Abstract
Objective
Caspase-2 is an initiator caspase involved in multiple apoptotic pathways, particularly in response to specific intracellular stressors (eg. DNA damage, ER stress). We recently reported that caspase-2 was pivotal for the induction of cell death triggered by excessive intracellular accumulation of long chain fatty acids, a response known as lipoapoptosis. The liver is particularly susceptible to lipid-induced damage, explaining the pandemic status of non-alcoholic fatty liver disease (NAFLD). Progression from NAFLD to non-alcoholic steatohepatitis (NASH) results, in part, from hepatocyte apoptosis and consequential paracrine-mediated fibrogenesis. We evaluated the hypothesis that caspase-2 promotes NASH-related cirrhosis.
Design
Caspase-2 was localized in liver biopsies from NASH patients. Its expression was evaluated in different mouse models of NASH, and outcomes of diet-induced NASH were compared in wild type (WT) and caspase-2 deficient mice. Lipotoxicity was modeled in vitro using hepatocytes derived from WT and caspase-2 deficient mice.
Results
We showed that caspase-2 is integral to the pathogenesis of NASH-related cirrhosis. Caspase-2 is localized in injured hepatocytes and its expression was markedly up-regulated in patients and animal models of NASH. During lipotoxic stress, caspase-2 deficiency reduced apoptosis, inhibited induction of pro-fibrogenic Hedgehog target genes in mice, and blocked production of Hedgehog ligands in cultured hepatocytes.
Conclusion
These data point to a critical role for caspase-2 in lipid-induced hepatocyte apoptosis in vivo, for the production of apoptosis-associated fibrogenic factors and in the progression of lipid-induced liver fibrosis. This raises the intriguing possibility that caspase-2 may be a promising therapeutic target to prevent progression to NASH.
Keywords: nonalcoholic steatohepatitis, caspase-2, apoptosis, fibrosis
Introduction
Because mice lacking the initiator caspase, caspase-2, exhibit no overt developmental phenotypes aside from accumulation of excess oocytes, the physiological importance of caspase-2 in controlling apoptosis has been the subject of some debate. It has been shown that caspase-2 is important for cell death in response to several stress-inducing agents, including ER stress, taxanes and DNA damage [1, 2, 3]. Additionally, we have recently reported that caspase-2 activation is essential for lipoapoptosis, a cell death pathway triggered by excess intracellular accumulation of lipids [4, 5].
Extracts prepared from Xenopus laevis eggs accumulate long chain fatty acids (FA) upon prolonged incubation at room temperature, and undergo the molecular events of apoptosis following caspase-2 activation. Blocking accumulation of long chain FA blunted the caspase-2 response [5]. Other lines of evidence also link caspase-2 activation with lipid metabolism. Addition of palmitate to hepatocytes in culture induced apoptotic cell death that was significantly dampened by siRNA-mediated knock-down of caspase-2 [5]. Rats fed high fat diets demonstrated increased caspase-2 expression in adipose tissue [6]. These reports raised the intriguing possibility that caspase-2 might play a role in diseases in which lipoapoptosis has been implicated in the pathogenic mechanism.
Non-alcoholic fatty liver disease (NAFLD) is the number one cause of chronic liver disease in the western world, with more than one third of the population affected [7]. It strongly associates with the metabolic syndrome and its components, namely obesity and insulin resistance/type 2 diabetes mellitus [8]. Although usually benign, in 20-30% of cases, fatty liver may result in serious injury with inflammation and hepatocyte necro-apoptosis, dubbed nonalcoholic steatohepatitis (NASH). NASH may eventuate in progressive fibrosis and cirrhosis [9]. NASH-related cirrhosis is already the third leading cause of liver transplantation in the United States [10].
Correlative histopathological studies of human liver biopsies, as well as research in cell and animal models [11], provide strong evidence of increased hepatocyte apoptosis in NASH. Indeed, apoptosis is believed to be crucial in the pathogenesis of NASH-related cirrhosis. Special attention has been given to effector caspases-3 and -7 [12], and more recently, to initiator caspases-8 and -9 [13, 14]. A prevailing concept is that injured hepatocytes behave as undead cells, initiating the apoptotic process but failing to complete it, thereby providing a sustained source of apoptosis-associated molecular signals, including Hedgehog, that drive liver inflammation and fibrosis [14, 15, 16].
Hepatocyte lipotoxicity is a hallmark of NASH [17]. In NASH, lipotoxicity has been attributed to insulin resistance [18]. Insulin resistance deprives hepatocytes of major trophic signals, inducing metabolic stress despite exposure to abundant nutrients [19]. Consistent with a role for caspase-2 in this process, immunoblotting of liver samples from morbidly obese NAFLD patients showed increased cleaved caspase-2, though it was not clear if this was a cause or consequence of cell death [20]. Here we evaluate the hypothesis that caspase-2 is an apical caspase required for lipotoxicity-related hepatocyte apoptosis, critical for driving the pathogenesis of NASH-related cirrhosis. We have found that increased caspase-2 expression is correlated with NASH in human patients and also that caspase-2 is elevated in several animal models of NAFLD. More importantly, we have found that caspase-2 knock-out mice are protected from hepatocyte apoptosis triggered during diet-induced NASH. This decreased apoptosis is accompanied by a reduced ductular reaction and decreased liver fibrosis. In aggregate, these data link caspase-2 to lipotoxicity and fibrogenesis in the liver and raise the intriguing possibility that caspase-2 might be a novel therapeutic target in NASH.
Material and Methods
Human Samples
Liver biopsies from liver transplant donors (n=4) and adult patients with simple steatosis (n=4), NASH with no or mild fibrosis (n=5), and NASH-cirrhosis (n=5) were randomly selected from Duke University Health System NAFLD Clinical Database and Biorepository. To validate findings in NASH, biopsies from a second independent cohort of 23 NASH patients from Duke University and Liver-Gastroenterology Department, University Hospital, Angers, France were also examined. Patients with histological NASH had no significant alcohol consumption or other chronic liver disease. Studies were conducted in accordance with National Institutes of Health and institutional guidelines for human subject research.
