SUMMARY
Genetic defects in the microRNA (miRNA) generating enzyme, dicer, are increasingly linked to disease. Loss of miRNA in dicer deficiency is thought to be due to loss of miRNA-generating activity. Here, we demonstrate a previously unknown catabolic mechanism driving miRNA depletion in dicer deficiency. We developed a Dicer-antagonist assay revealing a pre-miRNA degrading enzyme that competes with pre-miRNA processing. We purified this pre-miRNA degrading activity using an unbiased chromatographic procedure and identified the ribonuclease complex Translin/Trax (TN/TX). In wild type dicer backgrounds, pre-miRNA processing was dominant. However, in dicer deficient contexts, TN/TX broadly suppressed miRNA. These findings indicate that miRNA depletion in dicer deficiency is due to the combined loss of miRNA-generating activity and catabolic function of TN/TX. Importantly, inhibition of TN/TX mitigated loss of both miRNA and tumor suppression with dicer haploinsufficiency. These studies reveal a potentially druggable target for restoring miRNA function in cancers and emerging dicer deficiencies.
INTRODUCTION
As the central miRNA-generating enzyme, Dicer is essential for proper physiological function (Bersnstein et al., 2003). miRNA originate as long primary transcripts that are processed to precursor (pre-) miRNA by a complex comprised of the RNaseIII Drosha and RNA binding partner DGCR8/Pasha (Lee et al., 2003; Denli et al., 2004; Gregory et al., 2004; Han et al., 2004). Following export from nucleus to cytoplasm (Yi et al., 2003; Lund et al., 2004), the miRNA-generating enzyme, consisting of the RNaseIII Dicer and RNA binding partner TRBP, processes pre-miRNA to mature miRNA (Bernstein et al., 2001; Hutvagner et al., 2001; Lee et al., 2004; Chendrimada et al., 2005; Haase et al., 2005; Lee et al., 2006; Paroo et al., 2009). Mature miRNA program Argonaute proteins to effect sequence specific transcript silencing (Hammond et al., 2001; Liu et al 2004., Meister et al., 2004).
miRNA expression is a net product of synthesis and degradation at each stage of maturation. Catabolic regulation of RNA Interference was introduced through a mutagenesis screen in C. elegans, which identified eri-1 as a siRNA-degrading nuclease (Kennedy et al 2004). Subsequently, related members of the DEDD family, sdn1-4, were found to promote turnover of a subset of miRNA in Arabidopsis (Ramachandran and Chen, 2008). A candidate approach also revealed xrn-2 as a catabolic regulator of select miRNA in C. elegans (Chatterjee and Grosshans, 2009). In mammals, a targeted study of immune response genes found that MCPIP1 aberrantly cleaved and inactivated pre-miRNA (Suzuki et al., 2011). The ER-stress responsive IRE1α was shown to digest precursors of miRNA that govern apoptosis (Upton et al., 2012). The Dis3l2 exonuclease was found to degrade Lin28-TUT-4 polyuridylated pre-let-7 (Viswanathan et al., 2008; Heo et al., 2008; Heo et al., 2009; Hagan et al., 2009; Chang et al., 2013). Further understanding of catabolic regulation of the miRNA pathway would be facilitated through unbiased, functional approaches.
A striking molecular signature of tumors is widespread loss of miRNA relative to non-tumor tissue (Lu et al., 2005; Lee et al., 2008; Ozen et al., 2008; Maillot et al., 2009; Dvinge et al., 2013). Experimental depletion of miRNA through genetic disruption of the biogenesis machinery promoted cellular transformation and tumorigenesis in mice (Kumar et al., 2007; 2009). Clinically, hemizygous deletion of dicer is observed in up to 40% of human tumors and is associated with poor patient prognoses (Table S1; Merritt et al., 2008; Kumar et al., 2009). These and other studies have established Dicer as a haploinsufficient tumor suppressor (Kumar et al., 2009; Heravi-Moussavi et al., 2012; Lambertz et al., 2010; Nittner et al., 2012; Ravi et al., 2012; Mito et al., 2013). Medical genetics is rapidly expanding the scope of dicer disorders.
Loss of miRNA in dicer deficiency is thought to be due to loss of miRNA-generating activity. Here, we demonstrate that depletion of miRNA in dicer deficiency is due to both loss of miRNA-generating activity and catabolic function of TN/TX. Inhibition of TN/TX mitigated loss of both miRNA and tumor suppression with dicer haploinsufficiency. A complementary catabolic mechanism integral to dicer deficiency advances understanding of miRNA depletion in tumor development and may represent a general mechanism for emerging dicer deficiencies.
RESULTS
Identification of a pre-miRNA degrading enzyme
In developing a biochemical purification scheme to isolate the human miRNA-generating complex (Paroo et al., 2009), a number of observations suggested that dicing activity is subject to negative regulation. For example, base line miRNA-generating activity from HeLa cell extract was relatively weak (Figure 1A, lane 1). Fractionation of extract by ammonium sulfate precipitation shifted miRNA-generating activity and the miRNA-generating machinery (Dicer/TRBP) to the pellet fraction (Figure 1A and 1B). Notably, miRNA production in the pellet fraction was greater than that of the original extract (Figure 1A, compare lanes 1 and 3). This suggested the presence of a miRNA-generating inhibitor in the soluble fraction. Reconstitution of supernatant and pellet resulted in lower levels of miRNA production relative to pellet alone (Figure 1A, compare lanes 3 and 4) and comparable to that of the original extract. This further suggested a negative regulator in the supernatant. Interestingly, upon overexposure and analysis of full length autoradiograms, pre-miRNA degrading activity became evident in the soluble fraction (Figure 1C, lane 3). That both dicing and pre-miRNA degrading activity were detected at relatively low levels in the starting extract (Figure 1C, lane 2) and that both activities were enhanced following separation (lanes 3 and 4), suggested that these enzymes compete for pre-miRNA substrate. Consistent with this, reconstitution of supernatant and pellet fractions reduced both dicing and pre-miRNA degradation (lane 5).
Figure 1.
Biochemical fractionation reveals a pre-miRNA-degrading enzyme that competes with pre-miRNA processing.
(A) In vitro miRNA-generating assays performed with HeLa extract or soluble and pellet fractions following ammonium sulfate precipitation.
(B) Immunoblotting of fractions for Dicer and TRBP.
(C) Full-length, overexposed autoradiogram depicting pre-miRNA-degradation products.
To identify this pre-miRNA degrading enzyme we developed an unbiased, multi-step chromatographic purification scheme (Figure 2A). In the first stage of the protocol, we made use of ammonium sulfate precipitation to isolate pre-miRNA degrading activity from miRNA-generating activity. Following the final Q sepharose column, fractions were assayed for pre-miRNA degrading activity (Figure 2B), resolved on a SDS-polyacrylamide gel and proteins visualized by silver staining (Figure 2C). Two bands exhibited perfect correlation with pre-miRNA degrading activity. That two proteins exhibited identical chromatographic behavior following such a stringent purification procedure suggested the possibility of an enzymatic complex. Mass spectrometry and sequencing analysis identified these constituents as Translin (TN) and Trax (TX; Table S2). This is consistent with recent studies demonstrating that TN and TX function as a ribonuclease complex (Liu et al., 2009; Tian et al., 2011; Ye et al., 2011). Subsequent immunoblotting of fractions confirmed perfect chromatographic correlation between pre-miRNA degrading activity and TN/TX (Figure 2D).
Figure 2.
Chromatographic purification of a pre-miRNA-degrading enzyme.
