Abstract
Background
We have reported that proBNP1-108 circulates and is processed to mature BNP1-32 in human blood. Building on these findings, we sought to determine whether proBNP1-108 processed forms in normal circulation are biologically active and stimulate cGMP, and whether proBNP1-108 processing and activity are altered in human heart failure (HF) compared to normal. Since BNP1-32 is deficient while proBNP1-108 is abundant in HF, we hypothesize that proBNP1-108 processing and degradation are impaired in HF patients ex vivo.
Methods and Results
We measured circulating molecular forms including BNP1-32, proBNP1-108, and NT-proBNP and all were significantly higher in HF patients compared to normals. Fresh serum samples from normals or HF patients were incubated with or without exogenous non-glycosylated proBNP1-108 tagged with 6 C-terminal Histidines to facilitate peptide isolation. His-tag ProBNP1-108 was efficiently processed into BNP1-32/3-32 at 5 min in normal serum, persisted for 15 min, then disappeared. Delayed processing of proBNP1-108 was observed in HF samples and the degradation pattern differed depending on LV function. The 5 min processed forms from both normal and HF serums were active and generated cGMP via GC-A receptors, however the 180 min samples were not active. The proBNP1-108 processing enzyme corin and BNP degrading enzyme DPPIV were reduced in HF versus normal, perhaps contributing to differential BNP metabolism in HF.
Conclusions
Exogenous proBNP1-108 is processed into active BNP1-32 and ultimately degraded in normal circulation. The processing and degradation of BNP molecular forms was altered but complete in HF which may contribute the pathophysiology of HF.
Keywords: natriuretic peptide, circulation, enzymes, heart failure
The endogenous cardiac hormone, B-type natriuretic peptide (BNP), possesses cardiovascular and renal protective actions through guanylyl cyclase receptors (GC)-A.1 In human heart failure (HF), BNP plays important roles in reducing preload to the heart by natriuresis and vasodilatation and in protecting against cardiac remodeling, especially prior to the activation of the renin-angiotensin-aldosterone system. The conventional concept is that BNP is produced in cardiac cells as proBNP1-108 and is secreted into the circulation as a mature peptide. After signal peptides cleavage from preproBNP1-134, the proBNP1-108 is cleaved by proteases to active states.2 ProBNP1-108 is cleaved into NT-proBNP1-76 and BNP1-32 by corin3 and furin.4 The active BNP1-32 can then be degraded to BNP3-32 by Dipeptidyl peptidase-4 (DPPIV),5, 6 to BNP5-32 by neutral endopeptidase (=neprilysin, NEP),7 and/or to smaller degradation peptides by other enzymes including insulin degrading enzyme (IDE).8–10 BNP3-32 has cGMP generating capabilities in human cell lines,11 however, has reduced cardiorenal actions compared to BNP1-32 in vivo in normal canines.6 Thus, it would be important to understand the patterns of BNP molecular forms and their activity in physiological versus pathophysiological states.
A hallmark of HF is the enhanced production of BNP from the heart with increased circulating BNP molecular forms, such as BNP and NT-proBNP, which are specific biomarkers for diagnosis and prognosis in human HF. However, the biological significance of the elevation in circulating BNP has raised questions regarding the BNP system in HF.12 We have reported that despite an elevation in immunoreactive BNP1-32, the active form of BNP, as measured by point-of-care testing in subjects with HF and New York Heart Association (NYHA) Class IV symptoms, quantitative mass spectral analysis demonstrated an absence of biologically active BNP1-32.13 Recent investigations have demonstrated reduced biological activities with other molecular forms of BNP, which contribute to the increased BNP immunoreactivity.11, 14 Also, we reported that in HF much of plasma BNP immunoreactivity measured by commonly used assays is due to altered circulating molecular forms with reduced cGMP-activating properties.13, 15 Studies suggest that the major non-biologically active immunoreactive BNP form in plasma of HF patients is proBNP1-108,16 however, we have recently reported that proBNP1-108 is present in the circulation of normal humans17, and that circulating proBNP1-108 predicts adverse cardiovascular outcomes in the general population.18
The impairment in processing of proBNP1-108 into active BNP1-32 may play a key role in HF. To better understand the role of proBNP1-108 processing and activation in normal and HF circulation, we examined circulating BNP molecular forms as well as the timing of processing of proBNP1-108 in normal and HF circulation ex vivo, then examined any processed forms for biologically activity. We hypothesized that circulating proBNP1-108 levels would be high and processing of proBNP1-108 would be impaired in HF, by either failure to process, delayed processing, or processing to inactive forms. We also hypothesized that proBNP1-108 processing enzyme corin and BNP1-32 degrading enzyme DPPIV circulating levels would be altered in HF.
Methods
All human experimental protocols used in the current study were approved by the Institutional Review Board at Mayo Clinic.
Study Population and Blood Sampling
Studies were performed on blood samples from healthy residents (=normals) of Olmsted County, Minnesota without cardiovascular, renal, pulmonary disease or diabetes and patients with HF, NYHA Class II-IV which were obtained at admission to the emergency room or clinic. Samples were collected, immediately stored on ice and examined, or stored at −80° until measured.