Animal Studies
Male obese, diabetic mice (ob/ob and db/db mice), and wild-type (WT) mice C57Bl/6 were obtained from Jackson Laboratory (Bar Harbor, ME). At least 5 animals per group were fed methionine choline-deficient (MCD) diets (MP Biomedicals, Solon, OH) for 8 weeks. WT mice were also fed MCD diet for 4 weeks. Equal numbers of mice were fed standard rodent food (4.7 kcal/g: 13% calories as fat, 62% carbohydrates and 24% proteins).
In a second experiment, caspase-2-/- (B6.129SY-casp2tm1Yuan/J) and appropriate control mice were purchased from Jackson Laboratory (Bar Harbor, ME). Five animals per group were fed high fat (HF)/MCD diets or the same HF diet supplemented with methionine and choline (MP Biomedicals, Solon, OH) for 8 weeks. The HF diets provided 4.5 kcal/g: 20% calories as fat, 58% carbohydrates and 22% proteins. At the end of treatment, mice were fasted for 6 hours and sacrificed. Livers were harvested and either formalin-fixed or snap frozen.
Animal care and procedures were approved by the Duke University Institutional Animal Care and fulfilled National Institutes for Health and Duke University IACUC requirements for humane animal care.
Cell Isolation and Culture
Mouse hepatocytes were isolated from 3 caspase-2-/- and 3 control mice as previously described [21]. Hepatocytes were seeded onto plastic culture dishes (Sigma, St. Louis, MO) in Dulbelcco's modified Eagle's medium (DMEM)/F12 (Invitrogen, Carlsbad, CA) supplemented with 10% fetal bovine serum (FBS), ITS (1:200; Invitrogen), dexamethasone (1 mM; Sigma, ST. Louis, MO) and antibiotics (Life Technologies). Cells were treated with similar media but with 2% FBS and 2% bovine serum albumin (Sigma) with or without palmitate (1 mM, Sigma) based on prior studies that defined the optimal dose and duration of palmitate treatment (Supplemental Figure 4).
Histopathological analysis
Formalin-fixed, paraffin-embedded liver biopsies were cut into 5 μm serial sections. NAFLD severity was assessed with hematoxylin and eosin staining using criteria described by Brunt et al. [22].
For immunohistochemistry, sections were deparafinized with xylene and rehydrated. Slides were incubated in 3% hydrogen peroxide/methanol. Antigen retrieval was performed by heating in 10 mM sodium citrate (pH 6.0). Sections were incubated overnight with caspase-2 antibody. Horseradish peroxidase-conjugated IgG secondary antibody was used. Antigens were demonstrated by diaminobenzidine (K3466; Dako Envision, Carpinteria, CA). Omitting primary antibodies from reactions eliminated staining, demonstrating specificity. Tissue sections were counterstained with Aqua Hematoxylin-INNOVEX (Innovex Biosciences). Morphometric analysis was done with Metamorph Software (Molecular Devices Corporation, Downingtown, PA). Immunohistochemistry antibodies are specified in Supplemental Table 1.
Liver fibrosis was assessed by Picrosirius red (Sigma, ST. Louis, MO) staining. Quantification was done by morphometric analysis in randomly chosen sections (x10 magnification, 20 fields/sample) [23].
Terminal deoxynucleotidyl transferase-mediated dUTP nick-end labeling (TUNEL) assay (Boehringer Mannheim, Germany) was performed according to the manufacturer's suggestions.
Serum and tissue analysis
Blood was collected when sacrificing the animals and serum was obtained. A biochemical panel was outsourced to Veterinary Diagnostic Laboratory, Division of Laboratory Animal Resources, Duke University Medical Center. Insulin was measured with Ultrasensitive Mouse Insulin ELISA kit (Crystal Chem Inc: #90080); Shh was measured by ELISA (Sigma:RAB0431); and triglycerides were measured in the serum and in the liver, with Triglyceride Colorimetric Assay kit (Cayman Chemical Company: #10010303), according to manufacturer instructions.
Molecular Studies
mRNA quantification by Real-time Reverse Transcription-PCR (RT-PCR)
Total RNA was extracted from cells or livers using TRIzol (Invitrogen). RNA was reverse transcribed to cDNA templates using random primer and Super Script RNAse H-Reverse Transcriptase (Invitrogen) and amplified. Semiquantitative qRT-PCR was performed using iQ-SYBR Green Supermix (Bio-Rad, Hercules, CA) and StepOne Plus Real-Time PCR Platform (ABI/Life Technologies), as previously described [20]. For primers, see Supplemental Table 3.
Western Blotting
Total proteins were extracted from hepatocytes and whole liver using RIPA buffer (Sigma) supplemented with phosphatase and protease inhibitors (Roche, Madison, WI). Equal amounts of protein were separated by electrophoresis on 4%-20% Criterion gels (BioRad, Hercules, CA), transblotted into polyvinylidene difluoride membranes, and incubated with antibodies against Caspase-2, Shh, Gli-2, and α-tubulin (Supplemental Table 2).
Apoptosis and Cell viability assays
Apoptosis and cytotoxicity were measured using ApoTox-Glo™ Triplex Assay (Promega, Madison, WI). Cell viability was measured using the Cell Counting Kit-8 (Dojindo Molecular Technologies, Gaithersburg, MD).
Statistics
Results were expressed as mean±SEM. Significance was established using Student's test or Mann-Whitney Test, and Spearman correlation, with significance at p<0.05.
Results
Patients with NASH demonstrate increased expression of caspase-2, with predilection for ballooned hepatocytes
Immunohistochemistry for caspase-2 in liver samples from NAFLD patients showed consistent increases in caspase-2 compared to controls, with strongest caspase-2 expression in the sub-group with NASH-cirrhosis (Figure 1). Caspase-2 localized predominantly in hepatocytes and the ratio of positive/negative cells was higher in ballooned compared to non-ballooned hepatocytes. Staining was also more intense near areas of lobular inflammation and near fibrotic septa. In NASH, Sonic hedgehog (Shh) is expressed in lipotoxic hepatocytes, as opposed to healthy ones, and is particularly enriched in ballooned cells [24]. Double immunohistochemistry for Shh and caspase-2 showed frequent double-positive cells, with strong co-localization of Shh and caspase-2 in ballooned hepatocytes.
