Abstract
Background
In preclinical stroke models, improvement in motor performance is associated with reorganization of cortical motor maps. However, the temporal relationship between performance gains and map plasticity is not clear.
Objective
This study was designed to assess the effects of rehabilitative training on the temporal dynamics of behavioral and neurophysiological endpoints in a rat model of focal cortical infarct.
Methods
Eight days after an ischemic infarct in primary motor cortex, adult rats received either rehabilitative training or were allowed to recover spontaneously. Motor performance and movement quality of the paretic forelimb was assessed on a skilled reach task. Intracortical microstimulation mapping procedures were conducted to assess the topography of spared forelimb representations either at the end of training (post-lesion day 18) or at the end of a three week follow-up period (post-lesion day 38).
Results
Rats receiving rehabilitative training demonstrated more rapid improvement in motor performance and movement quality during the training period that persisted through the follow-up period. Motor maps in both groups were unusually small on post-lesion day 18. On post-lesion day 38, forelimb motor maps in the rehabilitative training group were significantly enlarged compared with the no-rehab group, and within the range of normal maps.
Conclusions
Post-infarct rehabilitative training rapidly improves motor performance and movement quality after an ischemic infarct in motor cortex. However, training-induced motor improvements are not reflected in spared motor maps until substantially later, suggesting that early motor training after stroke can help shape the evolving post-stroke neural network.
Keywords: cerebral cortex, neuronal plasticity, stroke, rats, Long-Evans, rehabilitation
Introduction
A substantial body of research directed towards the resolution of motor impairments after stroke focuses on therapeutic interventions to optimize the expression of adaptive neural plasticity.1,2 Plasticity phenomena in spared tissue have been examined at many levels of analysis, from altered gene expression to structural reorganization of axonal pathways to reorganization of motor representations. Changes in motor map topography have been particularly relevant in translational studies since they can be examined in clinical populations with noninvasive imaging approaches,3 and can be addressed in more detail in preclinical studies using invasive approaches.4,5
Map expansion is thought to be related to functional restoration, as both develop in parallel during the early weeks to months following cortical injury. However, the temporal relationship of map plasticity and functional improvement is complex, as behavioral gains can plateau prior to expansion of motor maps.6 Many early changes in motor maps may be independent of functional capabilities, presumably related to pathophysiological processes including diaschisis, edema and hyperexcitability. Faced with a complex interplay of early pathophysiological events, spontaneous improvements in motor function, development of compensatory motor strategies and initiation of regenerative processes, understanding the role of rehabilitative interventions in shaping neuroplastic events that ultimately will support recovered performance seems daunting.
To better understand how post-injury motor experience affects map plasticity and behavioral performance, a rat model of ischemic cortical injury was used. An ischemic infarct was directed at the forelimb representation within the primary motor cortex (caudal forelimb area; CFA). Motor maps were derived within the spared territory rostral to the infarct, including the rostral forelimb area (RFA), a motor field with many similarities to premotor cortex in primate species.7 In different groups of rats, motor maps were derived either the day after a 10-day rehabilitative training period, or after a three week follow-up period. Map plasticity was compared to improvements in motor performance and movement kinematics on a skilled reaching task. While a previous study in rats demonstrated reduced forelimb representations in the ipsilesional RFA after a traumatic injury to CFA,8 this study represents the first examination of map changes in this area after an ischemic injury. It is also unique in its demonstration of how motor experience modulates map plasticity beyond the timeframe of rehabilitative training.
Materials and Methods
Subjects and Group Assignments
A total of 25 adult, male, Long-Evans hooded rats (Harlan; 4–5 month old, 300–400g) was used in accordance with National Institutes of Health regulations, and approved by the Institutional Animal Care and Use Committee of the University of Kansas Medical Center. Rats were singly housed in a Plexiglas cage with ad libitum food and water on a 12h:12h light:dark cycle, with ambient temperature maintained at 68–71°F. Animals were randomly assigned to one of four post-lesion groups that varied by survival time (18 or 38 days) and post-injury behavioral experience (rehabilitative training, referred to as “rehab” group, or no rehabilitative training, referred to as “no-rehab” group). Thus, there were four groups: 1) rehab/short-term survival (n=6), 2) rehab/long-term survival (n=7), 3) no-rehab/short-term survival (n=6), 4) no-rehab/long-term survival (n=6). Through post-lesion day (PLD) 18, rats in the two rehab groups received the same pre- and post-operative experiences; rats in the two no-rehab groups also received the same experiences. Therefore these groups were combined as rehab (n=13) and no-rehab (n=12) groups for analyses through PLD 18.
Pre-infarct Behavioral Training and Motor Assessment
Single-pellet retrieval task training
This task has been used in numerous studies after focal cortical infarcts in rodents and non-human primates due to its high sensitivity and reliability.9 High-resolution video recordings were made of training and assessment sessions for subsequent slow-motion and frame-by-frame analysis. Each animal was placed within a Plexiglas reaching box with a 1cm-wide slot. Rats reached through the slot a distance of 2cm to retrieve a single food pellet (45mg, Bioserve) from a horizontal shelf. Forelimb preference was determined for each rat prior to training. A removable wall was inserted into the Plexiglas box to restrict the rat to reach only with the preferred forelimb (the forelimb used for more than 50% of the reaches). Pre-infarct training proceeded for 10 days (60 single-pellet trials/day).
Single-pellet retrieval task assessment
Pre-infarct motor performance was assessed on the day following the 10-day training period. The total number of successful retrievals and total number of reaching attempts (limb advances through reaching slot) were tallied based on 20 single-pellet trials. A successful retrieval required the rats to grasp and transport the food pellet to their mouth. Each trial ended with a successful retrieval or five unsuccessful attempts.
Post-infarct motor performance was assessed on the single-pellet retrieval task on PLD 7, 12, 17 and in the long-term survival groups, every 5 days through PLD 37. Performance was based on a 20-trial session. On assessment days that coincided with rehabilitative training days, assessment trials were conducted prior to rehabilitative training trials.
Kinematic Analysis
In the long-term survival groups, kinematics of forelimb use during the retrieval task were assessed using the Eshkol-Wachmann Movement Notation adapted by Whishaw et al.10 For each trial resulting in a successful retrieval, the quality of forelimb movements was analyzed. Specific movements consisted of pronation, grasp, supinate I (acquiring the pellet), supinate II (retrieving the pellet), and release.11,12 For each movement, a score of 0 was assigned if the movement was normal, 0.5 if the movement was abnormal, but present, or 1 if the movement was absent.
Footfault task
To assess forelimb performance during locomotion, a footfault task was conducted on the day prior to the lesion, PLD 7, 12, 17 and in the long-term survival groups, every 5 days through PLD 37. Each rat was placed onto an elevated grid (57cm × 44cm with 4cm × 4cm grid opening) and allowed to locomote freely for 3 min. A footfault was defined as extension of the forepaw through the grid openings without any of the digits catching on a grid. The number of steps and the number of footfaults made with each forelimb were recorded. Performance was defined as the percentage of footfaults per step made with the forelimb contralateral to the lesion.
