Abstract
Plant cell culture is emerging as an alternative bioproduction system for recombinant pharmaceuticals. Growing plant cells in vitro under controlled environmental conditions allows for precise control over cell growth and protein production, batch-to-batch product consistency and a production process aligned with current good manufacturing practices. With the recent US FDA approval and commercialization of the world’s first plant cell-based recombinant pharmaceutical for human use, β-glucocerebrosidase for treatment of Gaucher’s disease, a new era has come in which plant cell culture shows high potential to displace some established platform technologies in niche markets. This review updates the progress in plant cell culture processing technology, highlights recent commercial successes and discusses the challenges that must be overcome to make this platform commercially viable.
The term ‘biopharmaceuticals’ refers to therapeutic proteins produced by modern biotechnological techniques [1]. Biopharmaceuticals have revolutionized modern medicine and represent the fastest growing sector within the pharmaceutical industry. There are over 200 protein biopharmaceuticals currently on the market [2], used for the treatment of diabetes, anemia, hepatitis, cancer and cardiovascular diseases [3,4], and in excess of 400 under development. Included among this group of protein therapeutics are mainly antibodies and antibody derivatives, vaccines and some serum-derived proteins, for example, cytokines, growth hormones, interleukins and interferon. The world market for bio-pharmaceuticals was valued at approximately US$199.7 billion in 2013, and is estimated to reach US$497.9 billion by 2020, representing a compound annual growth rate of 13.5% [5]. Monoclonal antibodies constitute the largest segment in the biopharmaceuticals market, accounting for an estimated share of 25.6% in 2013. In terms of therapeutic areas, neurology applications is the largest market with an estimated share of 28.2% in 2013 [5].
Currently, the biopharmaceutical industry relies mainly on microbial fermentation and mammalian cell-based production. It has been estimated that 45% of recombinant proteins in the USA and Europe are made in mammalian cells (35% in Chinese hamster ovary or CHO cells, and 10% in others), 40% in bacteria (39% in Escherichia coli and 1% in others) and 15% in yeasts [6]. These established production platforms will continue to be the focus of most biopharmaceutical companies who may not look beyond these for regulatory reasons or simply due to inertia borne from unfamiliarity. However, there are limitations associated with these systems in terms of cost, scalability, safety and quality/authenticity of proteins. For example, the mammalian cell-based system suffers from limitation in culture scalability, high production cost and risk of contamination with human pathogens. The prokaryotic nature of bacteria (e.g., E. coli) limits the complexity of the proteins (cannot be correctly processed, such as glycosylation), and the appearance of inclusion bodies increases the cost of production. For yeast-based systems, low product yields, inefficient protein secretion and hyperglycosylation of proteins (addition of a large number of mannose residues) are common problems. These limitations have prompted research into alternative bioproduction platforms.
‘Molecular farming’ in plants is emerging as a promising approach for the production of recombinant pharmaceuticals; its potential for low-cost production of high quality, safe and biologically active mammalian proteins has been well reported [7]. Unlike other expression systems, molecular farming has different forms, including cultivation of whole plants in fields, transient expression by agroinfiltration of plants or virus-infected plants, in vitro culture of plant tissues or organs and plant cell suspension culture. All of them have been investigated as economical alternative bio-production platforms in the past two to three decades [7,8]. Attention is now shifting from basic research toward commercial exploitation of the molecular farming system.
Compared with cultivation of whole plants or plant tissues or organs, plant cell suspension culture has more immediate potential for industrial application, as it is analogous to traditional microbial fermentation and mammalian cell culture with less regulatory and environmental concerns. In fact, the plant cell culture system has long been exploited for its unique biosynthetic potential for secondary metabolites or therapeutic proteins, but with limited success [9]. This is mainly because the characteristics of growth and metabolism of plant cells differ considerably from those of microbial and mammalian cells. An important breakthrough was achieved in May 2012, when the carrot cell-produced therapeutic enzyme, taliglucerase alfa (commercially known as ELELYSO™, a hydrolytic lysosomal glucocerebrosidase for intravenous infusion) was finally approved by the US FDA as an orphan drug for treatment of Gaucher’s disease, and thereby became the world’s first plant-made pharmaceutical used in humans [10]. Taliglucerase alfa was developed by Protalix Biotherapeutics [11], an Israel-based biopharmaceutical company (Karmiel, Israel), and marketed by Pfizer.
Before Protalix’s landmark success, Dow AgroSciences (IN, USA; [12]) received in 2006 the world’s first regulatory approval by the US Department of Agriculture (USDA) for a tobacco cell-based vaccine against Newcastle disease virus [13]. The commercial success by these two companies undoubtedly ushers in a new era in the biopharmaceutical industry that promises to provide growth opportunity for this new platform. Other plant cell-made pharmaceuticals, including monoclonal antibodies [14–16], vaccines [17–20], growth factors [21] and cytokines [22–25] that are in pre- and early clinical stages, as well as more therapeutic enzymes in the Protalix’s development pipeline [11], are expected to enter into the marketplace in the future. Plant cell culture is now reaching the stage at which it may challenge those established bioproduction systems that use bacterial, yeast and mammalian cells, though major problems with plant cell culture still exist with regards to low product yields, inherent production variability and nonmammalian glycosylation. This review highlights the recent advancements and commercial success of the plant cell-based bioproduction platform, and discusses the prospect and challenges that must be overcome to make this platform commercially viable.
Plant cell culture as an attractive bioproduction platform
Basics of plant cell culture system
Similar to the microbial and mammalian cells, undifferentiated plant calli can be dispersed in liquid media and propagated in perpetuity under a sterile and controlled environment. Plant cell suspension culture was originally developed for production of valuable secondary metabolites, such as paclitaxel, shikonin, arte-misinin, digoxin, ginsenosides and ajmalicine with a few commercial successes [26–29]. Only in the last two decades has the production potential of the plant cell culture for heterologous proteins been recognized and it has now become a viable alternative bioproduction platform for pharmaceutical proteins.
The most widely used plant cell lines for recombinant biopharmaceutical production are those derived from tobacco (Nicotiana tabacum), such as cultivars BY-2 (N. tabacum cv. Bright Yellow 2) cells (Figure 1) and NT-1 (N. tabacum-1) cells. They have appealing features, including being fast-growing, robust and able to readily undergo Agrobacterium-mediated transformation and cell cycle synchronization [30–32]. Other commonly used plant cell lines are those derived from common edible crop species, such as rice (Oriza sativa), soybean (Glycine max), alfalfa (Medicago sativa), carrot (Daucus carota) and tomato (Lycopersicon esculentum). In fact, these cell lines may be more favorable than tobacco cells in terms of by-product levels and regulatory compliance [32]. Notably, carrot cells are used by Protalix for the production of recombinant human glucocerebrosidase, the first plant cell-made biopharmaceutical approved for the market.
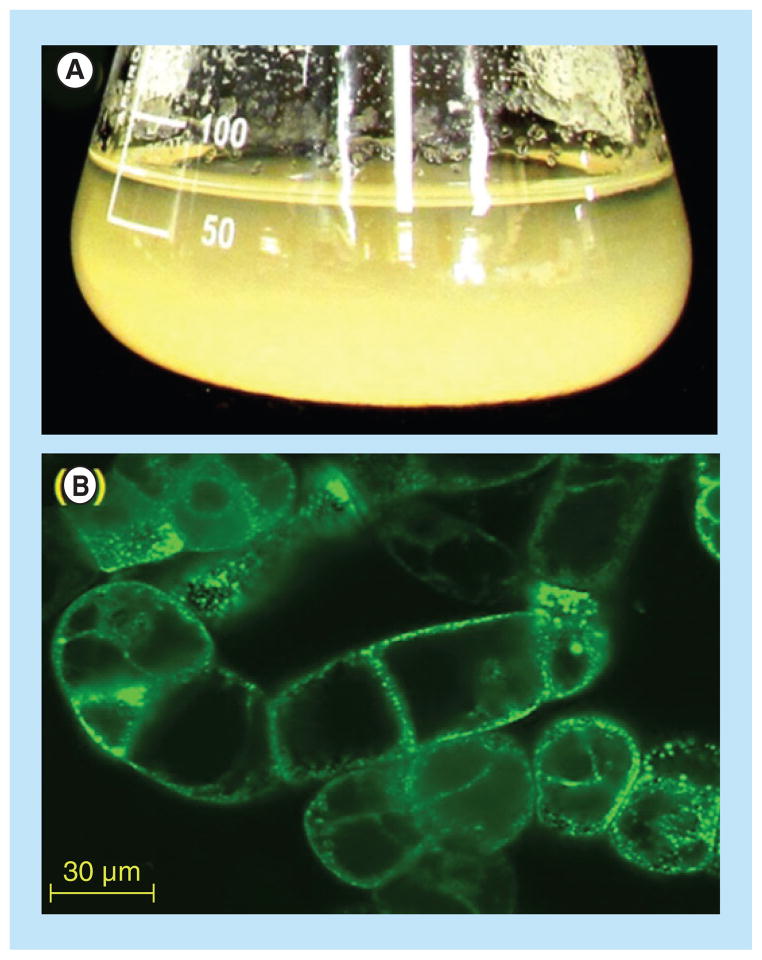
Figure 1. Suspension cultured tobacco BY-2 cells for recombinant protein expression.
(A) BY-2 cells grown in a shake flask for a week; (B) Fluorescence micrographs of BY2 cells expressing enhanced green fluorescence protein. The cells were inspected for green fluorescence using a Zeiss LSM 510 laser-scanning confocal microscope (Carl Zeiss AG, Jena, Germany) (488 nm excitation; 510 nm emission). The cultured plant cells are clustered, and most of the expressed enhanced green fluorescence protein protein is accumulated at the cell wall-cytoplasm membrane interface.