(A) Purification scheme of pre-miRNA-degrading activity from HeLa cytoplasmic extract (S100). Numbers indicate salt concentration (mM).
(B) Individual fractions from the final Q sepharose column were assayed for pre-miRNA-degrading activity.
(C) Fractions were subjected to SDS-polyacrylamide gel electrophoresis and silver stained. See also Table S2 for peptides identified by mass spectrometry.
(D) ractions were immunoblotted for Translin and Trax.
Pre-miRNA degradation competes with pre-miRNA processing
To authenticate TN/TX as a pre-miRNA degrading enzyme, we generated recombinant TN/TX complexes. Previous structure-function studies indicated that TN serves as a scaffold for the catalytic TX subunit (Liu et al., 2009; Tian et al., 2011; Ye et al., 2011). We used a dual expression system to produce wild type and catalytically defective (E126A) TN/TX in E. coli (Figure 3A). Wild type, but not mutant TN/TX, yielded dose-dependent pre-miRNA degrading activity (Figure 3B). These findings indicate that our activity guided chromatographic purification correctly identified TN/TX as a pre-miRNA degrading enzyme.
Figure 3.
Pre-miRNA degradation by TN/TX competes with pre-miRNA processing by Dicer/TRBP in vitro.
(A) Colloidal blue-stained polyacrylamide gel depicting wild type (WT) and catalytic mutant (E126A) recombinant TN/TX complexes.
(B) Pre-miRNA degradation assays using indicated amounts of WT or catalytic mutant TN/TX. Assays were performed with synthetic pre-miR-16, pre-miR-21 or pre-let-7a as indicated.
(C) Sequences and structures of pre-miRNA and short-hairpin RNA (shRNA) were obtained from miRBase and RNAfold. Arrows indicate cleavage sites determined through mapping studies.
(D) Stem-loop degradation assays performed with indicated amounts of TN/TX and pre-miR-16, sh-miR-16, pre-miR-21 or sh-miR-21 as indicated.
(E) Pre-miRNA processing reactions were performed with 50 nM recombinant Dicer/TRBP and indicated amounts of either WT or catalytic mutant TN/TX.
(F) Stem-loop processing reactions performed with either pre-miR-16 or sh-miR-16, 50 nM Dicer/TRBP and indicated amounts of TN/TX.
Interestingly, TN/TX cleaved pre-miR-16 and pre-miR-21 efficiently relative to pre-let7a (Figure 3B). Analysis of stem loop structures curated in miRBase (Griffith-Jones, 2004), indicated a distinguishing feature. Pre-miR-16 and pre-miR-21 bear mismatch bulges in the stem regions, a characteristic shared by most pre-miRNA (Figure 3C). In contrast, the pre-let-7a stem harbors greater complementarity, lacking these canonical bulges. RNase A / T1 guided mapping studies indicated that TN/TX cleaved pre-miR-16 and pre-miR-21 at these unpaired regions of the stem (Figure 3C, arrows). This is consistent with the single-strand specific endonuclease function of TN/TX (Liu et al., 2009; Tian et al., 2011; Ye et al., 2011). As a direct test, we removed mismatches on the stems of pre-miR-16 and pre-miR-21 to generate short hairpin sh-miR-16 and sh-miR-21 with complementary stems (Figure 3C). These shRNA constructs were cleaved much less efficiently by TN/TX (Figure 3D). Thus, TN/TX degrades pre-miRNA by cleaving mismatch bulges in their stems.
As our initial fractionation studies indicated that pre-miRNA degradation competes with pre-miRNA processing (Figure 1C), we sought to determine whether TN/TX could inhibit miRNA production. We performed miRNA-generating assays with a constant level of recombinant Dicer/TRBP and titrated in wild type or catalytically defective TN/TX. miRNA production was inhibited with wild type but not mutant complex, indicating that TN/TX inhibits dicing in a nuclease dependent manner (Figure 3E). Moreover, TN/TX inhibited processing of pre-miR-16 but not sh-miR-16, further indicating that substrate cleavage is required for inhibiting dicing (Figure 3F). Dicer/TRBP and TN/TX were found to compete similarly for pre-miRNA binding (Figure S1). Collectively, these findings indicate that pre-miRNA degradation by TN/TX competes with pre-miRNA processing by Dicer/TRBP.
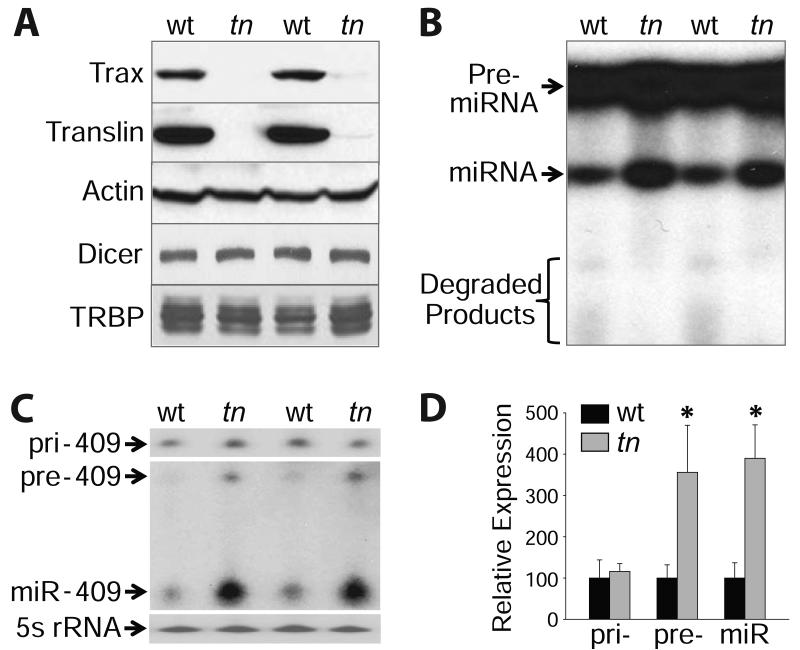
To investigate a potential role for TN/TX in regulating miRNA expression in vivo, we performed experiments with wild type and translin (tn) −/− mice (Fukuda et al., 2008; Wu et al., 2011). Given that TN/TX is abundant in testis and cerebellum (Chennathukuzhi et al., 2003), these tissues were selected for study. As the scaffolding function of TN is required to stabilize the catalytic TX subunit, tn−/− mice are defective for both subunits (Figure 4A; Chennathukuzhi et al., 2003; Wu et al., 2011). Consistent with our recombinant studies, extracts prepared from tn−/− mice yielded lower levels of pre-miRNA degradation and higher levels of miRNA-generating activity compared with wild type (Figure 4B). We then performed miRNA profiling analysis of cerebellar RNA from wild type and tn−/− animals. These studies revealed a small subset of miRNA suppressed by TN/TX (Table S3). Consistent with in vitro studies, elevated miRNA in tn−/− mice was accompanied by higher levels of pre-miRNA relative to wild type (Figure 4C and 4D). Taken together, these findings demonstrate that TN/TX functions as a pre-miRNA degrading enzyme, thereby reducing substrate for the miRNA-generating machinery.
Figure 4.
Pre-miRNA degradation by TN/TX competes with pre-miRNA processing by Dicer/TRBP in vivo.
(A) Immunoblotting performed with testis extracts from two pairs of wild type (wt) and translin (tn) −/− mice.
(B) Pre-miRNA processing assays performed with 25 μg of testis extract from two pairs of wt and tn−/− mice.
(C) Northern blotting performed with cerebellar RNA from two pairs of wt and tn−/− mice. Blots were hybridized with probes for miR-409 and 5s rRNA.