Immunoprecipitation (IMPT) and Western Blot (WB) of Histidine-tagged proBNP1-108 in Human Serum
Fresh serum samples, with or without proBNP1-108 added, were incubated for varying time points up to 180 min at 37°C. His-tag proBNP1-108 (non-glycosylated form, Abcam, MA) is a recombinant fusion protein with 6 Histidines added to the C-terminus (Figure 1A). Following incubation, His-tag proBNP1-108 (500 ng/100 ul) was immunoprecipitated using His-tag antibody coated beads (Dynabeads His-tag Isolation & Pulldown, Invitrogen Dynal AS, Norway) for 30 min at 4°C. The beads were washed four times with Binding/Wash Buffer and then His-Elution Buffer was added. For WB analysis, the IMPT beads were loaded onto polyacrylamide gels and transferred onto PVDF membranes. Membranes were blocked and incubated with rabbit polyclonal anti-6xHis antibody (Abcam, 1:5000 dilution) overnight at 4°C. Membranes were washed and incubated with secondary antibody (anti-rabbit antibody, Cell signaling Inc., MA, 1:10000 dilutions) for 1 hour at room temperature (RT). Bands were visualized by ECL Prime (GE Healthcare Life Sciences, NJ) and exposure to X-ray film. Band intensity was quantified using the Fluor-S MultiImager (Bio-Rad, Hercules, CA). Protein bands were trypsin digested and sequenced using Edman sequencing by the Mayo Proteomics Core Facility as previously described.17, 19
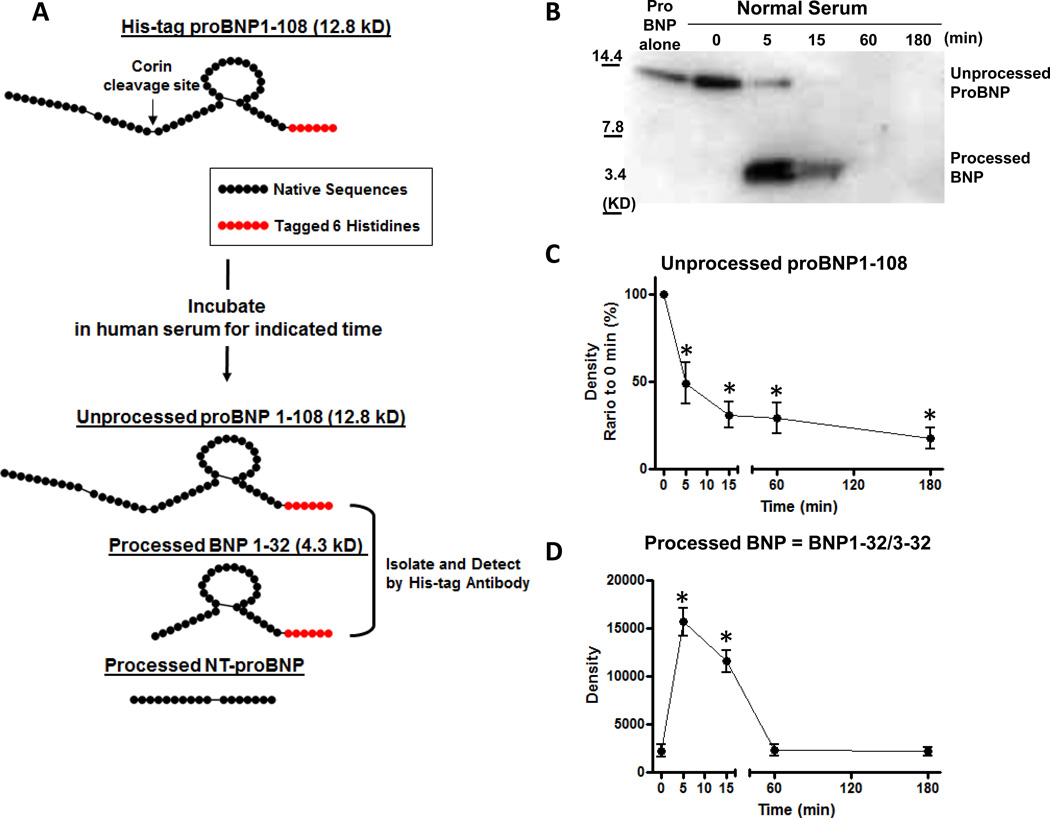
Figure 1. ProBNP1-108 processing and degradation in normal human serum ex vivo.
A: A schema of ex vivo study method. Exogenous proBNP1-108 was incubated in fresh human serum for indicated times at 37°C. Unprocessed or processed proBNP1-108 were isolated by IMPT using His-tag beads, and detected by WB with 6xHis antibody. B: Representative WB for His-tag proBNP1-108 incubated in serum samples from normal subjects for indicated times. C and D: Densitometric analysis of unprocessed proBNP1-108 (C) and processed form (BNP1-32/3-32) (D) at indicated times. Values are mean ± SEM. *p<0.05 vs 0 min, 1-way ANOVA with Bonferroni multiple comparison test.
Assays for ProBNP1-108, NT-proBNP and BNP
Plasma proBNP1-108, NT-proBNP, and BNP levels were determined by immunoassays as previously described.20–23 ProBNP1-108 concentrations were determined using Bio-Rad proBNP1-108 assay with automated two-step sandwich fluorescence immunoassay on the BioPlex™2200 analyzer (Bio-Rad, Hercules, CA). The proBNP1-108 intra- and inter-assay coefficient variations (CVs) using proBNP1-108 EDTA plasma pool were 6.0% and 7.7%, 5.4% and 6.7%, and 2.7% and 5.5%, at 140, 400, and 1900 pg/ml, respectively. The specifications of this assay indicate <0.05% cross-reactivity with BNP1-32 and NT-proBNP. NT-proBNP was measured on the Elecsys™2010 Analyzer (Roche Diagnostics; lower limit of detection, 5 µg/l with inter- and intra-assay CVs 3.1% and 2.5%, respectively) and the cross-reactivity with proBNP1-108 and BNP1-32/3-32 were 30% and <2%, respectively. BNP was measured by the Shionoria assay method (inter- and intra-assay CVs 7.2 ± 1.7 µg/l and 8.0 ± 1.4 µg/ml, respectively) which detects 82% of BNP1-32 and 100% of BNP3-32, and cross-reactivity with proBNP1-108 and NT-proBNP of <2% and <1%, respectively.
Cell Culture and cGMP assay
Human embryonic kidney 293 (HEK293) cells were stably transfected with either human GC-A or GC-B using Lipofectamine (Invitrogen, Grand Island, NY). Transfected cells were maintained in Dulbecco’s Modified Eagle’s medium supplemented with 10% fetal bovine serum, 100U/ml penicillin, 100U/ml streptomycin, and 250ug/ml G418. Cells on 6 well plates were incubated in Hank's balanced salt solution (Invitrogen) containing 20 mmol/l HEPES, 0.1% BSA, and 0.5 mmol/l 3-isobutyl-1-methylxanthine (Sigma-Aldrich Co., St Lois, MO) and treated with BNP1-32 (Phoenix Pharmaceuticals, Inc., CA), proBNP1-108, immunoprecipitated proBNP1-108 or serum treated (processed) proBNP1-108 for 10 min. Treated cells were lysed in 6% TCA and sonicated for 10 min. The samples were ether extracted four times in 4 volumes of ether, dried, and reconstituted in 300 µl cGMP assay buffer. The samples were assayed using a competitive RIA cGMP kit (Perkin-Elmer, Santa Clara, CA) as previously described.24, 25 There is no cross-reactivity with ANP, BNP, CNP, ET, and <0.001% cross-reactivity with cAMP, GMP, GDP, ATP, and GTP.
Corin and DPPIV Assays
Serum corin or DPPIV levels were determined using commercially available enzyme-linked immunosorbent assays (ELISA) (Quantikine, R&D Systems, MN). In brief, samples and standards of recombinant human corin or DPPIV protein were added to microtiter plate wells precoated with anti-human corin or DPPIV antibody and incubated for 2 hours at RT. Each well was washed, then incubated with corin or DPPIV conjugate for 2 hours at RT. Wells were washed and substrate solution was added. After incubation for 30 min at RT, the enzyme reactions were stopped. Human corin or DPPIV concentrations were determined by comparison to the standard curve. Intra-/Inter-assay CVs for corin and DPPIV were 2.9/3.6%, 5.6/8.5%, respectively.