Post-Infarct Rehabilitative Training
Rehabilitative training was initiated in the rehab groups on PLD 8. On PLD 8–12, a tray-reaching task was used. This task requires less precise reaching and grasping than the single-pellet retrieval task since rats retrieve pellets for 20 minutes from a tray filled with pellets.13 On PLD 13–17, the single-pellet retrieval task was implemented (60 training trials per day). Rats assigned to the no-rehab groups had a similar number of food pellets available from the floor of the reaching box. Randomly selected videos of motor performance and movement kinematics were independently scored by separate examiners blind to the experimental condition as a reliability check.
Cortical Infarct Procedure
Anesthesia was induced with 3% isoflurane gas followed by ketamine (100mg/kg, IP) and xylazine (5mg/kg, IM). Additional doses of ketamine (20mg/kg IM) were used as needed. Six 0.7-mm diameter holes were drilled over the CFA contralateral to the dominant forelimb at anteroposterior +1.5, +0.5, and −0.5 mm and mediolateral +2.5 and +3.5 mm from bregma.14 To induce cortical ischemia, 0.33 µL of endothelin-1 (ET-1; Bachem Laboratories, 0.3mg/mL) was injected into each hole at a depth of 1.5mm from the cortical surface, through a micropipette (160µm o.d.) attached to a Hamilton syringe using a microsyringe injector (UltraMicro Pump III, World Precision Instruments). Appropriate postoperative care was provided under veterinary supervision.
Post-Infarct Neurophysiological Assessment
Standard intracortical microstimulation (ICMS) techniques were used to derive forelimb movement maps in the cortex rostral to the ischemic lesion.8 Rats in short-term and long-term survival groups underwent an ICMS mapping procedure on PLD 18 and 38, respectively. Rats were anesthetized with ketamine/xylazine and secured in a stereotaxic frame. Anesthesia was maintained throughout the procedure with bolus injections of ketamine (20mg/kg, i.m.) as needed to minimize spontaneous movements and toe pinch reflex. A craniectomy was performed over the frontal cortex. A digital image of the surface vasculature was obtained and imported into a graphics program to guide the placement of the microelectrode on a 250µm grid pattern. A glass microelectrode (tapered to 15–20µm o.d. with beveled tip; impedance = 500–700kΩ) was filled with 3.5M NaCl and connected to a constant-current stimulator (Model BSI-2, BAK Electronics) through a platinum wire. The electrode was lowered to 1700µm (approximately Layer V) using a hydraulic microdrive (Model 650, David Kopf Instruments). The ICMS stimulus consisted of 13 cathodal pulses (200µs each) delivered at 350Hz. Movements and their threshold current levels (maximum = 80µA) were recorded for each stimulation site. ICMS-evoked movements were defined via visual observation by an observer blind to the electrode placement. A second observer, blind to the experimental condition, verified the evoked movement. Distal forelimb movements were defined as visually observable movements of the wrist (extension, flexion, supination, pronation) and/or digits (extension, flexion). Proximal forelimb movements were defined as visually observable movements of the elbow (extension, flexion) and/or shoulder (extension, flexion). Movement representation maps were reconstructed from movement topography and areal extents measured with imaging software (NIH IMAGE vl.61). Rostral, caudal, medial and lateral extents of forelimb representations were recorded based on the boundaries of reconstructed maps (rostral, caudal relative to bregma; medial, lateral relative to midline).
Histology
Immediately following the ICMS mapping procedure, rats were euthanized by an overdose of Buthanasia and perfused transcardially with normal saline followed by 4% paraformaldehyde in 0.1M PBS. Brains were postfixed in 20% glycerol, sectioned coronally (30µm), and stained with cresyl violet. Lesion volume estimation was obtained by the difference of the cortical volume of the injured hemisphere subtracted from that of the intact hemisphere,8 using the Cavalieri method in StereoInvestigator (Microbrightfield, Inc.).
Statistical Analysis
Statistical analyses were performed using JMP v10.0 (SAS Institute, Inc., Cary, NC). Two-way ANOVAs were used to examine the effects of Group, Time, and Group × Time interactions on lesion volume, motor performance, kinematic scores, topography of forelimb motor maps, and ICMS current thresholds. Post-hoc comparisons were performed with Tukey tests when appropriate (α = 0.05).
Results
Histological Results
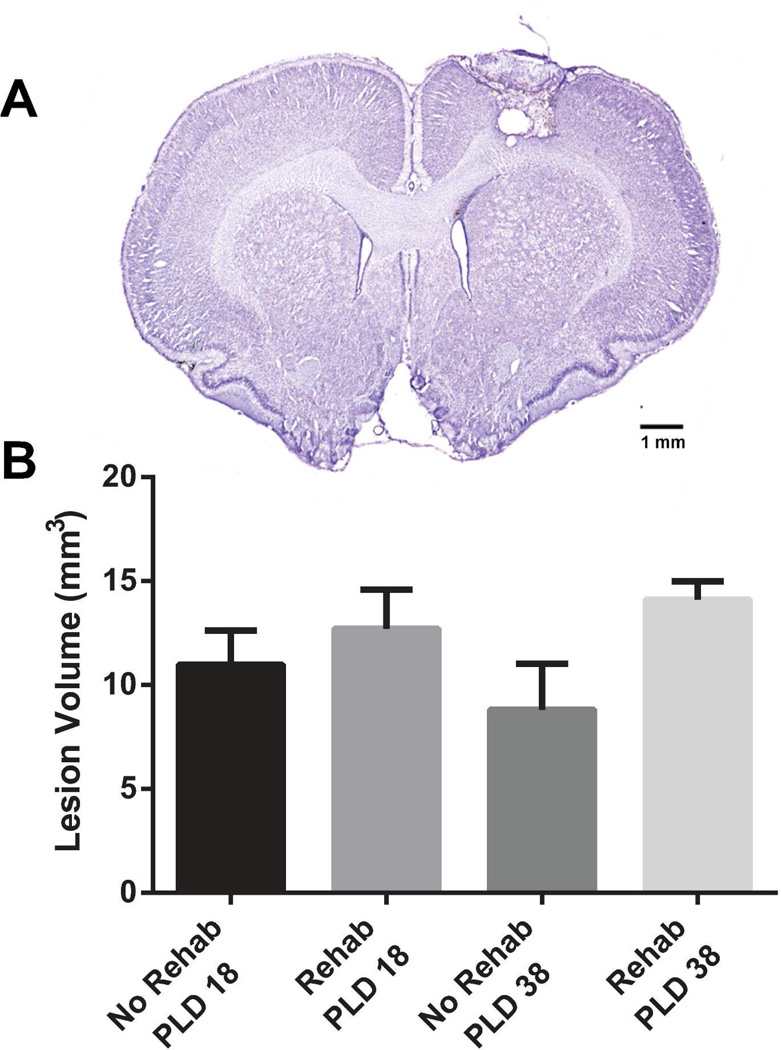
The lesion extended through all cortical layers leaving the underlying white matter intact in all infarcted rats (Fig. 1). No cortical damage was evident at the level of RFA in any of the cases. While the main effect of Group was not statistically significant (F1, 21 = 3.9206; p = 0.0603), mean lesion volume in the rehab group was 36% larger than in the no-rehab group.
