Abstract
Objective
The objective of this observational open label trial was to characterize changes in diabetes self-management and psychological distress associated with a mobile health (mHealth) interactive voice response (IVR) self-management support program.
Methods
For three to six months, 301 patients with diabetes received weekly IVR calls assessing health status and self-care and providing tailored pre-recorded self-management support messages. Patients could participate together with an informal caregiver who received suggestions on self-management support, and patients’ clinicians were notified automatically when patients reported significant problems.
Results
Patients completed 84% of weekly calls, providing 5,682 patient-weeks of data. Thirty nine percent participated with an informal caregiver. Outcome analyses adjusted for study design factors and sociodemographics indicated significant pre-post improvement in medication adherence, physical functioning, depressive symptoms, and diabetes-related distress (all p values < 0.001). Analyses of self-management problems indicated that as the intervention proceeded, there were significant improvements in patients’ IVR-reported frequency of weekly medication adherence, SMBG performance, checking feet, and frequency of abnormal self-monitored blood glucose readings (all p values < 0.001).
Conclusions
We conclude that the combined program of automated telemonitoring, clinician notification, and informal caregiver involvement was associated with consistent improvements in medication adherence, diabetes self-management behaviors, physical functioning, and psychological distress. A randomized controlled trial is needed to verify these encouraging findings.
Keywords: diabetes, mHealth, primary care, care management
Introduction
Inadequate self-management of blood glucose and blood pressure among patients with type 2 diabetes are associated with subsequent chronic hyperglycemia, microvascular complications, and heart disease.1 Although care management improves outcomes,2 such services depend upon scarce personnel to provide between-visit monitoring and patient education.3 Mobile health (mHealth) services, including interactive voice response (IVR) calls in which patients respond to automated prompts, may help address these barriers to effective care management.4,5
Support from informal caregivers also is likely to improve diabetes outcomes. However, in-home caregivers often lack the tools they need to systematically monitor changes in patients’ health status and support self-care;6 and caregivers are at risk for burnout in part due to the high demands and insufficient clinical support for their role.6,7 Many patients live alone, with up to 7 million Americans receiving “long-distance” caregiving;8 and caregivers at a distance may receive insufficient information about the patient’s day-to-day self-management.9 mHealth monitoring and feedback to caregivers may enable these geographically-distant supportive individuals to be more involved and effective.
To address the gaps in informational support for people with diabetes and their social networks, we developed an mHealth service that makes weekly automated IVR calls to patients in order to closely monitor their self-management and provide immediate problem-tailored support. The service notifies health care teams when patients experience significant difficulties and provides caregivers from outside the patients’ home with automatic, structured updates about the patient’s status along with guidance on self-management support. In a prior report describing our implementation of this intervention,10 we demonstrated that the system detects abnormal glycemia and blood pressure levels that might otherwise go unreported; provides clinical information that is reliable, valid, and actionable; and increases patients’ access to between-visit monitoring and diabetes self-management support. In this present report, we test the hypothesis that the intervention yields long term improvements in functional status, depressive symptoms, and diabetes-related distress increases, and that it improves three self-management behaviors (medication adherence, self-monitoring of blood glucose (SMBG), and checking one’s feet for tissue damage) as well as the frequency of high and low blood glucose values as indicated by SMBG.
Methods
Patient eligibility and recruitment
Patient participants were recruited from 16 Department of Veterans Affairs (VA) outpatient clinics in Michigan, Illinois, Indiana, and Ohio between March 2010 and December 2012. Eligibility criteria were: an ICD-9 diagnosis of type 2 diabetes; one or more outpatient VA primary care visits in the prior 12 months; and one or more current VA prescriptions for an antihyperglycemic medication. We excluded patients with diagnoses indicating cognitive impairment or severe mental illness or who were living in a supervised residential facility. Potential participants were mailed an introductory letter and then further screened for eligibility by telephone. After providing written informed consent, patients received information about using the IVR system and communicating effectively with informal caregivers and clinicians. Participants were paid $20 for each of two 45-minute interviews, which occurred at baseline and at study completion. The study was approved by human subjects committees at the Ann Arbor VA Healthcare System and University of Michigan.