Figure 1. Increased caspase-2 staining in patients with NASH.
A. Caspase-2 immunohistochemistry (brown) in representative human healthy livers (n=4), patients with simple steatosis (n=4), NASH with no or mild fibrosis (n=5) and NASH-associated cirrhosis (n=5). B. Morphometric analysis of caspase-2 staining in all patients. Caspase-2 staining was also performed in a replication cohort of patients with NASH and low fibrosis (n=12) or cirrhosis (n=11). Results are expressed as fold change over data in healthy liver and graphed as mean±SEM.*P<0.05; **P<0.01 for normal livers versus all other groups. C. Caspase-2 positive cells were counted in non-ballooned hepatocytes (NBH) and ballooned hepatocytes (BH) in 20 randomly selected fields/section in patients with NASH (n=5). Mean±SEM are graphed. D. Double immunohistochemistry for Sonic hedgehog (Shh, green) and caspase-2 (brown) in NASH.
Different animal models of NASH also demonstrate increased liver expression of caspase-2, independent of obesity and insulin resistance
We studied caspase-2 expression by immunohistochemistry in several different animal models of NASH: ob/ob, db/db, and WT mice fed chow or MCD diet for 8 weeks. Chow-fed ob/ob and db/db mice are models of mild NASH, obesity and type 2 diabetes mellitus, whereas the MCD diet model leads to more severe NASH but has features of starvation, including significant weight loss and increased insulin sensitivity [25]. Relative to respective controls, caspase-2 staining was increased in all models of NASH, with highest expression in more severe NASH, as modeled by feeding the MCD diet (Figure 2A). Moreover, caspase-2 expression increased with the duration of MCD diet exposure in WT mice (Figure 2C).
Figure 2. Increased caspase-2 levels in mouse models of NASH correlates with fibrosis.
A. Caspase-2 immunohistochemistry in livers from representative WT mice, ob/ob and db/db mice fed regular chow or methionine choline deficient (MCD) diet. Morphometric analysis of caspase-2 staining in all mice (n=5/treatment group). Results are expressed relative to data in chow-fed WT mice and graphed as mean±SEM. B. qRT-PCR analysis of Caspase-2 mRNA in WT mice fed chow (n=7) or MCD diet (n=6). Representative western blot analysis of Caspase-2. Caspase-2 was quantified by densitometry, normalized to α-tubulin in the same sample, expressed relative to chow-fed controls and graphed as mean±SEM. *<0.05 and **<0.01, control versus MCD diet; †<0.05 and ‡<0.01, WT versus obese mice. C. Caspase-2 immunohistochemistry from representative WT mice fed chow diet or MCD diet for 4 weeks or 8 weeks. Morphometric analysis of caspase-2 staining in all mice (n=3/group). **<0.01, control versus MCD diet; ‡<0.01 8 versus 4 weeks MCD diet. D. Spearman correlation between Sirius red morphometry and caspase-2 immunohistochemistry.
Additional analyses were done in MCD diet-fed mice with NASH. WT animals fed MCD diet showed a more than 50% increase in total caspase-2 mRNA and protein as compared to control chow-fed mice (Figure 2B). As expected, MCD diets caused fibrosing NASH in WT mice, with hepatic steatosis, intense lobular inflammation, ductular reaction, and bridging fibrosis. Also as reported previously [26], MCD diets increased hepatic Hedgehog pathway activity, demonstrated by induction of Hedgehog ligands, Hedgehog-regulated transcription factors, and osteopontin, a pro-fibrogenic Hedgehog-target gene, as well as accumulation of fibrosis markers (α-SMA and collagen-1α1) (Supplemental Figure 1).
Caspase-2 expression in hepatocytes correlates with the severity of fibrosis in animal models of NASH
Models of mild NASH (chow-fed ob/ob and db/db mice) and severe NASH (MCD diet fed WT, ob/ob or db/db mice) were also assessed for liver fibrosis using Sirius Red staining (Supplemental Figure 1). The pattern of fibrotic changes mimicked the pattern of caspase-2 staining changes. In fact, morphometric analysis demonstrated that caspase-2 and fibrosis were strongly correlated (r=0.818, P=0.00019) (Figure 2D).
Caspase-2 KO mice are protected from hepatocyte apoptosis during diet-induced NASH
WT and caspase-2 KO mice were fed HF+MCD diet or similar HF diet supplemented with methionine and choline for 8 weeks. On the control HF diet, WT mice developed mild steatosis and some inflammatory foci, whereas caspase-2 KO mice maintained normal histology except for rare inflammatory foci. After consuming HF+MCD diet, WT mice exhibited typical features of severe NASH, with severe steatosis, lobular inflammation, cellular degeneration, ductular reaction and fibrosis. Caspase-2 KO mice appeared to be protected from MCD diet-induced NASH, demonstrating less severe liver injury on H&E stained liver sections (Figure 3A). The protective effect of caspase-2 deficiency was confirmed by analysis of serum alanine aminotransferase levels, which were more than twice as high in MCD diet-fed WT mice as similarly treated caspase-2 KO mice (Figure 3B). Reduced liver injury in caspase-2 KO mice was not explained by improvements in hepatic steatosis or inflammation. As assessed by oil red O staining (Figure 3B) and hepatic triglyceride quantification (Supplemental Figure 2), both WT mice and caspase-2 KO mice developed similar levels of liver fat when fed MCD diets. The two groups also developed similar levels of MCD diet-induced inflammation, as measured by number of inflammatory foci (Figure 3. B), immunohistochemistry for macrophage markers, and qRT-PCR for inflammatory cytokines (Supplemental Figure 3). However, caspase-2 deficiency had a dramatic effect on apoptosis, significantly reducing numbers of hepatocytes with activated caspase-3 (Figure 3C), a key downstream effector caspase in lipoapoptosis [14], as well as apoptotic liver cells themselves, as assessed by TUNEL assay (Figure 3D).