In addition to undifferentiated cells of higher plants, suspension cultures of a lower plant – moss – have received increasing interest as a new bioproduction platform. While plant cells do not need light and grow on sugar-based media, moss (e.g., Physcomitrella patens) requires light but needs only water and inorganic salts as a medium, which reduces the production cost and facilitates product recovery from the culture media [17]. A unique feature of the moss P. patens is its ability to promote efficient homologous recombination [33], which means that new genes can be transformed into the moss genome and endogenous genes can be disrupted by gene targeting [17]. The gene targeting approach was efficiently used to modify the glycosylation pathway in moss by knocking out genes encoding enzymes that add nonhuman glycans to proteins, thus allowing the production of humanized glycoproteins [34,35]. The moss production system is being developed by a German biopharmaceutical company, Greenovation Biotech GmbH (Heilbronn, Germany) [36], for the production of complex pharmaceutical proteins.
Advantages over other expression systems
Compared with other expression systems, the advantages and disadvantages of plant cell culture platform are summarized in Table 1. Plant cell culture inherits most of the advantages of plant-based expression systems, particularly, the ability of being able to produce complex proteins that are properly glycosylated, folded and assembled without the risk of contamination by pathogens and endotoxins [9]. Although suspension culture of plant cells does not share the perspectives of unlimited scalability of whole-plant cultivation in fields, it is totally devoid of the problems associated with the vagaries of weather, pest, soil and gene flow in the environment [37]. Because of short growth cycles of suspension cultured cells, the timescale needed for the production of recombinant proteins in plant cell culture can be counted in days or weeks compared with months needed for the production in transgenic whole plants [37]. In addition, growing plant cells in sterile and controlled environments, such as the bioreactor system, allows for precise control over cell growth conditions, batch-to-batch product consistency, utilization of chemically inducible promoters or viral vectors and a production process aligned with cGMP [9,31,38]. As such, the regulatory concerns regarding plant cell-made pharmaceuticals are reduced, and the production platform is readily accepted by the current biopharmaceutical producers owing to its consistency with those established production systems. One additional advantage of plant cell culture is that recombinant proteins can be secreted into culture media and, therefore, the downstream processing of recovering and purifying proteins from culture media becomes much less expensive than from whole plants. These advantages may partly outweigh the lower protein yields of the plant cell culture system along with its potentially higher capital costs [17,37].
Table 1.
Summary of the advantages and disadvantages of plant cell culture platform compared with other expression systems.
| Compared with: | Plant cell culture | |
|---|---|---|
| Advantages | Disadvantages | |
| Whole plant cultivation |
|
|
| Plant transient expression |
|
|
| Mammalian/insect cell culture |
|
|
| Yeast fermentation |
|
|
| Escherichia coli fermentation |
|
|
On the other hand, plant cell culture resembles traditional microbial fermentation or mammalian cell culture; all propagate in bioreactors as homogeneous suspensions for large-scale production. Similar to microbial fermentation, plant cells have relatively rapid doubling times (as fast as 16 h) and can grow in simple synthetic media using conventional bioreactors. However, as higher eukaryotic organisms, plant cells are more comparable to mammalian cells that can execute nearly all post-translational modifications and synthesize complex proteins, for example, glycoproteins similar to their native counterparts [39]. Nevertheless, plant cells offers an unique attractive feature compared with these systems – safety. This is because plant cells do not harbor any known human pathogens and bacterial endotoxins, which are important considerations for therapeutics production. This issue has received significant consideration after Genzyme (MA, USA) experienced interruption (June, 2009) in their CHO cell production of Cerezyme® (Genzyme) due to infection of the cell line with a calicivirus. Such a risk is completely absent from plant cell culture system. Therefore, plant cell culture is regarded as integrating the merits of whole-plant systems with those of microbial and mammalian cell cultures, and holds great promise as a new ‘biofactory’ for valuable therapeutic proteins [31–32,40].
Pharmaceutical proteins produced by plant cell culture
Since the first human protein (serum albumin) was expressed in tobacco cells in 1990 [41], a wide array of biologically active proteins has been successfully produced in plant cell culture in the past 20–25 years. These mainly include antibodies, vaccine antigens, growth hormones and factors, cytokines and therapeutic enzymes. A comprehensive list of these proteins has been published recently [31]. Besides the first two recombinant proteins that have been approved for commercial production (i.e., Newcastle disease vaccine and β-glucocerebrosidase), some other representative pharmaceutical proteins that show potential for commercialization are listed in Table 2. Of the different groups of therapeutic proteins expressed in plant cell culture system, antibodies remain the most frequently chosen because they represent the dominant class of recombinant proteins for the pharmaceutical industry, and they are also relatively stable, thus can accumulate to high levels (>100 mg/l) [42]. In addition, antibodies can be purified easily from the media or cell extracts by Protein A affinity chromatography [17].
Table 2.
Representative plant cell-produced pharmaceutical proteins.
| Product | Plant species | Promoter | Ref. |
|---|---|---|---|
| Antibody | |||
|
| |||
| Anti-HBsAg mAb | Nicotiana tabacum cv. BY-2 | CaMV35S | [14] |
|
| |||
| Anti-rabies virus mAb | N. tabacum cv. Xanthi | CaMV35S | [15] |
|
| |||
| Anti-HIV antibody 2G12 | N. tabacum cv. BY-2 | CaMV35S | [16] |
|
| |||
| Antigen (vaccine) | |||
|
| |||
| HBsAg | Glycine max cv. Williams 82 | (ocs)3mas | [18] |
| N. tabacum cv. NT-1 | CaMV35S | [43] | |
| N. tabacum cv. BY-2 | (ocs)3mas † | [19] | |
|
| |||
| Hemagglutinin-neuraminidase of Newcastle disease virus | N. tabacum cv. BY-2 | CaMV35S | [17] |
|
| |||
| Escherichia coli O157:H7 intimin | N. tabacum cv. BY-2 | CaMV35S | [44] |
|
| |||
| Therapeutic enzyme | |||
|
| |||
| Glucocerebrosidase | Daucus carota | CaMV35S | [45,46] |
|
| |||
| Recombinant α-galactosidase-A | D. carota | CaMV35S | [11] |
|
| |||
| DNase I | D. carota | CaMV35S | [11] |
|
| |||
| Growth hormone & factor | |||
|
| |||
| hGH | N. tabacum cv. BY-2 | CaMV35S | [25] |
| Oryza sativa L. cv. Donjin | RAmy3D | [47] | |
|
| |||
| Cytokines | |||
|
| |||
| hIL-12 | O. sativa | RAmy3D | [48] |
|
| |||
| hIL-10 | N. tabacum cv. BY-2 | CaMV35S | [49] |
|
| |||
| hGM-CSF | O. sativa | RAmy3D | [24] |
|
| |||
| hIFNα2 | N. tabacum cv. BY-2 | CaMV35S | [22] |
|
| |||
| hEPO | Physcomitrella patens | PpUbq1‡ | [23,50] |
|
| |||
| Others | |||
|
| |||
| Bryodin-1 | N. tabacum cv. NT-1 | CaMV35S | [51] |
|
| |||
| Human α1-antitrypsin | O. sativa | RAmy3D | [52] |
| D. carota | CaMV35S | [11] | |
|
| |||
| Human lactoferrin | Acanthopanax senticosus | SWPA2§ | [53] |
Hybrid promoter constructed from octopine synthase (ocs) and mannopine synthase (mas) promoter sequences.
5′ promoter region of a moss (Physcomitrella patens) ubiquitin gene.
Sweet potato peroxidase anionic 2 promoter, an oxidative stress-inducible peroxidase promoter.
HBsAg: Hepatitis B surface antigen; hEPO: Human erythropoietin; hGH: Human growth hormone; hGM-CSF: Human GM-CSF; hIFNα2: Human interferon α2b; hIL: Human interleukin; mAB: Monoclonal antibody
Plant cell oral delivery system
One of the unique features of plant cell-produced pharmaceuticals is the concept that plant cells not only serve as the production system but also as the delivery vehicle for oral medications [54,55]. Oral drug delivery must overcome several hurdles, such as the acidic and digestive gastric environment, to improve the bio-availability of biopharmaceuticals [56]. Plant cells have fibrous walls made of cellulose, which cannot be broken down by human enzymes in the gastrointestinal tract, but they can be degraded by the microbes that colonize in the gut. This feature enables biopharmaceuticals expressed inside plant cells (or bioencapsulated) to be protected in the stomach from acids/enzymes, but released in the intestines to the immune or blood circulatory system when plant cell walls are digested by gut-residing microbes [56].
Oral delivery of plant cell-expressed biopharmaceuticals is currently being developed for treating a number of human and animal diseases. Of special interest is the edible vaccine, in which an antigenic protein is bioencapsulated in plant cells. It has been regarded as a cost-effective, easy-to-store, easy-to-administer and socioculturally readily acceptable vaccine delivery system, especially for developing countries [57]. Many edible vaccines, such as those against Dengue, polio, malaria, tuberculosis, cholera, anthrax and plague, have been shown to confer both mucosal and systemic immunity and protection against bacterial, viral or protozoan pathogens and toxin challenge [56,58–61]. In addition to edible vaccines, oral delivery of auto-antigens (diabetes, hemophilia, etc.) was found to be effective against complications of Type I diabetes and hemophilia [62–64]; oral delivery of proinsulin or exendin-4 could regulate blood glucose levels similar to injections [65]. Most noticeably, oral glucocerebrosidase (PRX-112) bioencapsulated in carrot cells is being developed by Protalix for the treatment of Gaucher’s disease. The Phase I clinical trial data just released (February 2014) demonstrated that active glucocerebrosidase was detected in the patients’ blood circulation and continuously present over 30 h following oral administration [11]. Thus, with daily administration of oral glucocerebrosidase, a steady-state level of active glucocerebrosidase in the patients’ blood circulation is expected to be achieved [66]. These results demonstrate that a plant cell-based oral delivery system can offer a low-cost alternative for delivering different therapeutic proteins to combat infectious or inherited diseases by eliminating inactivated pathogens, expensive purification, cold storage/transportation and sterile injections [56]
One of the major challenges for the plant cell oral delivery system lies in the accumulation of a sufficient amount of biopharmaceuticals in plant cells so that a required dose can be consumed easily. Progress has been made toward the improvement of protein expression in plant cell culture [31], as discussed below. In addition, downstream processing technologies have also advanced; for example, the lyophilization process has been shown to increase the therapeutic protein contents up to 25-fold (on a per gram basis) and maintain therapeutic protein’s stability for more than 15 months at room temperature [65]. However, before the oral delivery of plant cell-based vaccine antigens/biopharmaceuticals becomes a practical reality, some issues, such as the uniformity and quality control of the products and public acceptance of genetically modified plants, still need be addressed.