Statistical Analysis
Descriptive statistics are reported as mean ± SEM. Unpaired t-test was performed for comparison between groups. Comparisons within a group in time course processing experiments were made by 1-way ANOVA followed by the Bonferroni multiple comparison posttest analysis when the global test was significant. Two-way ANOVA with main effects of time point and group was used to compare the results between normals and HF, followed by Bonferroni posttests. GraphPad Prism 5 (GraphPad Software, La Jolla, CA) and JMP 10 were used for the above calculations. Statistical significance was accepted as p<0.05.
Results
Circulating BNP Molecular Forms in HF Patients Compared to Age- and Gender-Matched Normal Subjects
We examined circulating levels of proBNP1-108, NT-proBNP and BNP in patients with HF (n=51) compared to normal subjects of similar age and gender, by selecting from the available group of normals using an age cut off of >65 (n=22) (Table 1). All 3 molecular forms, including proBNP1-108, were significantly higher in HF compared to normals, suggesting proBNP1-108 production is enhanced or its processing is impaired in HF.
Table 1.
The difference of parameters between normal and HF
| Normal (n=22) |
Heart Failure (n=51) |
p value | |
|---|---|---|---|
| Age (yo) | 71.2 ± 0.9 | 75.2 ± 1.5 | 0.9477 |
| Gender, % Male | 63.6 | 63 | 0.9423 |
| ProBNP (pg/ml) | 20.5 ± 4.0 | 475.0 ± 49.0 | <0.0001 |
| NT-proBNP (pg/ml) | 85.5 ± 13.2 | 3622.0 ± 589.6 | 0.0002 |
| BNP (pg/ml) | 19.8 ± 3.4 | 373.9 ± 42.3 | <0.0001 |
| Creatinine (mg/dl) | 0.90 ± 0.05 | 1.37 ± 0.07 | <0.0001 |
Data are expressed as mean ± SEM. ProBNP; pro B-type natriuretic peptides, and NT-proBNP; amino-terminal proBNP.
Patient Characteristics for Ex Vivo Study
Table 2 reports characteristics for normal (n=13) and HF patients (n=14) used for the ex vivo proBNP1-108 processing and degradation studies while Supplemental Table 1 reports individual characteristics in HF patients. Most patients were in-hospital and in unstable phase, and had moderate to severe HF.
Table 2.
Normal and HF patient characteristics for ex vivo study
| Normal (n=13) |
Heart failure (n=14) |
p | |
|---|---|---|---|
| Age (yo) | 41.2 ± 2.8 | 74.8 ± 3.1 | <0.0001 |
| Male (%) | 61.5 | 85.7 | 0.15 |
| NYHA II / III / IV | — | 1/8/(5) | — |
| EF (%) | — | 43.9 ± 5.7 | — |
| NT-proBNP (pg/ml) | 38.4 ±5.6 | 8511.9 ± 1885.2 | 0.0006 |
| Cre (pg/ml) | 0.82 ± 0.05 | 1.51 ± 0.12 | <0.0001 |
Data are expressed as mean ± SEM. NYHA, New York Heart Association Class; EF, ejection fraction; NT-proBNP, amino-terminal proBNP; Cre, serum creatinine.
ProBNP1-108 Processing in Human Serum from Normal Subjects Ex Vivo
We first examined proBNP1-108 processing in normal human serum treated with exogenous proBNP1-108. To identify processed BNP forms, 6 Histidines were tagged onto the C-terminus so 6xHis antibody could be used to detect unprocessed proBNP1-108 (predicted MW = 12.8 KD) and/or processed BNP1-32 (predicted MW = 4.3 KD), but not processed NT-proBNP (Figure 1A). As we previously reported,17 proBNP1-108 (approx. 12 KD) was processed at 5 min into a smaller molecular form (approx. 4 KD) in fresh serum from normal subjects (Figure 1B) which was confirmed as BNP1-32 and 3-32 by sequence analysis (Figure 1B). Processing to BNP1-32/BNP3-32 was not observed in samples pretreated with EDTA (Figure 1B, 0 min).
Densitometric Analysis of ProBNP1-108 Processing and Degradation in Fresh Serum from Normal Subjects Ex Vivo
Figure 1B shows a representative WB for the time course processing of proBNP1-108 up to 180 min in normal serum. We examined 5 different time points, 0, 5, 15, 60 and 180 min. Densitometric analysis of these time points showed rapid processing with a decrease of 50% unprocessed proBNP1-108 at 5 min, then disappearing completely over 180 min (Figure 1C). Processed BNP (=BNP1-32/3-32) was significantly increased at 5 min (p<0.0001 by 1-way ANOVA) (Figure 1D), and remained elevated for 15 min, then disappeared after 60 min (Figure 1D).
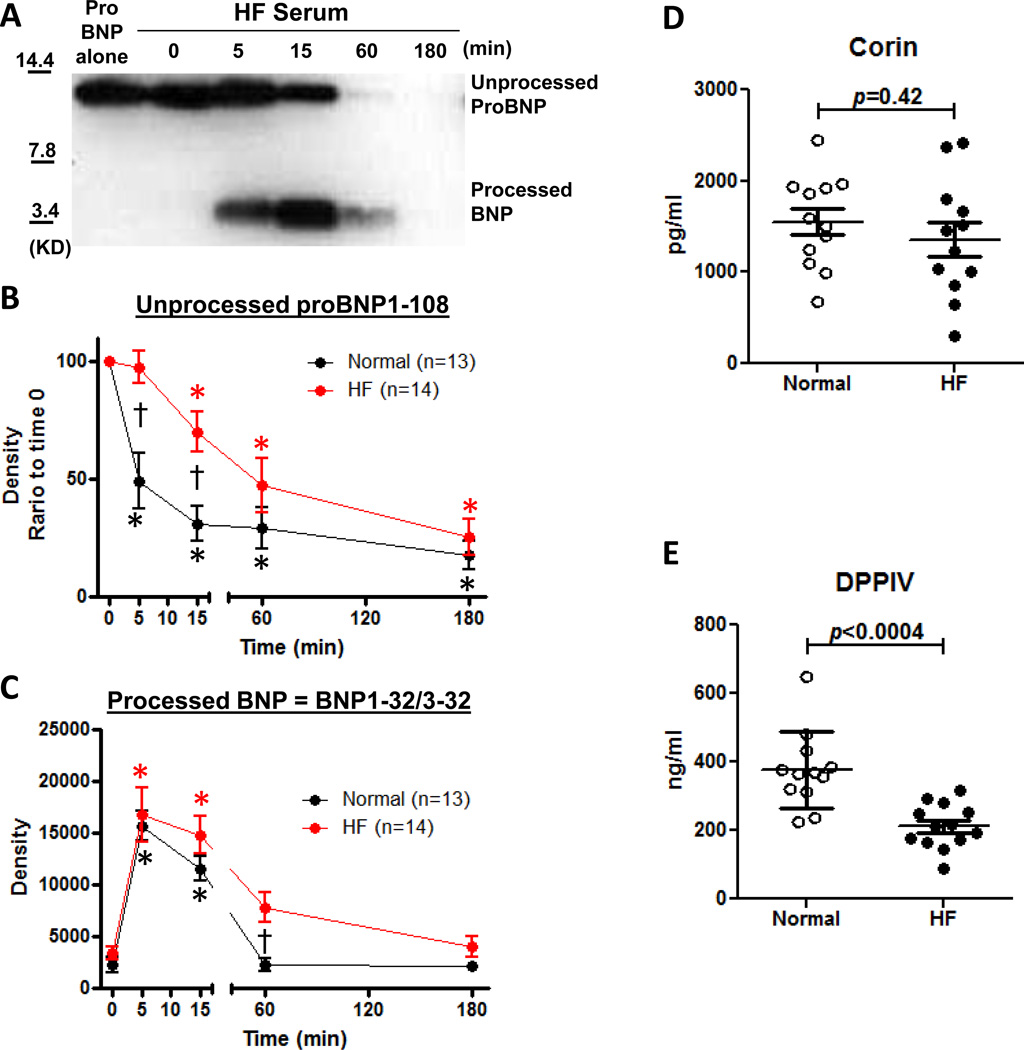
ProBNP1-108 Processing and Degradation Profile in Normal Subjects vs HF Patients
We repeated our analyses to compare proBNP1-108 processing and degradation in HF patient samples compared to normals (Figure 2A, 2B and 2C). The overall time course of unprocessed proBNP1-108 was significantly different between normal subjects and HF patients using 2-way ANOVA (p<0.0001, Figure 2B). Unprocessed proBNP1-108 levels in HF at 5 min and 15 min were significantly higher compared to normals, suggesting proBNP1-108 processing was delayed in HF compared to normal subjects. BNP1-32/3-32 in HF patients at 5 min to 15 min were similar to normal subjects, however, was significantly higher at 60 min than normals, then decreased to levels similar to normal subjects at 180 min (p=0.005 by 2-way ANOVA, Figure 2C), suggesting BNP1-32/3-32 processing was completed but delayed in HF.