Figure 3. Caspase-2 KO mice are protected from NASH-related apoptosis.
A. H&E stained liver sections from representative WT and caspase-2 KO mice fed either HF diet or HF+MCD diet as detailed in the Methods. B. Oil red O staining in representative liver sections; mean±SEM numbers of hepatic inflammatory foci and serum ALT in all mice (n=20). C. Immunohistochemistry for cleaved caspase-3: liver sections from representative mice; capase-3-positive cells were counted and graphed as mean±SEM (n=5 mice/group). D. TUNEL assay: sections from representative mice; TUNEL-positive cells were counted and graphed as mean±SEM (n=5 mice/group). White bars = HF diet; black bars = HF+MCD diet; *<0.05 and **<0.01, HF versus HF-MCD diet; †<0.05 and ‡<0.01, WT versus caspase-2 KO mice.
Reduced ductular reaction and liver fibrosis accompany decreased lipoapoptosis in caspase-2 KO mice
During NASH, liver cell death triggers compensatory regenerative responses that lead to activation of the hepatic progenitor compartment, resulting in the ductular reaction [16]. Because we found that capase-2 promotes NASH-related apoptosis (Figure 3C,D) we examined the effect of capase-2 deficiency on the ductular reaction. As expected [27], MCD diet exposure provoked a ductular reaction in WT mice, as demonstrated by induction of several markers of ductular-type progenitors, including Ker19 and Ker7 (Figure 4 A,B). In caspase-2 KO mice, all of these ductular reaction parameters were significantly reduced (Figure 4 A,B). The blunted ductular response in caspase-2 KO seems to be a consequence of decreased apopototic cell death (Figure 3 C,D) and not a primary defect in regeneration, since liver mass and liver to body mass was similar in both genotypes (data not shown) and there was no evidence of liver failure, namely no increase in bilirubin levels (data not shown). Because lipoapoptosis and the ductular reaction are strongly linked to fibrosis stage in NASH [27], we next compared the level of stellate cell activation and fibrosis severity in the two strains. Compared to WT mice, caspase-2 KO mice developed significantly less hepatic stellate cell activation and fibrosis when chronically challenged by MCD diets, as assessed by Sirius red staining, collagen-1α and αSMA immunohistochemistry, and qRT-PCR analysis of whole liver collagen-1α and α-sma gene expression (Figure 4C). The aggregate data, therefore, support the concept that caspase-2 deficiency constrains progression of NASH-related fibrosis by inhibiting hepatocyte apoptosis and resultant fibrogenic regenerative responses that often occur during lipotoxic stress.
Figure 4. Reduced NASH-related ductular reaction and liver fibrosis in Caspase-2 KO mice.
A. qRT-PCR analysis of progenitor markers, K19 and K7, in groups described in legend to Figure 3. Results in all mice (n=20) are graphed as mean±SEM. B. Immunohistochemistry for progenitor marker, Pan-CK: sections from representative mice; morphometric analysis in all mice expressed as mean±SEM. C. Fibrosis markers: sections from representative mice; morphometric analysis in all mice expressed as mean±SEM; qRT-PCR analysis of liver RNA normalized to expression in HF diet-fed WT mice and expressed as mean±SEM. Top panels = Sirius red staining, middle panels =collagen-1α, bottom panels = α-smooth muscle actin (aSMA). White bars = HF diet; black bars = HF+MCD diet. *<0.05 and ** <0.01, HF versus HF-MCD diet; †<0.05 and ‡<0.01, WT versus caspase-2 KO mice.
Caspase-2 deficiency decreases hedgehog pathway activation in response to liver lipotoxicity
Our findings in humans (Figure 1) and mice with NASH (Figures 2-4) demonstrate that caspase-2 is increased during lipotoxicity and plays an important role in NASH-related lipoapoptosis and its sequelae. Lipotoxicity has been modeled by treating cultured hepatocytes with palmitate to induce lipoapoptosis [14]. Dying cells are known to activate their production of various pro-regenerative fibrogenic factors, including Shh [15, 16, 24]. Moreover, blocking cell death after initiation of apoptosis prolongs Shh production [14, 15]. To determine if caspase-2 directly influenced hepatocyte production of Shh ligands, we compared the effects of palmitate on Shh expression in primary hepatocytes from WT and caspase-2 KO mice. Hepatocytes from caspase-2 KO mice expressed less Shh ligand in response to a lipotoxic stimulus than WT mice, at both the mRNA and protein level (Figure 5A). Moreover, caspase-2 KO hepatocytes were protected from caspase 3/7 activation and cell death induced by palmitate treatment (Figure 5A) Because Shh protein cannot be localized by immunohistochemistry in mice using available Shh antibodies, we compared serum levels of Shh in WT and caspase-2 KO mice to determine if caspase-2 deficiency also reduced Shh ligand production during lipotoxicity in vivo. Indeed, after MCD diet feeding, levels of Shh protein were significantly lower in caspase-2 KO mice than similarly treated WT mice (3.8±2.3 versus 14.5±2.1pg/mL, P<0.007). Reduced Shh ligand exposure was accompanied by less activation of the Hedgehog pathway in the livers of caspase-2 KO mice. Compared to WT mice, caspase-2 KO mice demonstrated lower levels of the Hedgehog-regulated transcription factor, gli-2, by qRT-PCR and western blot analysis (Figure 5B). Messenger RNA and protein expression of osteopontin, a profibrogenic gli2-target gene, were also reduced significantly in the caspase-2 KO mice (Figure 5C).
Figure 5. Decreased production of Shh ligand and Hedgehog pathway activation in Caspase-2 KO mice.