Companies devoted to commercialization of plant cell culture platforms
Even though plant cell culture has been shown as a promising alternative bioproduction platform for pharmaceutical proteins, there are only a few biotech or pharmaceutical companies that have ever focused or are focusing on the development and commercialization of this platform. In addition to Protalix, which successfully commercialized the plant cell-produced β-glucocerebrosidase enzyme for human use, Dow AgroSciences and Phyton Biotech (NJ, USA) are another two companies that have made efforts in commercializing the plant cell culture platform; a German biopharmaceutical company, Greenovation Biotech, is trying to commercialize the moss-based bioproduction system. These companies will be introduced in detail below. In addition, a nonprofit German research institute, Fraunhofer Institute for Molecular Biology and Applied Ecology (Fraunhofer IME; Aachen, Germany) [67], has conducted sophisticated plant cell fermentation strategies, such as fed-batch and continuous fermentation, for recombinant protein production. Fraunhofer IME also successfully established cryopreservation protocols for some plant cell lines.
Dow AgroScience, LLC
Dow AgroSciences [12] is a US company based in Indianapolis (IN). Dow AgroSciences developed the Concert™ Plant-Cell-Produced System as a leading edge platform for the production of vaccine antigen. In January 2006, Dow AgroSciences received regulatory approval for the world’s first plant-cell-produced vaccine against Newcastle disease virus in poultry from the USDA Center for Veterinary Biologics. This approval represents an innovative milestone for the company and the industry.
The plant-derived poultry vaccine is the recombinant hemagglutinin-neuraminidase glycoprotein, one of the surface glycoproteins of the Newcastle disease virus and the major surface antigen that induces neutralizing antibodies. The vaccine was expressed in tobacco BY-2 cells. In order to reduce the production cost and make a plant-derived veterinary vaccine economically viable, crude cell extract containing the recombinant hemagglutinin-neuraminidase glycoprotein was directly injected into chickens and full protection on the chickens when challenged with the virus was conferred [17,68]. Although the plant cell-produced poultry vaccine has been proven to be effective and received regulatory approval, it only remained a proof-of-concept. Dow AgroSciences has never intended to market this product. Instead, it used this animal vaccine as an example to completely run through the process However, it paved the way for future plant cell-made therapeutics.
Phyton Biotech, Inc
Phyton Biotech [69], based in East Windsor, NJ, USA (closed in 2008), used to be the pioneer and leader in commercializing plant cell culture for the production of small molecules as well as recombinant proteins. Its research and development center is located in Vancouver, Canada and plant cell culture manufacturing facility located in Ahrensburg, Germany, where the world’s largest commercial cGMP manufacturing facility for plant cell fermentation (bioreactors up to 75,000 l) is operated. With the proprietary plant cell culture fermentation (PCF™) platform, Phyton has developed and commercialized products with applications in the pharmaceutical and biotech industries, such as paclitaxel and docetaxel.
The significant commercial success for Phyton was developing a commercial production of paclitaxel with Taxus (T. chinensis) cell suspension culture [70], which since 1995 has provided Bristol-Myers Squibb with a secure, sustainable and environmentally-friendly source of paclitaxel for Taxol®, a mitotic inhibitor used in cancer chemotherapy. Later, Phyton expanded its PCF™ platform to include recombinant proteins. In 2007, Phyton acquired novel glyco-engineering technology from Dow Chemical Co. (MI, USA) to produce humanized glycoproteins (monoclonal antibodies) in cultured plant cells [69], but ended up without achieving commercial success. In addition, Phyton has developed proprietary cryopreservation technology for long-term storage of plant cells to stably express their traits, thereby ensuring resupply on demand.
Protalix BioTherapeutics, Inc
Protalix [11] is an Israel-based biopharmaceutical company that is leveraging its proprietary plant cell-based expression system, ProCellEx®, for the development and commercialization of recombinant biopharmaceuticals. Using ProCellEx, Protalix has been developing a proprietary pipeline of novel and biosimilar recombinant proteins that target large and established pharmaceutical markets. In May 2012, Protalix partnered with Pfizer to commercialize taliglucerase alfa for injection (ELELYSO), the world’s first plant cell-produced human therapeutic protein approved by the FDA for marketing. By 2013, approvals have been granted by the regulatory authorities of other countries including Israel, Brazil, Chile, Uruguay, Mexico and so on., and are expected to be granted in Canada, Australia and Argentina by 2014. In Latin America, ELEYSO is known as UPLYSO™ (alphataliglicerase).
Protalix’s taliglucerase alfa is a recombinant active form of the lysosomal enzyme, β-glucocerebrosidase, which is expressed in carrot root cells cultured in a disposable bioreactor system (Figure 2). The enzyme is used to treat Gaucher’s disease, the most common lysosomal disease caused by decreased activity of the lysosomal enzyme acid β-glucosidase, resulting in lysosomal accumulation of glucosylceramide [71]. Currently, the main treatment option for patients with severe Gaucher’s disease is enzyme therapy. Since 1994, Cerezyme®, an analog of the human enzyme β-glucocerebrosidase produced in CHO cells by Genzyme, has been used for enzyme therapy. Since proper glycosylation of β-glucocerebrosidase is required for optimal enzyme activity and targeting to macrophages, functional enzyme cannot be produced by prokaryotic E. coli [9]. Even for the CHO cell-expressed imiglucerase, it must be enzymatically processed in vitro to expose terminal mannose residues that are specifically recognized by the endocytic carbohydrate receptors on macrophages for efficient uptake [17,72]. The plant cell-produced glucocerebrosidase is regarded as a ‘biosimilar’ as it is structurally homologous to Cerezyme®, with comparable enzymatic activity and uptake in macrophages [46,73]. Because the newly synthesized glucocerebrosidase is targeted to the plant cell vacuoles where the complex type N-glycans are trimmed to the paucimannose form and expose terminal mannose residues, the recombinant enzyme does not require further modifications for clinical use after bioproduction, resulting in significant cost reduction, approximately 25% less expensive than its competitor Cerezyme® [45,46,74]. To some extent, such products are also known as ‘biobetters’ as an extension of ‘biosimilars’. In addition to ELELYSO, Protalix’s development pipeline also includes the following product candidates:
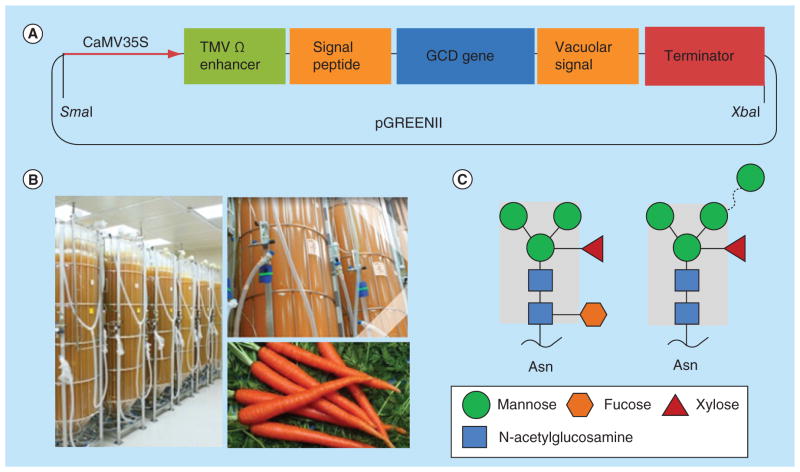
Figure 2. Recombinant human β-glucocerebrosidase production with carrot cell culture by Protalix BioTherapeutics.
(A) GCD expression cassette constructed in the binary vector pGREENII. The expression cassette comprises the CaMV35S promoter, the TMV omega translational enhancer, a signal peptide, the human GCD sequence, a vacuolar targeting signal and the octopine synthase terminator sequence from Agrobacterium tumefaciens [46]. (B) Carrot cell suspension culture in disposable plastic bioreactors for the production of GCD [11]. (C) Two major N-linked glycan structures detected on the recombinant GCD expressed in carrot cells. These N-glycans have a main core of two N-acetylglucosamine residues and a β1–4-linked Mannose, attached to two additional mannose residues in α1–3 and α1–6 linkages (shadowed) [46].
GCD: β-glucocerebrosidase; TMV: Tobacco mosaic virus.
PRX-102, a modified version of the recombinant human α-galactosidase A enzyme for the treatment of Fabry disease (Phase I/II clinical trial);
PRX-112, an orally delivered glucocerebrosidase enzyme that is produced and encapsulated within carrot cells for the treatment of Gaucher’s disease (Phase I clinical trial);
PRX-110, a DNase I enzyme for the treatment of cystic fibrosis (preclinical trial);
PRX-107, an α1-antitrypsin for the treatment of emphysema due to hereditary α1-antitrypsin deficiency (preclinical trial).
PRX-106, an oral formulation of anti-TNF-α for the treatment of immune and inflammatory mediated disorders (preclinical trial);
Obviously, Protalix is currently the world’s leader in development and commercialization of the plant cell-based production platform for biopharmaceuticals with great success.