Figure 2. Ex vivo ProBNP1-108 processing and degradation in HF and circulating corin and DPPIV levels.
A: Representative WB of proBNP1-108 processing in HF serum. B and C: Densitometric analysis of unprocessed proBNP1-108 (B) and processed form (BNP1-32/3-32) (C) at indicated times from normal vs HF patients. Values are mean ± SEM. *p<0.05 vs 0 min, 1-way ANOVA with Bonferroni multiple comparison test. †p<0.05 vs normals in indicated times, 2-way ANOVA with Bonferroni multiple comparison test. D and E: Circulating corin (D) and DPPIV (E) levels in normal vs HF serum. Values are mean ± SEM.
Corin and DPPIV Levels and in Normal Subjects vs HF Patients
We next examined circulating levels of processing enzyme corin and degrading enzyme DPPIV in our serum samples. In HF patients, circulating corin levels tended to be lower, and DPPIV levels were significantly lower than in healthy subject serum (Figure 2D and 2E), which may contribute to the slower processing of proBNP1-108 and removal of BNP from HF serum.
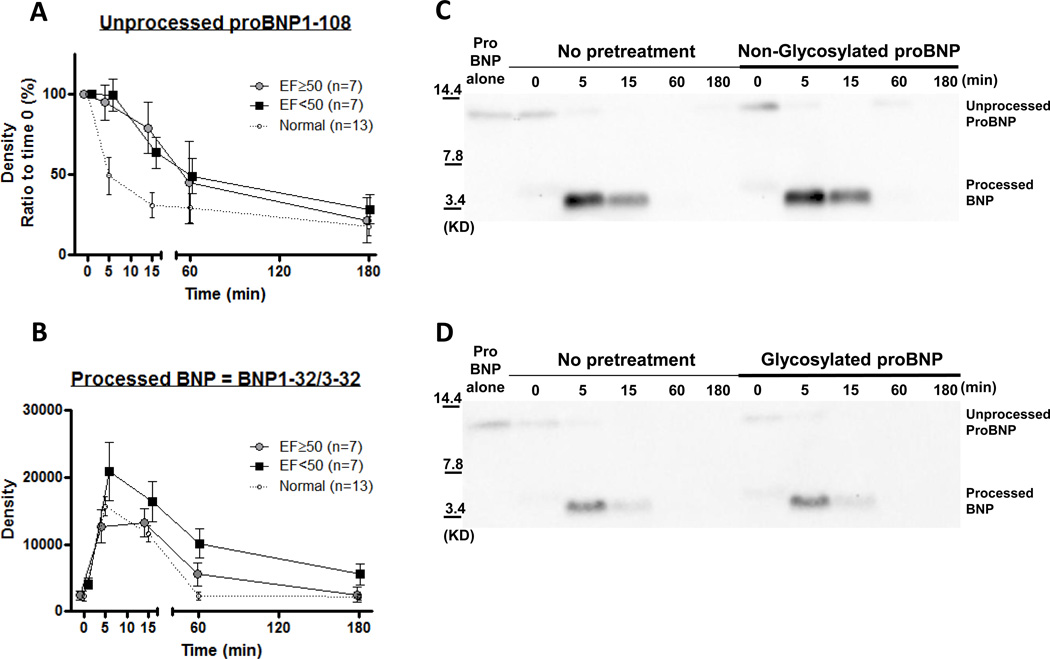
Effect of Ejection Fraction on ProBNP1-108 Processing and Degradation
We performed a sub-analysis of proBNP1-108 processing and degradation, dividing the HF samples into 2 groups; EF<50% or EF≥50% (Figure 3A & B). The group with EF≥50% revealed significantly lower values of BNP1-32/3-32 compared to EF<50% (Figure 3B, p=0.006 by two-way ANOVA), however unprocessed proBNP1-108 was similar in both groups (Figure 3A, p=ns by two-way ANOVA), suggesting the EF≥50% group may have rapid and accelerated degradation of BNP1-32/3-32 within 5 min, whereas the EF<50% group may have delayed degradation of BNP1-32/3-32. Because of the significant age difference between the normal and HF groups, we performed additional sub-analyses, dividing the normal group into two groups by median age (=38) and found no significant difference in either unprocessed or processed forms by two-way ANOVA (data not shown).
Figure 3. ProBNP1-108 processing and degradation based on %EF and high proBNP1-108 concentration.
A and B: Sub-analysis of ex vivo ProBNP1-108 processing and degradation in HF by %EF. Densitometric analysis of unprocessed proBNP1-108 (A) and processed form (BNP1-32/3-32) (B) at indicated times; EF<50% (closed square), EF≥50% (grayed circle), or normals (opened circle with breaking line). Values are mean ± SEM. p values were shown in graphs analyzed by 2-way ANOVA with Bonferroni multiple comparison test. No significant difference between groups at indicated times, 2-way ANOVA with Bonferroni multiple comparison test. C and D: Ex vivo ProBNP1-108 processing and degradation in normals with or without pretreatment with proBNP1-108. Representative WB for His-tag proBNP1-108 incubated in serum samples from normal subjects (n=4) for indicated times. Samples were pretreated with or without 500 pg/ml non-glycosylated proBNP1-108 (C) or glycosylated proBNP1-108 (D) before treatment with His-tag proBNP1-108.