Baseline assessment
We assessed patients’ characteristics at baseline by telephone. For the current analyses, we created dichotomous indicators for minority race/ethnicity, being married, and having more than a high school education. Self-reported annual household income was collapsed into approximate quartile brackets of <$15,000, $15,000 – $29,999, $30,000 – $54,999, and $55,000+. We computed a summed index of physician-diagnosed medical comorbidities based on a self-report checklist of common chronic conditions, and collapsed this into brackets for 0 – 3, 4 – 6, and 7+ comorbid conditions. The Morisky Medication Adherence Scale (MMAS) was used to measure long term medication nonadherence, and for descriptive purposes we applied the standard cut-off of 2 to indicate nonadherence.11,12 From the Medical Outcome Study 12-Item Short Form (SF-12),13 we calculated the Physical Composite Score (PCS) and Mental Composite Score (MCS). For both the PCS and the MCS, higher scores reflect better functioning in the respective domain, the potential range spans 0–100, and the population mean is 50.0 (SD = ± 10.0). We administered the 10-item version of the Center for Epidemiological Studies Depression Scale (CES-D),14 and for descriptive purposes we also created a binary indicator for clinically significant depressive symptoms using Irwin et al.’s cutoff for older adults.15 We applied the established cutoff of 40 to define diabetes distress using the Problem Areas in Diabetes (PAID),16 which measures diabetes-specific psychological distress.
Intervention
The focus of the mHealth service was based on the assumption that patients, informal caregivers, and healthcare teams would use frequent updates about the patient’s health and self-care along with automated tailored advice to address emerging problems and improve illness self-management.17 The overall goals of the intervention were to: (a) monitor patients’ symptoms and self-management problems, (b) provide patients with tailored messages about diabetes self-management and medical help-seeking, (c) generate guidance on self-management support for patients’ informal caregivers via structured emails, and (d) provide patients’ clinicians with actionable feedback via faxed updates about selected patient-reported health and self-care problems.
During each week that an IVR call was scheduled, the system made up to three attempts to contact each patient on up to three different patient-selected day/time combinations (i.e., up to nine attempts each week of participation). The calls followed tree-structured algorithms, and lasted between 5 and 10 minutes during which patients responded to questions about their experiences during the past week using their telephone touchtone keypad and heard messages that gave verbal reinforcement (e.g., “That's great! For a person with diabetes like you, it is important to look at your feet every day.”) and as-needed self-management messages based on their responses. The wording of questions and feedback messages was developed with input from experts in diabetes self-management, endocrinology, primary care, and mHealth service design. Queries focused on symptoms of hypoglycemia and hyperglycemia, performance of fasting SMBG, any SMBG results <90 mg/dL, hypoglycemia self-treatment, three or more instances of SMBG in the prior week with results > 300 mg/dL, possession of at least a two-week supply of antihypergycemic medication, adherence to antihyperglycemic medication, and foot inspection. All VA patients with hypertension receive a home blood pressure monitor; if a patient with diabetes and hypertension participating in the mHealth program had self-monitored their blood pressure ≥ 3 days that week, additional questions assessed: patient-reported systolic blood pressure levels of > 300 mmHg at least half the time during the prior week or < 90 mmHg on ≥ 2 days during the prior week, possession of at least a two-week supply of antihypertensive medication, adherence to antihypertensive medication, and whether the patient was following a low sodium diet. If the patient reported difficulty in any of these areas, the system provided pre-recorded self-management education about that issue. In addition, the system notified the patient’s primary care team when the patient reported clinically significant problems. Further details on item wording for the IVR patient calls or feedback notifications are available from the authors.
Patients had the option of designating a family member or close friend to receive emailed summaries of each completed call along with structured suggestions on supporting the patient’s diabetes self-management. These individuals were required to be living outside the patient’s residence, because our goal was to supplement any in-home informal caregiving that was already occurring. We used the Norbeck Social Support Questionnaire (NSSQ)18 to help patients identify the best individual for this role. To be eligible, informal caregivers needed to be ≥ 18 years old, have no history of cognitive or severe psychiatric impairment, and have access to email. After providing informed consent, participating caregivers underwent DVD-based training on communicating effectively with the patient and any in-home caregiver that may be involved.
Finally, whenever patients reported a pattern of either abnormal blood glucose or blood pressure levels, or significant medication nonadherence, the system responded automatically by faxing a clinician notification that explained the issue to patients’ primary care team. Based upon clinician input, the thresholds for generating notifications were selected to have a low false-positive rate for identifying urgent issues, provide actionable information, and efficiently use human resources for follow-up without burdening clinicians.