Greenovation Biotech, GmbH
Greenovation Biotech [36] is a German biopharmaceutical company that employs its proprietary moss (Physcomitrella)-based BryoTechnology™ for the commercialization of recombinant biopharmaceuticals. Similar to higher plants, P. patens is able to grow using light as a sole source of energy and can perform complex post-translational modifications of expressed proteins [23]. The moss N-glycans are generally free of the core α-1,6-fucose, a sugar-structure typically present on N-glycans of mammalian-cell-derived proteins. The absence of this sugar structure has been proven to drastically increase the efficacy of IgG-products by enhancing antibody-dependent cellular cytotoxicity [75]. In addition, genome engineering in the moss, which is based on a homologous recombination, is straightforward and very effective compared with that in other organisms (e.g., mammalian and insect cells, and other plants). Greenovation has used genome engineering extensively to optimize the N-Glycan structures of produced proteins [34–35,76].
Currently, Greenovation has two moss-derived products under preclinical development. Both of them are targeted for enzyme-replacement therapies: α-galactosidase for Fabry disease and β-glucocerebrosidase for Gaucher disease. The α-galactosidase is the company’s lead candidate, and the preclinical development of this enzyme is close to being completed. With the GMP-manufacturing and protein analytics having been fully established, greenovation plans to move this first ever moss-expressed biopharmaceutical candidate into clinical trial Phase I/II in Fall 2014.
Strategies for enhanced plant cell culture production
Although numerous studies have demonstrated the feasibility of plant cell culture for biopharmaceutical production, only a few examples have been commercially developed so far. Generally, low protein yields, typically ranging from 0.01 to 10 mg/l, remain the major bottleneck limiting the commercialization of this technology. A protein yield of 10 mg/l was generally regarded as the entry level for commercial process development [32]. Recent advances in plant molecular biology have greatly improved the yields of some heterologous proteins well beyond 10 mg/l. For example, a production yield of up to 247 mg/l of α1-antitrypsin was achieved in rice cell culture using a sucrose-inducible RAmy3D promoter [77].
For reaching a high protein expression in plant cell cultures, strategies not only at the molecular level but also at the process development level are required to maximize the efficiency of all stages of the production pipeline (Table 3) [7,28,31]. Because these strategies have been extensively reviewed recently [7,31], the following discussions only give a brief summarization and highlights those that resulted in high levels of protein production. In addition, a proprietary technology, termed HypGlyco technology, that dramatically facilitates the secretion of expressed proteins from cultured plant cells is also introduced [78].
Table 3.
Molecular and process development strategies used to improve recombinant protein yields in plant cell cultures.
| Strategies | Approaches | Ref. |
|---|---|---|
| Molecular approaches | ||
|
| ||
| Enhance transcription | Develop strong promoters, double enhanced promoters and hybrid promoters | [18,79–81] |
| Use inducible promoters | [53,77] | |
| Engineer better enhancers, activators or repressors | [82,83] | |
|
| ||
| Improve translation efficiency | Optimize 5′- and 3′-untranslated region | [84,85] |
| Design preferred genetic codon | [86,87] | |
|
| ||
| Minimizing post-translational degradation | Target nascent proteins to subcellular compartments such as endoplasmic reticulum. | [88,89] |
| Coexpress with protease inhibitor and protein cofactor/subunit; coexpress antibody with antigen | [90,91] | |
| Express as fusion to a highly expressed and stable peptide | [22,25,92] | |
|
| ||
| Process development | ||
|
| ||
| Improve cell culture methods | Optimize medium composition and supplement protein-stabilizing agents | [93–100] |
| Develop immobilized cell culture | [31,101] | |
| In situ remove expressed protein | [31,102–103] | |
|
| ||
| Optimize culture scale-up | Select and/or improve bioreactor design | [104–106] |
| Select culture strategy (e.g., batch vs fed-batch vs continuous culture) | [24,107–110] | |
Molecular approaches
Molecular approaches target mainly the two genetic information transfer processes defined in the central dogma: transcription and translation [111–113]. In the past decade, significant progress has been made to improve the recombinant protein expression in plant cells through enhancing gene transcription and improving translation efficiency [111,113–114], boosting protein yields by up two or three orders of magnitude [40,113]. In addition, improving post-translational protein stability is also critical in achieving high protein yields [115].
For enhanced gene transcription in plant cells, strong promoter systems (either constitutive or inducible) can be utilized. The most commonly used constitutive promoters, cauliflower mosaic virus 35S (CaMV 35S) promoter resulted in up to 35 mg/l hGH [25], 30 mg/l Bryodin-1 [51] and 28 mg/l hIFNα2 [22] expressions in tobacco cell culture. Alternatively, inducible promoters, particularly those regulated by chemical stimuli, such as alcohol, steroid, salts, sucrose and so on, have been increasingly used in recent years. The most successful example of an inducible promoter developed for plant cell expression is using the rice α-amylase 3D (RAmy3D) promoter, which is induced by sucrose starvation. The RAmy3D promoter has enabled high-level expression of many therapeutic proteins, such as α1-antitrypsin, hGM-CSF, hGH, Bryodin-1, hIL-12, lysozyme and human serum albumin (hSA), in rice cell culture with the highest secreted protein yields reaching 247 mg/l for α1-antitrypsin [77]. However, the growth characteristics of the rice cell line are inferior to those of tobacco BY-2 and NT-1 cell lines [32] and the viability of rice cells is significantly decreased when grown in a sucrose-starvation medium to activate the RAmy3D promoter [31,40]. More information about the characteristics of various promoters used for expressing foreign genes in plant cell culture system are summarized by Huang and McDonald [40].
Translation efficiency can be improved by manipulating the 5′- and 3′-untranslated region of the plant expression cassettes [112]. For example, utilization of the 5′-leader sequence, such as those from a tobacco etch virus, tobacco mosaic virus or alfalfa mosaic virus, enhanced the transgene expression by several-fold due to enhanced translation efficiency [31]. In addition, another commonly used approach to improve translation efficiency is through codon optimization of the transgene by using the preferred codon and/or removing the rare codon for the host plant cells [87]. A 5- to 10-fold increase in accumulation of the human acetylcholinesterase in tobacco cells has been shown by expressing the codon-optimized gene sequence as compared with expressing the native human sequence [86]. However, optimizing transgene codon does not always improve the yield of expressed proteins in plant cells.
In order to minimize post-translational protein degradation, targeting the foreign proteins to subcellular compartments, for example, the endoplasmic reticulum (ER), has been widely used. This can be achieved by linking an ER retention signal, such as the KDEL or HDEL tag at the C-terminus of the target protein. Retaining expressed proteins in the ER can effectively prevent the foreign proteins from proteolytic degradation [112–113,115] and meanwhile, many molecular chaperones contained in the ER help the nascent proteins fold and assemble correctly [116]. Recombinant protein yields could typically be improved by 10- to 100-fold with ER retention compared with those entering the secretory pathway [32,89,115,117]. The expression of KDEL-tagged human EGF in tobacco cells resulted in a 104-fold increase in protein yield [88]. In addition, other strategies were also developed for reducing the effects of proteolytic degradation in plant cells, which include: coexpression of a recombinant protein with protease inhibitors, knockout mutations in the genes encoding specific proteolytic enzymes and removal of protease-specific sites from foreign proteins using genetic engineering techniques [31,115,118].
Process development approaches
Because plant cells are cultivated in bioreactors for process scale-up, the culture conditions can be altered and manipulated much more easily than those for cultivation of whole plants in fields. Enhanced protein productivity can be achieved through optimization of bioreactor culture conditions and development of advanced bioreactor culture strategies [31,40,119]. With the optimization of various operating conditions in a batch culture bioreactor (e.g., agitation speed, aeration rate, pH and dissolved oxygen, higher protein yields than those obtained from shake flasks were achieved [108,120]. However, the inherent limitations of the batch culture mode, such as long lag phase, depletion of key nutrients and the accumulation of inhibitory substances/metabolites, prevent the batch culture from achieving the desired productivity. Therefore, advanced culture strategies, such as fed-batch culture [108], two-stage culture [24], perfusion culture [107], semi-continuous culture [109] and continuous culture [121], have been developed for plant cell culture to further improve cell density and productivity. In fact, these culture strategies have been successfully utilized for mammalian and microbial cell culture processes for commercial production of various biobased products. However, only a few studies related to advanced culture strategies for plant cell culture have been reported and most of them directed to the production of secondary metabolites [31]. More information on adoption of advanced bioreactor culture strategies for enhanced plant cell-based production can be found in some recent reviews [31,40,119].
HypGlyco technology for high-yield secretion of recombinant proteins
HypGlyco technology exploits the glycosylation ‘code’ of plant hydroxyproline (Hyp)-rich glycoproteins for de novo design of short biopolymer tags [122,123], such as 5 to 50 tandem repeats of the ‘Ser–Pro’ dipeptide motif, which are targeted for extensive Hyp-O-glycosylation with arabinogalactan polysaccharides in plant cells [122]. Such biopolymer tags appear to function as a ‘molecular carrier’ in promoting efficient transport of the tagged recombinant proteins into culture media as well as protecting the proteins from proteolytic degradation (Figure 3). HypGlyco technology has been shown to dramatically enhance the yields of secreted proteins as high as 1500-fold compared with control systems [22,78]. A series of proteins, including reporter protein enhanced green fluorescence protein (EGFP) and human proteins such as hIFNα2, hGH, growth hormone antagonist and hSA have been expressed in plant cells with the HypGlyco technology; high secreted protein yields up to 250 mg/l EGFP were achieved [22,25,78]. Furthermore, the extensively Hyp-O-glycosylated HypGlyco carriers greatly extended the serum half-life of small therapeutic proteins, for example, hGH and hIFNα2, by as much as 13-fold without significantly affecting their bioactivity [22,25]. In addition, the HypGlyco carriers decorated with many Hypglycans (arabinogalactan polysaccharides) were found to be not immunogenic when injected into mice and only mildly so when injected as a fusion protein [22,25].