Effect of High Glycosylated and Non-glycosylated ProBNP1-108 Concentrations on ProBNP1-108 Processing and Degradation
To assess whether high circulating levels of proBNP1-108 interferes with His-tag proBNP1-108 processing and degradation ex vivo, we pretreated normal serum with 500pg/ml glycosylated or non-glycosylated proBNP1-108. Neither of these pretreatments affected His-tag proBNP1-108 processing or degradation (Figure 3C and 3D) in normal serum, suggesting the delay in processing and degradation seen in HF is not simply an over production of proBNP1-108, but may reflect a deficiency in enzyme level or activity.
cGMP Activity in Vivo in GC-A or GC-B Expressing HEK293 Cells
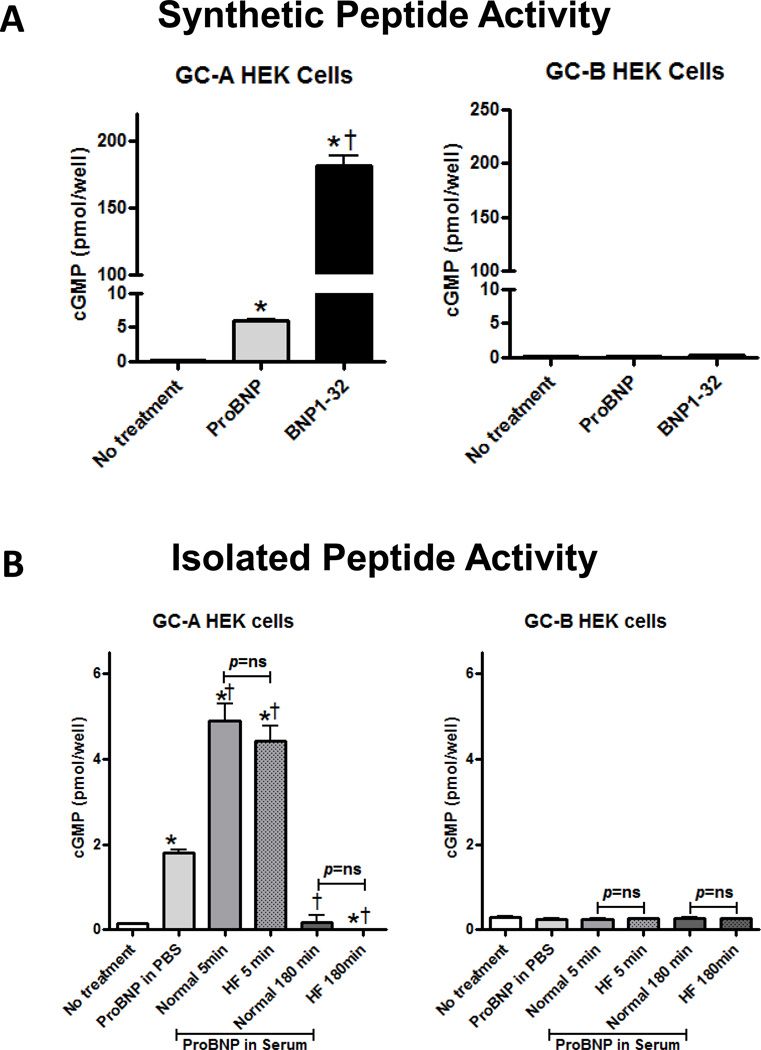
We examined the cGMP generating activity of proBNP1-108 and immunoprecipitated serum processed BNP forms in GC-A or GC-B expressing HEK293 cells. First, to verify activity levels of proBNP1-108 and BNP1-32, cells were treated with equimolar doses (10−8 M) of synthetic BNP1-32 or synthetic proBNP1-108 for 10 min. As we have previously reported, BNP1-32 significantly increased cGMP production (Figure 4A), while proBNP1-108 significantly increased cGMP production, but to only 1/30th the level of BNP1-32 (Figure 4A) in GC-A expressing cells. Neither proBNP1-108 nor BNP1-32 stimulated cGMP production in GC-B cells (Figure 4A).
Figure 4. cGMP response in GC-A or GC-B expressing HEK293 cells.
A: GC-A or GC-B transfected HEK cells were treated with synthetic BNP1-32 and proBNP1-108 at 10−8M concentration for 10 min. B: GC-A or GC-B transfected HEK cells were treated with isolated peptides from 10−8M proBNP1-108 incubated serums as indicated for 10 min. Values are mean ± SEM from 3 samples from normals or HF patients. *p<0.05 vs no treatment. †p<0.05 vs ProBNP1-108 in PBS.
Next, we assessed cGMP production with immunoprecipitated proteins from fresh normal serum treated with proBNP1-108 (10−8 M) at 5 or 180 min, which may contain both unprocessed proBNP1-108 and processed proBNP1-108 (=BNP1-32/3-32) and compared them to immunoprecipitated proBNP1-108 (10−8 M) incubated in phosphate buffered saline (PBS) as a control. ProBNP1-108 incubated in normal serum for 5 min stimulated significantly more cGMP production in GC-A cells as compared to either 180 min serum-treated proBNP1-108 or proBNP1-108 in PBS (Figure 4B). ProBNP1-108 incubated in normal serum for 180 min did not stimulate cGMP production in GC-A cells (Figure 4B). ProBNP1-108 incubated in HF serum for 5 min or 180 min generated cGMP levels similar to normals (Figure 4B). None of the samples stimulated cGMP production in GC-B cells (Figure 4B).
Discussion
The paradox of high circulating BNP levels in patients with HF has been a continuing source of consternation. Recent studies from our laboratory suggested that the increased BNP in HF patients is in the form of the prohormone, proBNP1-108, which has little biological activity, suggesting proBNP1-108 may not be processed in HF.11, 13 This study demonstrates that exogenous proBNP1-108 processing occurs in the ex vivo human circulation of both normal and HF subjects, with degradation of all forms complete within 180 min. Delayed processing of proBNP1-108 was observed in HF compared to normals. Further, accelerated degradation of BNP1-32/3-32 was observed in HF with preserved EF (HFPEF), and delayed degradation of BNP1-32/3-32 was observed in HF with reduced EF (HFREF) relative to normals. The processed forms from both normal and HF samples were biologically active and generated cGMP through GC-A activation. Because of our surprise finding of delayed processing of BNP in HF serum, we examined the proBNP1-108 processing enzyme corin and the BNP degrading enzyme DPPIV and found both were reduced in HF compared to normal serum. Our important new insights into BNP processing and activity in physiologic and HF conditions include delayed but not absent proBNP1-108 processing in HF and retained cGMP generating activity of mature BNP1-32 in HF patient samples together with a reduction in two key BNP modifying enzymes, corin and DPPIV, in HF.