Weekly assessment
The system logged all attempted IVR calls. For completed calls, we created a binary indicator for adhering to antihyperglycemic medications less often than “always.” This approach was chosen because of this variable’s strongly skewed distribution (skewness index = 3.3), and also because the use of a stringent cutoff compensates for the general upward bias that characterizes self-reported adherence.19 We also analyzed the presence versus absence of four additional issues: not performing preprandial SMBG at least once that week, not performing daily foot inspection, obtaining at least two SMBG results above 300 mg/dl, and obtaining at least one SMBG result below 90 mg/dl.
Post intervention assessment
Patients were enrolled in two waves, with the first wave receiving IVR calls weekly for three months and the second wave receiving IVR calls weekly for six months. This methodological change was driven by our decision to extend the protocol after observing promising initial findings. At the end of intervention, we re-administered the MMAS, SF-12 (PCS and MCS), CES-D, and PAID.
Data analysis
Data were analyzed using Stata v.13.1.20 We computed descriptive statistics (frequency, mean, SD) on patients’ sociodemographic and clinical characteristics. The outcome analysis evaluated pre-post changes in scores for the PCS, MCS, CES-D, PAID, and MMAS. Specifically we conducted five mixed model linear regression analyses that used all available observations and thus represented an “intent-to-treat” analysis. Time effects were estimated after adjusting for study duration, whether or not the patient participated with an informal caregiver, age, income bracket, and number of comorbid conditions. We then analyzed the effect of study week (ranged from 1 to 26) on five IVR-reported self-management problems (weekly nonadherence, performing SMBG, checking feet, and obtaining SMBG values indicating either high or low blood glucose). As above, these analyses included all available observations, and were adjusted for the same set of control covariates. All statistical tests were adjusted for the clustering of observations within patients. Finally, we adjusted the conventional alpha of 0.05 for potential inflation within each set of five multiple comparisons, so that statistical significance was evaluated against a Bonferroni-adjusted alpha criterion (0.05/5) of p < 0.01.
Results
Participant characteristics
Of 422 eligible patients, 301 (71.3%) consented to participate, including 108 in the three month program and 193 in the six month program. Sample demographic characteristics are displayed in Table 1. The typical participant was a Caucasian male, as would be expected in the VA population. The majority were at least 60 years old, and 30% were at least 70 years old. Fifty-three percent of participants had at least some college education, 26% had annual household incomes < $15,000, 68% were married or cohabitating, and 18% were employed (consistent with the high rate of retirement expected in this population). Sixty nine percent had at least four comorbid medical conditions, and 86% had a hypertension diagnosis. Thirty-nine percent of participants opted to participate with an informal caregiver. A total of 261 (87%) patient participants completed the study. Attrition was more likely among those who were enrolled into the six-month versus the three-month program (p < 0.001), but was not significantly associated with any other baseline variable.
Table 1.
Sample characteristics (n=301).
| Variable | Mean ± SD or percent |
|---|---|
| Age | 66.7 ± 9.8 |
| Male | 97.0 |
| Caucasian | 92.8 |
| Married or cohabitating | 68.0 |
| At least some college education | 53.0 |
| Employed | 18.0 |
| Yearly household income | |
| < $15,000 | 26.3 |
| $15,000 – $29,000 | 24.6 |
| $30,000 – $54,000 | 28.9 |
| > $55,000 | 20.3 |
| Participated with an informal caregiver a | 39.2 |
| Number of comorbid conditions b | |
| 0 – 3 | 30.6 |
| 4 – 6 | 46.5 |
| 7 or more | 22.9 |
| Hypertension diagnosis | 85.7 |
Notes:
. From outside patient’s home.
. Based on presence of self-reported hypertension, cardiovascular disease, hyperlipidemia, cancer, stroke, arthritis, chronic lung disease, migraine, asthma, and low back pain.
As shown in Table 2, baseline medication nonadherence was somewhat prevalent (MMAS mean 1.2 ± 1.0, with scores falling in the “nonadherent” range for 34% of participants), and baseline levels of physical functioning tended to be poor (PCS mean ± SD: 32.3 ± 12.2). While some participants reported at least mild depressive symptoms at baseline (CES-D: 8.29 ± 6.27, falling in elevated range for 30%), the mean MCS (50.0 ± 11.7) suggested that most patients were unimpaired by psychological distress and the PAID indicated little evidence of diabetes-specific distress (13.2 ± 13.1, and elevated for 4%).
