Abstract
Background
Patients with heart failure (HF) have high rates of cognitive impairment and depressive symptoms. Depressive symptoms have been associated with greater cognitive impairments in HF; however, it is not known whether particular clusters of depressive symptoms are more detrimental to cognition than others.
Objective
To identify whether somatic and/or nonsomatic depressive symptom clusters were associated with cognitive function in persons with HF.
Methods
Participants were 326 HF patients (40.5% female, 26.7% race-ethnicity, aged 68.6±9.7 years). Depressive symptoms were measured using a depression questionnaire commonly used in medical populations: the Patient Health Questionnatire-9 (PHQ-9). Somatic and Nonsomatic subscales scores were created using previous factor analytic results. A neuropsychological battery tested attention, executive function, and memory. Composites were created using averages of age-adjusted scaled scores. Regressions adjusting for demographic and clinical factors were conducted.
Results
Regressions revealed that PHQ-9 Total was associated with Attention (β=−.14, p=.008) and Executive Function (β=−.17, p=.001). When analyzed separately, the Nonsomatic subscale – but not the Somatic symptoms subscale (ps ≥.092) – was associated with Attention scores (β=−.15, p=.004) and Memory (β=−.11, p=.044). Both Nonsomatic (β=−.18, p<.001) and Somatic symptoms (β=−.11, p=.048) were related to Executive Function. When included together, only the Nonsomatic symptom cluster was associated with Attention (β=−.15, p=.020) and Executive Function (β=−.19, p=.003).
Conclusions
Greater overall depressive symptom severity was associated with poorer performance on multiple cognitive domains, an effect driven primarily by the nonsomatic symptoms of depression.
Clinical Implications
These findings suggest that screening explicitly for nonsomatic depressive symptoms may be warranted and that the mechanisms underlying the depression-cognitive function relationship HF are not solely related to sleep or appetite disturbance. Thus, interventions which target patients’ somatic symptoms only (e.g., poor appetite or fatigue) may not yield maximum cognitive benefit compared to a comprehensive treatment which targets depressed mood, anhedonia, and other nonsomatic symptoms.
Keywords: depressive symptoms, cognition, heart failure, neuropsychological testing
Introduction
Patients with heart failure (HF) have been found to have a high prevalence of cognitive impairment (up to 73%).1–3 Sauve and colleagues4 report that HF patients have four times greater risk of cognitive impairment compared to matched community controls. These HF-related cognitive deficits have been implicated in poorer HF outcomes, such as impaired self-care, greater disability, and increased mortality among patients with HF.5, 6 In addition to prevalent cognitive impairment, individuals with HF have also high rates of depressive symptoms7, 8 with 20–50% presenting with elevated depressive symptoms or major depressive disorder.9–12 Depressive symptoms are also associated with adverse HF outcomes, including increased hospital readmission rates and decreased survival.9, 12, 13 Importantly, evidence indicates that cognitive impairment and depressive symptoms are related in patients with HF.14, 15 For example, greater depressive symptoms have been shown to predict poorer cognitive function in persons with HF after controlling for demographic and medical variables.14, 15 Pathways linking depression and cognitive function in HF may involve pathological changes in brain structure, function, and/or perfusion, as individuals with HF demonstrate atrophy, white matter hyperintensities, and cerebral hypoperfusions,14, 16 all factors that are associated with both impaired cognition16–18 and elevated depressive symptoms.19, 20
One limitation of the studies examining the link between cognitive impairment and depressive symptoms in HF is that they typically examine depression as a unidimensional construct. However, evidence suggests that depression is comprised of multiple dimensions, including affective, cognitive, behavioral, and somatic symptoms.21 Indeed, these symptom clusters have been shown to have differential impact on HF outcomes22, 23 as well as other indices of cardiovascular function.24–29 For example, some studies found that the somatic symptoms cluster (e.g., sleep disturbance, appetite changes, and low energy) was the strongest predictor across a variety of outcomes,22, 25, 29 whereas other studies reported similar results for the nonsomatic symptom clusters (e.g., cognitive, affective, or behavioral symptoms).23, 26–28 For example, Schiffer et al.22 found that individuals with high somatic symptom scores had a greater incidence of mortality than those with low scores (31% vs. 15%; hazard ratio = 2.3). Given these conflicting findings and the paucity of studies examining depressive symptom clusters in HF populations, it is not clear whether specific depressive symptom clusters are most damaging to HF outcomes.30 Additionally, no study to date has specifically examined whether certain depressive symptoms clusters are better predictors of cognitive impairment in HF than are others.
Addressing this limitation can help to determine the relative importance of depressive symptom clusters in predicting cognitive impairment in HF and could have significant scientific and clinical implications. First, identifying which clusters are the most detrimental to cognitive function in HF patients could help to identify the mechanisms underlying the relationship between depression and cognitive impairment in HF. Second, systematically targeting the most harmful clusters and their mechanisms could result in more effective HF treatment strategies, which adequately address depression and cognitive deficits. Accordingly, the primary objective of this study was to compare the relative importance of somatic and nonsomatic depressive symptom clusters in predicting cognitive function in a large sample of patients with HF.
Method
Participants of the Heart Failure Adherence, Behavior, and Cognition Study (Heart ABC)
The sample was 326 persons with HF enrolled in the larger, ongoing Heart ABC study.31 Study eligibility requirements were as follows: (1) Aged 50–85 years at enrollment, (2) Documented systolic HF diagnosis within 36 months of study enrollment, (2) physician-documented New York Heart Association class II or III ≥ 3 months duration at the time of study enrollment, (3) No cardiac surgery within last 3 months, (4) No history of neurological disorder or injury (e.g., Alzheimer’s disease, dementia, stroke, seizures), (5) No history of moderate or severe head injury, (6) No past or current history of psychotic disorders, bipolar disorder, learning disorder, developmental disability, renal failure requiring dialysis, or untreated sleep apnea, (7) No current substance abuse or within the past 5 years, and (8) No current use of home tele-health monitoring program for HF. Participants with complete data on the measures of depressive symptoms and cognitive function were selected.