Figure 1.
Histological results. (A) Cresyl-violet stained coronal section showing a representative lesion 1.2mm rostral to bregma (at the level of CFA). The lesion infarcted all cortical layers, while largely sparing the underlying white matter. (B) Lesion volume. While the mean lesion volume was somewhat larger in the rehab groups, there was no main effect of Group (p = 0.0654).
Effects of Rehabilitative Training on Motor Behavior
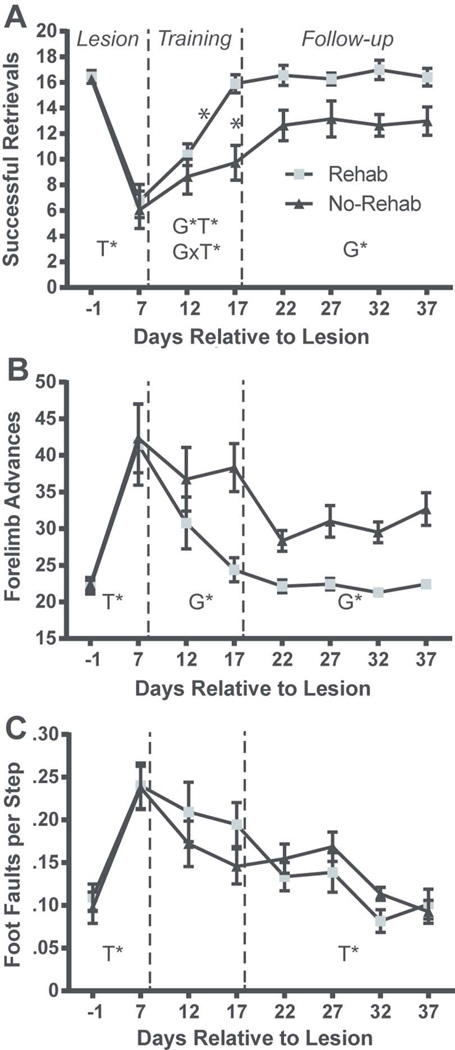
Behavioral results are described separately for three post-lesion phases (Fig. 2): 1) Lesion phase (PLD −1 to PLD 7); 2) Rehabilitative training phase (single pellet retrieval training; PLD 12 to PLD 17); 3) Follow-up phase: (PLD 22 to 37).
Figure 2.
Motor performance results. (A) Mean number of successful retrievals (from a maximum of 20 (± SEM). (B) Mean number of reaching attempts (forelimb advances) (± SEM). (C) Mean number of foot faults per step (± SEM). The lesion resulted in significant impairment in retrievals, forelimb advances and foot faults on PLD 7 in both rehab and no-rehab groups (Lesion phase). The rehab group displayed significantly greater performance gains in retrievals and advances during the rehabilitative training phase (Training) that persisted during the follow-up period (Follow-up phase). Both rehab and no-rehab groups improved in foot-faults at similar rates during the training and follow-up periods. SEM = standard error of the mean; G* = significant Group effect; T* = significant Time effect; G×T* = significant Group × Time interaction.
Successful retrievals
(Fig. 2A). Lesion phase: The analysis revealed a significant effect of Time (F1, 23 = 105.908, p < 0.0001), demonstrating that the lesion resulted in a deficit in both the rehab and no-rehab groups. Rehabilitative training phase: There was a significant effect of Group (F1, 23 = 8.465, p = 0.008), Time (F1, 23 = 20.828, p = 0.0001) and Group × Time interaction (F1, 23 = 9.428, p = 0.005). Post-hoc tests revealed that the rehab group retrieved significantly more pellets on PLD 17, and also compared with both groups on PLD 12. Follow-up phase: There was a significant effect of Group (Group F1, 33 = 6.808, p = 0.024), demonstrating that the benefit of rehabilitative training persisted throughout the follow-up phase.
Reaching attempts (forelimb advances; Fig. 2B)
Lesion phase: Similar to the retrieval analysis, there was a significant effect of Time (F1, 23 = 31.064, p < 0.0001), demonstrating a lesion effect in both groups. Rehabilitative training phase: There was a significant effect of Group (F1, 23 = 6.688, p = 0.017), indicating that the rehab group made fewer reaching attempts to retrieve pellets compared with the no-rehab group. Follow up phase: There was a significant effect of Group (F1, 33 = 27.353, p = 0.0003), demonstrating that the benefit of rehabilitative training persisted after training was discontinued.
Footfaults
(Fig. 2C). Lesion phase: The ANOVA revealed a significant effect of Time (F1, 23 = 43.195, p < 0.0001), demonstrating a deficit as a result of the infarct in both groups. Rehabilitative training phase: There were no statistically significant effects. Follow up phase: There was a significant effect of Time (F3, 33 = 3.945, p = 0.0163), demonstrating improvement in both groups on the foot fault task during the follow-up phase.