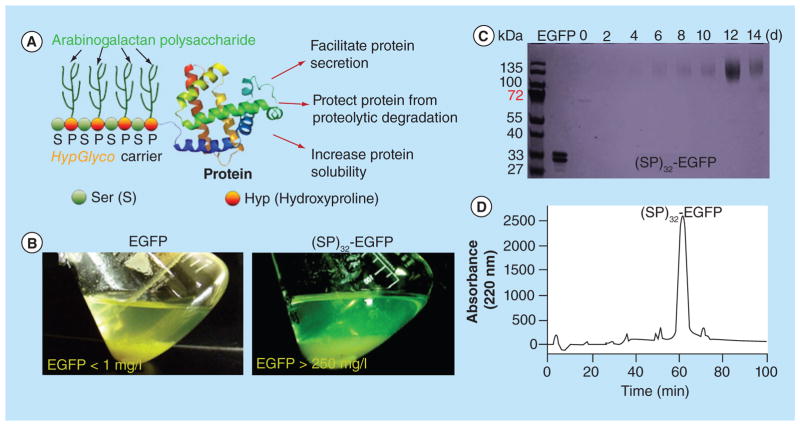
Figure 3. Enhanced secreted protein yields by the HypGlyco technology in plant cell culture.
(A) Schematic of HypGlyco technology. Here, all the ‘Pro’ residues in the ‘Ser–Pro’ module, or (SP) for short, are hydroxylated to be Hyp and subsequently O-glycosylated with arabinogalactan polysaccharides in plant cells; (B) Enhanced secretion of EGFP by an N-terminal HypGlyco carrier (SP)32 in tobacco BY-2 cell culture. The (SP)32 refers to 32 tandem repeats of the ‘Ser–Pro’ dipeptide motif, which dramatically enhanced the secretion of the tagged EGFP from the culture tobacco cells with more than 250 mg/l of secreted EGFP detected. By comparison, EGFP expressed without a HypGlyco carrier was barely detectable in the culture medium (<1.0 mg/l); (C) SDS-PAGE separation of the culture media of the tobacco cells expressing (SP)32–EGFP. The media were harvested every other day for 14 days. The Coomassie blue-stained SDS-PAGE gel showed the (SP)32–EGFP fusion protein dominated the cell culture media; (D) Reversed-phase HPLC detection of the dominant (SP)32–EGFP peak in the cell culture medium (after 12 days’ culture).
d: Days; EGFP: Enhanced green fluorescence protein.
While the HypGlyco carriers have been shown to improve the clinical effectiveness and the yields of some protein therapeutics [22,25], other pharmaceutical proteins might not function when they possess a glycosylated carrier or tag. For many pharmaceutical applications ‘equivalency’ is critical to acceptance. Therefore, in practical applications, a site-specific cleave site between the target protein and the HypGlyco carrier can be designed for postharvest cleavage of the carrier to recover the native recombinant protein. Although this will incur increased downstream processing costs, the HypGlyco technology is extremely promising for overcoming the bottleneck of low protein yields, potentially making molecular farming in plant cell culture system economically feasible.
Ongoing challenges and solutions
In addition to the major obstacle of low productivity in plant cell culture, which could be improved by molecular and process development approaches as discussed above, other major challenges remain to be addressed, including nonmammalian glycosylation, genetic instability and cell culture scale-up in bioreactors [38,98,124], which are discussed briefly below.
Nonmammalian glycosylation
While plant-produced proteins and native human proteins have similar post-translational modifications, some differences in glycosylation do exist. Alterations of the glycosylation pattern may not specifically affect the activity of a protein, but it is regarded as potentially generating an immunogenicity response as well as reducing functionality of the protein [98,117,125–126]. A comprehensive review of the N- and O-glycosylation of proteins in plants and the limitations and advantages of plant-specific glycosylation on therapeutic proteins was published recently [39].
N-glycosylation is the most important post-translational modification as 30% of all approved biopharmaceuticals contain N-linked glycans [17]. Although the glycosylation machinery in plants is similar to its mammalian counterpart, the final complex-type N-glycans differ between plants and mammals owing to different processing and modifications of the core glycan in the Golgi apparatus [117,127]. N-linked glycans produced in plants usually contain the α(1,3)-fucose and β(1,2)-xylose residues, two epitopes not found in mammalian glycans are known to be responsible for inducing immunogenicity [98,128–129]; whereas, the β(1,4)-galactose and terminal sialic acids contained in mammalian glycoproteins are not synthesized in plants, which may reduce the clinical efficiency of the plant-produced glycoproteins owing to decreased serum half-life [39,130]. Considerable progress has been made toward the humanization of protein N-glycosylation in plants. Some strategies that turned out to be feasible include retrieving of expressed proteins in the ER by adding a C-terminal tetrapeptide H/KDEL motif [131,132], knockout of endogenous plant glycosyltransferases that transfer β(1,2)-xylose and α(1,3)-fucose residues onto nascent proteins [133–136], and engineering of the mammalian glycosyltransferases, such as β(1,4)-galactosyltransferase or β (1,4)-N-acetylglucosaminyl transferase III into host plants [127,137].
However, plant-derived N-glycans are more of a problem in theory than in practice because nonmammalian glycosylation does not necessarily always convey a negative impact on plant cell-expressed glycoproteins. In fact, it can affect the solubility, stability and biological activity of a protein positively as well as negatively [17]. In contrast to mammalian cell-based production where a mixture of N-glycans is often present on recombinant proteins, the N-glycans produced in plants are very homogenous within/along a given protein molecule as well as between batches [16,134,138]. This opens opportunities for N-glycan-dependent therapeutics, for example, those for treatment of lysosomal storage diseases. Plants are also amazingly amenable to glyco-engineering, which provides an intriguing opportunity for designing new N-glycans normally not found in the target proteins, but will improve therapeutic performance [138]. A good example is the recombinant glucocerebrosidase produced in carrot cells, which was poised for FDA approval. The N-glycan structures of the therapeutic enzyme were trimmed in plant cells to expose mannose residues, leading to the correct mannose glycosylation pattern [45–46,74]. Another example of beneficial plant glycosylation is a desialylated form of human erythropoietin (hEPO) produced in plants, termed asialo-hEPO, that lacks hematopoietic activity but can serve as a safe drug with neuro- and tissue-protective functions after stroke and additional hypoxia stress [139,140]. In addition, plant-specific glycans might also be advantageous for the formulation of more potent vaccines, because the glycans might help increase the immune visibility of the antigen [17].
In contrast to N-glycosylation, which has significant structural and functional implications, much less attention has been paid on O-glycosylation and its impacts on the clinical function of plant-derived bio-pharmaceuticals [31]. Unlike N-glycosylation occurring at a consensus sequence (Asn–X–Ser/Thr), there is no well-defined consensus sequence for O-glycosylation. In plant cells, O-glycosylation has been described mainly for the hydroxyl groups of Hyp, Ser and Thr residues. Of which, the O-glycosylation on Hyp residue is unique to higher plants and green algae. The Hyp-O-linked sugars are abundant in plant cells and make a major contribution to the structural properties of the extracellular matrix. Therapeutic proteins produced in plant cells could possibly bear Hyp-O-glycans that could be a source of immunogenicity [141–143]. In fact, Hyp-O-glycosylation has been demonstrated to occur in the maize-expressed human IgA1 [144]. In addition, hEPO expressed in moss and N. benthamiana was shown to be hydroxylated within the ‘SPP’ motif, but O-glycosylation was not observed [23,50]. Further research is needed to understand the impacts of the plant O-glycans on the stability, biological activity and efficacy of the therapeutic proteins [145]. On the other hand, genetic glycoengineering can be applied to avoid the plant-specific O-glycosylation [35]. A straightforward approach is to eliminate the O-glycan attachment sites - the Hyp residues on the recombinant proteins. This was achieved for the production of hEPO in the moss (P. patens) production system by ablation or downregulation of a single prolyl-4-hydroxylases (P4H) gene [23]. This paved the way to a further humanization of plant-made biopharmaceuticals in the moss bioreactor.
Genetic instability
Suspension cultured plant cells have been frequently shown to suffer from genetic instability, resulting in the loss of transgene expression. This poses another challenge to plant cell culture technology. It has been found that the expression of a recombinant IgG1 in tobacco cell culture dramatically decreased for a period of 3 years compared with the relatively constant levels of the antibody expressed in tobacco hairy root culture [102]. In another example, the expression of hGM-CSF in tobacco NT-1 cell culture decreased by more than 80% following 250 subculture events [124]. Epigenetic transcriptional silencing is thought to be the dominant contributing factor to the unstable protein expression in plant cell culture [146,147]. Other possible causes include gene drift and transgene loss [31].
In order to overcome the issue of genetic instability, an efficient technique to preserve elite plant cell lines, namely, cell banking, is required. This can be achieved by cryopreservation of the elite cell lines, usually in liquid nitrogen at −196°C, in the form of Master and Working cell banks [17,31]. Several plant cell lines have been successfully frozen and restored from cryopreservation, for example, a transgenic BY-2 cells producing hSA has been cryopreserved for 1 week and the growth and recombinant protein productivity remained stable after cryopreservation [148]. However, there is no universal technique for cryopreservation developed so far. A specific cryopreservation protocol needs to be adapted to each individual cell line. Alternative approaches used to maintain high productivity of plant cell lines include rescreening of high-producing cell lines when the reduction of protein yield is observed and coexpression of gene silencing suppressors [31,40].
Cell culture scale-up
Scale-up of cell cultures in bioreactors is the critical step to achieve commercial productivity of plant cell culture technology. Although plant cells are readily cultured in most standard bioreactors, and those well-established principles for the cultures of microbial and mammalian cells also apply to plant cell culture, the transition from shake flasks to bioreactors is still complicated and problematic; poor cell growth and low protein production have been reported when the plant cell culture was scaled up in bioreactors [149,150]. The engineering considerations of scaling up plant cell culture and important features of various types of bioreactors have been well reviewed by Huang and McDonald recently [40,119].