Measures
Depressive Symptoms
Depressive symptoms were assessed using the Patient Health Questionnaire-9 (PHQ-9).32 The PHQ-9 consists of 9 items assessing the following symptoms of depression: (1) anhedonia, (2) depressed mood, (3) sleep difficulties, (4) fatigue, (5) appetite changes, (6) poor self-esteem, (7) concentration problems, (8) psychomotor retardation/agitation, and (9) suicidal ideation. Items are rated 0=not at all, 1=several days, 2=more than half the days, and 3=nearly every day. We calculated the PHQ-9 Total (sum of all items; range: 0–27) and two subscale scores based on previous factor analytic results in patient populations.33, 34 The PHQ-9 Somatic subscale was computed by summing the sleep disturbance, fatigue, and appetite changes items (Items 3–5; range: 0–9), and the PHQ-9 Nonsomatic subscale was computed by summing the remaining six items (Items 1–2 and 6–9; range: 0–18). Higher total and subscale scores indicate more severe symptom levels with the following clinical cut-offs for the total score: No depression: 0–4; Mild depression: 5–9; Moderate depression: 10–14, and Moderately Severe-to-Severe depression: 15 or greater. The PHQ-9 has demonstrated good reliability and validity.32 Cronbach’s α was .84 for the PHQ-9 Total, .80 for the Somatic subscale, and .71 for the Nonsomatic subscale. The subscales were strongly correlated at r(326) = .63, p < .001.
Cognitive Function
Cognitive function across multiple domains was assessed using a comprehensive neuropsychological test battery, comprised of tests with strong psychometric properties. These tests were selected because they comprise the gold standard assessment of neuropsychological functioning across a variety of cognitive domains and ensured that our assessment of cognitive functioning was comprehensive and accurate. Several tests for each domain were selected to maximize our assessment of each neuropsychological construct by utilizing unique assessment techniques. The three cognitive domains were the following:
Attention: The capacity to attend to and process information was measured by four tests. First, for both the Stoop Word and Stroop Color subtests, participants read lists of colored words as quickly as possible.35 Next, for Trail Making Test A, patients connect 25 numbers in ascending order, as quickly and accurately as possible, and are timed.36 Last, for Letter-Number Sequencing, patients are asked to repeat a series of letters and numbers in a specific order.37
Executive function: The capacity to problem-solve, plan, inhibit, and reason was assessed using three tests. First, for the Stroop Color-Word subtest, participants are asked to identify the ink color (e.g., red ink) of a written list of color words (e.g., “blue”) as quickly as possible.35 Second, for the Trail Making Test B, patients connect 25 alternating numbers and letters in ascending order and are timed.36 Third, for the Frontal Assessment Battery (FAB), participants complete six subtests, including conceptualization, mental flexibility, motor programming, sensitivity to interference, inhibitory control, and environmental autonomy.38
Memory: The capacity to retain and recall verbal information was measured using the Rey Auditory Verbal Learning Test Learning Over Time, True Hits, Short Delay, and Long Delay scores.39 For this test, participants are read a 15-item word list five times and are asked to repeat as many words as they can remember each time. After the fifth trial, participants are read an interference list and then asked to recall words from the original list. They are also asked to recall the original words after a 20-minute delay.
Covariates
The following variables were included as covariates/potential confounders of any observed relationship between cognitive function and depression: gender (0 = male, 1 = female), race-ethnicity (0=white, 1= non-white), education level (1 = 8th grade or less, 2 = 9–11th grade, 3 = high school, 4 = technical or trade school, 5 = some college, 6 = bachelor’s degree, 7 = master’s degree), socioeconomic status (SES), Charlson Comorbidity Index (CCI) score 40, and baseline self-reported HF severity. SES was estimated using subjects’ zip code.41 The SES score was calculated as a z-score using indicators of income and education for each zip code41 with zero as the sample mean and higher scores indicating higher socioeconomic status. The CCI is a summary score of comorbid medical conditions (e.g., diabetes, peripheral vascular disease, myocardial infarction, etcetera).40 Baseline self-reported heart failure severity was assessed by asking participants’ questions about their current symptoms/limitations. An example item is: Do you markedly reduce physical activity due to tiredness, heart fluttering, shortness of breath, anginal pain? Based on their responses, we assigned them to the corresponding NYHA class, ranging from Class I (Mild) and Class II – (Mild) to Class III (Moderate) and Class IV (Severe).42 Thus, we categorized some patients’ HF severity as class I or IV based on their current self-reported symptoms/limitations at the time of their baseline assessment despite our aforementioned inclusion criteria of physician-documented NYHA class II or III at the time of enrollment. We also assessed participants’ body mass index using measured height and weight and their ejection fraction from their medical record.
Procedure
All patients enrolled in Heart ABC31 were recruited from inpatient and/or outpatient cardiology practices in northeast Ohio and gave their written, informed consent to participate. All procedures were approved by the Institutional Review Boards of Kent State University, Summa Health Systems, Inc., and Case Western Research University and were completed in accordance with the Helsinki Declaration. After recruitment and written consent, a trained, qualified research assistant conducted the series of self-report questionnaires and neuropsychological testing for the baseline line visit either at the medical center or at the patient’s home. All research assistants (5 at each site) were trained to perform standardized test administration of the neuropsychological tests by a research coordinator supervised by a licensed clinical neuropsychologist (J.G.). Research coordinators conducted quarterly evaluations of research assistants to ensure standardization of test administration. Inter-rater reliability was assessed and determined to be satisfactory (90.3% concordance).