(D) Quantitative analysis of primary (pri-), precursor (pre-) and mature miR-409 from wt (black bars) and tn−/− mice (gray bars). * indicates greater than wt (p ≤ 0.02; n = 3; means ± SD). See also Table S3.
Loss of miRNA in dicer deficiency is due to loss of miRNA-generating activity and TN/TX
The modest differences in miRNA expression between wild type and tn−/− mice indicated that pre-miRNA were preferentially processed to mature miRNA by Dicer/TRBP rather than degraded by TN/TX. Yet, TN/TX markedly suppressed in vitro miRNA-generating activity. We reasoned that when dicer is fully functioning, miRNA-generating capacity sufficiently compensates for TN/TX. However, when miRNA-generating activity is limiting, TN/TX effectively competes with dicing. To address this possibility, we made use of previously established HCT116 human colorectal carcinoma cells encoding either wild type or dicer hypomorph alleles, in which exon 5 within the helicase domain was genetically disrupted (dicerex5; Cummins et al., 2006). As expression of Dicer and TRBP is interdependent (Chendrimada et al., 2005; Haase et al., 2005; Lee et al., 2006; Paroo et al., 2009), dicer hypomorph cells exhibited attenuated expression of both subunits of the miRNA-generating enzyme and reduced miRNA-generating activity (Figure S2A and S2B).
We established stable knockdown of TN/TX through lentivirus-mediated shRNA expression in dicerwt and dicerex5 cells. As observed with tn−/− mice, knockdown of TN resulted in loss of both the structural TN and catalytic TX subunits, whereas knockdown of TX reduced expression of only the catalytic subunit (Figure 5A and 5B). Relative to control, depletion of TN and TX attenuated pre-miRNA degradation and enhanced miRNA-generating activity for both dicerwt and dicerex5 cells (Figure 5C and 5D). The slight functional differences between shTN and shTX correspond to differences in knockdown efficiency.
Figure 5.
Loss of miRNA in dicer deficiency is due to loss of miRNA-generating activity and TN/TX.
HCT116 human colon carcinoma cells encoding wild type (wt) or dicer hypomorph alleles (ex5) were used to generate sub-cell lines stably expressing shRNA against luciferase (shluc), translin (shTN) or trax (shTX).
(A) Immunoblotting performed with extracts from dicerwt and (B) dicerex5 cells.
(C) Pre-miRNA processing assays performed with 20 μg of extract from dicerwt and (D) dicerex5 cells.
(E) Histogram summary of miRNA profiling studies. Data are presented as ratios of miRNA expression for shTN relative to shluc for dicerwt (blue bars) and dicerex5 cells (red bars). These values represent the degree of miRNA suppression by TN/TX. The dashed line indicates equivalent miRNA levels for shTN and shluc. See also Table S4.
(F) Scatter plot representation of miRNA responsiveness to Dicer and TN/TX. Data are fold change in miRNA for dicerex5 versus dicerwt (horizontal axis) and shTN versus shluc for dicerex5 cells (vertical axis). Closed circles represent miRNA with two-fold or greater decrease in dicerex5 versus dicerwt. Open circles represent miRNA with less than two-fold decrease in dicerex5 versus dicerwt. The correlation coefficient of the regression line for all data points is 0.94 (p < 0.0001).
(G) Relative expression of miR-21 and (H) a miR-21 target reporter. * indicates higher miRNA expression for shTN and shTX compared with shluc in dicerex5 cells (p < 0.025). # indicates lower miRNA-target expression in shTN and shTX versus shluc in dicerex5 cells (p < 0.001; n = 4; means ± SD).
To determine the importance of TN/TX in regulating cellular miRNA we conducted miRNA profiling studies. Consistent with modest differences between wild type and tn−/− mice (in which dicer was intact), TN/TX was found to have only minor influence on miRNA expression in dicerwt cells (Figure 5E, blue bars). However, in dicerex5 cells, TN/TX broadly suppressed miRNA expression (Figure 5E, red bars; Table S4). Interestingly, miRNA that were most strongly downregulated in dicerex5 versus dicerwt cells, were those most strongly suppressed by TN/TX (Figure 5F, upper left quadrant). That is, loss of miRNA in dicer defective cells was due to the combined effects of, a) the expected loss of miRNA-generating activity, and b) catabolic function of TN/TX.
We subsequently authenticated profiling studies using miRNA-specific assays. In dicerwt cells, TN/TX had minor influence on miRNA expression (Figure S2C). However, in dicerex5 cells, depletion of TN/TX partially restored miRNA expression relative to dicerwt. This further indicated that loss of miRNA in dicerex5 cells was due to both loss of miRNA-generating activity and TN/TX. Thus, although TN/TX inhibited in vitro miRNA-generating activity for both dicerwt and dicerex5 cells (Figure 5C and 5D), dicing capacity in dicerwt cells was sufficient to compensate for TN/TX, whereas in dicerex5 cells, impaired miRNA-generating activity revealed TN/TX function. Collectively, these findings indicate that TN/TX is integral to miRNA depletion in dicer deficiency.
To determine if the observed differences in miRNA expression impacted downstream miRNA function, miRNA-mediated silencing assays were conducted. dicerwt cells expressed relatively high levels of miR-21 and strongly suppressed expression of a miR-21 reporter relative to dicerex5 cells (Figure 5G and 5H). Knockdown of TN/TX in dicerex5 cells partially restored miR-21 and miR-21-mediated silencing. Collectively, these findings indicate that loss of miRNA in dicer deficiency is due to both loss of miRNA-generating activity and catabolic function of TN/TX. Importantly, inhibition of TN/TX can, at least in part, reverse these defects.
Inhibition of TN/TX rescues dicer haploinsufficiency
Laboratory and clinical studies have established dicer as a haploinsufficient tumor suppressor (Kumar et al., 2007; Merritt et al., 2008; Kumar et al., 2009; Hill et al., 2009). Our findings of a catabolic cause of miRNA loss in dicer deficiency raised the possibility that TN/TX may also underlie loss of tumor suppressor activity. In HCT116 colon carcinoma cells, Dicer did not exhibit tumor suppressor activity. That is, we did not observe differences in cell proliferation or colony formation between dicerwt and dicerex5 cells and importantly, these outcomes were also unaltered by TN/TX (Figure S2D and S2E).