Plant cells exhibit unique biological and morphological features that are distinctive from bacterial and mammalian cells, as summarized in Table 4, which might impose limitations on their applications in large-scale growth and process development. Two distinctive properties of plant cell culture that call for a special consideration in bioreactor process development include large cell size and complex morphology [31,119]. Plant cells (20–50 μm in diameter and 100–500 μm in length) are significantly larger than bacteria (<1 μm in diameter), yeasts (3–5 μm in diameter) and mammalian cells (10–100 μm in diameter), with a large intracellular vacuole accounting for up to 90% of the cell volume and a rigid, inflexible cellulose-based cell wall [40,151]. Thus, plant cells are susceptible to shear stresses, limiting the mechanical agitation techniques available to meet oxygen demands for cell growth. The general solution to the shear-sensitivity property of plant cells involves growing cells in low-shear stress environments, such as pneumatic bioreactors (e.g., airlift and bubble column bioreactor), centrifugal impeller bioreactors [152] or stirred tank bioreactors with decreased impeller agitation speeds or with a specially designed low-shear impeller [31]. The concept of ‘critical shear stress’, above which cell viability is lost, has been an important factor in establishing guidance for plant cell bioreactor design [40]. A critical shear stress between 50 and 200 N/m for plant cell culture was earlier reported [153].
Table 4.
Comparison of plants cells with mammalian cells, yeasts and bacteria with regard to the characteristics calling for special considerations in bioreactor process development.†
| Characteristics | Plant cells | Mammalian cells | Yeasts | Bacteria |
|---|---|---|---|---|
| Size | 20–50 μm in diameter and 100–500 μm in length | 10–50 μm | 3–5 μm | <1 μm |
| Shape | Spherical/cylindrical | Spherical | Spherical to ellipsoidal | Spherical |
| Cell aggregation | Aggregated to form cell clusters from <100 μm to over 2 mm | Single cells; not aggregated | Single cells; not aggregated | Single cells; not aggregated |
| Doubling time | 20–100 h | 24–48 h | 2–3 h | 30 min to 1 h |
| Shear sensitivity | High | Extremely high | Low | Low |
| Oxygen uptake rate | 2–10 mmol/l/h | 0.05–10 mmol/l/h | 10–200 mmol/l/h | 10–90 mmol/l/h |
| Required kLα value in bioreactor operation | 10–50/h | 0.25–10/h | 100–1000/h | 100–500/h |
| Protein localization | Intracellular/secreted | Secreted | Intracellular/secreted | Usually intracellular |
kLα: Volumetric oxygen transfer coefficient.
Data adapted from [31].
In terms of morphology, suspension cultured plant cells tend to form aggregates ranging from two to thousands of cells (from <100 μm to over 2 mm) and sometimes even display cellular differentiation. The sizes of cell aggregate are dependent on plant species, medium composition, inoculum, cell growth stage and culture conditions [119]. On the one hand, formation of moderate cell aggregates (e.g., 100–1000 μm), known as self-immobilization of cells, may protect the shear-sensitive plant cells from shear damage and enhance sedimentation rates of the cultured cells, thus facilitating media exchange as well as in situ recovery of culture broth. On the other hand, there are mixing and rheological problems with the cultures of plant cell aggregates in bioreactors because the cell aggregates tend to sediment or stick to the reactor surfaces forming extensive wall growth or crusts and they can also block the openings and pipes of a bioreactor. In addition, mass transfer of the cell culture system is influenced; the inner cells of the large aggregates (>1 mm) may become oxygen and nutrient deficient, resulting in adverse effects on cell growth and foreign protein production [119]. However, still other research indicated the mass transfer in living plant cell aggregates is actually facilitated by the mechanisms, which depend on metabolic activity and which do not function in deactivated cells [95,154]. Therefore, mass transfer limit may not occur readily in living cell aggregates.
General criteria for choosing a suitable bioreactor design for plant cell culture should consider a low shear stress to cells and an adequate oxygen transfer. Bioreactors typically employed for large-scale plant cell culture include those of stirred tank, airlift and bubble column [31,40,119]. Currently, increasing attention has been paid to the use of disposable bioreactors for efficient plant cell cultures. This type of bioreactor has been successfully implemented by Protalix with its ProCellEx™ production platform [11]. Disposable bio-reactors provide benefits such as high flexibility, ease of handling, reduction in cross-contamination and savings in both time and cost [9], which are attributed to the presterility of the disposable containers (usually plastic bags) in which plant cells are grown [105,155–156]. So far, many different types of disposable bioreactors have been developed for plant cell cultures, including wave-mixed, stirred and bubble column-styled [157]. These disposable bioreactors and issues regarding their scaling-up were described in greater detail in some recent reviews [28,156,158].
Conclusion & future perspective
Plant cell suspension culture, which integrates the merits of whole plant systems with those of microbial fermentation or mammalian cell culture, provides a number of unique advantages for production of recombinant therapeutics. However, the commercialization potential of this production platform has long been a controversial subject in the biotechnology industry. As the world’s first plant cell-produced human therapeutic (β-glucocerebrosidase) has become a commercial success and several others are under pre-clinical and clinical trials, plant cell culture can now be said to have ‘come of age’, which will usher in a new era in the biopharmaceutical industry. The key areas to ensure advancement of this technology will be in leveraging the molecular and process engineering approaches to further increase the recombinant protein expression levels, to facilitate protein secretion and prevent proteolytic degradation, to optimize bioreactor operational strategies for maximizing cellular productivity and to humanize or take advantage of the unique glycans of plant glycoproteins for improved protein efficacy. In addition, continuing efforts should be made toward utilizing the low-cost, highly efficient and safe bioreactor configuration – disposable bioreactor – for large-scale plant cell culture, which can easily fulfill cGMP requirements. If all the major challenges, including low protein productivity, nonmammalian glycosylation and genetic instability, can be met through systematic and concerted research efforts that are both biologically and engineering-based, there is no doubt that plant cell culture will become commercially competitive with the currently established mammalian and microbial cell culture platforms for the production of recombinant biopharmaceuticals.
Key terms.
Molecular farming
A new biotechnology that uses plants as a host to produce recombinant therapeutics, such as vaccines and antibodies, as well as industrial proteins in large quantities.
Plant cell culture
Techniques used to maintain or grow undifferentiated plant cells under sterile conditions on a nutrient culture medium of known composition. Plant cell culture has been used to produce valuable secondary metabolites and recombinant proteins.
Protalix
An Israel-based biopharmaceutical company that uses plant cell cultures as a bioproduction platform for recombinant therapeutic proteins. In May 2012, Protalix partnered with Pfizer to commercialize the world’s first plant cell-based recombinant pharmaceutical for human use, taliglucerase alpha for treatment of Gaucher’s disease.
Edible vaccine
A vaccine in which an antigenic protein is engineered into cells of an edible plant, such as lettuce, spinach, broccoli and potatoes. After ingestion, the antigen is released from plant cells and recognized by the immune system. For edible vaccines, plant cells not only serve as the production system but also as the delivery vehicle for an antigen.
Disposable bioreactor
A single-use bioreactor equipped with a disposable bag (typically made from plastic) instead of a stainless steel or glass vessel. Disposable bioreactors provide benefits such as high flexibility, ease of handling, reduction in cross-contamination and savings in both time and cost.
Executive summary.
Background
Plant cell culture is emerging as an alternative bioproduction system for recombinant pharmaceuticals.
The world’s first plant cell-made pharmaceutical used in humans, taliglucerase alfa, was approved by the US FDA for marketing in May 2012.
Plant cell culture is now reaching the stage at which it may challenge those established bioproduction systems.
Plant cell culture as an attractive bioproduction platform
Plant cell culture integrates the merits of the whole plant system with those of microbial fermentation or mammalian cell culture.
A wide array of biologically active proteins has been successfully produced in plant cell culture.
The plant cell-based oral delivery system offers a low-cost alternative to deliver therapeutic proteins to combat infectious or inherited diseases.
Companies devoted to commercializing plant cell culture platform
Dow AgroSciences (IN, USA), Phyton Biotech (NJ, USA), Protalix (Karmiel, Israel) and Greenovation Biotech (Heilbronn, Germany) have been or are currently devoted to commercializing the plant cell culture platform.
Strategies for enhanced plant cell culture production
Significantly improved protein expression has been achieved through enhancing gene transcription, improving translation efficiency and reducing post-translational protein degradation.
Enhanced protein productivity can also be achieved through optimization of bioreactor culture conditions and development of advanced bioreactor culture strategies.
HypGlyco technology dramatically enhances the yields of secreted proteins as high as 1500-fold.
Ongoing challenges & solutions
In addition to low productivity, other major challenges that remain to be addressed include nonmammalian glycosylation, genetic instability and cell culture scale-up in bioreactors.
Nonmammalian glycosylation does not necessarily always convey a negative impact on plant cell-expressed proteins.
Large cell size and complex morphology represent two distinctive properties of plant cell culture that call for special considerations in bioreactor process development.
Future perspective
Systematic and concerted research efforts that are both biologically and engineering-based will be critical to the commercial success of the plant cell-based bioproduction platform.