Data Analyses
Raw neuropsychological test scores were converted to age-adjusted scaled scores using normative data for each test (M = 10, SD = 3). To facilitate interpretation, the scaled scores for each test were then converted to T-scores (M = 50, SD = 10). The T-scores for the tests in each domain were then averaged to create a composite score for that domain: attention, executive function, and memory (e.g., the T-scores for the Stroop Color-Word, Trails B, and FAB tests were averaged together to create the Executive Function composite score). T-scores ≤ 35 are indicative of cognitive impairment. To examine the relationship between depressive symptoms and cognitive function, a series of linear regressions was conducted. Each analysis was conducted with the Attention, Executive Function, or Memory composite score as the dependent variable in separate regressions, and the following covariates were always included on Step 1: gender, race-ethnicity, education level, SES, CCI score, and NYHA HF severity. Two primary types of regressions were run: independent-entry and simultaneous-entry. For independent-entry models, either the PHQ-9 Total, the Somatic subscale, or the Nonsomatic subscale was entered alone on Step 2. In the simultaneous-entry models, the Somatic and Nonsomatic subscales were entered together on Step 2 in order to determine whether the depressive symptom clusters had unique effects on the cognitive variables. If the continuous PHQ-9 Total score was related to a cognitive variable in the regression models, an analysis of covariance (ANCOVA) was run to compare the variable across the PHQ-9 severity categories, adjusting for the same covariates as the regression models. All data analyses were conducted using IBM SPSS version 20.0 statistical software.
Results
Participants
Participants were predominantly older (age = 68.6, SD = 9.7), white (73.3%), male (59.5%), and had at least a high school diploma (88.3%) (See Table 1). On average, overall depressive symptom severity was subclinical (PHQ-9 < 5), but the standard deviation was adequate, indicating considerable individual differences in severity (PHQ-9 Total M = 4.6, SD = 4.9). Additionally, the sample had a wide range of depressive symptom severity levels, including 27.9% Mild (n = 91), 7.4% Moderate (n = 24), and 5.8% Moderately Severe-to-Severe (n = 19). Bivariate correlations between the PHQ-9 Total score and covariates indicates that PHQ-9 was associated with age (r(326) = −.13, p = .02), gender (r(326) = .13, p = .02), race-ethnicity (r(326) = .12, p = .03), SES (r(326) = −.19, p = .001), education level (r(326) = −.18, p = .001), and self-reported HF severity (r(326) = .37, p < .001). At the group level, participants had average performance in Attention (M = 44.3), Executive Function (M = 45.9), and Memory (M = 47.9) (See Table 1). Participants exhibited the following percentages of cognitive impairment (defined as T-score ≤ 35) on the composite domains: 12% for Attention, 11% for Executive Function, and 6.1% for Memory.
Table 1.
Characteristics of Participants (N = 326)
| M(SD) or N(%) | |
|---|---|
| Demographic and Clinical Factors | |
| Age | 68.6(9.7) |
| Female | 132(40.5) |
| Non-whitea | 87(26.7) |
| Education Level | |
| 8th Grade or Less | 8(2.5) |
| 9–11th Grade | 30(9.2) |
| High School | 94(28.8) |
| Technical or Trade School | 36(11.0) |
| Some College | 86(26.4) |
| Bachelor’s Degree | 41(12.6) |
| Master’s Degree | 31(9.5) |
| SES Score | .08(4.2) |
| Body mass index (kg/m2) | 30.3(6.7) |
| Charlson Comorbidity Indexb | 3.3(1.7) |
| Ejection Fraction | 29.3(8.4) |
| Self-reported HF Severity at Baseline (NYHA) | |
| Class I | 31(9.5) |
| Class II | 74(22.7) |
| Class III | 205(62.9) |
| Class IV | 16(4.9) |
| Patient Health Questionnaire-9 | |
| Total | 4.6(4.9) |
| Somatic | 2.5(2.3) |
| Nonsomatic | 2.1(3.1) |
| Cognitive Variable Scores | |
| Attention Composite Score | 44.3(7.5) |
| Stroop Word | 42.8(9.3) |
| Stroop Color | 45.1(9.6) |
| Trails A | 42.4(10.3) |
| Letter-Number Sequencing | 47.0(10.4) |
| Executive Function Composite Score | 45.9(8.1) |
| Stroop Color-Word | 45.1(10.1) |
| Trails B | 41.6(12.0) |
| Frontal Assessment Battery | 51.0(8.2) |
| Memory Composite Score | 47.9(7.8) |
| Learning Over Time | 49.5(10.7) |
| True Hits | 49.1(9.1) |
| Short Delay | 45.6(10.9) |
| Long Delay | 47.3(9.4) |
Note. SES = socioeconomic status. NYHA = New York Heart Association. HF = heart failure. Means and standard deviations are presented for continuous variables. Sample size and percentages are presented for categorical variables.
Of the non-white participants, 97% identified as African American.
Most common comorbidities reported on the Charlson and % of participants: myocardial infarction (50.3%), diabetes (44.5%), and chronic obstructive pulmonary disease; COPD (26.7%).
Demographic and Clinical Factors Associated with Cognitive Function
Regressions revealed that the following covariates were significantly associated with Attention: gender (β = .18, p = .001), race-ethnicity (β = −.24, p < .001), education level (β = .30, p < .001), and SES (β = .12, p = .029). Together, all covariates accounted for 22.5% of the variance in Attention (p < .001). A similar pattern was found for Executive Function with all the covariates accounting for 24.2% of the variance (p < .001) and significant associations observed with gender (β = .15, p = .004), race-ethnicity (β =−.26, p < .001), and education level (β = .35, p < .001). The covariates accounted for 18.3% of the variance in Memory (p < .001) with gender (β = .43, p < .001), race-ethnicity (β = −.13, p = .020), and education level (β = .14, p = .013) reaching significance. With the exception of SES and Attention, SES, medical comorbidity, and HF severity were not significantly related to any cognitive variables (all ps ≥ .09).