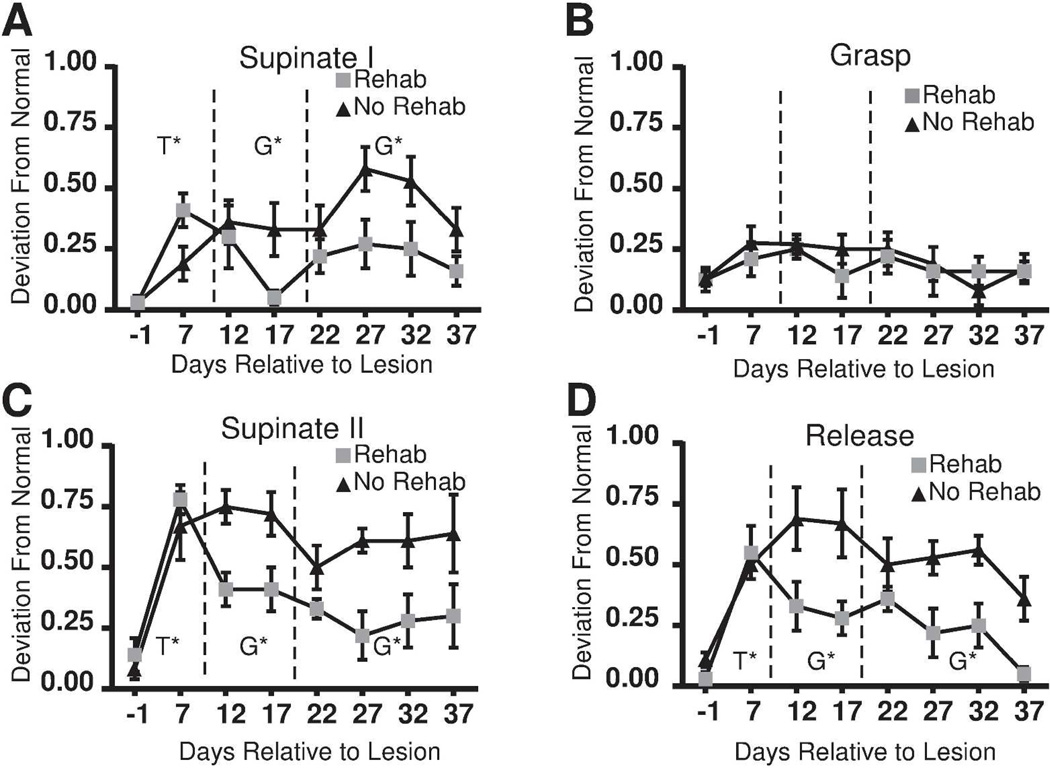
Movement kinematics on single pellet retrieval task
Lesion phase: There was a significant effect of Time (lesion effect) for supinate I (F1, 10= 21.922, p = 0.0009), supinate II (F1, 10 = 44.705, p < 0.0001) and release (F1, 10 = 26.406, p = 0.0004) (Fig. 3). The effect of Time for grasp approached significance (F1, 10 = 4.755, p = 0.054). There was no effect of Time for pronate, advance or digit extend. Thus, the remaining analyses were limited to supinate I, supinate II, release and grasp. Rehabilitative training phase: The effects of Time on supinate I approached significance (F1, 10 = 4.54, p = 0.059). Significant Group differences were found for supinate I (F1, 10 =6.47, p = 0.029), supinate II (F1, 10 = 23.17, p = 0.0007), and release (F1, 10 = 30.39, p = 0.0003) reflecting superior kinematic endpoints in the rehab group. Follow-up phase: Group differences favoring the rehab group persisted for supinate I (F3, 30 = 15.5, p = 0.0005), supinate II (F3, 30 = 18.40, p = 0.0002) and release (F3, 30 = 25.42, p < 0.0001). There was an effect of Time for release (F3, 30 = 3.62, p = 0.024). Post-hoc analysis comparing PLD22 to PLD37 revealed that both rehab and no-rehab groups showed significant improvement in release.
Figure 3.
Kinematic results. Mean scores based on kinematic notation analysis (± SEM). (A) Supinate I. (B) Grasp. (C) Supinate II. (D) Release. Kinematic scores showed significant impairment in both groups on PLD 7 for (A) Supinate I (p=0.0009) (B) Supinate II (p=0.0001) and (C) Release (p=0.0004). Kinematic scores (supinate I, supinate II and release) were superior in the rehab group during both the training and follow-up phases. SEM = standard error of the mean; G* = significant Group effect; T* = significant Time effect; G×T* = significant Group × Time interaction.
Effects of Rehabilitative Training on Motor Output Maps
Location of forelimb representations
ICMS maps were successfully obtained in 21 of 25 rats. Two rats died prior to the mapping procedure (long-term rehab group = 1, long-term no-rehab group = 1). In two additional rats (long-term rehab group = 1, long-term no-rehab group = 1), no evoked movements were observed from ICMS stimulation at the maximum current level (80µA). Such outcomes are not uncommon in ICMS experiments, and are typically attributable to improper anesthetic depth that cannot be corrected during the course of the procedure.8 Subsequent analyses focused on the 21 rats with successful maps.
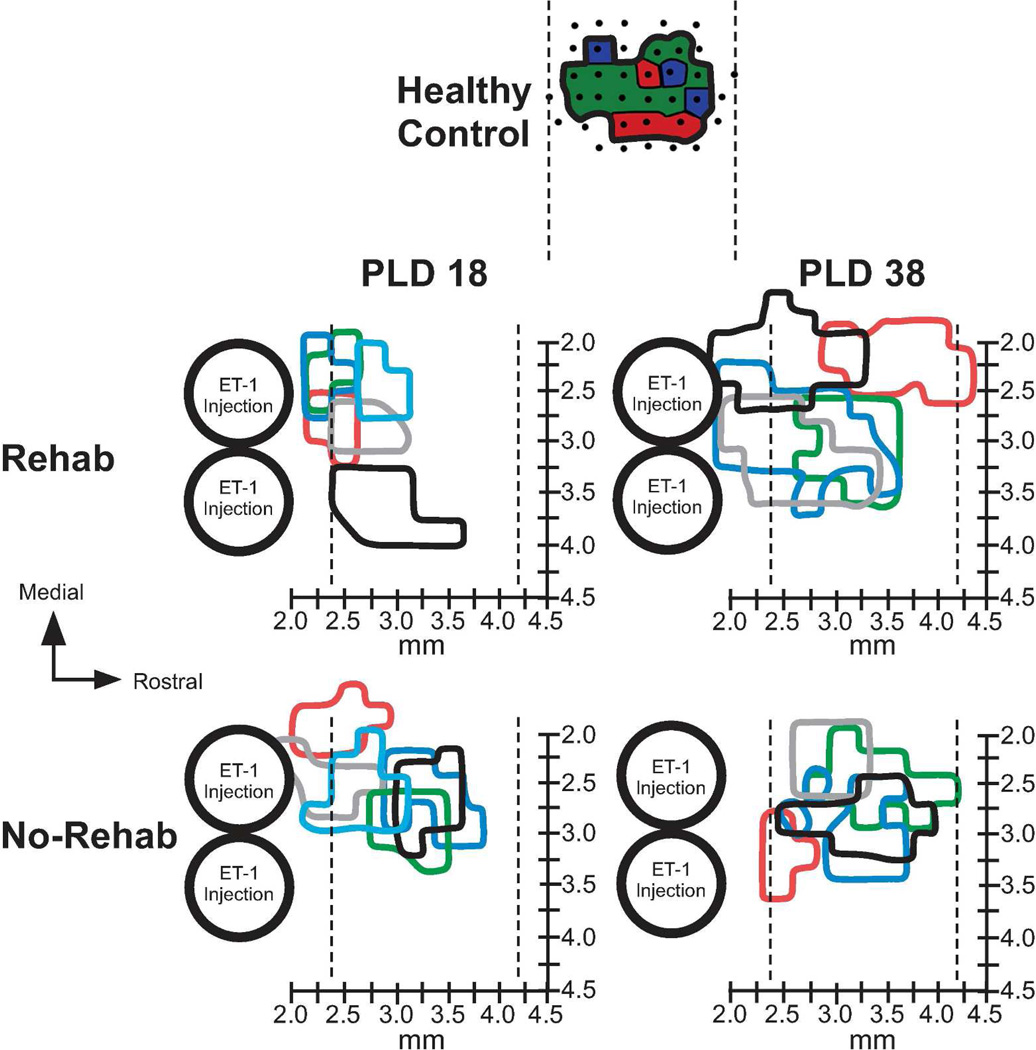
On both PLD 18 and PLD 38, the spared forelimb motor representations were largely contained within the typical RFA location (Fig 4).8 In both rehab and no-rehab groups, neck, jaw, orofacial and vibrissae movements were evoked on the medial, rostral and lateral borders of the forelimb representation (as well as the caudal border in rats with more rostrally located forelimb maps). Because only the forelimb representation was mapped in its entirety, we did not examine these additional representations systematically.