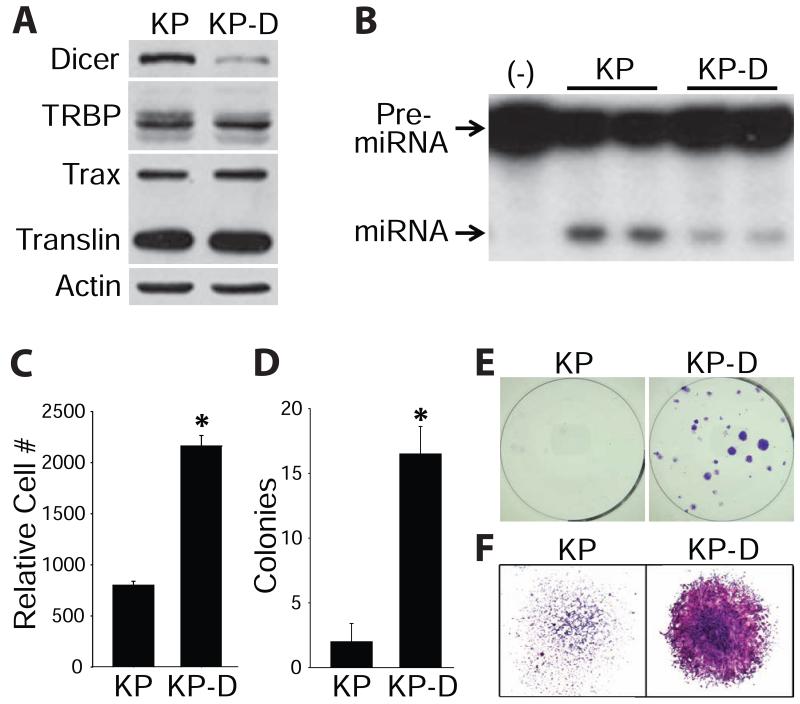
To determine if TN/TX promotes loss of tumor suppressor activity in dicer deficiency, we made use of a previously established model wherein Dicer functions as a tumor suppressor (Kumar et al., 2009; Ravi et al., 2012; Mito et al., 2013). KrasG12D; p53−/− sarcoma cells encoding either dicer+/+ (KP) or dicer+/− (KP-D) were isolated from primary mouse tumors (Mito et al., 2013). Monoallelic deletion of dicer was reflected in lower levels of Dicer and miRNA-generating activity in KP-D versus KP cells (Figure 6A and 6B). KP-D cells proliferated more rapidly (Figure 6C), formed colonies more efficiently (Figure 6D and 6E) and more densely relative to KP cells (Figure 6F). These findings are consistent with impaired tumor suppressor function of dicer in KP-D versus KP cells and mirror the more aggressive pathology observed for KP-D versus KP tumors (Kumar et al., 2009; Ravi et al., 2012; Mito et al., 2013).
Figure 6.
Dicer functions as a haploinsufficient tumor suppressor in KP sarcoma cells.
KrasG12D; p53−/− mouse sarcoma cells encoding either dicer+/+ (KP) or dicer+/− (KP-D).
(A) Immunoblots and (B) in vitro miRNA-generating activity from KP and KP-D cells.
(C) For proliferation studies, cells were plated, incubated overnight and baseline counts taken the next day (0 hours). Cell populations at 72 hours were normalized to those at 0 hours. * indicates greater cell numbers for KP-D versus KP cells (p < 0.0001; n = 3; means ± SD).
(D and E) Cells were plated at clonal density (500 cells per 6-well plate) and incubated for 8 days. Colonies were fixed and visualized with crystal violet. * indicates greater colony formation for KP-D versus KP cells (p < 0.0001; n = 3; means ± SD).
(F) Representative images of colony density at 2.5× magnification.
We established stable knockdown of TN/TX using lentivirus-mediated shRNA expression in KP and KP-D cells (Figure 7A and 7B). Similar to HCT116 cells, depletion of TN and TX attenuated pre-miRNA degradation and enhanced miRNA-generating activity for both KP and KP-D cells (Figure 7C and 7D). miRNA profiling also revealed patterns similar to those for HCT116 cells. In dicer+/+ KP cells, TN/TX had only minor influence on miRNA expression (Figure 7E, blue bars). However, in dicer deficient KP-D cells, miRNA that were most strongly downregulated relative to KP cells, were those most markedly suppressed by TN/TX (Figure 7E and 7F; Table S5). miRNA-specific assays supported these profiling studies (Figure S3).
Figure 7.
Inhibition of TN/TX rescues loss of both miRNA and tumor suppressor activity in dicer haploinsufficiency.
KrasG12D; p53−/− mouse sarcoma cells encoding either dicer+/+ (KP) or dicer+/− (KP-D) were used to generate sub-cell lines stably expressing shRNA against luciferase (shluc), translin (shTN) or trax (shTX).
(A) Immunoblotting performed with extracts from KP and (B) KP-D cells.
(C) Pre-miRNA processing assays performed with 20 μg of extract from KP and (D) KP-D cells.
(E) Histogram summary of miRNA profiling studies. Data are presented as ratios of miRNA expression for shTN relative to shluc for KP (blue bars) and KP-D cells (red bars). These values represent the degree of miRNA suppression by TN/TX. The dashed line indicates equivalent miRNA levels for shTN and shluc. See also Table S5.
(F) Scatter plot representation of miRNA responsiveness to Dicer and TN/TX. Data are fold change for miRNA downregulated in KP-D versus KP cells (horizontal axis) and shTN versus shluc for KP-D cells (vertical axis). Closed circles represent miRNA with two-fold or greater decrease in KP-D versus KP. Open circles represent miRNA with less than two-fold decrease in KP-D versus KP. The correlation coefficient of the regression line for all data points is 0.89 (p < 0.0001).
(G) For proliferation studies, cells were plated, incubated overnight and baseline counts taken the next day (0 hours). Cell populations at 72 hours were normalized to those at 0 hours. * indicates higher cell counts for shluc versus shTN and shTX in KP-D cells (p < 0.003; n = 3; means ± SD).
(E) Cells were plated at clonal density (500 cells per 6-well plate) and incubated for 8 days. * indicates greater colony formation for shluc versus shTN and shTX in KP-D cells (p < 0.025; n = 3; means ± SD).
(F) Representative images of colony density at 2.5× magnification.
Importantly, TN/TX promoted loss of tumor suppressor activity in dicer deficient KP-D cells. Inhibition of TN/TX reduced cell proliferation in KP-D, but not KP cells (Figure 7G). Similarly, depletion of TN/TX attenuated efficiency and density of colony formation in KP-D cells (Figure 7H and 7I). Collectively, these findings indicate that loss of both miRNA and tumor suppressor function with dicer haploinsufficiency was due to the combined loss of miRNA-generating activity and TN/TX. Notably, inhibition of TN/TX, at least in part, reversed these defects.
DISCUSSION
Human diseases are increasingly linked to genetic defects in dicer. Loss of miRNA in dicer deficiency is thought to be due to loss of miRNA-generating activity. In the current study, we demonstrate that loss of miRNA in dicer deficiency is due to both the expected loss of miRNA-generating activity and TN/TX. This catabolic mechanism, integral to dicer deficiency, advances understanding of miRNA depletion in tumor development and may represent a general mechanism for other dicer disorders.
Wholesale miRNA depletion promotes tumor development through de-repression of proliferative and embryonic cellular programming (Lu et al., 2005; Kanellopoulou et al., 2005; Thomson et al., 2006; Wang et al 2007). Varied mechanisms contributing to miRNA downregulation in diverse tumor types have been reported (He et al., 2007; Chang et al., 2008; Heo et al., 2008; Viswanathan et al., 2008; Hagan et al., 2009; Heo et al., 2009; Paroo et al., 2009; Viswanathan et al., 2009; Martello et al., 2010; Xhemalce et al., 2012; Chang et al., 2013; Mori et al., 2014). However, genetic defects of the miRNA-generating machinery represents a general mechanism. Indeed, loss of function mutations have been reported for trbp (Melo et al., 2009), exportin-5 (Melo et al., 2010) and dicer (Hill et al., 2009; Foulkes et al., 2011; Rio Frio et al., 2011; Heravi-Moussavi et al., 2012). However, these mutations are very rare. In contrast, hemizygous deletion of dicer is frequently found across tumor types (Table S1; Kumar et al., 2009). Our studies reveal that loss of both miRNA and tumor suppression with dicer haploinsufficiency is due to the expected loss of miRNA-generating activity and complementary catabolic function of TN/TX.
Historically, TN/TX was known to function as a non-catalytic nucleic acid interacting complex. Advanced structural modeling suggested the presence of a possible nucleolytic motif (Liu et al., 2009). Biochemical studies established TN/TX as an endonuclease with a role in siRNA programming of Argonaute2 (Liu et. al., 2009; Ye et al., 2011). However, this function of TN/TX may be limited to specific experimental contexts. For example, TN/TX was not found to influence siRNA-mediated silencing in Neurospora (Li et al., 2012). Similarly, we found no role for TN/TX (or Dicer) in promoting siRNA-induced silencing in HCT116 dicerwt, dicerex5, KP or KP-D cells (Table S6).