Depressive Symptoms Clusters Associated with Cognitive Function
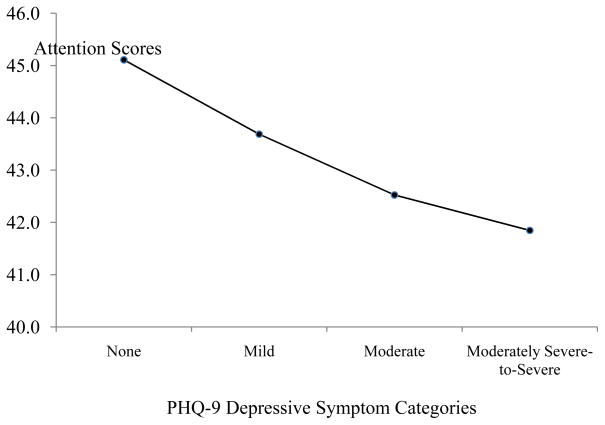
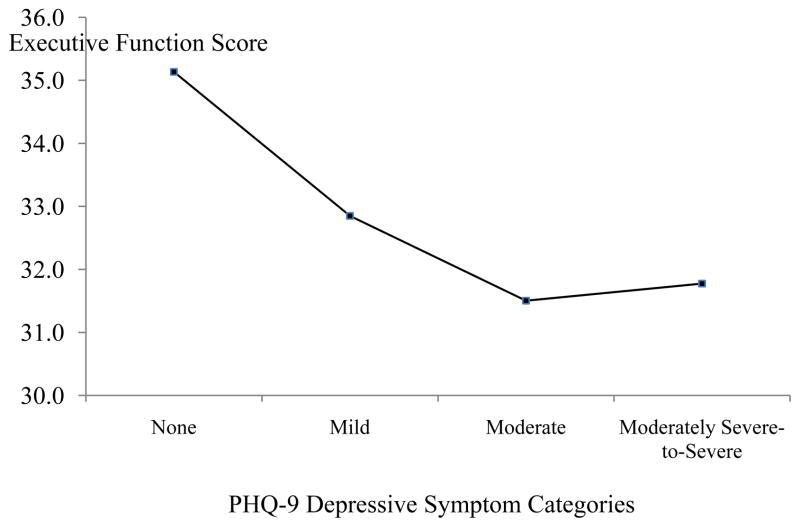
Independent-entry regression analyses adjusting for all demographic and medical covariates revealed that the PHQ-9 Total was associated with Attention (p =.008) and Executive Function scores (p = .001) (see Table 2) and accounted for an additional 2% and 3% of the variance, respectively. A trend was detected between the PHQ-9 Total score and Memory (p = .067) (see Table 2) and accounted for an additional 1% of the variance. Given that the PHQ-9 Total was negatively associated with Attention and Executive Function, we ran an ANCOVA to determine whether attention (Figure 1) and executive function (Figure 2) differed across the PHQ-9 severity categories. The ANCOVA omnibus test showed a trend for attention, F(3, 311) = 2.13, p = .096 and was significant for executive function, F(3, 311) = 4.60, p = .004.
Table 2.
Regressions of Depressive Symptom Clusters Predicting Domains of Cognitive Function (N= 326)
| Model Entry | Attention | Executive Function | Memory | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | ΔR2 | β | p | R2 | ΔR2 | β | p | R2 | ΔR2 | β | p | |
| Independenta | ||||||||||||
| PHQ-9 Total | .24 | .02 | −.14 | .008* | .27 | .03 | −.17 | .001* | .19 | .01 | −.10 | .067† |
| Somatic | .23 | .01 | −.09 | .092 | .25 | .01 | −.11 | .048* | .19 | .00 | −.06 | .259 |
| Non-somatic | .25 | .02 | −.15 | .004* | .27 | .03 | −.18 | .000* | .19 | .01 | −.11 | .044* |
| Simultaneousb | .25 | .02 | .27 | .03 | .19 | .01 | ||||||
| Somatic | .00 | .995 | .01 | .907 | .01 | .943 | ||||||
| Non-somatic | −.15 | .020* | −.19 | .003* | −.11 | .096 | ||||||
Note. Covariates included in all models were gender, minority status, education level, socioeconomic status, Charlson score, and NYHA heart failure severity class. All covariates were included on Step 1 of each model.
The PHQ-9, Somatic Subscale, or Non-somatic Subscale was entered on Step 2 of three separate models.
The Somatic and Non-somatic subscales were entered together on Step 2 of the same model.
Significant at p < .05
Significant at p < .07
Figure 1.
Average Attention Scores across PHQ-9 Depressive Symptom Categories. Note. None = PHQ-9 score 0–4. Mild = PHQ-9 score 5–9. Moderate: PHQ-9 score = 10–14. Moderately Severe-to-Severe: PHQ-9 score: 15 or greater. Covariates appearing in the model were: gender, race-ethnicity, SES, education level, Charlson score, and HF severity level.
Figure 2.
Average Executive Function Scores across PHQ-9 Depressive Symptom Categories. Note. None = PHQ-9 score 0–4. Mild = PHQ-9 score 5–9. Moderate: PHQ-9 score = 10–14. Moderately Severe-to-Severe: PHQ-9 score: 15 or greater. Covariates appearing in the model were: gender, race-ethnicity, SES, education level, Charlson score, and HF severity level.
Independent-entry analyses for the Somatic and Nonsomatic symptoms subscales indicated that the Nonsomatic subscale (p = .004) – but not the Somatic symptoms subscale (p = .092) – was associated with Attention scores (see Table 2). A similar pattern emerged for Memory, with the Nonsomatic subscale (p = .044) significantly related to Memory but no relationship detected between Somatic symptoms (p = .259) (see Table 2). Both the Nonsomatic (p < .001) and Somatic symptoms (p = .048) predicted Executive Function.