Figure 4.
Forelimb movement representations rostral to infarct. On PLD 18 (one day after rehabilitative training), forelimb representations were reduced in size in both groups compared with healthy (historical) controls. On PLD 38 (20 days after rehabilitative training), forelimb representations were significantly larger in the rehab group compared with the no-rehab group. Large circles represent predicted spread of the two most rostral ET-1 injections, corresponding to infarcted tissue. Colored outlines represent boundaries of total forelimb representations (distal and proximal) for individual cases. Vertical dotted lines indicate rostral and caudal limits of motor maps in RFA in historical controls (95% confidence interval; same rat strain and methodology). X-axis represents rostrocaudal distance from bregma. Y-axis represents mediolateral distance from midline. At top of figure, a representative healthy control map is illustrated to show the typical size and location of RFA8 (dots – microelectrode penetrations; red – digit movement; green – wrist/forearm movement; blue – proximal forelimb movement.
On PLD 18, in two rats in each group, movements could not be evoked rostral to the forelimb representation using the maximum current (80 µA). In three rats in each group, the forelimb representation extended further caudally to include the peri-infarct area immediately rostral to the infarct, where neck and orofacial representations typically are found11–13. As this location shift was relatively small (< 500µm), we cannot rule out the roles of tissue cavitation (in the infarcted area), edema or inflammation in such shifts. Thus, since it could not be determined that these forelimb representations were entirely within RFA, we conservatively refer to forelimb representations rostral to the lesion.
On PLD 38, in three rats in the rehab group, forelimb movements were evoked caudal to the typical RFA territory. There was a significant effect of Time in the rostral extent of forelimb movement maps (F3, 20 = 5.7161, p = 0.0287), but no effect of Group nor Group × Time interaction. The map extended more rostrally in long-term vs. short-term survival groups. There were no significant differences in medial or lateral extent of the maps.
Size of forelimb representations
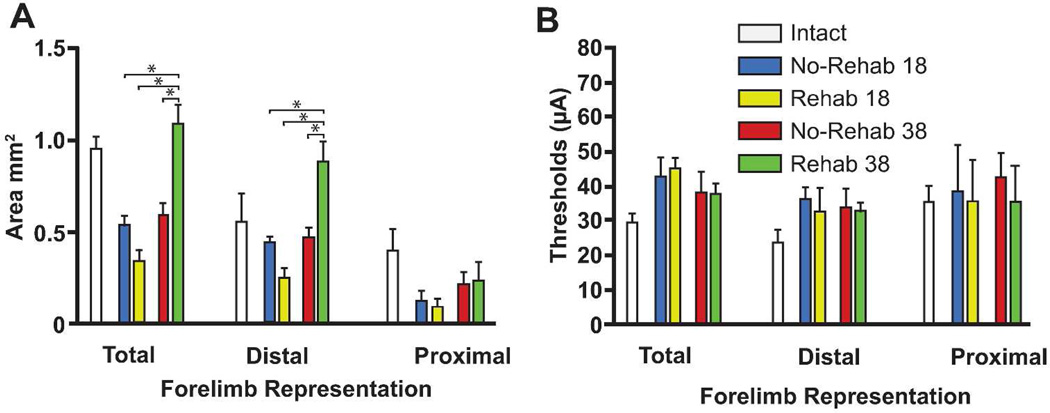
On PLD 18, the total forelimb area rostral to the lesion in the rehab and no-rehab groups was 0.34 ± 0.06 mm2 (mean ± SEM) and 0.54 ± 0.05 mm2, respectively. This contrasts with the much larger RFA area in historical controls (0.95 ± 0.7 mm2).8,15 On PLD 38, the total RFA area was 1.08 ± 0.11 mm (14% larger than historical controls) and 0.60 ± 0.09 mm (37% smaller than historical controls) in the rehab and no-rehab groups, respectively.
There was a significant effect of Time in the total forelimb area rostral to the lesion (F3, 20 = 23.4417, p = 0.0002) and a significant Group × Time interaction (F3, 20 = 19.5069, p = 0.0004). Post-hoc tests indicate that the forelimb area of the rehab/PLD 38 group was larger than any of the other three groups (p < 0.05, Tukey’s HSD; Fig. 5A).
Figure 5.
Motor map areas and thresholds. (A) Mean area for total distal forelimb, distal forelimb and proximal forelimb movement representations in each of the four groups (±SEM). Total forelimb area in the rehab rats on PLD 38 was significantly larger than any of the other groups. This was primarily due to expansion of the distal forelimb area. (B) Mean RFA movement threshold (±SEM). There was no difference in current threshold. Mean area and threshold (±SEM) from historical controls is shown for descriptive comparison.
Further analysis to differentiate distal vs. proximal representations revealed that most of the change in the total forelimb area was due to changes in the distal area. While the effect of Group approached significance (F3, 20 = 3.9535, p = 0.0631), there was a significant effect of Time (F3, 20 = 16.6762, p = 0.0008) and a significant Group × Time interaction (F3, 20 = 18.4697, p = 0.0005). Post-hoc tests showed that the distal forelimb area was larger in the rehab/38 day group than any of the other three groups (p < 0.05). There were no significant effects for proximal forelimb area.
There were no significant linear correlations between map size and final performance or kinematic scores. The strongest relationship was between the distal forelimb area and release scores (PLD 38 rats; F = 2.5018, p = 0.1524).
ICMS current thresholds
There were no significant differences in the threshold currents required to evoke distal or proximal forelimb movements (Fig 5B).
Discussion
After a focal ischemic infarct in the rat’s primary motor cortex forelimb representation, rehabilitative training was effective in improving motor performance and movement quality on a single-pellet retrieval task. Motor scores returned to near-baseline performance by the end of the rehabilitative training phase, and gains were retained during a three week follow-up period. Spared forelimb motor maps were unusually small in both groups on PLD 18. Maps were significantly larger in the rehab group on PLD 38, tripling in size compared to PLD 18. These results demonstrate that rehabilitative training results in rapid improvements in motor performance and movement quality, and delayed expansions in spared motor maps.
Effects of Rehabilitative Training on Motor Performance and Movement Quality
The performance deficits and training-induced performance gains observed in this study are similar to those reported previously in rats after motor cortex lesions, though the severity of initial deficits and the time course of improvements are largely dependent upon lesion size and location.16–19 Altered movement kinematic patterns during the single-pellet retrieval task also have been demonstrated frequently in rats after focal cortical lesions in motor cortex, especially in supination/pronation, aim, grasp and release.11,16,17,20 Some impairments, such as grasp kinematics, are much more severe in rats with subcortical infarcts.21 Since the rehabilitative training was focused on the retrieval task, requiring skilled use of the digits, it is not surprising that rehabilitative training did not affect performance on the footfault task. This suggests that training effects are task-specific, and do not generalize to tasks that do not require skilled digit use.