Previously, we reported that proBNP1-108 was processed within 30 min in normal human serum and plasma.17 Extending that study, we show that proBNP1-108 processing occurs very rapidly in normal serum, with 50% of proBNP1-108 processed within the first 5 min and both active and unprocessed forms completely absent by 60 min. This suggests the enzymes necessary to cleave proBNP1-108, generally believed to be furin and/or corin, are present and active in the normal circulation. Indeed, it has been reported that soluble corin is present in the circulation due to shedding of the membrane-bound form of corin,26 and we have demonstrated both here and previously, the presence of corin in normal plasma.17 To date, there are no studies to confirm circulating forms of furin in human serum, and we were unable to detect furin in our serum samples (data not shown), however soluble circulating forms of furin cannot be excluded. It is also possible that other, as yet undetermined enzymes process proBNP1-108 to active forms in normal human serum.
We and others have previously shown the presence of various BNP forms in the normal circulation, consisting of what was believed to be largely inactive forms (proBNP1-108; BNP4-32; BNP5-32).13, 14, 27 Similar to findings by Dickey and colleagues, we show that in fact, proBNP1-108 activates GC-A, although with much less potency than BNP1-32.28 Additionally, we show that active BNP1-32 is produced in normal serum, but is rapidly used or degraded to the aforementioned smaller forms, implying that its presence in the serum is simply too short lived to be detected in normal sample assay situations. This hypothesis is supported by studies by Vanderheyden et al which showed that BNP1-32 is degraded into BNP3-32 by DPPIV in the circulation of either healthy or HF patients.29
We then hypothesized that HF patients would demonstrate reduced processing of proBNP1-108 in HF serum as it has been reported that there may be impaired proBNP1-108 processing in HF30 with decreased soluble corin levels.31,32 Indeed, proBNP1-108 was processed completely and degraded in HF serum, but the processing was delayed compared to normals. There are several possible scenarios to explain our findings of delayed proBNP1-108 processing in HF: 1) reduced or delayed circulating proBNP1-108 processing enzyme presence or activity in HF. This theory is supported by the aforementioned studies of decreased soluble corin in HF. Indeed corin levels trended lower in our HF samples, however the contribution of furin or other as yet unknown proteases cannot be disregarded and additional studies are needed to better understand the aberrant processing; 2) excessive production of proBNP1-108 from the heart overwhelming the proBNP1-108 processing system in HF, though here we show that high concentrations of proBNP1-108 added to our normal samples did not overwhelm the processing system, suggesting that under normal circumstances, the circulating enzymes are capable of dealing with excessive proBNP1-108 production; or 3) increased release of glycosylated vs non-glycosylated proBNP1-108, which is not cleaved by furin or corin, in HF.4, 33 Although our current study shows that addition of either non-glycosylated or glycosylated proBNP1-108 to normal serum does not delay proBNP1-108 processing, we cannot eliminate this possibility, which is supported by studies by Semenov and colleagues demonstrating that non-glycosylated proBNP1-108 made up less than 50% of the total proBNP1-108 of 23 HF patients.34 If indeed too much glycosylated proBNP1-108 is being released into the circulation of HF patients, or there is a defect in removing critical glycosylation for access to cleavage, exogenous non-glycosylated proNPs might be useful as administrative therapy in cardiovascular disease. Additional studies are needed.
To better understand the degradation of BNP forms in HF, we compared the levels of DPPIV in normal vs HF samples. DPPIV degrades BNP1-32 to BNP3-32 with high specificity while it is a relatively poor and slow degrading substrate for NEP.5, 29 The level of DPPIV in normal vs HF patients is not known, although BNP3-32 levels, the product of DPPIV cleavage of BNP1-32, rise significantly in HF patients.29 We found a significantly lower level in our HF samples. The delayed processing of proBNP1-108 to BNP1-32 seen in our HF samples may mean less DPPIV is needed to handle the slower accumulation of BNP1-32. Conversely, DPPIV enzymatic activity was shown to be increased in the serum of HF patients,35 which may suggest higher active but lower circulating levels of DPPIV are present in HF.
Among patients with HF, approximately half have preserved ejection fractions. The clinical manifestations of HFPEF and HFREF are similar, however there are distinct differences in pathophysiology.36 We therefore did a sub-analysis of proBNP1-108 processing and degradation according to %EF. Interestingly, both patients with HFREF and HFPEF had delayed processing of proBNP1-108, however, HFPEF had accelerated (immediate) degradation of BNP1-32/3-32 and HFREF had delayed degradation of BNP1-32/3-32 compared to normals (Figure 3A and 3B), suggesting the BNP degradation system might differ in HFPEF and HFREF patients. BNP1-32 is degraded by several enzymes, including, as mentioned previously, DPPIV, NEP and IDE, and potentially yet unknown enzymes. Therapeutic blockade of BNP1-32 degrading enzymes, especially in HFPEF, may improve BNP1-32 availability and outcomes in this sub-group of HF patients. Interestingly LCZ696, an angiotensin receptor neprilysin inhibitor, improved NT-proBNP levels as well as left atrial volume compared to valsartan, an angiotensin receptor blocker, in the PARAMOUNT study which investigated the actions of LCG696 in patients with HFPEF.37 These favorable effects of LCZ 696 may suggest blocking accelerated degradation of BNP1-32 in HFPEF patients will lead to better outcomes.
Finally, although we saw proBNP1-108 was cleaved in HF samples, it was possible that the cleaved products were inactive. We therefore examined these forms for functional activation, and found that the HF processed BNP forms are functional. This may suggest that early or supplemental NP therapy would be helpful in meeting the demands of an impaired NP processing system in HF.
A limitation to the current study is that it was done in isolated serum, which may differ from the in vivo situation. Our ex vivo study reflects extracellular, circulating proBNP1-108/BNP1-32/BNP3-32 metabolism, and may not reflect the tissue/intracellular mechanism. ProBNP1-108 processing and activity may be influenced by other circulating materials such as blood cells, cytokines, and enzymes present in the circulation or on cells found in whole blood circulation, but not in isolated serum. Further studies of proBNP1-108 processing in animal models are warranted. It is possible that the additional 6 histidine on the C-terminus of proBNP1-108 may change its processing and degradation compared to native proBNP1-108, however these 6 histidines would not affect the comparison between normal and HF as we used the same His-tag proBNP1-108 for both groups. Finally, our population in the normal and HF ex vivo study differed in age because of the difficulty to collect fresh samples from age-matched normals. Our sub-analysis showed age did not have a strong impact on proBNP1-108 processing and degradation, but additional studies that are more closely matched in terms of age are required to confirm these findings.