When the Somatic and Nonsomatic symptoms were included in the simultaneous-entry model to determine their unique effects on cognitive function, only the Nonsomatic symptom cluster remained associated with Attention (p = .020) and Executive Function (p = .003). The relationship between Nonsomatic symptoms and Memory was reduced to a trend (p = .096). The Somatic symptom cluster was not related to any cognitive variables in the simultaneous-entry models (all ps ≥ .312). Thus, the relationship observed between the PHQ-9 Total and cognitive variables was largely due to the Nonsomatic subscale.
Of note, we conducted a sensitivity analysis in which we added participants’ ejection fraction percentages and body mass index as covariates in Step 1 of the models but the pattern of results remained unchanged (data not shown; available upon request). Given the strong correlation between the Somatic and Nonsomatic subscales, we also checked the collinearity statistics for each simultaneous- entry regression. The tolerance and variance inflation factor scores were all ≥ .83 and ≤ 1.21, respectively, indicating that multicollinearity was not an issue in the analyses.
Discussion
In a large sample of patients with HF, we found that greater overall depressive symptom severity predicted poorer attention and executive function with a trending effect for poorer memory function. The observed effects of depressive symptom severity on multiple cognition domains were driven primarily by the nonsomatic symptoms of depression and not the somatic symptoms. These findings indicate that the physical symptoms of depression, such as fatigue and appetite disturbance, may not be as strongly implicated in depression-related cognitive deficits in HF, whereas cognitive, affective, and behavioral symptoms, such as poor self-esteem, anhedonia, and psychomotor slowing, may play a larger role in cognitive performance.
Our findings are consistent with investigations documenting a relationship between greater overall depressive symptom severity and cognitive impairment in persons with HF. For example, Garcia et al. 15 found that greater depressive symptom severity was associated with poorer performance across multiple cognitive domains, including attention, executive function, psychomotor speed, and language. Our study extends this work by suggesting that the effects may be have been driven by the nonsomatic symptoms of depression. To our knowledge, our study is the first to examine specific depressive symptoms clusters as predictors of cognitive function in a HF sample. Two studies of older adults 43, 44 also suggest that nonsomatic symptoms of depression (e.g., dysphoria, meaninglessness, apathy) predict neuropsychological functioning, but these studies did not directly examine persons with HF or adjust for somatic symptoms of depression. Studies that have directly investigated somatic versus nonsomatic depressive symptom clusters in persons with HF did not examine cognitive function as the outcome but instead focused on physical outcomes, such as cardiac event-free survival, all-cause mortality, and/or health status 22, 23. The results of these studies are mixed, with some indicating that somatic symptoms are more health toxic 22 while others suggest that nonsomatic symptoms are better predictors of poor health outcomes 23.
Several factors may explain why the nonsomatic scores were better predictors of cognitive function in our study as well as clarify the discrepant results across studies. First, the somatic items on measures like the PHQ-9 may be measuring HF severity level instead of true depressive symptoms in persons with HF, as previously suggested by Lee and colleagues.23 For instance, in our sample, the correlation of HF severity level with somatic symptoms was stronger than with nonsomatic symptoms (r = .38 vs. .30, respectively). Additionally, the somatic subscale predicted attention when entered into a model without covariates, an effect that was eliminated only after including HF severity (results not shown; available upon request). The stronger association between somatic depressive symptoms and health status may explain why the somatic subscale is often found to be the better predictor in previous studies which have examined physical outcomes, such as cardiac event-free survival, all-cause mortality, and/or health status 22, 45, rather than the cognitive outcomes that were analyzed in our study. Future studies would benefit from examining both cognitive and physical HF outcomes and their relationship with specific depressive symptoms clusters.
Next, differences in the measurement of depressive symptoms might have contributed to our results. For instance, the use of the PHQ-9 versus other symptom measures of depression (e.g., the Beck Depression Inventory; BDI) can impact which items/symptoms are represented on the somatic vs. nonsomatic subscales as well as the number of items and corresponding variability. The study with findings consistent with ours (i.e., nonsomatic symptoms predicted cardiac event-free survival in HF patients)23 also utilized the PHQ-9 and equivalent subscale computations. The fewer number of items on the Somatic subscale (3-items) compared to the Nonsomatic subscale (6-items) of the PHQ-9 may yield less variability in scores, which, in turn, may contribute to the failure to detect an association between the Somatic subscale and outcomes. In contrast, the study that showed that somatic symptoms were better predictors of mortality used the BDI,22 which contains a different representation of depressive symptoms than the PHQ-9 (e.g., greater number of somatic than nonsomatic items). Furthermore, the somatic subscale of the BDI was calculated using items that were included in our nonsomatic subscale (e.g., psychomotor changes). These differences highlight the importance of clearly describing how symptom clusters are calculated within and across studies.
Lastly, our finding that nonsomatic symptoms instead of somatic symptoms were associated with cognitive function may be explained by the differential association of these symptom clusters with specific brain regions and/or function. For example, a study using resting state fMRI data examined somatic (e.g., sleep disturbance, weight loss) and nonsomatic (e.g., hopelessness, cognitive problems) depressive symptoms in patients with major depressive disorder. The authors found that more nonsomatic symptoms than somatic symptoms were associated with abnormal brain activity across a variety of brain regions, such as the orbitofrontal cortex, cingulate cortex, and insula. Importantly, these frontal, limbic, and insular regions are closely implicated in attention, executive function, and/or memory 46–49. A related line of evidence indicates that cerebral hypoperfusion, a documented concomitant of HF 50, is related to nonsomatic depressive symptoms in the same or closely related brain regions (e.g., cingulate cortex, dorsolateral prefrontal cortex, medial prefrontal cortex) 51. Such findings emphasize the need for studies that use advanced neuroimaging techniques to determine how depressive symptom clusters may be differentially associated with brain changes in HF.