A key finding of our studies was rescuing loss of both miRNA and tumor suppressor activity with dicer haploinsufficiency through inhibition of TN/TX. As is the case for some nucleases, TN/TX may act on multiple classes of substrates. For example, TN/TX was found to influence tRNA processing in Neurospora and mouse embryonic fibroblasts (Li et al., 2012). Importantly, the cellular effects of TN/TX reported here were entirely Dicer-dependent. First, TN/TX did not alter cell proliferation or colony formation in fully functional dicer contexts including dicerwt HCT116 colon carcinoma cells or dicer+/+ KP sarcoma cells. Second, Dicer did not exhibit tumor suppressor activity in HCT116 cells and despite broadly suppressing miRNA in dicerex5 cells, TN/TX exhibited little influence on cell behavior. Third, loss of tumor suppressor activity in dicer deficient KP-D sarcoma cells was reversed by TN/TX inhibition. Collectively, our findings indicate that the molecular and cellular functions of TN/TX specifically oppose those of Dicer and are integral to loss of both miRNA and tumor suppressor function with dicer insufficiency.
In advancing understanding of the mechanisms driving miRNA depletion in dicer deficiency, our studies reveal a possible therapeutic solution. Although there is much hope for oligonucleotide-based miRNA therapeutics, pharmacodynamic challenges persist. In contrast, TN/TX represents a potentially druggable target for restoring global miRNA function for dicer disorders. Moreover, as TN/TX exhibited limited effects in wild type dicer contexts, such therapy may enable selective action in dicer impaired cells for the treatment of dicer deficiencies including aging, cancer, diabetes and neurodegeneration.
EXPERIMENTAL PROCEDURES
General procedures
Antibodies against Actin, Dicer, Translin and Trax were obtained from Abcam. TRBP antiserum was raised against purified full length recombinant TRBP. Site directed mutagenesis was performed using the "QuikChange" system from Stratagene. RNase A and RNase T1 were purchased from Invitrogen.
Pre-miRNA processing and pre-miRNA complex formation assays
Synthetic pre-miRNA and sh-miRNA were synthesized by Dharmacon and IDT. Pre-miRNA was 5` end-labeled with γ-P32 ATP using T4 polynucleotide kinase (NEB) and PAGE purified. Assays were performed with pre-miR-16 except for experiments reported in Figure 3 as specified. Assays were conducted in 100 mM KCl, 20 mM Tris, 3 mM MgCl2 at 37 °C for 15 min. RNA was phenol-chloroform extracted, ethanol precipitated and resolved on 7 M urea, 16% polyacrylamide gels. Pre-miRNA complex formation assays were performed as in vitro miRNA-generating assays but without MgCl2 and with 2.5 mM EDTA to prevent substrate cleavage. Following incubation, reactions were immediately loaded on to 4.5 % native polyacrylamide gels.
Fractionation and purification of pre-miRNA degrading activity
HeLa cell pellet was obtained from National Cell Culture Center. Ammonium sulfate precipitation was performed by saturating HeLa S100 extract with 40% salt. Following rotation for one hour at 4 °C, soluble and pellet fractions were resolved by centrifugation at 20,000 × g at 4 °C for 20 minutes. The pellet fraction was resuspended in the original volume with buffer A. Following dialysis, equal volumes of each fraction were used for pre-miRNA processing assays.
Fifty liters of culture was used to develop the purification scheme and twenty-five liters of culture was used for the final purification procedure. Cells were lysed in four times pellet volume of buffer A with protease inhibitors including 1 mM Pefabloc SC, 5 μg/ml Leupeptine, and 0.7 μg/ml Pepstatin (Roche). After sitting on ice for 20 minutes, cells were broken with 40 strokes in a douncer followed by a 20,000g spin at 4 °C for 30 minutes. The supernatant was further centrifuged at 100,000g for one hour at 4 °C to make S100.
All purification steps were carried out at 4°C. All columns were purchased from Pharmacia except for the fluoroapatite column which was obtained from BioRad. S100 was precipitated by ammonium sulfate at 40% saturation. After a 30-min 20,000g spin, the supernatant was diluted with 20% ammonium sulfate and loaded on to a 10 ml HiTrap phenyl column over 14 column runs. Pre-miRNA degrading activity was eluted with 7% ammonium sulfate and fractions were precipitated by 60% ammonium sulfate saturation. After a 30-min 20,000g spin, the pellet was resuspended in 20% ammonium sulfate and loaded on to a 10 ml HiTrap octyl column over three column runs. Pre-miRNA degrading activity was eluted with 8% ammonium sulfate and loaded on to a 26/60 Superdex200 column. Fractions were assayed for pre-miRNA processing and active fractions were loaded on to a 5 ml fluoroapatite column. Peak activity was eluted between 70 and 100 mM potassium phosphate and directly loaded on a Q sepharose column. Fractions were assayed for pre-miRNA degrading activity, resolved on SDS-polyacrylamide gels and silver stained (Invitrogen). Bands depicted in Figure 2C were excised with a razor blade and destained. Protein identification was performed by Applied Biomics.
Preparation of recombinant protein complexes
Wild type and catalytic mutant (E126A) His-Trax and Translin complexes were co-expressed in BL21 cells using the pETDuet-1 vector. Cultures were expanded at 37°C to an OD600 of 0.4. Induction was carried out in 1 mM IPTG for 10 h at 16°C. Cells were harvested and lysed by sonication in 20 mM Tris-HCl, pH 8.0, 50 mM NaCl, 5 mM ß-mercaptoethanol and protease inhibitors. S20 (20,000g supernatant) lysates were incubated with Ni-NTA beads (Qiagen) overnight, loaded on to a collecting column and washed sequentially with 75 vol buffer B (buffer A + 1 M NaCl) and 25 vol buffer A containing 20 mM imidazole. After a final 50 mM imidazole wash, proteins were eluted in Buffer A + 250 mM imidazole. Complexes were further purified by Q sepharose and Superdex 200 columns and stored in aliquots containing 10% glycerol at −80 °C. Recombinant Dicer/TRBP was prepared as described (Paroo et al., 2009).
Animal procedures
A colony of translin −/− mice was established at Johns Hopkins from the line generated in Dr. M. Kasai’s laboratory (Fukuda et al., 2008). These mice had been backcrossed to C57/BL6 for over 10 generations. The translin KO mice (Nbio055) were provided by the JCRB Laboratory Animal Resource Bank of the National Institute of Biomedical Innovation (Osaka, Japan). Genotyping of mice was performed on DNA isolated from tail snips. PCR was conducted using the primers and conditions described by Fukuda et al. (Fukuda et al., 2008). 3-4 month old male mice were sacrificed by decapitation with a small animal guillotine. Cerebella were rapidly removed and frozen on a dry ice/ethanol slurry. Animal housing and procedures adhered to IACUC guidelines.
shRNA, lentivirus and cell culture procedures
DNA oligos for shRNA were cloned in to the pGreenPuro vector (SBI). Four sequences against each of TN and TX were tested for knockdown efficiency in transient transfection studies. Transfections were performed using Lipofectamine 2000 (Invitrogen). The most potent sequence for each was selected for lentivirus production. Sequences used were as follows:
shluc1: 5` gatcgatttcgagtcgtcttaatttcaagagaattaagacgactcgaaatctttttg 3`;
shluc2: 5` gatcgattatgtccggttatgtattcaagagatacataaccggacataatctttttg 3`;
shTN (human/mouse): 5` gatctgctaaacactgcgctttatttcaagagaataaagcgcagtgtttagcatttttg 3`;
shTX (human): 5` gatcgctttcctattctagcatttattcaagagataaatgctagaataggaaagctttttg 3`.
shTX (mouse): 5'- gatctgtatatgtgctcgctctattttcaagagaaatagagcgagcacatatacatttttg 3`.
shRNA vectors were co-transfected with packaging vectors (SBI) in 293TN cells. Pseudoviral particles were collected at 48 and 72 hours post-transfection and concentrated. Viral titers were assessed in target cells using the Global UltraRapid Lentiviral Titering kit (SBI). To achieve single copy integration per cell, cells were transduced with viral particles and Transdux (SBI) at a multiplicity of infection (MOI) of 0.25. After 72 hours, cell selection was initiated with growth media containing 2 μg/ml puromycin for ten days.