Table 2.
Results of mixed linear regression analyses of health outcomes.
| Mean ± SD | Effect of time (pre vs. post)a | ||||
|---|---|---|---|---|---|
| Dependent variable | Pre (n=301) | Post (n=261) | bb | 95% c.i. | p value c |
| Medication nonadherence (long term) d | 1.20 ± 0.95 | 0.87 ± 0.88 | −0.30 | −0.42 – −0.18 | < 0.001 |
| Physical functioning e | 32.3 ± 12.2 | 36.2 ± 11.9 | 3.16 | 2.13 – 4.18 | < 0.001 |
| Psychological functioning f | 50.0 ± 11.7 | 51.4 ± 11.1 | 1.03 | −0.13 – 2.19 | 0.083 |
| Depressive symptoms g | 8.29 ± 6.27 | 6.52 ± 6.06 | −1.54 | −2.08 – −1.01 | < 0.001 |
| Diabetes-related distress h | 13.2 ± 13.1 | 9.5 ± 10.8 | −3.28 | −4.75 – −1.81 | < 0.001 |
Notes:
. After adjustment for study duration, having an informal caregiver co-participant, age, income bracket, and medical comorbidity.
. Unstandardized regression coefficient for the effect of time (pre vs. post).
. Unadjusted p values are listed, but were evaluated against a Bonferroni – corrected criterion of p(crit) = 0.01.
. As measured by the Morisky Medication Adherence Scale (MMAS); higher scores reflect poorer adherence.
. As measured by the Physical Component Scale (PCS); higher scores reflect better functioning
. As measured by the Mental Component Scale (MCS); higher scores reflect better functioning.
. As measured by the Center for Epidemiological Studies – Depression; higher scores reflect greater depressive symptoms.
. As measured by the Problem Areas in Diabetes; higher scores reflect greater distress.
Intervention implementation
The details of our implementation of this intervention have been described elsewhere,21 and therefore only overall characteristics pertinent to the present analysis are presented herein. Patients participated for a total of 5,682 patient-weeks, and completed an IVR monitoring and self-care support call during 4,759 (84%) of these. As can be seen in Table 3, the most frequently IVR-reported problem was medication nonadherence, which was reported during 17.4% of intervention weeks. This was followed in order of decreasing incidence by reported problems with low blood glucose (8.8% of weeks), not checking one’s feet (7.6% of weeks), not performing SMBG at least once (7.4% of weeks), and high blood glucose (1.4% of weeks). A total of 1,198 clinician notifications were generated (equating to 21 notifications per 100 patient-weeks), with the most common reasons being high blood pressure (55% of notifications) and low blood glucose (42%). Further details on clinical notifications have been previously published.21
Table 3.
Results of logistic regression analyses of self-management problems (n = 5682 patient-weeks).
| Adjusted effect of intervention week a | ||||
|---|---|---|---|---|
| Dependent variable b | Rate c | AORd | 95% c.i. | p value e |
| Medication nonadherence (short term) f | .174 | 0.98 | 0.96 – 0.99 | 0.008 |
| Not performing SMBG g | .074 | 0.95 | 0.92 – 0.98 | 0.002 |
| Not checking feet h | .076 | 0.95 | 0.92 – 0.97 | < 0.001 |
| High blood glucose i | .014 | 0.93 | 0.89 – 0.96 | < 0.001 |
| Low blood glucose j | .088 | 0.94 | 0.93 – 0.96 | < 0.001 |
Notes:
. Estimated after adjusting the model for 3 vs. 6 month study duration, participating with an informal caregiver, age, income bracket, and medical comorbidity. For ease of interpretabiity and comparability across dependent variables, the effect of week on nonadherence was estimated without the quadratic term (square root of week) in the model.
. All dependent variables are based upon IVR-reported data (coded as 0 = “problem did not occur” vs. 1 = “problem occurred”).
. Mean rate of problem across all available patient-weeks of participation.
. Adjusted odds ratio.
