Abstract
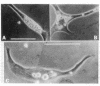
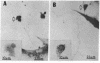
Nerve and muscle cells differentiated morphologically, in cultures of dissociated cells prepared from amphibian neural plate and underlying mesoderm (Xenopus laevis, Nieuwkoop and Faber stage 15). Cultures were grown in a defined medium containing sterile Steinberg's salt solution and 0.1% bovine serum albumin, and maintained for periods up to 5 days.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- HUGHES A. The development of the primary sensory system in Xenopus laevis (Daudin). J Anat. 1957 Jul;91(3):323–338. [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Hayashi H., Takahashi K. Calcium and potassium currents of the membrane of a barnacle muscle fibre in relation to the calcium spike. J Physiol. 1969 Nov;205(1):115–129. doi: 10.1113/jphysiol.1969.sp008955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966 Mar;49(4):793–806. doi: 10.1085/jgp.49.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauschka S. D., Konigsberg I. R. The influence of collagen on the development of muscle clones. Proc Natl Acad Sci U S A. 1966 Jan;55(1):119–126. doi: 10.1073/pnas.55.1.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JONES K. W., ELSDALE T. R. The culture of small aggregates of amphibian embryonic cells in vitro. J Embryol Exp Morphol. 1963 Mar;11:135–154. [PubMed] [Google Scholar]
- Jackson P. A., Messenger E. A., Warner A. E. Differentiation of amphibian embryonic cells in vitro. J Physiol. 1975 Mar;246(2):9P–10P. [PubMed] [Google Scholar]
- Kidokoro Y. Development of action potentials in a clonal rat skeletal muscle cell line. Nat New Biol. 1973 Jan 31;241(109):158–159. doi: 10.1038/newbio241158a0. [DOI] [PubMed] [Google Scholar]
- Sachs H. G., McDonald T. F., DeHaan R. L. Tetrodotoxin sensitivity of cultured embryonic heart cells depends on cell interactions. J Cell Biol. 1973 Jan;56(1):255–258. [PMC free article] [PubMed] [Google Scholar]
- Sperelakis N., Lehmkuhl D. Insensitivity of cultured chick heart cells to autonomic agents and tetrodotoxin. Am J Physiol. 1965 Oct;209(4):693–698. doi: 10.1152/ajplegacy.1965.209.4.693. [DOI] [PubMed] [Google Scholar]
- Spitzer N. C. The ionic basis of the resting potential and a slow depolarizing response in Rohon-Beard neurones of Xenopus tadpoles. J Physiol. 1976 Feb;255(1):105–135. doi: 10.1113/jphysiol.1976.sp011272. [DOI] [PMC free article] [PubMed] [Google Scholar]