It is typically assumed that motor performance gains are due to a combination of compensation (use of alternative kinematic patterns) and true recovery (return of baseline kinematic patterns).16,17,22–27 One of the key translational questions regarding post-stroke rehabilitation is the following: Can rehabilitative interventions result in more extensive recovery of normal kinematic patterns, i.e., movement quality? In the present study, rehabilitative training resulted in a sustained improvement in kinematics, in addition to greater and more rapid gains in functional performance. In a previous study after motor cortex lesions in rats, neither motor performance nor normalization of kinematic patterns was aided by post-stroke practice.16 Also, a recent study of chronic human stroke survivors undergoing a constraint-induced movement therapy intervention demonstrated improvement in functional outcomes, but no improvement in kinematic outcomes.28 However, other studies have demonstrated that behavioral experience can alter not only the trajectory of motor recovery, but can at least partially reduce compensatory movement patterns.17,18
It can be argued that performance gains by rats in the no-rehab group in the present study were largely compensatory. But the present results suggest that rehabilitative training provides benefits beyond simply improving compensatory skills; and may promote true recovery. The results parallel those in a human stroke population in which motor performance improved over time, but those subjects with the greatest recovery of normal kinematic patterns had the most extensive functional improvement.29
Temporary Disruption of Spared Motor Maps after Motor Cortex Lesions
The reduced size of forelimb representations on PLD 18 suggests that, at least for a few weeks after the infarct, the functional integrity of cortex rostral to the lesion (including RFA) is disrupted, perhaps by a diaschisis-like effect. Focal cortical infarcts produce hypometabolism throughout a large region of ipsilesional cortex, closely corresponding to areas with known corticocortical connections with the infarct core.30 Thus, the functional integrity of neurons connected to the infarct is likely to be compromised. Since the CFA and RFA have dense reciprocal interconnections, neurons involved with motor control of the forelimb are most likely to be disrupted, allowing a competitive advantage for neurons more involved with motor control of more proximal (neck, orofacial, vibrissae) musculature. Similar hypotheses have been proposed to explain map changes after focal cortical impact injuries.8 Such injuries result in widespread sub-lethal effects in the cortex ipsilateral to the damage.31 It has long been known that the overlap of face/neck and forelimb representations in motor cortex provides a substrate for rapid map plasticity. Forelimb motor sites can convert to face/neck motor sites and vice versa within hours.32,33 If sub-lethal effects differentially compromise forelimb related neurons in RFA due to the dense reciprocal connections with CFA, then more proximal movement fields are likely to emerge. The present results suggest that this bias toward face/neck representations persists at least for a few weeks after the injury.
Temporal Relationship between Cortical Plasticity and Behavioral Recovery
While forelimb motor maps were substantially smaller on PLD 18, motor performance had already plateaued. Thus, behavioral improvement preceded map expansion. Similar results were found in the supplementary motor area of monkeys undergoing spontaneous recovery after infarcts that damaged primary motor cortex and nearby premotor areas.6 It was suggested that undetected changes in behavior, such as improvement in kinematic patterns, may have occurred during later stages, and that late motor map reorganization may reflect improved movement quality.
Part of the rationale for conducting the present study was to determine whether late motor map changes reflect late improvements in kinematic patterns. However, as with motor performance measures, kinematic endpoints had already improved by the end of the rehabilitative training period. As there continued to be improvements in release during the follow-up period, it is still possible that some of the late map expansion was due to refinement in movement quality. It is possible that the smaller PLD 38 forelimb maps in the no-rehab group reflect the continued use of maladaptive compensatory movement strategies, sometimes called “learned-bad use”.34 Such compensation may have dampened both motor performance gains and forelimb map expansion.
The lack of an effect of rehabilitative training on motor maps on PLD 18 contrasts with the large number of neuroimaging studies in stroke survivors demonstrating rapid structural and functional changes after rehabilitative interventions.35 However, human studies correlating the effects of interventions on neuroimaging endpoints are typically done in a more chronic state. The present results suggest that as clinical trials are conducted at earlier time points after stroke, neuroimaging results may not be entirely predictable from results in chronic populations. Changes in functional maps may only be observable at later time points.
There is considerable evidence that cortical injury initiates a plethora of presumably regenerative neurophysiologic and neuroanatomic events in the peri-infarct and remote tissue that last for at least a few weeks.27,36 A time-dependent expression of both neuronal growth-promoting and growth-inhibiting genes occurs within the first week after stroke. Altered gene expression profiles are found in the peri-infarct tissue37 and in neurons in the RFA that project to the infarcted zone.14 Other early changes include synaptogenesis, axonal sprouting, dendritic arborization and dendritic spine remodeling.38–44 Peri-infarct neuronal excitability changes occur rapidly and are mediated by extrasynaptic GABAA (γ-aminobutyric acid type A) receptors.45–47 Pharmacologic blockade of tonic GABAergic transmission during this early stage can rapidly improve recovery in mice after stroke.47 Also, decreased excitation in the peri-infarct cortex is mediated by altered NMDA (N-methyl-D-aspartate) and AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors. Modulation of AMPA receptors five days after stroke enhances functional recovery mediated by release of BDNF (brain-derived neurotrophic factor).48 Thus, while motor maps (and sensory maps36,49) are disrupted and disorganized at these early time points, repair processes are already initiated, and are amenable to therapeutic interventions.
In conclusion, during the first few weeks after injury, two competing processes are at play: 1) Diaschisis results in sub-lethal, presumably reversible functional disruption of local and connected neurons; less affected neurons in these same regions gain a competitive advantage, resulting in altered map topography. 2) Regenerative processes are set into motion, and are modifiable via behavioral or pharmacological interventions. Thus, rehabilitative training during the first few weeks post-injury results in rapid behavioral gains, but such gains typically are not expressed in motor output maps due to the ongoing diaschisis. However, cortical reorganization continues long after behavioral recovery has stabilized. It is likely that rehabilitative training guides the eventual neuroanatomical and neurophysiological changes that will persist in chronic stages, much like motor training in healthy rats induces synaptogenesis that is reflected later.50 The implication of these results for clinical stroke rehabilitation is that the process of post-injury neural plasticity can be guided in a powerful way by the type and quality of early post-injury motor experience.
Acknowledgments
Source of Funding
This research was funded by NIH R37 NS030853 to R.J.N.