For phenotypic assays, cells were trypsinized, washed with PBS and resuspended in DMEM containing 0% FBS. For proliferation assays, 500 cells were added to 96-well plates containing 100 μl of media with a 2× serum concentration (10% FBS for HCT116 cells, 5% FBS for sarcoma cells) in a volume of 100 μl. Cells were incubated overnight and counted the next day for baseline readings. Counts at 72 hours were normalized to those at 0 hours. Cell counts were assayed using a Cell Titer Glo luminescent cell viability assay (Promega). For colony formation assays, 500 cells were added to 6-well plates containing the minimum serum concentration required for clonal growth (5% FBS for HCT116 cells, 2.5% FBS for sarcoma cells). Colonies were typically cultured for 8 days, fixed, stained with crystal violet and clones greater than 1 mm in diameter counted.
miRNA analysis
As cell culture parameters have been shown to influence miRNA expression (Hwang et al., 2009, Kim et al., 2011), RNA was prepared from cultures at 60% confluency and 48 hours post-plating. Total RNA was isolated using Trizol (Invitrogen). miRNA profiling was performed by Exiqon using miRCURY LNA Universal RT miRNA PCR human or mouse panel I. For cell lines, analysis was performed using pooled replicates for each experimental group. Statistical analyses were performed by Exiqon.
miRNA qPCR was performed using the Universal cDNA synthesis kit II, LNA PCR primer sets and ExiLENT SYBR green master mix (Exiqon). Small RNA northern blotting was performed with 10 μg of total RNA from each mouse cerebellum. RNA was resolved on 7 M urea, 16% polyacrylamide gels, transferred to Zeta-Probe membrane (Bio-Rad) and subjected to UV crosslinking. Membranes were prehybridized using Ultrahyb-Oligo buffer (Invitrogen) at 42 °C. Hybridization was conducted at 42 °C overnight followed by two washes in 2× SSC and 0.1% SDS and one wash in 0.5× SSC and 0.1% SDS. RNA oligos were used as probes for hybridization. Oligos were 5`-end-labeled with γ-32P-ATP by T4 polynucleotide kinase (NEB). Probes were stripped by pouring boiling 0.1× SSC and 0.1% SDS over membranes.
Silencing assays
miRNA-mediated silencing assays were conducted by co-transfecting cells with constructs encoding either firefly luciferase (FL) or firefly luciferase under the control of miR-21 (FL-21) and Renilla luciferase (RL) as a transfection control (Yi et al., 2003). Reporter activity was assayed using a Dual Luciferase Reporter System (Promega). Reporter expression was determined by the ratio of (FL-21 / RL) to (FL / RL). siRNA-induced silencing assays were conducted by co-transfecting cells with constructs encoding FL, RL and 0 to 1 nM siRNA against FL. FL activity was normalized to RL and percent inhibition was plotted against siRNA concentration.
Statistical Analysis
Experiments were typically run in triplicate and repeated in a minimum of three independent trials. Image quantitation was performed using ImageJ analysis software (NIH). Data are represented as means ± standard deviation, unless otherwise stated. Student’s t-tests were employed where the minimum level of significance was p < 0.05. Regression analyses and IC50 calculations were performed with Sigma Plot 11.0.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Dr. Bert Vogelstein for the generous gift of dicerwt and dicerex5 colon carcinoma cells. We thank Dr. Bryan Cullen for sharing miRNA reporter constructs. We thank Dr. Mollie Meffert and Dr. Larisa Nonn for providing critical feedback and helpful suggestions. We thank Drs. Eiji Furuta and Ghata Singhal for technical assistance. This work was supported by grants from the National Institutes of Health (DA-00266 to J.B.) and American Cancer Society (279336 to Z.P.). Z.P. is a Junior Investigator of the Chicago Biomedical Consortium with support from The Searle Funds at The Chicago Community Trust (R-005).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Bamford S, Dawson E, Forbes S, Clements J, Pettett R, Dogan A, Flanagan A, Teague J, Futreal PA, Stratton MR, et al. The COSMIC (Catalogue of Somatic Mutations in Cancer) database and website. Br J Cancer. 2004;91:355–358. doi: 10.1038/sj.bjc.6601894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein E, Caudy AA, Hammond SM, Hannon GJ. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature. 2001;409:363–366. doi: 10.1038/35053110. [DOI] [PubMed] [Google Scholar]
- Bernstein E, Kim SY, Carmell MA, Murchison EP, Alcorn H, Li MZ, Mills AA, Elledge SJ, Anderson KV, Hannon GJ. Dicer is essential for mouse development. Nature genetics. 2003;35:215–217. doi: 10.1038/ng1253. [DOI] [PubMed] [Google Scholar]
- Chang HM, Triboulet R, Thornton JE, Gregory RI. A role for the Perlman syndrome exonuclease Dis3l2 in the Lin28-let-7 pathway. Nature. 2013;497:244–248. doi: 10.1038/nature12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang TC, Yu D, Lee YS, Wentzel EA, Arking DE, West KM, Dang CV, Thomas-Tikhonenko A, Mendell JT. Widespread microRNA repression by Myc contributes to tumorigenesis. Nature genetics. 2008;40:43–50. doi: 10.1038/ng.2007.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S, Grosshans H. Active turnover modulates mature microRNA activity in Caenorhabditis elegans. Nature. 2009;461:546–549. doi: 10.1038/nature08349. [DOI] [PubMed] [Google Scholar]
- Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chennathukuzhi V, Stein JM, Abel T, Donlon S, Yang S, Miller JP, Allman DM, Simmons RA, Hecht NB. Mice deficient for testis-brain RNA-binding protein exhibit a coordinate loss of TRAX, reduced fertility, altered gene expression in the brain, and behavioral changes. Mol Cell Biol. 2003;23:6419–6434. doi: 10.1128/MCB.23.18.6419-6434.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins JM, He Y, Leary RJ, Pagliarini R, Diaz LA, Jr., Sjoblom T, Barad O, Bentwich Z, Szafranska AE, Labourier E, et al. The colorectal microRNAome. Proc Natl Acad Sci U S A. 2006;103:3687–3692. doi: 10.1073/pnas.0511155103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denli AM, Tops BB, Plasterk RH, Ketting RF, Hannon GJ. Processing of primary microRNAs by the Microprocessor complex. Nature. 2004;432:231–235. doi: 10.1038/nature03049. [DOI] [PubMed] [Google Scholar]
- Dvinge H, Git A, Graf S, Salmon-Divon M, Curtis C, Sottoriva A, Zhao Y, Hirst M, Armisen J, Miska EA, et al. The shaping and functional consequences of the microRNA landscape in breast cancer. Nature. 2013;497:378–382. doi: 10.1038/nature12108. [DOI] [PubMed] [Google Scholar]