In conclusion, proBNP1-108 is processed in the circulation of both normal and HF human subjects to biologically active cGMP-activating molecular forms. There is delayed processing and altered degradation of exogenous non-glycosylated proBNP1-108 in HF, providing new information about BNP molecular forms in the circulation of both normal and disease states.
Supplementary Material
Acknowledgments
We would like to thank Dr. Kent R Bailey and Mr. Christopher G Scott for their input on the statistical analysis. We also deeply appreciate of healthy volunteers in our institution and patients who participated in our study.
Sources of Funding
This work was supported by grants from the National Institute of Health (RO1 HL36634 and PO1 HL76611) awarded to Dr. John C. Burnett Jr., an American Heart Association Post-Doctoral Fellowship (10POST3600045) and Scientist Development Grant (12SDG11460017) awarded to Dr. Tomoko Ichiki, and the Mayo Foundation.
Footnotes
Disclosures
None.
References
- 1.van den Akker F. Structural insights into the ligand binding domains of membrane bound guanylyl cyclases and natriuretic peptide receptors. J Mol Biol. 2001;311:923–937. doi: 10.1006/jmbi.2001.4922. [DOI] [PubMed] [Google Scholar]
- 2.Sudoh T, Kangawa K, Minamino N, Matsuo H. A new natriuretic peptide in porcine brain. Nature. 1988;332:78–81. doi: 10.1038/332078a0. [DOI] [PubMed] [Google Scholar]
- 3.Yan W, Wu F, Morser J, Wu Q. Corin, a transmembrane cardiac serine protease, acts as a pro-atrial natriuretic peptide-converting enzyme. Proc Natl Acad Sci U S A. 2000;97:8525–8529. doi: 10.1073/pnas.150149097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Semenov AG, Tamm NN, Seferian KR, Postnikov AB, Karpova NS, Serebryanaya DV, Koshkina EV, Krasnoselsky MI, Katrukha AG. Processing of pro-b-type natriuretic peptide: Furin and corin as candidate convertases. Clin Chem. 2010;56:1166–1176. doi: 10.1373/clinchem.2010.143883. [DOI] [PubMed] [Google Scholar]
- 5.Brandt I, Lambeir AM, Ketelslegers JM, Vanderheyden M, Scharpe S, De Meester I. Dipeptidyl-peptidase iv converts intact b-type natriuretic peptide into its des-serpro form. Clin Chem. 2006;52:82–87. doi: 10.1373/clinchem.2005.057638. [DOI] [PubMed] [Google Scholar]
- 6.Boerrigter G, Costello-Boerrigter LC, Harty GJ, Lapp H, Burnett JC., Jr Des-serine-proline brain natriuretic peptide 3-32 in cardiorenal regulation. Am J Physiol Regul Integr Comp Physiol. 2007;292:R897–R901. doi: 10.1152/ajpregu.00569.2006. [DOI] [PubMed] [Google Scholar]
- 7.Pankow K, Wang Y, Gembardt F, Krause E, Sun X, Krause G, Schultheiss HP, Siems WE, Walther T. Successive action of meprin a and neprilysin catabolizes b-type natriuretic peptide. Circ Res. 2007;101:875–882. doi: 10.1161/CIRCRESAHA.107.153585. [DOI] [PubMed] [Google Scholar]
- 8.Ralat LA, Guo Q, Ren M, Funke T, Dickey DM, Potter LR, Tang WJ. Insulin-degrading enzyme modulates the natriuretic peptide-mediated signaling response. The Journal of biological chemistry. 2011;286:4670–4679. doi: 10.1074/jbc.M110.173252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toll L, Brandt SR, Olsen CM, Judd AK, Almquist RG. Isolation and characterization of a new atrial peptide-degrading enzyme from bovine kidney. Biochem Biophys Res Commun. 1991;175:886–893. doi: 10.1016/0006-291x(91)91648-v. [DOI] [PubMed] [Google Scholar]
- 10.Muller D, Schulze C, Baumeister H, Buck F, Richter D. Rat insulin-degrading enzyme: Cleavage pattern of the natriuretic peptide hormones anp, bnp, and cnp revealed by hplc and mass spectrometry. Biochemistry. 1992;31:11138–11143. doi: 10.1021/bi00160a026. [DOI] [PubMed] [Google Scholar]
- 11.Heublein DM, Huntley BK, Boerrigter G, Cataliotti A, Sandberg SM, Redfield MM, Burnett JC., Jr Immunoreactivity and guanosine 3',5'-cyclic monophosphate activating actions of various molecular forms of human b-type natriuretic peptide. Hypertension. 2007;49:1114–1119. doi: 10.1161/HYPERTENSIONAHA.106.081083. [DOI] [PubMed] [Google Scholar]
- 12.Ichiki T, Huntley BK, Burnett JC., Jr Bnp molecular forms and processing by the cardiac serine protease corin. Adv Clin Chem. 2013;61:1–31. doi: 10.1016/b978-0-12-407680-8.00001-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkridge AM, Heublein DM, Bergen HR, 3rd, Cataliotti A, Burnett JC, Jr, Muddiman DC. Quantitative mass spectral evidence for the absence of circulating brain natriuretic peptide (bnp-32) in severe human heart failure. Proc Natl Acad Sci U S A. 2005;102:17442–17447. doi: 10.1073/pnas.0508782102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liang F, O'Rear J, Schellenberger U, Tai L, Lasecki M, Schreiner GF, Apple FS, Maisel AS, Pollitt NS, Protter AA. Evidence for functional heterogeneity of circulating b-type natriuretic peptide. J Am Coll Cardiol. 2007;49:1071–1078. doi: 10.1016/j.jacc.2006.10.063. [DOI] [PubMed] [Google Scholar]
- 15.Andrews GL, Shuford CM, Burnett JC, Jr, Hawkridge AM, Muddiman DC. Coupling of a vented column with splitless nanorplc-esi-ms for the improved separation and detection of brain natriuretic peptide-32 and its proteolytic peptides. J Chromatogr B Analyt Technol Biomed Life Sci. 2009;877:948–954. doi: 10.1016/j.jchromb.2009.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller WL, Phelps MA, Wood CM, Schellenberger U, Van Le A, Perichon R, Jaffe AS. Comparison of mass spectrometry and clinical assay measurements of circulating fragments of b-type natriuretic peptide in patients with chronic heart failure. Circ Heart Fail. 2011;4:355–360. doi: 10.1161/CIRCHEARTFAILURE.110.960260. [DOI] [PubMed] [Google Scholar]
- 17.Ichiki T, Huntley BK, Heublein DM, Sandberg SM, McKie PM, Martin FL, Jougasaki M, Burnett JC., Jr Corin is present in the normal human heart, kidney, and blood, with pro-b-type natriuretic peptide processing in the circulation. Clin Chem. 2011;57:40–47. doi: 10.1373/clinchem.2010.153908. [DOI] [PubMed] [Google Scholar]