Several limitations of our study should be noted. First, we were not able to use the aforementioned neuroimaging techniques and, consequently, cannot elucidate the anatomic correlates of our findings and verify that the depressive symptoms clusters were differentially associated with brain structure or function. Second, our study was cross-sectional, so we are unable to determine whether depressive symptoms promote cognitive impairment or vice versa. Third, the PHQ-9 was our single measure of depressive symptoms. Use of multiple measures (e.g., the BDI and/or CES-D) may provide more comprehensive information about the depressive symptoms clusters and their associations with cognitive function. Fourth, detailed information regarding participants medication use (e.g., duration and dose of antidepressants) was not collected and such information may be important given that some antidepressants have been shown to effect cognitive function.52 Lastly, use of a comprehensive neuropsychological battery to assess cognitive impairment is a strength of our study design, but we also recognize that such a time-intensive assessment would not be feasible for most clinical settings. Given that the PHQ-9 is fast and easy to administer, we suggest that providers could use the PHQ-9 in conjunction with a shorter cognitive screening test (e.g., the Montreal Cognitive Assessment; MOCA) in order to determine whether referral for more extensive neuropsychological testing is warranted.
Despite these limitations, our study has several potential implications. First, the findings suggest that persons with HF who have elevated nonsomatic symptoms are at risk for poorer cognitive function, which may impact their ability to adhere to the complex HF treatment regimen, given evidence that cognitive deficits and greater depressive symptoms predict poorer HF outcomes.5, 6, 9, 13 Thus, if our findings our replicated, screening explicitly for nonsomatic depressive symptoms may be warranted. Next, our findings imply that the mechanisms underlying the depression-cognitive function relationship HF are not solely related to sleep or appetite disturbance. Thus, interventions which may primarily target patients’ somatic symptoms (e.g., fatigue medications like modafinil53 or appetite-stimulants like mirtazipine54) may not yield maximum cognitive benefit compared to a comprehensive treatment which targets depressed mood, anhedonia, and other nonsomatic symptoms. Examples of interventions which can target both somatic and nonsomatic symptoms include cognitive behavioral therapies (CBT)55 and exercise.56 The impact of depression treatments on cognitive function in patients with HF is not yet known; however, exercise training is a promising treatment for both depression57, 58 and cognition.59, 60 Such possibilities should be fully explored in prospective, controlled trials of depression interventions and cognition in HF.
In brief summary, our study sought to compare the relative importance of somatic and nonsomatic depressive symptom clusters in predicting cognitive function in a sample of patients with HF. We found that overall depressive symptom severity was associated with poorer performance on multiple cognition domains, an effect driven primarily by the nonsomatic symptoms of depression. Our results suggest that future research is needed to determine how depressive symptoms clusters may be differentially associated with brain changes in HF. Such findings may clarify the pathways from depression to poorer cognitive function in persons with HF and inform the development of effective depression interventions, which may ultimately improve cognitive function in this population.
Summary and Implications.
| What’s New? |
|---|
|
|
|
Acknowledgments
This work was supported by the National Heart, Lung, and Blood Institute at the National Institute of Health [R01 HL096710-01A1 to M.A.D. and J.W.H]. The authors thank all members of the Heart ABC team for their technical assistance on this project.
Footnotes
Conflict of Interest: None declared.
The authors declare no conflict of interest.
References
- 1.Pressler SJ, Subramanian U, Kareken D, et al. Cognitive deficits in chronic heart failure. Nursing Research. 2010;59:127. doi: 10.1097/NNR.0b013e3181d1a747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogels R, Scheltens P, Schroeder-Tanka JM, et al. Cognitive impairment in heart failure: A systematic review of the literature. European Journal of Heart Failure. 2007;9:440–9. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Harkness K, Demers C, Heckman GA, et al. Screening for cognitive deficits using the Montreal cognitive assessment tool in outpatients ≥ 65 years of age with heart failure. The American journal of cardiology. 2011;107:1203–7. doi: 10.1016/j.amjcard.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 4.Sauvé MJ, Lewis WR, Blankenbiller M, et al. Cognitive impairments in chronic heart failure: a case controlled study. Journal of cardiac failure. 2009;15:1–10. doi: 10.1016/j.cardfail.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 5.Zuccala G, Onder G, Pedone C, et al. Cognitive dysfunction as a major determinant of disability in patients with heart failure: results from a multicentre survey. Journal of Neurology, Neurosurgery & Psychiatry. 2001;70:109–12. doi: 10.1136/jnnp.70.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zuccalà G, Pedone C, Cesari M, et al. The effects of cognitive impairment on mortality among hospitalized patients with heart failure. The American Journal of Medicine. 2003;115:97–103. doi: 10.1016/s0002-9343(03)00264-x. [DOI] [PubMed] [Google Scholar]
- 7.Trojano L, Incalzi RA, Acanfora D, et al. Cognitive impairment: a key feature of congestive heart failure in the elderly. Journal of neurology. 2003;250:1456–63. doi: 10.1007/s00415-003-0249-3. [DOI] [PubMed] [Google Scholar]