. Unadjusted p values are listed, but were evaluated against a Bonferroni – corrected criterion of p(crit) = 0.01.
. Adhering to antihyperglycemic medication less often than “always” during prior week.
. Not performing preprandial SMBG at least once during prior week.
. Not performing daily foot inspection during prior week.
. Obtaining at least two SMBG results above 300 mg/dl during prior week.
. Obtaining at least one SMBG result below 90 mg/dl during prior week.
Intervention effects on health outcomes
The results of the linear regression analyses for the five health outcome variables are shown in Table 2. The key predictor of interest in each model was time (pre versus post intervention), which was tested after adjusting for: study duration (three vs. six months), whether the patient participated together with an informal caregiver, patient age, number of comorbid conditions, and income bracket. As shown in Table 2, time was associated with significant improvements in four health outcome variables: long term medication nonadherence (b = −0.30, 95% c.i.: −0.42 – −0.18, p < 0.001), physical functioning (b = 3.16, 95% c.i.: 2.13 – 4.18, p < 0.001), depressive symptoms (b = −1.54, 95% c.i.: −2.08 – −1.01, p < 0.001), and diabetes-related distress (b = −3.28, 95% c.i.: −4.75 – −1.81, p < 0.001). However, we did not observe significant changes over time for the fifth outcome variable, psychological functioning as measured by the MCS (b = 1.03, 95% c.i.: −0.13 – 2.19, p = 0.083). Study duration (3 vs. 6 months) did not have any significant effects on IVR outcomes (all p values ≥ 0.105). Cohen’s d effect size statistic ranged from a maximum of 0.31 (long term medication nonadherence) down to 0.23 (diabetes related distress), except for psychological functioning was trivial (−0.08).
Trends in IVR-reported self-management problems during intervention
This second set of analyses focused upon variation across time in the weekly incidence of five self-management problems as measured by weekly IVR: weekly medication nonadherence, not performing SMBG, not checking feet, and obtaining SMBG values indicating both high and low blood glucose. The results of the logistic regression analyses are summarized in Table 3. As in the above analyses of health outcomes, the effect of intervention week (1–26) on each self-management problem was tested after adjusting for the effects of study duration, participating with an informal caregiver, patient age, number of comorbid conditions, and income bracket. As can be seen in Table 3, as intervention progressed there were significant decreases in medication nonadherence, not performing SMBG, not checking feet, and obtaining SMBG values indicating both high and low blood glucose (all p values < 0.001).
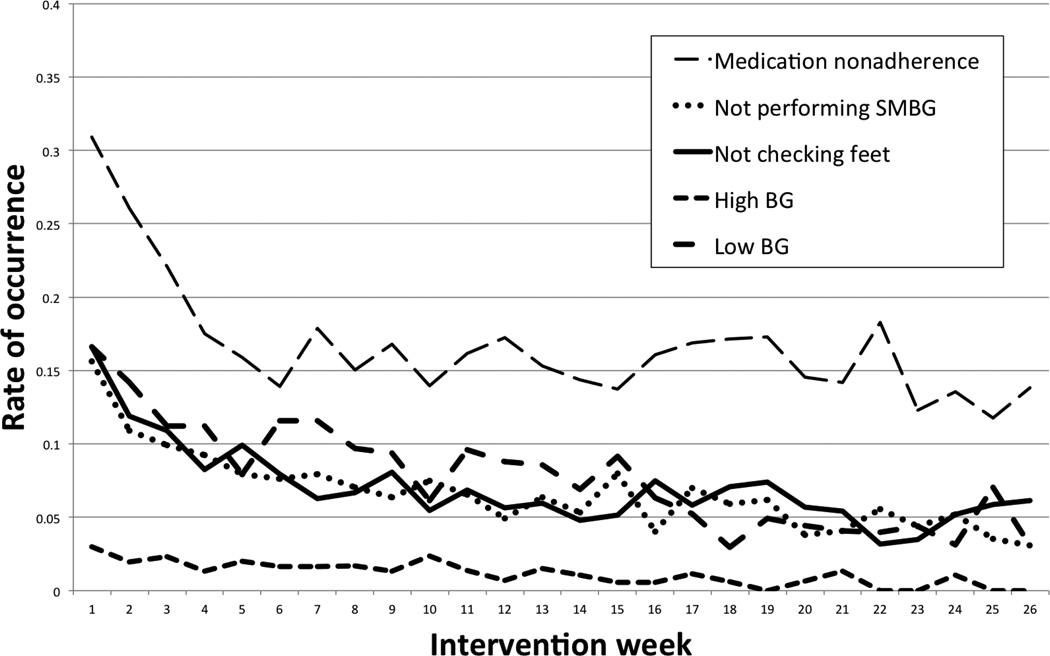
Finally, we plotted the mean probability of a negative patient report for each self-management problem by intervention week. As can be seen in Figure 1, rate of improvement in most problems seemed to follow a linear pattern over time. A notable exception was that weekly medication nonadherence rate dropped precipitously during the first twelve weeks of intervention, after which it became fairly constant for the remainder of patients’ follow-up. Given the apparent curvilinear trend in nonadherence over time, we subsequently added a quadratic term (square root of week) to the logistic model predicting weekly nonadherence (data not shown). The quadratic term had a significant effect (AOR = 0.40, 95% c.i.: 0.28 – 0.57, p < 0.001), confirming the presence of a marked curvilinear association, while the linear effect of time remained significant (p < 0.001).