A. Shh mRNA and protein, Caspase-3/7 activation, and viability of hepatocytes from WT and caspase-2 KO mice before and after treatment with palmitate or bovine serum albumin (control). Mean±SEM data from triplicate cultures are graphed. Results shown are representative of triplicate experiments. *<0.05 and **<0.01, BSA versus Palmitate; †<0.05 and ‡<0.01, WT versus caspase-2 KO mice. B. Markers of hedgehog pathway activity in WT and caspase-2 KO mice either HF or HF+MCD diets. Top panels = qRT-PCR and Western blot analysis of Gli2 mRNA and protein. Bottom panels = osteopontin immunohistochemistry: sections from representative mice; morphometric analysis of all mice;qRT-PCR analysis of osteopontin mRNA. All results are graphed as mean±SEM. White bars = HF diet; black bars = HF+MCD diet. *<0.05 and **<0.01, HF versus HF+MCD diet; †<0.05 and ‡<0.01, WT versus caspase-2 KO mice.
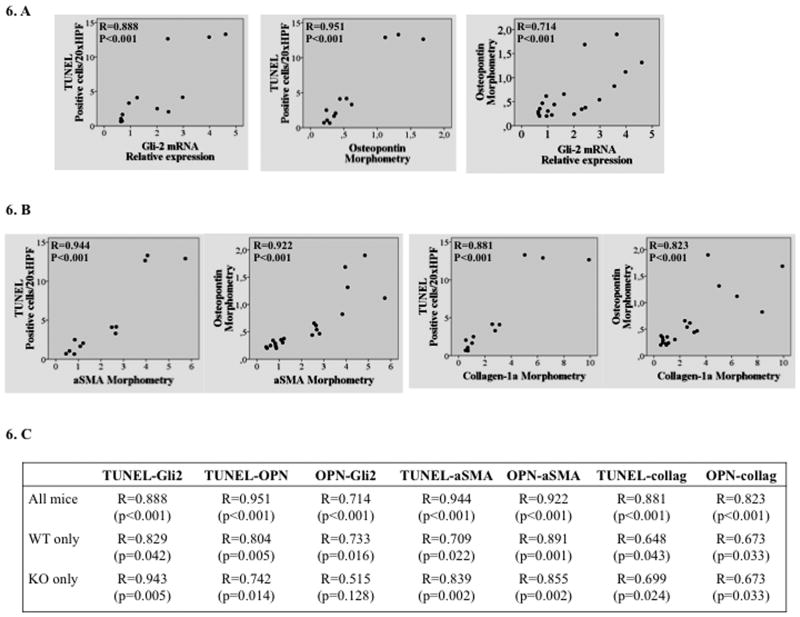
Apoptosis strongly correlates with hedgehog pathway activation and fibrogenesis in WT and caspase-2 KO mice with NASH
The aggregate data, therefore, suggest a mechanism that might explain why caspase-2 correlated with fibrosis severity in various animal models of NASH (Figure 2C). Namely, caspase-2 is a major driver of lipoapoptosis; cells undergoing lipoapoptosis produce Hedgehog ligands; Hedgehog ligands stimulate fibrogenic regenerative responses. Indeed, we found strong correlations between numbers of apoptotic liver cells (assessed by TUNEL assay) and gli-2 mRNA levels, as well as osteopontin expression (quantified by histomorphometry). Also, apoptosis by TUNEL and osteopontin immunohistochemistry strongly correlated with immunohistochemistry for markers of hepatic stellate cell activation (α-SMA) and fibrosis, (collagen-1α)(Figure 6). These results support the hypothesis that lipotoxicity activates caspase-2, thereby initiating hepatocyte lipoapoptosis with consequent production of Hedgehog ligands that promote liver fibrosis.
Figure 6. Apoptosis correlates with hedgehog pathway activation and fibrogenesis during NASH.
Data from HF or HF+MCD diet fed WT and caspase-2 KO mice were evaluated by Spearman correlation analysis. A. Apoptosis (as assessed by TUNEL assay) and Gli2 mRNA expression (left); Apoptosis and Osteopontin morphometry (middle); Osteopontin morphometry and Gli2 mRNA expression. B. Apoptosis and Osteopontin also correlated with aSMA and collagen 1a immunohistochemistry. C. Summary table with correlations performed in all groups, and separately in WT or caspase-2 KO mice.
Discussion
In this study we showed that caspase-2 expression is increased in NASH, and demonstrated the cell types in which that expression occurs, namely fatty hepatocytes and particularly, ballooned hepatocytes. Evidence for enrichment of caspase-2 in ballooned hepatocytes was intriguing to us because hepatocyte ballooning is a consequence of cytoskeletal disruption and caspase-2 has been shown to cleave certain cytoskeletal proteins [28]. Accumulation of ballooned hepatocytes has been linked to fibrosis stage in NASH [29], and we found that caspase-2-positive cells were enriched near fibrotic septa in both NASH patients and several animal models of NASH. Moreover, we discovered that the level of caspase-2 expression correlated strongly with the degree of fibrosis, suggesting that the caspase-2-positive hepatocytes themselves might be contributing to the fibrogenic process, though we cannot exclude the converse, i.e., that activated stellate cells, inflammatory cells or progenitors release factors that activate caspase-2 and decrease hepatocyte viability. In any case, these findings were noted in obese, diabetic models of NASH, as well as NASH models that are characterized by weight loss and insulin sensitivity, suggesting that caspase-2 induction and related fibrogenesis are driven by hepatocyte lipotoxicity per se, rather than associated co-morbidities.
The possibility that caspase-2 induction might be directly linked to lipotoxicity is intriguing because lipoapoptosis is considered a key driver of NASH pathogenesis and progression [12]. Indeed, it has already been shown that long chain FA, particularly saturated ones, induce and activate caspase-2 in Xenopus laevis eggs and mammalian cells [5]. A plausible model is suggested by the fact that FA decrease the free CoA/acyl-CoA ratio, resulting in reduced activation of calcium/calmodulin regulated protein kinase II (CAMKII) and consequent decreases in CAMKII-mediated phosphorylation of caspase-2 at Serine-135. Because such phosphorylation typically inactivates caspase-2, its activity might increase as FA accumulate [30]. Caspase-2 may also be activated by tumor necrosis factor (TNF)-α. In endothelial cells, for example, TNF-α induces apoptosis through TNFRSF1A-mediated activation of caspase-2 [31]. Though this pathway has not been studied in hepatocytes, TNFRSF1A is expressed by all cell types, and TNF-α is increased in obesity and NAFLD, two diseases in which this cytokine is believed to play a major role [32]. In the present study, we showed that caspase-2 deficiency protected mice from hepatocyte apoptosis despite diet-related increases in hepatic steatosis and TNF-α production, suggesting that caspase-2 is an important down-stream effector of liver cell death due to these factors during NASH.