- 8.Haworth J, Moniz-Cook E, Clark A, et al. Prevalence and predictors of anxiety and depression in a sample of chronic heart failure patients with left ventricular systolic dysfunction. European Journal of Heart Failure. 2005;7:803–8. doi: 10.1016/j.ejheart.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 9.Jiang W, Alexander J, Christopher E, et al. RElationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Archives of Internal Medicine. 2001;161:1849–56. doi: 10.1001/archinte.161.15.1849. [DOI] [PubMed] [Google Scholar]
- 10.Koenig HG. Depression in hospitalized older patients with congestive heart failure. General hospital psychiatry. 1998;20:29–43. doi: 10.1016/s0163-8343(98)80001-7. [DOI] [PubMed] [Google Scholar]
- 11.Freedland KE, Rich MW, Skala JA, et al. Prevalence of depression in hospitalized patients with congestive heart failure. Psychosom Med. 2003;65:119–28. doi: 10.1097/01.psy.0000038938.67401.85. [DOI] [PubMed] [Google Scholar]
- 12.Rutledge T, Reis VA, Linke SE, et al. Depression in heart failure: A meta-analytic review of prevalence, intervention effects, and associations with clinical outcomes. Journal of the American College of Cardiology. 2006;48:1527–37. doi: 10.1016/j.jacc.2006.06.055. [DOI] [PubMed] [Google Scholar]
- 13.Adams J, Kuchibhatla M, Christopher EJ, et al. Association of depression and survival in patients with chronic heart failure over 12 years. Psychosomatics. 2012;53:339–46. doi: 10.1016/j.psym.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alosco ML, Spitznagel MB, Raz N, et al. The interactive effects of cerebral perfusion and depression on cognitive function in older adults with heart failure. Psychosom Med. 2013;75:632–9. doi: 10.1097/PSY.0b013e31829f91da. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garcia S, Spitznagel MB, Cohen R, et al. Depression is associated with cognitive dysfunction in older adults with heart failure. Cardiovascular Psychiatry and Neurology. 2011;2011:6. doi: 10.1155/2011/368324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vogels R, Oosterman JM, van Harten B, et al. Neuroimaging and correlates of cognitive function among patients with heart failure. Dementia and geriatric cognitive disorders. 2007;24:418–23. doi: 10.1159/000109811. [DOI] [PubMed] [Google Scholar]
- 17.Jefferson AL, Poppas A, Paul RH, et al. Systemic hypoperfusion is associated with executive dysfunction in geriatric cardiac patients. Neurobiology of aging. 2007;28:477–83. doi: 10.1016/j.neurobiolaging.2006.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rosenberg GA. Inflammation and white matter damage in vascular cognitive impairment. Stroke. 2009;40:S20–S3. doi: 10.1161/STROKEAHA.108.533133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Machale SM, Lawrie SM, Cavanash J, et al. Cerebral perfusion in chronic fatigue syndrome and depression. The British Journal of Psychiatry. 2000;176:550–6. doi: 10.1192/bjp.176.6.550. [DOI] [PubMed] [Google Scholar]
- 20.Köhler S, Thomas AJ, Lloyd A, et al. White matter hyperintensities, cortisol levels, brain atrophy and continuing cognitive deficits in late-life depression. The British Journal of Psychiatry. 2010;196:143–9. doi: 10.1192/bjp.bp.109.071399. [DOI] [PubMed] [Google Scholar]
- 21.Davidson KW, Rieckmann N, Rapp MA. Definitions and distinctions among depressive syndromes and symptoms: implications for a better understanding of the depression–cardiovascular disease association. Psychosom Med. 2005;67:S6–S9. doi: 10.1097/01.psy.0000162257.19266.fc. [DOI] [PubMed] [Google Scholar]
- 22.Schiffer AA, Pelle AJ, Smith O, et al. Somatic versus cognitive symptoms of depression as predictors of all-cause mortality and health status in chronic heart failure. The Journal of clinical psychiatry. 2009;70:1667–73. doi: 10.4088/JCP.08m04609. [DOI] [PubMed] [Google Scholar]
- 23.Lee KS, Lennie TA, Heo S, et al. Association of physical versus affective depressive symptoms with cardiac event–free survival in patients with heart failure. Psychosom Med. 2012;74:452–8. doi: 10.1097/PSY.0b013e31824a0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roest AM, Carney RM, Freedland KE, et al. Changes in cognitive versus somatic symptoms of depression and event-free survival following acute myocardial infarction in the Enhancing Recovery In Coronary Heart Disease (ENRICHD) study. Journal of Affective Disorders. 2013;149:335–41. doi: 10.1016/j.jad.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deverts DJ, Cohen S, Dilillo VG, et al. Depressive symptoms, race, and circulating C-reactive protein: The Coronary Artery Risk Development in Young Adults (CARDIA) Study. Psychosom Med. 2010;72:734–41. doi: 10.1097/PSY.0b013e3181ec4b98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kubzansky LD, Sparrow D, Vokonas P, et al. Is the glass half empty or half full? A prospective study of optimism and coronary heart disease in the normative aging study. Psychosom Med. 2001;63:910–6. doi: 10.1097/00006842-200111000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Matthews KA, Raikkonen K, Sutton-Tyrrell K, et al. Optimistic attitudes protect against progression of carotid atherosclerosis in healthy middle-aged women. Psychosom Med. 2004;66:640–4. doi: 10.1097/01.psy.0000139999.99756.a5. [DOI] [PubMed] [Google Scholar]
- 28.Stewart J, Zielke D, Hawkins M, et al. Depressive symptom clusters and 5-year incidence of coronary artery calcification: The CARDIA study. Circulation. 2012:410–7. doi: 10.1161/CIRCULATIONAHA.112.094946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stewart JC, Janicki DL, Muldoon MF, et al. Negative emotions and 3-year progression of subclinical atherosclerosis. Archives of General Psychiatry. 2007;64:225–33. doi: 10.1001/archpsyc.64.2.225. [DOI] [PubMed] [Google Scholar]
- 30.Carney RM, Freedland KE. Are somatic symptoms of depression better predictors of cardiac events than cognitive symptoms in coronary heart disease? Psychosom Med. 2012;74:33–8. doi: 10.1097/PSY.0b013e3182405ac4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Clinicaltrials.gov. Self-management and Cognitive Function in Adults With Heart Failure (Heart ABC) - Identifier: NCT01461629. Bethesda, MD: National Library of Medicine; 2011. [cited 2013 8/22/2013]; Available from: http://clinicaltrials.gov/ct2/show/NCT01461629?term=heart+abc&rank=1. [Google Scholar]