Figure 1.
Mean rate of problem occurrence by intervention week.
Discussion
In summary, we described the results of a three-to-six month program of weekly IVR calls to assess and improve diabetes self-management, with automated feedback to patients and clinicians, and (when applicable) informal caregivers. Intervention seemed to be well accepted by patients, given that 84% of scheduled IVR calls were completed and only 13% of patients dropped out. During intervention, patients became less likely over time to report problems adhering to medication, performing SMBG, checking their feet, and obtaining abnormally high or low values from SMBG. After intervention, patients showed improvements in long-term medication adherence, physical functioning, depressive symptoms, and diabetes specific distress.
These highly encouraging results indicate that the combination of automated telemonitoring with clinician notification and optional caregiver involvement may benefit day-to-day self-management, both short and long-term medication adherence, and long term functional and psychological distress outcomes. Furthermore, these findings appear to apply regardless of patient age and income level, medical comorbidity, and caregiver co-participation. Although we did not assess long-term control of blood glucose or blood pressure, our findings provide a firm basis for hypothesizing that these biomedical indices may also respond favorably to this and similar interventions.
As can be seen in Figure 1, most of the improvement in weekly medication adherence occurred during the first eight to twelve weeks of intervention participation after which adherence stabilized. In contrast, problems reported in the other four areas of self-management appeared to follow a pattern of linear cumulative change over time. Furthermore, improvements in all five self-management problems tended to be maintained throughout the trial, suggesting that the benefits of this intervention might extend beyond six months. Although it is fairly unrealistic to expect newly adopted health behaviors to be maintained indefinitely in lieu of ongoing support, their apparent stability suggests that there may be an opportunity to offer a less frequent calling schedule for patients who achieve specific clinical stability benchmarks. This feature could minimize assessment burden for appropriately selected patients and possibly enhance the overall efficiency of the system for covering larger patient populations.22 Alternatively, a de-intensified focus upon low-incidence self-management problems would create new opportunities to focus upon alternative behavioral targets such as weight loss, fruit and vegetable consumption, or stress management.
Although the statistically significant improvements in health outcomes are noteworthy, all of the associated effect magnitudes fell in the “small” range. This implies that, despite the speedy and stable response seen in self-management problems, there may be a ceiling effect on how much long term improvement in health outcomes could realistically be expected with the intervention as it currently designed. As noted above, further work might extend the range of issues that could be monitored. It may also be more effective to offer individualized IVR assessments that are tailored to specific patients’ needs and preferences based upon some combination of patient choice and expert-designed algorithms. Although this expanded system would be considerably more elaborate to develop and maintain, it might have the benefit of fitting a broader range of patients and better enhancing motivation by adopting new health behaviors. Further innovations in tailoring IVR interventions to patient situation and dynamic progress are also possible via the integration of machine learning principles and exploitation of the rapidly expanding capabilities and prevalence of smart phone technologies.
Interestingly, less than 40% of patients chose to participate together with an informal caregiver. Based on patients’ feedback at baseline, the lack of caregiver involvement was usually due to personal preference as opposed to the unavailability of a person to play this role. There were few clues in our data that might help explain this, except that participation with an informal caregiver was less likely among patients from higher income brackets (p = .010). However, this association could be spurious, given that 10 comparisons were conducted. Moreover, we were unable to identify any published studies linking income to social connectedness.