Liver cell death is a key factor that differentiates NASH from hepatic steatosis [32]. Furthermore, the level of hepatocyte death strongly predicts the severity of liver fibrosis during NASH [12]. Various mechanisms have been proposed to explain these observations, including the possibility that initiation of lipoapoptosis stimulates afflicted hepatocytes to produce fibrogenic factors until they die [33]. Hedgehog ligand is one such pro-fibrogenic, death-associated molecular signal. Hedgehog ligands stimulate outgrowth of stromal cells that are typically associated with wound healing, such as progenitors and myofibroblasts [26, 34]. In flies, experimental interventions that delay the demise of cells that have initiated apoptosis prolongs Hedgehog ligand production, thereby amplifying local accumulation of factors that exacerbate fibrogenic repair [15].
Our group and Gores' lab have provided evidence that a similar process contributes to fibrosis progression during NASH. We showed that triggering apoptosis by inhibiting nuclear localization of NF-kB provoked mammalian hepatocytes to produce Shh in vitro and linked this to hepatic accumulation of Hedgehog-responsive progenitors and myofibroblasts, as well as liver fibrogenesis, in mice with genetically-impaired nuclear localization of NFkB in hepatocytes[16]. Subsequently, we reported that human ballooned hepatocytes are particularly enriched with Hedgehog ligands and demonstrated that accumulation of Hedgehog-positive hepatocytes correlated significantly with fibrosis severity in human NASH[24, 35]. Shortly thereafter, Gores' group discovered that ballooned hepatocytes were deficient in caspase-9, thereby identifying a mechanism that likely delayed the death of such cells and prolonged their production of Hedgehog ligands [14]. This helped to explain why Shh expression was particularly enriched in ballooned hepatocytes [24, 35], and why accumulation of ballooned hepatocytes was a robust predictor of fibrosis severity in NASH [29]. However, although Gores and colleagues also observed caspase-2 expression in ballooned hepatocytes, they concluded that such cells were not likely to be producing Hedgehog ligand because lipoapoptosis had been initiated per se. Rather, they suggested that it was the deficiency of caspase-9 that promoted Hedgehog ligand production by ballooned hepatocytes based on studies in cultured hepatoma cells demonstrating detectable Shh ligand production in response to palmitate treatment only when caspase-9 was depleted with a short hairpin RNA [14].
The present study provides a novel in vivo function for caspase-2 and clarifies the role of caspase-2 in NASH-related lipoapoptosis, as well as the role of lipoapoptosis itself as a stimulus for Hedgehog ligand production. We demonstrated that caspase-2 KO mice had significantly fewer cells with activated caspase-3, as well as significantly reduced numbers of TUNEL-positive cells during NASH. These findings show that caspase-2 is an important up-stream caspase in hepatocyte lipoapoptosis. Moreover, we found that caspase-2 deficient hepatocytes produce significantly less Shh mRNA and Shh protein than wild-type hepatocytes when they are directly exposed to relatively high doses of palmitate, a well-established trigger of lipoapoptosis in wild type hepatocytes [36]. The aggregate new information, therefore, support the concept that the mechanisms initiating recovery of tissue integrity after injury are highly conserved across species and include fundamental integration of mechanisms that program cell death with those that program replacement of the cell that is destined to die. More importantly, the present data extend a growing body of evidence that links tissue regeneration to scarring [37] and support the concept that morphogens released from dying cells drive both responses. In adult organisms, as in developing embryos, both the concentration and the duration of exposure to Hedgehog ligands seem to inform tissue construction, with excessive and/or sustained exposure to Shh favoring tissue accumulation of fibrogenic stromal cells while constraining terminal epithelial differentiation [26, 38]. Consistent with that concept, we found that progenitors, myofibroblasts and fibrosis increased when caspase-2-mediated apoptosis and Hedgehog ligand production increased during NASH, and that all of these processes were significantly attenuated by caspase-2 depletion.
These results identify caspase-2 as a potential therapeutic target in NASH. This possibility merits further study because preventing fibrosis progression is likely to require prolonged treatment in human NASH, and we found no evidence that chronically inhibiting caspase-2 was unsafe in our mouse model of MCD diet-induced NASH. MCD diet-fed mice do not develop obesity or insulin resistance, key correlates of NASH in humans, however. Hence, validation of these concepts will require future studies in mouse models that better model human NASH, such as prolonged feeding of Western diets [39]. Nevertheless, despite initial concerns about the possible tumorigenic effect of caspase-2 depletion [40], caspase-2 KO mice are reported to be generally healthy [41, 42]. If confirmed by further research, the aggregate data would suggest that caspase-2 inhibition might be beneficial for many of the adverse clinical outcomes that result from NASH.
Supplementary Material
Summary Box.
What is already known
NAFLD, the main cause of liver disease in the Western world, results from excessive lipid accumulation in the liver.
Apoptosis plays a crucial role in the progression from simple steatosis to NASH, a more serious type of liver pathology that can lead to cirrhosis.
Caspase-2 is an initiator caspase that is essential for palmitate-induced lipoapotosis in cultured HepG2 cells.
New Findings
Caspase-2 is up-regulated in humans and rodents with NASH, and strongly localizes in injured/ballooned hepatocytes.
Caspase-2 deficient mice are protected from apoptosis, Hedgehog pathway activation, and fibrosis during diet-induced NASH.
Hepatocytes isolated from caspase-2 deficient mice are protected from palmitate-mediated induction of Hedgehog ligand synthesis and cell death by apoptosis.
Future Impact in Clinical Practice
Caspase-2 may be a new therapeutic target for NASH.
Acknowledgments
We thank Manal Abdelmalek for kindly selecting the human samples evaluated.
This research is supported by NIH DK0077794 (Diehl AM), Duke Endowment: The Florence McAlister Professorship (Diehl AM), and NIH GM080333 (Kornbluth S).