Footnotes
Financial & competing interest disclosure
This work was supported by the Arkansas Center for Plant-Powered Production funded through an NSF RII Arkansas ASSET Initiative grant, the National Institute of Health SBIR I grant [1 R43 GM 093621–01] and the Arkansas Biosciences Institute, the major research component of the Arkansas Tobacco Settlement Proceeds Act of 2000. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest;
•• of considerable interest
- 1.Walsh G. Biopharmaceuticals: recent approvals and likely directions. Trends Biotechnol. 2005;23(11):553–558. doi: 10.1016/j.tibtech.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Anne J, Maldonado B, Van Impe J, Van Mellaert L, Bernaerts K. Recombinant protein production and streptomycetes. J Biotechnol. 2012;158(4):159–167. doi: 10.1016/j.jbiotec.2011.06.028. [DOI] [PubMed] [Google Scholar]
- 3.Leader B, Baca QJ, Golan DE. Protein therapeutics: a summary and pharmacological classification. Nat Rev Drug Discov. 2008;7(1):21–39. doi: 10.1038/nrd2399. [DOI] [PubMed] [Google Scholar]
- 4.Woodnutt G, Violand B, North M. Advances in protein therapeutics. Curr Opin Drug Discov Devel. 2008;11(6):754–761. [PubMed] [Google Scholar]
- 5.Anonymous. In: Biopharmaceuticals – A Global Market Overview. , editor. Industry Experts; Dublin, Ireland: 2013. pp. 1–486. [Google Scholar]
- 6.Sanchez S, Demain A. Special issue on the production of recombinant proteins. Biotechnol Adv. 2012;30(5):1100–1101. doi: 10.1016/j.biotechadv.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 7.Xu J, Dolan MC, Medrano G, Cramer CL, Weathers PJ. Green factory: plants as bioproduction platforms for recombinant proteins. Biotechnol Adv. 2012;30(5):1171–1184. doi: 10.1016/j.biotechadv.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Obembe OO, Popoola JO, Leelavathi S, Reddy SV. Advances in plant molecular farming. Biotechnol Adv. 2011;29(2):210–222. doi: 10.1016/j.biotechadv.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 9•.Wilson SA, Roberts SC. Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol J. 2012;10(3):249–268. doi: 10.1111/j.1467-7652.2011.00664.x. Discusses recent advancements in plant cell culture processing technology, focusing on progress towards overcoming the problems associated with commercialization of these production systems and highlighting recent commercial successes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.US FDA. FDA approves new orphan drug to treat a form of Gaucher disease. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm302549.htm.
- 11.Protalix Biotherapeutics. www.protalix.com.
- 12.Dow AgroSciences. www.dowagro.com.
- 13.Fox JL. Turning plants into protein factories. Nat Biotechnol. 2006;24(10):1191–1193. doi: 10.1038/nbt1006-1191. [DOI] [PubMed] [Google Scholar]
- 14.Yano A, Maeda F, Takekoshi M. Transgenic tobacco cells producing the human monoclonal antibody to hepatitis B virus surface antigen. J Med Virol. 2004;73(2):208–215. doi: 10.1002/jmv.20077. [DOI] [PubMed] [Google Scholar]
- 15.Girard LS, Fabis MJ, Bastin M, Courtois D, Petiard V, Koprowski H. Expression of a human anti-rabies virus monoclonal antibody in tobacco cell culture. Biochem Biophys Res Commun. 2006;345(2):602–607. doi: 10.1016/j.bbrc.2006.03.219. [DOI] [PubMed] [Google Scholar]
- 16.Holland T, Sack M, Rademacher T, et al. Optimal nitrogen supply as a key to increased and sustained production of a monoclonal full-size antibody in BY-2 suspension culture. Biotechnol Bioeng. 2010;107(2):278–289. doi: 10.1002/bit.22800. [DOI] [PubMed] [Google Scholar]
- 17••.Schillberg S, Raven N, Fischer R, Twyman RM, Schiermeyer A. Molecular farming of pharmaceutical proteins using plant suspension cell and tissue cultures. Curr Pharm Des. 2013;19(31):5531–5542. doi: 10.2174/1381612811319310008. Comprehensive review discussing various types of in vitro plant cell and tissue cultures system, including suspension cells, hairy roots, moss, microalgae and duckweed, for the production of therapeutic proteins. The advantages and disadvantages of different culture systems are described, and novel strategies for system and process optimization to increase yields and scalability are presented. [DOI] [PubMed] [Google Scholar]
- 18.Smith ML, Mason HS, Shuler ML. Hepatitis B surface antigen (HBsAg) expression in plant cell culture: kinetics of antigen accumulation in batch culture and its intracellular form. Biotechnol Bioeng. 2002;80(7):812–822. doi: 10.1002/bit.10444. [DOI] [PubMed] [Google Scholar]
- 19.Sojikul P, Buehner N, Mason HS. A plant signal peptide-hepatitis B surface antigen fusion protein with enhanced stability and immunogenicity expressed in plant cells. Proc Natl Acad Sci USA. 2003;100(5):2209–2214. doi: 10.1073/pnas.0438037100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dreesen IA, Charpin-El Hamri G, Fussenegger M. Heat-stable oral alga-based vaccine protects mice from Staphylococcus aureus infection. J Biotechnol. 2010;145(3):273–280. doi: 10.1016/j.jbiotec.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 21.Greenovation Products. www.greenovation.com/products.html.
- 22•.Xu J, Tan L, Goodrum KJ, Kieliszewski MJ. High-yields and extended serum half-life of human interferon alpha 2b expressed in tobacco cells as arabinogalactan-protein fusions. Biotechnol Bioeng. 2007;97(5):997–1008. doi: 10.1002/bit.21407. First report on the development of HypGlyco technology. The HypGlyco carriers were shown to dramatically enhance the yields of secreted interferon α2 as high as 1500-fold, and greatly extended the serum half-life of the cytokine by as much as 13-fold without significantly affecting its bioactivity. [DOI] [PubMed] [Google Scholar]
- 23.Parsons J, Altmann F, Graf M, Stadlmann J, Reski R, Decker EL. A gene responsible for prolyl-hydroxylation of moss-produced recombinant human erythropoietin. Sci Rep. 2013;3:3019. doi: 10.1038/srep03019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shin YJ, Hong SY, Kwon TH, Jang YS, Yang MS. High level of expression of recombinant human granulocyte-macrophage colony stimulating factor in transgenic rice cell suspension culture. Biotechnol Bioeng. 2003;82(7):778–783. doi: 10.1002/bit.10635. [DOI] [PubMed] [Google Scholar]
- 25.Xu J, Okada S, Tan L, Goodrum KJ, Kopchick JJ, Kieliszewski MJ. Human growth hormone expressed in tobacco cells as an arabinogalactan-protein fusion glycoprotein has a prolonged serum life. Transgenic Res. 2010;19(5):849–867. doi: 10.1007/s11248-010-9367-8. [DOI] [PubMed] [Google Scholar]
- 26.Rao SR, Ravishankar GA. Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv. 2002;20(2):101–153. doi: 10.1016/s0734-9750(02)00007-1. [DOI] [PubMed] [Google Scholar]
- 27.Georgiev MI, Weber J, Maciuk A. Bioprocessing of plant cell cultures for mass production of targeted compounds. Appl Microbiol Biotechnol. 2009;83(5):809–823. doi: 10.1007/s00253-009-2049-x. [DOI] [PubMed] [Google Scholar]
- 28.Weathers PJ, Towler MJ, Xu JF. Bench to batch: advances in plant cell culture for producing useful products. Appl Microbiol Biotechnol. 2010;85(5):1339–1351. doi: 10.1007/s00253-009-2354-4. [DOI] [PubMed] [Google Scholar]
- 29.Smetanska I. Production of secondary metabolites using plant cell cultures. Food Biotechnol. 2008;111:187–228. doi: 10.1007/10_2008_103. [DOI] [PubMed] [Google Scholar]
- 30.Ozawa K, Takaiwa F. Highly efficient Agrobacterium-mediated transformation of suspension-cultured cell clusters of rice (Oryza sativa L.) Plant Sci. 2010;179(4):333–337. [Google Scholar]
- 31••.Xu J, Ge X, Dolan MC. Towards high-yield production of pharmaceutical proteins with plant cell suspension cultures. Biotechnol Adv. 2011;29(3):278–299. doi: 10.1016/j.biotechadv.2011.01.002. Comprehensive review discussing all aspects of the issues relevant to plant cell culture technology, outlining viable strategies at both the biological and process engineering levels for advancing the economic feasibility of plant cell-based protein production. [DOI] [PubMed] [Google Scholar]
- 32.Hellwig S, Drossard J, Twyman RM, Fischer R. Plant cell cultures for the production of recombinant proteins. Nat Biotechnol. 2004;22(11):1415–1422. doi: 10.1038/nbt1027. [DOI] [PubMed] [Google Scholar]
- 33.Schaefer DG. Gene targeting in Physcomitrella patens. Curr Opin Biotechnol. 2001;4(2):143–150. doi: 10.1016/s1369-5266(00)00150-3. [DOI] [PubMed] [Google Scholar]
- 34.Schuster M, Jost W, Mudde GC, et al. In vivo glyco-engineered antibody with improved lytic potential produced by an innovative non-mammalian expression system. Biotechnol J. 2007;2(6):700–708. doi: 10.1002/biot.200600255. [DOI] [PubMed] [Google Scholar]
- 35.Huether CM, Lienhart O, Baur A, et al. Glyco-engineering of moss lacking plant-specific sugar residues. Plant Biol. 2005;7(3):292–299. doi: 10.1055/s-2005-837653. [DOI] [PubMed] [Google Scholar]
- 36.Greenovation. www.greenovation.com.