It was somewhat puzzling that the intervention did not lead to an improvement in psychological functioning as assessed by the MCS, given the improvement in diabetes-related distress and physical functioning. This might be due to a partial ceiling effect, insofar as the mean baseline MCS score fell at the center of the normal range, from which it may be relatively difficult to improve. Additionally, the MCS explained only 40% of the variance in the distress measures. Because the constructs of functional limitation and distress symptoms are conceptually as well as empirically distinct,23 it is quite plausible that they could respond differently to intervention.
Limitations
The largest limitations of this study its lack of a control condition and its reliance upon self report. For the first issue, an attention placebo or waitlist control would have helped us definitively rule out the possibility that the observed improvements in patients’ health and self-care are attributable to some nonspecific aspect of study participation, the passage of time, or regression to the mean. However, values on the given outcome measures are not usually expected to change significantly over a three to six month time period. Moreover, we are unaware of the existence of any concomitant intervention that could explain the improvements, such as a system-wide quality improvement initiative. The self-reported date may be influenced by recall and social desirability report biases. Although we used validated instruments and previously established that the information provided by the system is reliable and valid10 future research should incorporate data from additional sources such as archival medical record data, biometric assessments, and corroborative reports by significant others. Because IVR call durations tended to be longer when patients reported self-management difficulties, patients may have been motivated to abbreviate their calls by denying difficulties. On the other hand, exit interviews indicated that 89% of patients wanted the intervention to become part of their usual care, suggesting that the calls were not unduly burdensome. Finally, due to the research setting the patient sample predominantly consisted of males over 60 years of age. Thus, caution is warranted when generalizing the findings to women and younger patients.
Conclusions
Based upon these encouraging results, we believe that this and similar interventions combining automated telemonitoring with clinician notification and caregiver involvement can benefit patients with type 2 diabetes. These benefits include not only improved day-to-day self-management and medication adherence, but also long term improvements in medication adherence, functional impairment, and psychological distress. Although the absolute magnitudes of the effects were modest, the current intervention is relatively inexpensive to implement and maintain because the majority of its costs were related to its development. The findings also suggest several potentially fruitful areas for further innovation and investigation, such as the development of mHealth services that automatically adapt their focus based on patients’ unique trajectory of response. Finally, we hope that most of the limitations noted above will be addressed by our follow-up work, as we are currently conducting a large randomized controlled trial in community based primary care clinics in order to verify and extend these findings.
Highlights.
-
-
Diabetes patients who received a weekly mHealth intervention showed improvements in five weekly self-management indicators.
-
-
Three-to-six month improvements were seen in adherence, physical function, and psychological adjustment.
-
-
This inexpensive intervention could lead to broad benefits and prevent diabetes complications.
Acknowledgements
JDP is a VA Senior Research Career Scientist and AMR is a VA HSR&D Career Development Awardee. Other financial support came from the Michigan Diabetes Translational Research Center (grant P30DK092926) and grant R18DK088294 from the National Institute of Diabetes and Digestive and Kidney Diseases. We appreciate the helpful statistical guidance that was generously provided by Ananda Sen, Ph.D. at the University of Michigan. Ms. Katie Grode at the University of Michigan provided editorial assistance. The views expressed in this manuscript are those of the authors and do not necessarily represent the opinions of the Department of Veterans Affairs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
All authors have read the journal's authorship agreement. We confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us. JEA researched the data and wrote the manuscript. AMR edited the manuscript and contributed to the Discussion. JDP reviewed and edited the manuscript, and contributed to the Discussion.
