, Gygi SP, Sabatini DM. The Sestrins Interact with GATOR2 to Negatively Regulate the Amino-Acid-Sensing Pathway Upstream of mTORC1. Cell reports. 2014 doi: 10.1016/j.celrep.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dann SG, Selvaraj A, Thomas G. mTOR Complex1-S6K1 signaling: at the crossroads of obesity, diabetes and cancer. Trends in molecular medicine. 2007;13:252–259. doi: 10.1016/j.molmed.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Dibble CC, Manning BD. Signal integration by mTORC1 coordinates nutrient input with biosynthetic output. Nature cell biology. 2013;15:555–564. doi: 10.1038/ncb2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Efeyan A, Zoncu R, Chang S, Gumper I, Snitkin H, Wolfson RL, Kirak O, Sabatini DD, Sabatini DM. Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature. 2013;493:679–683. doi: 10.1038/nature11745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE, Shaw RJ. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Molecular cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie DG, Ross FA, Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nature reviews Molecular cell biology. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay N, Sonenberg N. Upstream and downstream of mTOR. Genes & development. 2004;18:1926–1945. doi: 10.1101/gad.1212704. [DOI] [PubMed] [Google Scholar]
- Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. doi: 10.1016/s0092-8674(03)00929-2. [DOI] [PubMed] [Google Scholar]
- Kalender A, Selvaraj A, Kim SY, Gulati P, Brule S, Viollet B, Kemp BE, Bardeesy N, Dennis P, Schlager JJ, et al. Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPasedependent manner. Cell metabolism. 2010;11:390–401. doi: 10.1016/j.cmet.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Park HW, Sciarretta S, Mo JS, Jewell JL, Russell RC, Wu X, Sadoshima J, Guan KL. Rag GTPases are cardioprotective by regulating lysosomal function. Nature communications. 2014;5:4241. doi: 10.1038/ncomms5241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Karin M. Sestrins orchestrate cellular metabolism to attenuate aging. Cell metabolism. 2013;18:792–801. doi: 10.1016/j.cmet.2013.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Park EJ, Birse R, Kim TE, Perkins GA, Ocorr K, Ellisman MH, Bodmer R, Bier E, et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science. 2009;327:1223–1228. doi: 10.1126/science.1182228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Budanov AV, Talukdar S, Park EJ, Park HL, Park HW, Bandyopadhyay G, Li N, Aghajan M, Jang I, et al. Maintenance of metabolic homeostasis by sestrin2 and sestrin3. Cell metabolism. 2012;16:311–321. doi: 10.1016/j.cmet.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- Menon S, Dibble CC, Talbott G, Hoxhaj G, Valvezan AJ, Takahashi H, Cantley LC, Manning BD. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156:771–785. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihaylova MM, Shaw RJ. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nature cell biology. 2011;13:1016–1023. doi: 10.1038/ncb2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro N, Rapley J, Avruch J. Amino acids activate mammalian target of rapamycin (mTOR) complex 1 without changing Rag GTPase guanyl nucleotide charging. The Journal of biological chemistry. 2014;289:2658–2674. doi: 10.1074/jbc.M113.528505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng M, Yin N, Li MO. Sestrins Function as Guanine Nucleotide Dissociation Inhibitors for Rag GTPases to Control mTORC1 Signaling. Cell. 2014;159:122–133. doi: 10.1016/j.cell.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sablina AA, Budanov AV, Ilyinskaya GV, Agapova LS, Kravchenko JE, Chumakov PM. The antioxidant function of the p53 tumor suppressor. Nat Med. 2005;11:1306–1313. doi: 10.1038/nm1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science. 2008;320:1496–1501. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullschleger S, Loewith R, Hall MN. TOR signaling in growth and metabolism. Cell. 2006;124:471–484. doi: 10.1016/j.cell.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Xu C, Min J. Structure and function of WD40 domain proteins. Protein & cell. 2011;2:202–214. doi: 10.1007/s13238-011-1018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.