Machado MV is a receiver of a PhD grant from Fundação para a Ciência e Tecnologia, FCT, Portugal
Footnotes
Authors contributions: Machado MV – study concept and design, acquisition of data, analysis and interpretation of data, drafting of the manuscript, statistical analysis
Michelotti GA – study concept and design, acquisition of data, analysis and interpretation of data
Pereira de Almeida T – acquisition of data, analysis and interpretation of data
Boursier, J – acquisition of human samples, analysis and interpretation of data
Kruger L – acquisition of data
Swiderska-Syn M – acquisition of data
Karaca G – acquisition of data
Xie G – acquisition of data, analysis and interpretation of data
Guy CD – analysis and interpretation of data
Bohinc B – acquisition of data
Lindblom KR – study concept and design, technical support
Johnson E – study concept and design, technical support
Johnson E – study concept and design, technical support
Kornbluth S – study concept and design, critical revision of the manuscript for important intellectual content, material support
Diehl AM – study concept and design, analysis and interpretation of data, manuscript writing, obtained funding
Disclosures: The authors have no conflict of interest to disclose.
References
- 1.Lamkanfi M, Declercq W, Kalai M, Saelens X, Vandenabeele P. Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell death and differentiation. 2002;9:358–61. doi: 10.1038/sj.cdd.4400989. [DOI] [PubMed] [Google Scholar]
- 2.Upton JP, Wang L, Han D, Wang ES, Huskey NE, Lim L, et al. IRE1alpha cleaves select microRNAs during ER stress to derepress translation of proapoptotic Caspase-2. Science. 2012;338:818–22. doi: 10.1126/science.1226191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouchier-Hayes L, Green DR. Caspase-2: the orphan caspase. Cell death and differentiation. 2012;19:51–7. doi: 10.1038/cdd.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu HY, Chen ZW, Li H, Zhou L, Liu F, Lv YY, et al. 12-Deoxyphorbol 13-palmitate mediated cell growth inhibition, G2-M cell cycle arrest and apoptosis in BGC823 cells. European journal of pharmacology. 2013;700:13–22. doi: 10.1016/j.ejphar.2012.11.015. [DOI] [PubMed] [Google Scholar]
- 5.Johnson ES, Lindblom KR, Robeson A, Stevens RD, Ilkayeva OR, Newgard CB, et al. Metabolomic profiling reveals a role for caspase-2 in lipoapoptosis. The Journal of biological chemistry. 2013;288:14463–75. doi: 10.1074/jbc.M112.437210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jobgen W, Fu WJ, Gao H, Li P, Meininger CJ, Smith SB, et al. High fat feeding and dietary L-arginine supplementation differentially regulate gene expression in rat white adipose tissue. Amino acids. 2009;37:187–98. doi: 10.1007/s00726-009-0246-7. [DOI] [PubMed] [Google Scholar]
- 7.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Digestive diseases. 2010;28:155–61. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 8.Loria P, Lonardo A, Carulli N. Should nonalcoholic fatty liver disease be renamed? Digestive diseases. 2005;23:72–82. doi: 10.1159/000084728. [DOI] [PubMed] [Google Scholar]
- 9.Angulo P, Keach JC, Batts KP, Lindor KD. Independent predictors of liver fibrosis in patients with nonalcoholic steatohepatitis. Hepatology. 1999;30:1356–62. doi: 10.1002/hep.510300604. [DOI] [PubMed] [Google Scholar]
- 10.Charlton MR, Burns JM, Pedersen RA, Watt KD, Heimbach JK, Dierkhising RA. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249–53. doi: 10.1053/j.gastro.2011.06.061. [DOI] [PubMed] [Google Scholar]
- 11.Machado MV, Cortez-Pinto H. Cell death and nonalcoholic steatohepatitis: where is ballooning relevant? Expert review of gastroenterology & hepatology. 2011;5:213–22. doi: 10.1586/egh.11.16. [DOI] [PubMed] [Google Scholar]
- 12.Feldstein AE, Canbay A, Angulo P, Taniai M, Burgart LJ, Lindor KD, et al. Hepatocyte apoptosis and fas expression are prominent features of human nonalcoholic steatohepatitis. Gastroenterology. 2003;125:437–43. doi: 10.1016/s0016-5085(03)00907-7. [DOI] [PubMed] [Google Scholar]
- 13.Bechmann LP, Gieseler RK, Sowa JP, Kahraman A, Erhard J, Wedemeyer I, et al. Apoptosis is associated with CD36/fatty acid translocase upregulation in non-alcoholic steatohepatitis. Liver Int. 2010;30:850–9. doi: 10.1111/j.1478-3231.2010.02248.x. [DOI] [PubMed] [Google Scholar]
- 14.Kakisaka K, Cazanave SC, Werneburg NW, Razumilava N, Mertens JC, Bronk SF, et al. A hedgehog survival pathway in ‘undead’ lipotoxic hepatocytes. Journal of hepatology. 2012;57:844–51. doi: 10.1016/j.jhep.2012.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ryoo HD, Gorenc T, Steller H. Apoptotic cells can induce compensatory cell proliferation through the JNK and the Wingless signaling pathways. Developmental cell. 2004;7:491–501. doi: 10.1016/j.devcel.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 16.Jung Y, Witek RP, Syn WK, Choi SS, Omenetti A, Premont R, et al. Signals from dying hepatocytes trigger growth of liver progenitors. Gut. 2010;59:655–65. doi: 10.1136/gut.2009.204354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuschwander-Tetri BA. Nontriglyceride hepatic lipotoxicity: the new paradigm for the pathogenesis of NASH. Current gastroenterology reports. 2010;12:49–56. doi: 10.1007/s11894-009-0083-6. [DOI] [PubMed] [Google Scholar]