- 32.Kroenke K, Spitzer RL. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:1–7. [Google Scholar]
- 33.Chilcot J, Rayner L, Lee W, et al. The factor structure of the PHQ-9 in palliative care. Journal of Psychosomatic Research. 2013 doi: 10.1016/j.jpsychores.2012.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Krause JS, Bombardier C, Carter RE. Assessment of depressive symptoms during inpatient rehabilitation for spinal cord injury: Is there an underlying somatic factor when using the PHQ? Rehabilitation Psychology. 2008;53:513. [Google Scholar]
- 35.Golden JC. Stroop Color and Word Test. Chicago, IL: Stoelting; 1978. [Google Scholar]
- 36.Reitan RM. Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills. 1958;8:271–6. [Google Scholar]
- 37.Wechsler D. Wechsler Adult Intelligence Scale. 3. San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- 38.Abood DA, Black DR, Birnbaum RD. Nutrition education intervention for college female athletes. Journal of nutrition education and behavior. 2004;36:135–9. doi: 10.1016/s1499-4046(06)60150-4. [DOI] [PubMed] [Google Scholar]
- 39.Lezak MD. Neuropsychological Assessment. New York, NY: Oxford University Press; 1995. [Google Scholar]
- 40.Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of Chronic Diseases. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 41.Roux AVD, Merkin SS, Arnett D, et al. Neighborhood of residence and incidence of coronary heart disease. New England Journal of Medicine. 2001;345:99–106. doi: 10.1056/NEJM200107123450205. [DOI] [PubMed] [Google Scholar]
- 42.Committee NYHAC, Association NYH. Nomenclature and criteria for diagnosis of diseases of the heart and great vessels. Little, Brown Medical Division; 1979. [Google Scholar]
- 43.O’Bryant SE, Hall JR, Cukrowicz KC, et al. The differential impact of depressive symptom clusters on cognition in a rural multi-ethnic cohort: a Project FRONTIER study. International journal of geriatric psychiatry. 2011;26:199–205. doi: 10.1002/gps.2514. [DOI] [PubMed] [Google Scholar]
- 44.Hall JR, O’Bryant SE, Johnson LA, et al. Depressive symptom clusters and neuropsychological performance in mild Alzheimer’s and cognitively normal elderly. Depression research and treatment. 2011 doi: 10.1155/2011/396958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith OR, Kupper N, Schiffer AA, et al. Somatic depression predicts mortality in chronic heart failure: Can this be explained by covarying symptoms of fatigue? Psychosom Med. 2012;74:459–63. doi: 10.1097/PSY.0b013e31824ef2f4. [DOI] [PubMed] [Google Scholar]
- 46.Nelson SM, Dosenbach NU, Cohen AL, et al. Role of the anterior insula in task-level control and focal attention. Brain Structure and Function. 2010;214:669–80. doi: 10.1007/s00429-010-0260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychology review. 2006;16:17–42. doi: 10.1007/s11065-006-9002-x. [DOI] [PubMed] [Google Scholar]
- 48.MacDonald AW, Cohen JD, Stenger VA, et al. Dissociating the role of the dorsolateral prefrontal and anterior cingulate cortex in cognitive control. Science. 2000;288:1835–8. doi: 10.1126/science.288.5472.1835. [DOI] [PubMed] [Google Scholar]
- 49.Frankland PW, Bontempi B, Talton LE, et al. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–3. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- 50.Gruhn N, Larsen FS, Boesgaard S, et al. Cerebral blood flow in patients with chronic heart failure before and after heart transplantation. Stroke. 2001;32:2530–3. doi: 10.1161/hs1101.098360. [DOI] [PubMed] [Google Scholar]
- 51.Bench CJ, Friston K, Brown R, et al. Regional cerebral blood flow in depression measured by positron emission tomography: the relationship with clinical dimensions. Psychological Medicine. 1993;23:579. doi: 10.1017/s0033291700025368. [DOI] [PubMed] [Google Scholar]
- 52.Amado-Boccara I, Gougoulis N, Poirier Littre M, et al. Effects of antidepressants on cognitive functions: a review. Neuroscience & Biobehavioral Reviews. 1995;19:479–93. doi: 10.1016/0149-7634(94)00068-c. [DOI] [PubMed] [Google Scholar]
- 53.DeBattista C, Lembke A, Solvason HB, et al. A prospective trial of modafinil as an adjunctive treatment of major depression. Journal of clinical psychopharmacology. 2004;24:87–90. doi: 10.1097/01.jcp.0000104910.75206.b9. [DOI] [PubMed] [Google Scholar]
- 54.Puzantian T. Mirtazapine, an antidepressant. American Journal of Health-System Pharmacy. 1998;55:44–9. doi: 10.1093/ajhp/55.1.44. [DOI] [PubMed] [Google Scholar]
- 55.Dobson KS. Handbook of cognitive-behavioral therapies. Guilford Press; 2009. [Google Scholar]
- 56.Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Archives of internal medicine. 1999;159:2349. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 57.Blumenthal JA, Babyak MA, Moore KA, et al. Effects of exercise training on older patients with major depression. Archives of internal medicine. 1999;159:2349–56. doi: 10.1001/archinte.159.19.2349. [DOI] [PubMed] [Google Scholar]
- 58.Lawlor DA, Hopker SW. The effectiveness of exercise as an intervention in the management of depression: systematic review and meta-regression analysis of randomised controlled trials. BMJ: British Medical Journal. 2001;322:763. doi: 10.1136/bmj.322.7289.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tanne D, Freimark D, Poreh A, et al. Cognitive functions in severe congestive heart failure before and after an exercise training program. International journal of cardiology. 2005;103:145–9. doi: 10.1016/j.ijcard.2004.08.044. [DOI] [PubMed] [Google Scholar]
- 60.Smith PJ, Blumenthal JA, Hoffman BM, et al. Aerobic exercise and neurocognitive performance: a meta-analytic review of randomized controlled trials. Psychosomatic Medicine. 2010;72:239–52. doi: 10.1097/PSY.0b013e3181d14633. [DOI] [PMC free article] [PubMed] [Google Scholar]