- 18.Macheret F, Boerrigter G, McKie PM, Costello-Boerrigter LC, Lahr BD, Heublein DM, Sandberg SM, Ikeda Y, Cataliotti A, Bailey KR, Rodeheffer RJ, Burnett JC., Jr Pro-b-type natriuretic peptide1-108 circulates in the general community: Plasma determinants and detection of left ventricular systolic dysfunction. J Am Coll Cardiol. 2010;57:1386–1395. doi: 10.1016/j.jacc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steen H, Mann M. The abc's (and xyz's) of peptide sequencing. Nat Rev Mol Cell Biol. 2004;5:699–711. doi: 10.1038/nrm1468. [DOI] [PubMed] [Google Scholar]
- 20.Miller WL, Burnett JC, Jr, Hartman KA, Hodge DO, Giuliani I, Minard F, Larue C, Jaffe AS. Role for precursor pro-b type natriuretic peptide in assessing response to therapy and prognosis in patients with decompensated heart failure treated with nesiritide. Clin Chim Acta. 2009;406:119–123. doi: 10.1016/j.cca.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Burnett JC, Jr, Kao PC, Hu DC, Heser DW, Heublein D, Granger JP, Opgenorth TJ, Reeder GS. Atrial natriuretic peptide elevation in congestive heart failure in the human. Science. 1986;231:1145–1147. doi: 10.1126/science.2935937. [DOI] [PubMed] [Google Scholar]
- 22.Macheret F, Boerrigter G, McKie P, Costello-Boerrigter L, Lahr B, Heublein D, Sandberg S, Ikeda Y, Cataliotti A, Bailey K, Rodeheffer R, Burnett JC., Jr Pro-b-type natriuretic peptide(1-108) circulates in the general community: Plasma determinants and detection of left ventricular dysfunction. Journal of the American College of Cardiology. 2011;57:1386–1395. doi: 10.1016/j.jacc.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Costello-Boerrigter L, Lapp H, Boerrigter G, Lerman A, Bufe A, Macheret F, Heublein D, Larue C, Burnett J. Secretion of prohormone of b-type natriuretic peptide, probnp1-108, is increased in heart failure. JACC: Heart Failure. 2013;1:207–212. doi: 10.1016/j.jchf.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin FL, Sangaralingham SJ, Huntley BK, McKie PM, Ichiki T, Chen HH, Korinek J, Harders GE, Burnett JC., Jr Cd-np: A novel engineered dual guanylyl cyclase activator with anti-fibrotic actions in the heart. PLOS ONE. 2012;7:e52422. doi: 10.1371/journal.pone.0052422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huntley BK, Ichiki T, Sangaralingham SJ, Chen HH, Burnett JC., Jr B-type natriuretic peptide and extracellular matrix protein interactions in human cardiac fibroblasts. J Cell Physiol. 2009;225:251–255. doi: 10.1002/jcp.22253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jiang J, Wu S, Wang W, Chen S, Peng J, Zhang X, Wu Q. Ectodomain shedding and autocleavage of the cardiac membrane protease corin. J Biol Chem. 2011;286:10066–10072. doi: 10.1074/jbc.M110.185082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seferian KR, Tamm NN, Semenov AG, Mukharyamova KS, Tolstaya AA, Koshkina EV, Kara AN, Krasnoselsky MI, Apple FS, Esakova TV, Filatov VL, Katrukha AG. The brain natriuretic peptide (bnp) precursor is the major immunoreactive form of bnp in patients with heart failure. Clin Chem. 2007;53:866–873. doi: 10.1373/clinchem.2006.076141. [DOI] [PubMed] [Google Scholar]
- 28.Dickey DM, Potter LR. Probnp(1-108) is resistant to degradation and activates guanylyl cyclase-a with reduced potency. Clin Chem. 2011;57:1272–1278. doi: 10.1373/clinchem.2011.169151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vanderheyden M, Bartunek J, Goethals M, Verstreken S, Lambeir AM, De Meester I, Scharpe S. Dipeptidyl-peptidase iv and b-type natriuretic peptide. From bench to bedside. Clin Chem Lab Med. 2009;47:248–252. doi: 10.1515/CCLM.2009.065. [DOI] [PubMed] [Google Scholar]
- 30.Rame JE, Tam SW, McNamara D, Worcel M, Sabolinski ML, Wu AH, Dries DL. Dysfunctional corin i555(p568) allele is associated with impaired brain natriuretic peptide processing and adverse outcomes in blacks with systolic heart failure: Results from the genetic risk assessment in heart failure substudy. Circ Heart Fail. 2009;2:541–548. doi: 10.1161/CIRCHEARTFAILURE.109.866822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ibebuogu UN, Gladysheva IP, Houng AK, Reed GL. Decompensated heart failure is associated with reduced corin levels and decreased cleavage of pro-atrial natriuretic peptide. Circ Heart Fail. 2011;4:114–120. doi: 10.1161/CIRCHEARTFAILURE.109.895581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dong N, Chen S, Yang J, He L, Liu P, Zheng D, Li L, Zhou Y, Ruan C, Plow E, Wu Q. Plasma soluble corin in patients with heart failure. Circ Heart Fail. 2010;3:207–211. doi: 10.1161/CIRCHEARTFAILURE.109.903849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Peng J, Jiang J, Wang W, Qi X, Sun XL, Wu Q. Glycosylation and processing of pro-b-type natriuretic peptide in cardiomyocytes. Biochem Biophys Res Commun. 2011;411:593–598. doi: 10.1016/j.bbrc.2011.06.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Semenov AG, Tamm NN, Seferian KR, Postnikov AB, Karpova NS, Serebryanaya DV, Koshkina EV, Krasnoselsky MI, Katrukha AG. Processing of pro-b-type natriuretic peptide: Furin and corin as candidate convertases. Clinical chemistry. 2010;56:1166–1176. doi: 10.1373/clinchem.2010.143883. [DOI] [PubMed] [Google Scholar]
- 35.dos Santos L, Salles TA, Arruda-Junior DF, Campos LC, Pereira AC, Barreto AL, Antonio EL, Mansur AJ, Tucci PJ, Krieger JE, Girardi AC. Circulating dipeptidyl peptidase iv activity correlates with cardiac dysfunction in human and experimental heart failure. Circulation. Heart failure. 2013;6:1029–1038. doi: 10.1161/CIRCHEARTFAILURE.112.000057. [DOI] [PubMed] [Google Scholar]
- 36.Komajda M, Lam CS. Heart failure with preserved ejection fraction: A clinical dilemma. Eur. Heart J. 2014;35:1022–1032. doi: 10.1093/eurheartj/ehu067. [DOI] [PubMed] [Google Scholar]
- 37.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ. The angiotensin receptor neprilysin inhibitor lcz696 in heart failure with preserved ejection fraction: A phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.