All authors have read the journal's policy on disclosure of potential conflict of interest. None of the authors have any financial or personal relationship to disclose with organizations that could potentially be perceived as influencing the described research. There are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- 1.Alberti KGMM, Zimmet P. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabetic medicine. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7<539::AID-DIA668>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 2.Anderson RM, Funnell MM, Aikens JE, et al. Evaluating the efficacy of an empowerment-based self-management consultant intervention: results of a two-year randomized controlled trial. Therapeutic Patient Education. 2009;1:3–11. doi: 10.1051/tpe/2009002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piette JD. Moving diabetes management from clinic to community: development of a prototype based on automated voice messaging. The Diabetes Educator. 1997;23:672–680. doi: 10.1177/014572179702300607. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz S. What works. IVR system helps diabetes center triple patient load; increases care level. Health management technology. 1998;19:41. [PubMed] [Google Scholar]
- 5.Piette JD. Interactive behavior change technology to support diabetes self-management: where do we stand? Diabetes Care. 2007;30:2425–2432. doi: 10.2337/dc07-1046. [DOI] [PubMed] [Google Scholar]
- 6.Rosland A-M, Heisler M, Janevic MR, et al. Current and potential support for chronic disease management in the United States: The perspective of family and friends of chronically ill adults. Families, Systems, & Health. 2013;31:119. doi: 10.1037/a0031535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armour T, Norris S, Jack L, Zhang X, Fisher L. The effectiveness of family interventions in people with diabetes mellitus: a systematic review. Diabetic medicine. 2005;22:1295–1305. doi: 10.1111/j.1464-5491.2005.01618.x. [DOI] [PubMed] [Google Scholar]
- 8.Talley RC, Crews JE. Framing the public health of caregiving. Am J Public Health. 2007;97:224–228. doi: 10.2105/AJPH.2004.059337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piette JD, Rosland AM, Silveira M, Kabeto M, Langa KM. The case for involving adult children outside of the household in the self-management support of older adults with chronic illnesses. Chronic illness. 2010;6:34–45. doi: 10.1177/1742395309347804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aikens JE, Zivin K, Trivedi R, Piette JD. Diabetes self-management support using mHealth and enhanced informal caregiving. Journal of Diabetes and its Complications. doi: 10.1016/j.jdiacomp.2013.11.008. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Medical Care. 1986;24:67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 12.Morisky DE, Ang A, Krousel-Wood M, Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. The Journal of Clinical Hypertension. 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34:220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 14.Kohout F, Berkman LF, Evans DA, Cornoni-Huntley J. Two shorter forms of the CES-D depression symptoms index. J Aging Health. 1993:179–193. doi: 10.1177/089826439300500202. [DOI] [PubMed] [Google Scholar]
- 15.Irwin M, Artin KH, Oxman MN. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D) Archives of Internal Medicine. 1999;159:1701. doi: 10.1001/archinte.159.15.1701. [DOI] [PubMed] [Google Scholar]
- 16.Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes Care. 2005;28:626–631. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- 17.Piette JD, Rosland AM, Marinec NS, Striplin D, Bernstein SJ, Silveira MJ. Engagement with automated patient monitoring and self-management support calls: experience with a thousand chronically-ill patients. Medical care. 2013;51:216–223. doi: 10.1097/MLR.0b013e318277ebf8. PMID: 23222527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Attkisson CC, Zwick R. The client satisfaction questionnaire. Psychometric properties and correlations with service utilization and psychotherapy outcome. Evaluation & Program Planning. 1982;5:233–237. doi: 10.1016/0149-7189(82)90074-x. [DOI] [PubMed] [Google Scholar]
- 19.Stirratt M, Dunbar-Jacob J, Crane H, Simoni J, Czajkowski S, Hillard M, Kahana S, Aikens JE, Hunter C, Velligan D, Huntley C, Rand C, Ogedegbe G, Nilsen W. Self-report measures of medication adherence behavior: Recommendations on optimal use. 2014 doi: 10.1007/s13142-015-0315-2. Manuscript under review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.StataCorp. Stata Statistical Software: Release 13.1. College Station, TX: StataCorp LP; 2014. [Google Scholar]
- 21.Aikens JE, Zivin K, Trivedi R, Piette JD. Diabetes self-management support using mHealth and enhanced informal caregiving. Journal of diabetes and its complications. doi: 10.1016/j.jdiacomp.2013.11.008. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piette JD, Aikens JE, Rosland AM, Sussman JB. Re-thinking the frequency of between-visit monitoring for patients with diabetes. Medical care. doi: 10.1097/MLR.0000000000000131. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ali S, Stone M, Skinner TC, Robertson N, Davies M, Khunti K. The association between depression and health-related quality of life in people with type 2 diabetes: a systematic literature review. Diabetes/metabolism research and reviews. 2010;26:75–89. doi: 10.1002/dmrr.1065. [DOI] [PubMed] [Google Scholar]