- Foulkes WD, Bahubeshi A, Hamel N, Pasini B, Asioli S, Baynam G, Choong CS, Charles A, Frieder RP, Dishop MK, et al. Extending the phenotypes associated with DICER1 mutations. Hum Mutat. 2011;32:1381–1384. doi: 10.1002/humu.21600. [DOI] [PubMed] [Google Scholar]
- Fukuda Y, Ishida R, Aoki K, Nakahara K, Takashi T, Mochida K, Suzuki O, Matsuda J, Kasai M. Contribution of Translin to hematopoietic regeneration after sublethal ionizing irradiation. Biol Pharm Bull. 2008;31:207–211. doi: 10.1248/bpb.31.207. [DOI] [PubMed] [Google Scholar]
- Gregory RI, Yan KP, Amuthan G, Chendrimada T, Doratotaj B, Cooch N, Shiekhattar R. The Microprocessor complex mediates the genesis of microRNAs. Nature. 2004;432:235–240. doi: 10.1038/nature03120. [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S. The microRNA Registry. Nucleic Acids Res. 2004;32:D109–D111. doi: 10.1093/nar/gkh023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase AD, Jaskiewicz L, Zhang H, Laine S, Sack R, Gatignol A, Filipowicz W. TRBP, a regulator of cellular PKR and HIV-1 virus expression, interacts with Dicer and functions in RNA silencing. EMBO reports. 2005;6:961–967. doi: 10.1038/sj.embor.7400509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan JP, Piskounova E, Gregory RI. Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat Struct Mol Biol. 2009;16:1021–1025. doi: 10.1038/nsmb.1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond SM, Boettcher S, Caudy AA, Kobayashi R, Hannon GJ. Argonaute2, a link between genetic and biochemical analyses of RNAi. Science. 2001;293:1146–1150. doi: 10.1126/science.1064023. [DOI] [PubMed] [Google Scholar]
- Han J, Lee Y, Yeom KH, Kim YK, Jin H, Kim VN. The Drosha-DGCR8 complex in primary microRNA processing. Genes & development. 2004;18:3016–3027. doi: 10.1101/gad.1262504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, He X, Lim LP, de Stanchina E, Xuan Z, Liang Y, Xue W, Zender L, Magnus J, Ridzon D, et al. A microRNA component of the p53 tumour suppressor network. Nature. 2007;447:1130–1134. doi: 10.1038/nature05939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heo I, Joo C, Cho J, Ha M, Han J, Kim VN. Lin28 mediates the terminal uridylation of let-7 precursor MicroRNA. Mol Cell. 2008;32:276–284. doi: 10.1016/j.molcel.2008.09.014. [DOI] [PubMed] [Google Scholar]
- Heo I, Joo C, Kim YK, Ha M, Yoon MJ, Cho J, Yeom KH, Han J, Kim VN. TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell. 2009;138:696–708. doi: 10.1016/j.cell.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Heravi-Moussavi A, Anglesio MS, Cheng SW, Senz J, Yang W, Prentice L, Fejes AP, Chow C, Tone A, Kalloger SE, et al. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N Engl J Med. 2012;366:234–242. doi: 10.1056/NEJMoa1102903. [DOI] [PubMed] [Google Scholar]
- Hill DA, Ivanovich J, Priest JR, Gurnett CA, Dehner LP, Desruisseau D, Jarzembowski JA, Wikenheiser-Brokamp KA, Suarez BK, Whelan AJ, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. doi: 10.1126/science.1174334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutvagner G, McLachlan J, Pasquinelli AE, Balint E, Tuschl T, Zamore PD. A cellular function for the RNA-interference enzyme Dicer in the maturation of the let-7 small temporal RNA. Science. 2001;293:834–838. doi: 10.1126/science.1062961. [DOI] [PubMed] [Google Scholar]
- Hwang HW, Wentzel EA, Mendell JT. Cell-cell contact globally activates microRNA biogenesis. Proc Natl Acad Sci U S A. 2009;106:7016–7021. doi: 10.1073/pnas.0811523106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanellopoulou C, Muljo SA, Kung AL, Ganesan S, Drapkin R, Jenuwein T, Livingston DM, Rajewsky K. Dicer-deficient mouse embryonic stem cells are defective in differentiation and centromeric silencing. Genes & development. 2005;19:489–501. doi: 10.1101/gad.1248505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy S, Wang D, Ruvkun G. A conserved siRNA-degrading RNase negatively regulates RNA interference in C. elegans. Nature. 2004;427:645–649. doi: 10.1038/nature02302. [DOI] [PubMed] [Google Scholar]
- Kim YK, Yeo J, Ha M, Kim B, Kim VN. Cell adhesion-dependent control of microRNA decay. Mol Cell. 2011;43:1005–1014. doi: 10.1016/j.molcel.2011.07.031. [DOI] [PubMed] [Google Scholar]
- Kumar MS, Lu J, Mercer KL, Golub TR, Jacks T. Impaired microRNA processing enhances cellular transformation and tumorigenesis. Nature genetics. 2007;39:673–677. doi: 10.1038/ng2003. [DOI] [PubMed] [Google Scholar]
- Kumar MS, Pester RE, Chen CY, Lane K, Chin C, Lu J, Kirsch DG, Golub TR, Jacks T. Dicer1 functions as a haploinsufficient tumor suppressor. Genes & development. 2009;23:2700–2704. doi: 10.1101/gad.1848209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambertz I, Nittner D, Mestdagh P, Denecker G, Vandesompele J, Dyer MA, Marine JC. Monoallelic but not biallelic loss of Dicer1 promotes tumorigenesis in vivo. Cell Death Differ. 2010;17:633–641. doi: 10.1038/cdd.2009.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee EJ, Baek M, Gusev Y, Brackett DJ, Nuovo GJ, Schmittgen TD. Systematic evaluation of microRNA processing patterns in tissues, cell lines, and tumors. RNA. 2008;14:35–42. doi: 10.1261/rna.804508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Ahn C, Han J, Choi H, Kim J, Yim J, Lee J, Provost P, Radmark O, Kim S, et al. The nuclear RNase III Drosha initiates microRNA processing. Nature. 2003;425:415–419. doi: 10.1038/nature01957. [DOI] [PubMed] [Google Scholar]
- Lee Y, Hur I, Park SY, Kim YK, Suh MR, Kim VN. The role of PACT in the RNA silencing pathway. The EMBO journal. 2006;25:522–532. doi: 10.1038/sj.emboj.7600942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Nakahara K, Pham JW, Kim K, He Z, Sontheimer EJ, Carthew RW. Distinct roles for Drosophila Dicer-1 and Dicer-2 in the siRNA/miRNA silencing pathways. Cell. 2004;117:69–81. doi: 10.1016/s0092-8674(04)00261-2. [DOI] [PubMed] [Google Scholar]
- Li L, Gu W, Liang C, Liu Q, Mello CC, Liu Y. The translin-TRAX complex (C3PO) is a ribonuclease in tRNA processing. Nat Struct Mol Biol. 2012;19:824–830. doi: 10.1038/nsmb.2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, Hammond SM, Joshua-Tor L, Hannon GJ. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- Liu Y, Ye X, Jiang F, Liang C, Chen D, Peng J, Kinch LN, Grishin NV, Liu Q. C3PO, an endoribonuclease that promotes RNAi by facilitating RISC activation. Science. 2009;325:750–753. doi: 10.1126/science.1176325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Getz G, Miska EA, Alvarez-Saavedra E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. doi: 10.1126/science.1090599. [DOI] [PubMed] [Google Scholar]