- 18.Birkenfeld AL, Shulman GI. Non alcoholic fatty liver disease, hepatic insulin resistance and type 2 diabetes. Hepatology. 2014;59:713–23. doi: 10.1002/hep.26672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leclercq IA, Da Silva Morais A, Schroyen B, Van Hul N, Geerts A. Insulin resistance in hepatocytes and sinusoidal liver cells: mechanisms and consequences. Journal of hepatology. 2007;47:142–56. doi: 10.1016/j.jhep.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 20.Michelotti GA, Xie G, Swiderska M, Choi SS, Karaca G, Kruger L, et al. Smoothened is a master regulator of adult liver repair. The Journal of clinical investigation. 2013;123:2380–94. doi: 10.1172/JCI66904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berry MN, Phillips JW. The isolated hepatocyte preparation: 30 years on. Biochemical Society transactions. 2000;28:131–5. doi: 10.1042/bst0280131. [DOI] [PubMed] [Google Scholar]
- 22.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 23.Choi SS, Sicklick JK, Ma Q, Yang L, Huang J, Qi Y, et al. Sustained activation of Rac1 in hepatic stellate cells promotes liver injury and fibrosis in mice. Hepatology. 2006;44:1267–77. doi: 10.1002/hep.21375. [DOI] [PubMed] [Google Scholar]
- 24.Rangwala F, Guy CD, Lu J, Suzuki A, Burchette JL, Abdelmalek MF, et al. Increased production of sonic hedgehog by ballooned hepatocytes. The Journal of pathology. 2011;224:401–10. doi: 10.1002/path.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rinella ME, Green RM. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. Journal of hepatology. 2004;40:47–51. doi: 10.1016/j.jhep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 26.Syn WK, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–88. e8. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Richardson MM, Jonsson JR, Powell EE, Brunt EM, Neuschwander-Tetri BA, Bhathal PS, et al. Progressive fibrosis in nonalcoholic steatohepatitis: association with altered regeneration and a ductular reaction. Gastroenterology. 2007;133:80–90. doi: 10.1053/j.gastro.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Vakifahmetoglu-Norberg H, Norberg E, Perdomo AB, Olsson M, Ciccosanti F, Orrenius S, et al. Caspase-2 promotes cytoskeleton protein degradation during apoptotic cell death. Cell death & disease. 2013;4:e940. doi: 10.1038/cddis.2013.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Guy CD, Suzuki A, Burchette JL, Brunt EM, Abdelmalek MF, Cardona D, et al. Costaining for keratins 8/18 plus ubiquitin improves detection of hepatocyte injury in nonalcoholic fatty liver disease. Human pathology. 2012;43:790–800. doi: 10.1016/j.humpath.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCoy F, Darbandi R, Lee HC, Bharatham K, Moldoveanu T, Grace CR, et al. Metabolic activation of CaMKII by coenzyme A. Molecular cell. 2013;52:325–39. doi: 10.1016/j.molcel.2013.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Espin R, Roca FJ, Candel S, Sepulcre MP, Gonzalez-Rosa JM, Alcaraz-Perez F, et al. TNF receptors regulate vascular homeostasis in zebrafish through a caspase-8, caspase-2 and P53 apoptotic program that bypasses caspase-3. Disease models & mechanisms. 2013;6:383–96. doi: 10.1242/dmm.010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Syn WK, Choi SS, Diehl AM. Apoptosis and cytokines in non-alcoholic steatohepatitis. Clinics in liver disease. 2009;13:565–80. doi: 10.1016/j.cld.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jung Y, Diehl AM. Non-alcoholic steatohepatitis pathogenesis: role of repair in regulating the disease progression. Digestive diseases. 2010;28:225–8. doi: 10.1159/000282092. [DOI] [PubMed] [Google Scholar]
- 34.Fleig SV, Choi SS, Yang L, Jung Y, Omenetti A, VanDongen HM, et al. Hepatic accumulation of Hedgehog-reactive progenitors increases with severity of fatty liver damage in mice. Laboratory investigation; a journal of technical methods and pathology. 2007;87:1227–39. doi: 10.1038/labinvest.3700689. [DOI] [PubMed] [Google Scholar]
- 35.Guy CD, Suzuki A, Zdanowicz M, Abdelmalek MF, Burchette J, Unalp A, et al. Hedgehog pathway activation parallels histologic severity of injury and fibrosis in human nonalcoholic fatty liver disease. Hepatology. 2012;55:1711–21. doi: 10.1002/hep.25559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei Y, Wang D, Topczewski F, Pagliassotti MJ. Saturated fatty acids induce endoplasmic reticulum stress and apoptosis independently of ceramide in liver cells. American journal of physiology Endocrinology and metabolism. 2006;291:E275–81. doi: 10.1152/ajpendo.00644.2005. [DOI] [PubMed] [Google Scholar]
- 37.Swiderska-Syn M, Syn WK, Xie G, Kruger L, Machado MV, Karaca G, et al. Myofibroblastic cells function as progenitors to regenerate murine livers after partial hepatectomy. Gut. 2013 doi: 10.1136/gutjnl-2013-305962. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sicklick JK, Li YX, Melhem A, Schmelzer E, Zdanowicz M, Huang J, et al. Hedgehog signaling maintains resident hepatic progenitors throughout life. American journal of physiology Gastrointestinal and liver physiology. 2006;290:G859–70. doi: 10.1152/ajpgi.00456.2005. [DOI] [PubMed] [Google Scholar]
- 39.Maher JJ. New insights from rodent models of fatty liver disease. Antioxidants & redox signaling. 2011;15:535–50. doi: 10.1089/ars.2010.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ho LH, Taylor R, Dorstyn L, Cakouros D, Bouillet P, Kumar S. A tumor suppressor function for caspase-2. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:5336–41. doi: 10.1073/pnas.0811928106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergeron L, Perez GI, Macdonald G, Shi L, Sun Y, Jurisicova A, et al. Defects in regulation of apoptosis in caspase-2-deficient mice. Genes & development. 1998;12:1304–14. doi: 10.1101/gad.12.9.1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Padalecki SS, Chaudhuri AR, De Waal E, Goins BA, Grubbs B, et al. Caspase-2 deficiency enhances aging-related traits in mice. Mechanisms of ageing and development. 2007;128:213–21. doi: 10.1016/j.mad.2006.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.