Footnotes
Disclosures: None
REFERENCES
- 1.Nudo RJ. Mechanisms for recovery of motor function following cortical damage. Curr Opin Neurobiol. 2006 Dec;16(6):638–644. doi: 10.1016/j.conb.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 2.Ward NS. The neural substrates of motor recovery after focal damage to the central nervous system. Arch Phys Med Rehabil. 2006 Dec;87(12 Suppl 2):S30–S35. doi: 10.1016/j.apmr.2006.08.334. [DOI] [PubMed] [Google Scholar]
- 3.Wittenberg GF. Experience, cortical remapping, and recovery in brain disease. Neurobiol Dis. 2010 Feb;37(2):252–258. doi: 10.1016/j.nbd.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dancause N, Barbay S, Frost SB, et al. Effects of small ischemic lesions in the primary motor cortex on neurophysiological organization in ventral premotor cortex. J Neurophysiol. 2006 Dec;96(6):3506–3511. doi: 10.1152/jn.00792.2006. [DOI] [PubMed] [Google Scholar]
- 5.Frost SB, Barbay S, Friel KM, Plautz EJ, Nudo RJ. Reorganization of remote cortical regions after ischemic brain injury: a potential substrate for stroke recovery. J Neurophysiol. 2003 Jun;89(6):3205–3214. doi: 10.1152/jn.01143.2002. [DOI] [PubMed] [Google Scholar]
- 6.Eisner-Janowicz I, Barbay S, Hoover E, et al. Early and late changes in the distal forelimb representation of the supplementary motor area after injury to frontal motor areas in the squirrel monkey. J Neurophysiol. 2008 Sep;100(3):1498–1512. doi: 10.1152/jn.90447.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nudo RJ, Frost SB. The evolution of motor cortex and motor systems. In: Kaas JH, Striedter GF, Bullock TH, Preuss TM, Rubenstein J, Krubitzer LA, editors. Evolution of Nervoius Systems. Oxford, UK: Academic Press; 2007. pp. 373–395. [Google Scholar]
- 8.Nishibe M, Barbay S, Guggenmos D, Nudo RJ. Reorganization of motor cortex after controlled cortical impact in rats and implications for functional recovery. J Neurotrauma. 2010 Dec;27(12):2221–2232. doi: 10.1089/neu.2010.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klein A, Sacrey LA, Whishaw IQ, Dunnett SB. The use of rodent skilled reaching as a translational model for investigating brain damage and disease. Neurosci Biobehav Rev. 2012 Mar;36(3):1030–1042. doi: 10.1016/j.neubiorev.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Whishaw IQ, Pellis SM. The structure of skilled forelimb reaching in the rat: a proximally driven movement with a single distal rotatory component. Behav Brain Res. 1990 Dec 7;41(1):49–59. doi: 10.1016/0166-4328(90)90053-h. [DOI] [PubMed] [Google Scholar]
- 11.Alaverdashvili M, Whishaw IQ. Compensation aids skilled reaching in aging and in recovery from forelimb motor cortex stroke in the rat. Neuroscience. 2010 Apr 28;167(1):21–30. doi: 10.1016/j.neuroscience.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 12.Whishaw IQ, Pellis SM, Gorny BP, Pellis VC. The impairments in reaching and the movements of compensation in rats with motor cortex lesions: an endpoint, videorecording, and movement notation analysis. Behav Brain Res. 1991 Jan 31;42(1):77–91. doi: 10.1016/s0166-4328(05)80042-7. [DOI] [PubMed] [Google Scholar]
- 13.Maldonado MA, Allred RP, Felthauser EL, Jones TA. Motor skill training, but not voluntary exercise, improves skilled reaching after unilateral ischemic lesions of the sensorimotor cortex in rats. Neurorehabil Neural Repair. 2008 May-Jun;22(3):250–261. doi: 10.1177/1545968307308551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Urban ET, 3rd, Bury SD, Barbay HS, Guggenmos DJ, Dong Y, Nudo RJ. Gene expression changes of interconnected spared cortical neurons 7 days after ischemic infarct of the primary motor cortex in the rat. Mol Cell Biochem. 2012 Oct;369(1–2):267–286. doi: 10.1007/s11010-012-1390-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kleim JA, Barbay S, Nudo RJ. Functional reorganization of the rat motor cortex following motor skill learning. J Neurophysiol. 1998 Dec;80(6):3321–3325. doi: 10.1152/jn.1998.80.6.3321. [DOI] [PubMed] [Google Scholar]
- 16.Whishaw IQ. Loss of the innate cortical engram for action patterns used in skilled reaching and the development of behavioral compensation following motor cortex lesions in the rat. Neuropharmacology. 2000 Mar 3;39(5):788–805. doi: 10.1016/s0028-3908(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 17.Moon SK, Alaverdashvili M, Cross AR, Whishaw IQ. Both compensation and recovery of skilled reaching following small photothrombotic stroke to motor cortex in the rat. Exp Neurol. 2009 Jul;218(1):145–153. doi: 10.1016/j.expneurol.2009.04.021. [DOI] [PubMed] [Google Scholar]
- 18.Knieling M, Metz GA, Antonow-Schlorke I, Witte OW. Enriched environment promotes efficiency of compensatory movements after cerebral ischemia in rats. Neuroscience. 2009 Oct 20;163(3):759–769. doi: 10.1016/j.neuroscience.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 19.Whishaw IQ, Alaverdashvili M, Kolb B. The problem of relating plasticity and skilled reaching after motor cortex stroke in the rat. Behav Brain Res. 2008 Sep 1;192(1):124–136. doi: 10.1016/j.bbr.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 20.Alaverdashvili M, Whishaw IQ. Motor cortex stroke impairs individual digit movement in skilled reaching by the rat. Eur J Neurosci. 2008 Jul;28(2):311–322. doi: 10.1111/j.1460-9568.2008.06315.x. [DOI] [PubMed] [Google Scholar]
- 21.Gharbawie OA, Auer RN, Whishaw IQ. Subcortical middle cerebral artery ischemia abolishes the digit flexion and closing used for grasping in rat skilled reaching. Neuroscience. 2006;137(4):1107–1118. doi: 10.1016/j.neuroscience.2005.10.043. [DOI] [PubMed] [Google Scholar]
- 22.Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain. 2000 May;123(Pt 5):940–953. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- 23.Alaverdashvili M, Whishaw IQ. A behavioral method for identifying recovery and compensation: hand use in a preclinical stroke model using the single pellet reaching task. Neurosci Biobehav Rev. 2013 Jun;37(5):950–967. doi: 10.1016/j.neubiorev.2013.03.026. [DOI] [PubMed] [Google Scholar]
- 24.Metz GA, Antonow-Schlorke I, Witte OW. Motor improvements after focal cortical ischemia in adult rats are mediated by compensatory mechanisms. Behav Brain Res. 2005 Jul 1;162(1):71–82. doi: 10.1016/j.bbr.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Lum PS, Mulroy S, Amdur RL, Requejo P, Prilutsky BI, Dromerick AW. Gains in upper extremity function after stroke via recovery or compensation: Potential differential effects on amount of real-world limb use. Top Stroke Rehabil. 2009 Jul-Aug;16(4):237–253. doi: 10.1310/tsr1604-237. [DOI] [PubMed] [Google Scholar]