- Maillot G, Lacroix-Triki M, Pierredon S, Gratadou L, Schmidt S, Benes V, Roche H, Dalenc F, Auboeuf D, Millevoi S, et al. Widespread estrogen-dependent repression of micrornas involved in breast tumor cell growth. Cancer Res. 2009;69:8332–8340. doi: 10.1158/0008-5472.CAN-09-2206. [DOI] [PubMed] [Google Scholar]
- Martello G, Rosato A, Ferrari F, Manfrin A, Cordenonsi M, Dupont S, Enzo E, Guzzardo V, Rondina M, Spruce T, et al. A MicroRNA targeting dicer for metastasis control. Cell. 2010;141:1195–1207. doi: 10.1016/j.cell.2010.05.017. [DOI] [PubMed] [Google Scholar]
- Meister G, Landthaler M, Patkaniowska A, Dorsett Y, Teng G, Tuschl T. Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell. 2004;15:185–197. doi: 10.1016/j.molcel.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Melo SA, Moutinho C, Ropero S, Calin GA, Rossi S, Spizzo R, Fernandez AF, Davalos V, Villanueva A, Montoya G, et al. A genetic defect in exportin-5 traps precursor microRNAs in the nucleus of cancer cells. Cancer Cell. 2010;18:303–315. doi: 10.1016/j.ccr.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Melo SA, Ropero S, Moutinho C, Aaltonen LA, Yamamoto H, Calin GA, Rossi S, Fernandez AF, Carneiro F, Oliveira C, et al. A TARBP2 mutation in human cancer impairs microRNA processing and DICER1 function. Nature genetics. 2009;41:365–370. doi: 10.1038/ng.317. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Merritt WM, Lin YG, Han LY, Kamat AA, Spannuth WA, Schmandt R, Urbauer D, Pennacchio LA, Cheng JF, Nick AM, et al. Dicer, Drosha, and outcomes in patients with ovarian cancer. N Engl J Med. 2008;359:2641–2650. doi: 10.1056/NEJMoa0803785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mito JK, Min HD, Ma Y, Carter JE, Brigman BE, Dodd L, Dankort D, McMahon M, Kirsch DG. Oncogene-dependent control of miRNA biogenesis and metastatic progression in a model of undifferentiated pleomorphic sarcoma. J Pathol. 2013;229:132–140. doi: 10.1002/path.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori M, Triboulet R, Mohseni M, Schlegelmilch K, Shrestha K, Camargo FD, Gregory RI. Hippo signaling regulates microprocessor and links cell-density-dependent miRNA biogenesis to cancer. Cell. 2014;156:893–906. doi: 10.1016/j.cell.2013.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nittner D, Lambertz I, Clermont F, Mestdagh P, Kohler C, Nielsen SJ, Jochemsen A, Speleman F, Vandesompele J, Dyer MA, et al. Synthetic lethality between Rb, p53 and Dicer or miR-17-92 in retinal progenitors suppresses retinoblastoma formation. Nat Cell Biol. 2012;14:958–965. doi: 10.1038/ncb2556. [DOI] [PubMed] [Google Scholar]
- Ozen M, Creighton CJ, Ozdemir M, Ittmann M. Widespread deregulation of microRNA expression in human prostate cancer. Oncogene. 2008;27:1788–1793. doi: 10.1038/sj.onc.1210809. [DOI] [PubMed] [Google Scholar]
- Paroo Z, Ye X, Chen S, Liu Q. Phosphorylation of the human microRNA-generating complex mediates MAPK/Erk signaling. Cell. 2009;139:112–122. doi: 10.1016/j.cell.2009.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V, Chen X. Degradation of microRNAs by a family of exoribonucleases in Arabidopsis. Science. 2008;321:1490–1492. doi: 10.1126/science.1163728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi A, Gurtan AM, Kumar MS, Bhutkar A, Chin C, Lu V, Lees JA, Jacks T, Sharp PA. Proliferation and tumorigenesis of a murine sarcoma cell line in the absence of DICER1. Cancer Cell. 2012;21:848–855. doi: 10.1016/j.ccr.2012.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rio Frio T, Bahubeshi A, Kanellopoulou C, Hamel N, Niedziela M, Sabbaghian N, Pouchet C, Gilbert L, O'Brien PK, Serfas K, et al. DICER1 mutations in familial multinodular goiter with and without ovarian Sertoli-Leydig cell tumors. JAMA. 2011;305:68–77. doi: 10.1001/jama.2010.1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki HI, Arase M, Matsuyama H, Choi YL, Ueno T, Mano H, Sugimoto K, Miyazono K. MCPIP1 ribonuclease antagonizes dicer and terminates microRNA biogenesis through precursor microRNA degradation. Mol Cell. 2011;44:424–436. doi: 10.1016/j.molcel.2011.09.012. [DOI] [PubMed] [Google Scholar]
- Thomson JM, Newman M, Parker JS, Morin-Kensicki EM, Wright T, Hammond SM. Extensive post-transcriptional regulation of microRNAs and its implications for cancer. Genes & development. 2006;20:2202–2207. doi: 10.1101/gad.1444406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y, Simanshu DK, Ascano M, Diaz-Avalos R, Park AY, Juranek SA, Rice WJ, Yin Q, Robinson CV, Tuschl T, et al. Multimeric assembly and biochemical characterization of the Trax-translin endonuclease complex. Nat Struct Mol Biol. 2011;18:658–664. doi: 10.1038/nsmb.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upton JP, Wang L, Han D, Wang ES, Huskey NE, Lim L, Truitt M, McManus MT, Ruggero D, Goga A, et al. IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science. 2012;338:818–822. doi: 10.1126/science.1226191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan SR, Daley GQ, Gregory RI. Selective blockade of microRNA processing by Lin28. Science. 2008;320:97–100. doi: 10.1126/science.1154040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan SR, Powers JT, Einhorn W, Hoshida Y, Ng TL, Toffanin S, O'Sullivan M, Lu J, Phillips LA, Lockhart VL, et al. Lin28 promotes transformation and is associated with advanced human malignancies. Nature genetics. 2009;41:843–848. doi: 10.1038/ng.392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Medvid R, Melton C, Jaenisch R, Blelloch R. DGCR8 is essential for microRNA biogenesis and silencing of embryonic stem cell self-renewal. Nature genetics. 2007;39:380–385. doi: 10.1038/ng1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YC, Williamson R, Li Z, Vicario A, Xu J, Kasai M, Chern Y, Tongiorgi E, Baraban JM. Dendritic trafficking of brain-derived neurotrophic factor mRNA: regulation by translin-dependent and -independent mechanisms. J Neurochem. 2011;116:1112–1121. doi: 10.1111/j.1471-4159.2010.07166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xhemalce B, Robson SC, Kouzarides T. Human RNA methyltransferase BCDIN3D regulates microRNA processing. Cell. 2012;151:278–288. doi: 10.1016/j.cell.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye X, Huang N, Liu Y, Paroo Z, Huerta C, Li P, Chen S, Liu Q, Zhang H. Structure of C3PO and mechanism of human RISC activation. Nat Struct Mol Biol. 2011;18:650–657. doi: 10.1038/nsmb.2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi R, Qin Y, Macara IG, Cullen BR. Exportin-5 mediates the nuclear export of pre-microRNAs and short hairpin RNAs. Genes & development. 2003;17:3011–3016. doi: 10.1101/gad.1158803. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.