- 26.Levin MF, Kleim JA, Wolf SL. What do motor "recovery" and "compensation" mean in patients following stroke? Neurorehabil Neural Repair. 2009 May;23(4):313–319. doi: 10.1177/1545968308328727. [DOI] [PubMed] [Google Scholar]
- 27.Krakauer JW, Carmichael ST, Corbett D, Wittenberg GF. Getting neurorehabilitation right: what can be learned from animal models? Neurorehabil Neural Repair. 2012 Oct;26(8):923–931. doi: 10.1177/1545968312440745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kitago T, Liang J, Huang VS, et al. Improvement after constraint-induced movement therapy: recovery of normal motor control or task-specific compensation? Neurorehabil Neural Repair. 2013 Feb;27(2):99–109. doi: 10.1177/1545968312452631. [DOI] [PubMed] [Google Scholar]
- 29.Roby-Brami A, Feydy A, Combeaud M, Biryukova EV, Bussel B, Levin MF. Motor compensation and recovery for reaching in stroke patients. Acta Neurol Scand. 2003 May;107(5):369–381. doi: 10.1034/j.1600-0404.2003.00021.x. [DOI] [PubMed] [Google Scholar]
- 30.Carmichael ST, Tatsukawa K, Katsman D, Tsuyuguchi N, Kornblum HI. Evolution of diaschisis in a focal stroke model. Stroke. 2004 Mar;35(3):758–763. doi: 10.1161/01.STR.0000117235.11156.55. [DOI] [PubMed] [Google Scholar]
- 31.Posmantur RM, Kampfl A, Taft WC, et al. Diminished microtubule-associated protein 2 (MAP2) immunoreactivity following cortical impact brain injury. J Neurotrauma. 1996 Mar;13(3):125–137. doi: 10.1089/neu.1996.13.125. [DOI] [PubMed] [Google Scholar]
- 32.Jacobs KM, Donoghue JP. Reshaping the cortical motor map by unmasking latent intracortical connections. Science. 1991 Feb 22;251(4996):944–947. doi: 10.1126/science.2000496. [DOI] [PubMed] [Google Scholar]
- 33.Nudo RJ, Jenkins WM, Merzenich MM. Repetitive microstimulation alters the cortical representation of movements in adult rats. Somatosens Mot Res. 1990;7(4):463–483. doi: 10.3109/08990229009144720. [DOI] [PubMed] [Google Scholar]
- 34.Alaverdashvili M, Foroud A, Lim DH, Whishaw IQ. "Learned baduse" limits recovery of skilled reaching for food after forelimb motor cortex stroke in rats: a new analysis of the effect of gestures on success. Behav Brain Res. 2008 Apr 9;188(2):281–290. doi: 10.1016/j.bbr.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 35.Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008 May;39(5):1520–1525. doi: 10.1161/STROKEAHA.107.502229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dijkhuizen RM, Singhal AB, Mandeville JB, et al. Correlation between brain reorganization, ischemic damage, and neurologic status after transient focal cerebral ischemia in rats: a functional magnetic resonance imaging study. J Neurosci. 2003 Jan 15;23(2):510–517. doi: 10.1523/JNEUROSCI.23-02-00510.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carmichael ST. Cellular and molecular mechanisms of neural repair after stroke: making waves. Ann Neurol. 2006 May;59(5):735–742. doi: 10.1002/ana.20845. [DOI] [PubMed] [Google Scholar]
- 38.Stroemer RP, Kent TA, Hulsebosch CE. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995 Nov;26(11):2135–2144. doi: 10.1161/01.str.26.11.2135. [DOI] [PubMed] [Google Scholar]
- 39.Hsu JE, Jones TA. Contralesional neural plasticity and functional changes in the less-affected forelimb after large and small cortical infarcts in rats. Exp Neurol. 2006 Oct;201(2):479–494. doi: 10.1016/j.expneurol.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Carmichael ST. Plasticity of cortical projections after stroke. Neuroscientist. 2003 Feb;9(1):64–75. doi: 10.1177/1073858402239592. [DOI] [PubMed] [Google Scholar]
- 41.Dancause N, Barbay S, Frost SB, et al. Extensive cortical rewiring after brain injury. J Neurosci. 2005 Nov 2;25(44):10167–10179. doi: 10.1523/JNEUROSCI.3256-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McNeal DW, Darling WG, Ge J, et al. Selective long-term reorganization of the corticospinal projection from the supplementary motor cortex following recovery from lateral motor cortex injury. J Comp Neurol. 2010 Mar 1;518(5):586–621. doi: 10.1002/cne.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carmichael ST, Chesselet MF. Synchronous neuronal activity is a signal for axonal sprouting after cortical lesions in the adult. J Neurosci. 2002 Jul 15;22(14):6062–6070. doi: 10.1523/JNEUROSCI.22-14-06062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brown CE, Aminoltejari K, Erb H, Winship IR, Murphy TH. In vivo voltage-sensitive dye imaging in adult mice reveals that somatosensory maps lost to stroke are replaced over weeks by new structural and functional circuits with prolonged modes of activation within both the peri-infarct zone and distant sites. J Neurosci. 2009 Feb 11;29(6):1719–1734. doi: 10.1523/JNEUROSCI.4249-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Witte OW, Stoll G. Delayed and remote effects of focal cortical infarctions: secondary damage and reactive plasticity. Adv Neurol. 1997;73:207–227. [PubMed] [Google Scholar]
- 46.Neumann-Haefelin T, Staiger JF, Redecker C, et al. Immunohistochemical evidence for dysregulation of the GABAergic system ipsilateral to photochemically induced cortical infarcts in rats. Neuroscience. 1998 Dec;87(4):871–879. doi: 10.1016/s0306-4522(98)00124-9. [DOI] [PubMed] [Google Scholar]
- 47.Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST. Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke. Nature. 2010 Nov 11;468(7321):305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clarkson AN, Overman JJ, Zhong S, Mueller R, Lynch G, Carmichael ST. AMPA receptor-induced local brain-derived neurotrophic factor signaling mediates motor recovery after stroke. J Neurosci. 2011 Mar 9;31(10):3766–3775. doi: 10.1523/JNEUROSCI.5780-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jablonka JA, Burnat K, Witte OW, Kossut M. Remapping of the somatosensory cortex after a photothrombotic stroke: dynamics of the compensatory reorganization. Neuroscience. 2010 Jan 13;165(1):90–100. doi: 10.1016/j.neuroscience.2009.09.074. [DOI] [PubMed] [Google Scholar]
- 50.Kleim JA, Hogg TM, VandenBerg PM, Cooper NR, Bruneau R, Remple M. Cortical synaptogenesis and motor map reorganization occur during late, but not early, phase of motor skill learning. J Neurosci. 2004 Jan 21;24(3):628–633. doi: 10.1523/JNEUROSCI.3440-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]