- 37.Plasson C, Michel R, Lienard D, et al. Production of recombinant proteins in suspension-cultured plant cells. Methods Mol Biol. 2009;483:145–161. doi: 10.1007/978-1-59745-407-0_9. [DOI] [PubMed] [Google Scholar]
- 38.Shih SMH, Doran PM. Foreign protein production using plant cell and organ cultures: advantages and limitations. Biotechnol Adv. 2009;27(6):1036–1042. doi: 10.1016/j.biotechadv.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 39.Gomord V, Fitchette AC, Menu-Bouaouiche L, et al. Plant-specific glycosylation patterns in the context of therapeutic protein production. Plant Biotechnol J. 2010;8(5):564–587. doi: 10.1111/j.1467-7652.2009.00497.x. [DOI] [PubMed] [Google Scholar]
- 40.Huang TK, McDonald KA. Bioreactor engineering for recombinant protein production in plant cell suspension cultures. Biochem Eng J. 2009;45:168–184. [Google Scholar]
- 41.Sijmons PC, Dekker BM, Schrammeijer B, Verwoerd TC, van den Elzen PJ, Hoekema A. Production of correctly processed human serum albumin in transgenic plants. Biotechnology (NY) 1990;8(3):217–221. doi: 10.1038/nbt0390-217. [DOI] [PubMed] [Google Scholar]
- 42.De Muynck B, Navarre C, Boutry M. Production of antibodies in plants: status after twenty years. Plant Biotechnol J. 2010;8(5):529–563. doi: 10.1111/j.1467-7652.2009.00494.x. [DOI] [PubMed] [Google Scholar]
- 43.Kumar GBS, Ganapathi TR, Srinivas L, Revathi CJ, Bapat VA. Hepatitis B surface antigen expression in NT-1 cells of tobacco using different expression cassettes. Biologia Plantarum. 2007;51(3):467–471. [Google Scholar]
- 44.Judge NA, Mason HS, O’Brien AD. Plant cell-based intimin vaccine given orally to mice primed with intimin reduces time of Escherichia coli O157:H7 shedding in feces. Infect Immun. 2004;72(1):168–175. doi: 10.1128/IAI.72.1.168-175.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aviezer D, Almon-Brill E, Shaaltiel Y, et al. Novel enzyme replacement therapy for Gaucher disease: ongoing Phase III clinical trial with recombinant human glucocerebrosidase expressed in plant cells. Mol Genet Metab. 2009;96(2):S13–S14. [Google Scholar]
- 46••.Shaaltiel Y, Bartfeld D, Hashmueli S, et al. Production of glucocerebrosidase with terminal mannose glycans for enzyme replacement therapy of Gaucher’s disease using a plant cell system. Plant Biotechnol J. 2007;5(5):579–590. doi: 10.1111/j.1467-7652.2007.00263.x. Paper published by Protalix reports that the human glucocerebrosidase expressed in the carrot cells and targeted to the storage vacuoles naturally contains terminal mannose residues on its complex glycans. Hence, the recombinant enzyme does not require exposure of mannose residues in vitro for biologically functioning, which is a requirement for the production of its counterpart in Chinese hamster ovary cells – Cerezyme. [DOI] [PubMed] [Google Scholar]
- 47.Kim TG, Baek MY, Lee EK, Kwon TH, Yang MS. Expression of human growth hormone in transgenic rice cell suspension culture. Plant Cell Rep. 2008;27(5):885–891. doi: 10.1007/s00299-008-0514-0. [DOI] [PubMed] [Google Scholar]
- 48.Shin YJ, Lee NJ, Kim J, An XH, Yang MS, Kwon TH. High-level production of bioactive heterodimeric protein human interleukin-12 in rice. Enzyme Microb Technol. 2010;46(5):347–351. [Google Scholar]
- 49.Kaldis A, Ahmad A, Reid A, et al. High-level production of human interleukin-10 fusions in tobacco cell suspension cultures. Plant Biotechnol J. 2013;11(5):535–545. doi: 10.1111/pbi.12041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weise A, Altmann F, Rodriguez-Franco M, et al. High-level expression of secreted complex glycosylated recombinant human erythropoietin in the Physcomitrella Delta-fuc-t Delta-xyl-t mutant. Plant Biotechnol J. 2007;5(3):389–401. doi: 10.1111/j.1467-7652.2007.00248.x. [DOI] [PubMed] [Google Scholar]
- 51.Francisco JA, Gawlak SL, Miller M, et al. Expression and characterization of bryodin 1 and a bryodin 1-based single-chain immunotoxin from tobacco cell culture. Bioconjugate Chem. 1997;8(5):708–713. doi: 10.1021/bc970107k. [DOI] [PubMed] [Google Scholar]
- 52.Trexler MM, McDonald KA, Jackman AP. A cyclical semicontinuous process for production of human alpha(1)-antitrypsin using metabolically induced plant cell suspension cultures. Biotechnol Prog. 2005;21(2):321–328. doi: 10.1021/bp0498692. [DOI] [PubMed] [Google Scholar]
- 53.Jo SH, Kwon SY, Park DS, et al. High-yield production of functional human lactoferrin in transgenic cell cultures of Siberian ginseng (Acanthopanax senticosus) Biotechnol Bioproc Eng. 2006;11(5):442–448. [Google Scholar]
- 54.Wang L, Coppel RL. Oral vaccine delivery: can it protect against non-mucosal pathogens? Expert Rev Vaccines. 2008;7(6):729–738. doi: 10.1586/14760584.7.6.729. [DOI] [PubMed] [Google Scholar]
- 55.Tiwari S, Verma PC, Singh PK, Tuli R. Plants as bioreactors for the production of vaccine antigens. Biotechnol Adv. 2009;27(4):449–467. doi: 10.1016/j.biotechadv.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kwon KC, Verma D, Singh ND, Herzog R, Daniell H. Oral delivery of human biopharmaceuticals, autoantigens and vaccine antigens bioencapsulated in plant cells. Adv Drug Delivery Rev. 2013;65(6):782–799. doi: 10.1016/j.addr.2012.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rosales-Mendoza S, Salazar-González JA. Immunological aspects of using plant cells as delivery vehicles for oral vaccines. Expert Rev Vaccines. 2014;13(6):737–749. doi: 10.1586/14760584.2014.913483. [DOI] [PubMed] [Google Scholar]
- 58.Arlen PA, Singleton M, Adamovicz JJ, Ding Y, Davoodi-Semiromi A, Daniell H. Effective plague vaccination via oral delivery of plant cells expressing F1-V antigens in chloroplasts. Infect Immun. 2008;76(8):3640–3650. doi: 10.1128/IAI.00050-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lakshmi PS, Verma D, Yang XD, Lloyd B, Daniell H. Low cost tuberculosis vaccine antigens in capsules: expression in chloroplasts, bio-encapsulation, stability and functional evaluation in vitro. PLoS ONE. 2013;8(1):e54708. doi: 10.1371/journal.pone.0054708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee SB, Li BC, Jin SX, Daniell H. Expression and characterization of antimicrobial peptides Retrocyclin-101 and Protegrin-1 in chloroplasts to control viral and bacterial infections. Plant Biotechnol J. 2011;9(1):100–115. doi: 10.1111/j.1467-7652.2010.00538.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davoodi-Semiromi A, Schreiber M, Nalapalli S, et al. Chloroplast-derived vaccine antigens confer dual immunity against cholera and malaria by oral or injectable delivery. Plant Biotechnol J. 2010;8(2):223–242. doi: 10.1111/j.1467-7652.2009.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Boyhan D, Daniell H. Low-cost production of proinsulin in tobacco and lettuce chloroplasts for injectable or oral delivery of functional insulin and C-peptide. Plant Biotechnol J. 2011;9(5):585–598. doi: 10.1111/j.1467-7652.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ruhlman T, Ahangari R, Devine A, Samsam M, Daniell H. Expression of cholera toxin B-proinsulin fusion protein in lettuce and tobacco chloroplasts – oral administration protects against development of insulitis in non-obese diabetic mice. Plant Biotechnol J. 2007;5(4):495–510. doi: 10.1111/j.1467-7652.2007.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Verma D, Moghimi B, LoDuca PA, et al. Oral delivery of bioencapsulated coagulation factor IX prevents inhibitor formation and fatal anaphylaxis in hemophilia B mice. Proc Natl Acad Sci USA. 2010;107(15):7101–7106. doi: 10.1073/pnas.0912181107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kwon KC, Nityanandam R, New JS, Daniell H. Oral delivery of bioencapsulated exendin-4 expressed in chloroplasts lowers blood glucose level in mice and stimulates insulin secretion in beta-TC6 cells. Plant Biotechnol J. 2013;11(1):77–86. doi: 10.1111/pbi.12008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zimran A. Oral administration of glucocerebrosidase for the treatment of Gaucher disease: a completely new approach. Presented at 10th Annual Meeting of the Lysosomal Disease Network: WORLD Symposium 2014; San Diego, CA, USA. 12–14 Februrary 2014. [Google Scholar]
- 67.Fraunhofer IME. www.ime.fraunhofer.de/en.html.
- 68.Ling HY, Pelosi A, Walmsley AM. Current status of plant-made vaccines for veterinary purposes. Expert Rev Vaccines. 2010;9(8):971–982. doi: 10.1586/erv.10.87. [DOI] [PubMed] [Google Scholar]
- 69.Phyton Biotech. www.phytonbiotech.com.
- 70.Mountford PG. The Taxol® story: development of a green synthesis via plant cell fermentation. In: Dunn PJ, Wells AS, Williams MT, editors. Green Chemistry in the Pharmaceutical Industry. Wiley-VCH Verlag GmbH & Co. KGaA; Weinheim, Germany: 2010. pp. 145–159. [Google Scholar]
- 71.Grabowski GA. Lysosomal storage disease 1 – Phenotype, diagnosis, and treatment of Gaucher’s disease. Lancet. 2008;372(9645):1263–1271. doi: 10.1016/S0140-6736(08)61522-6. [DOI] [PubMed] [Google Scholar]
- 72.Friedman B, Vaddi K, Preston C, Mahon E, Cataldo JR, McPherson JM. A comparison of the pharmacological properties of carbohydrate remodeled recombinant and placental-derived beta-glucocerebrosidase: implications for clinical efficacy in treatment of Gaucher disease. Blood. 1999;93(9):2807–2816. [PubMed] [Google Scholar]
- 73.Zimran A, Brill-Almon E, Chertkoff R, et al. Pivotal trial with plant cell-expressed recombinant glucocerebrosidase, taliglucerase alfa, a novel enzyme replacement therapy for Gaucher disease. Blood. 2011;118(22):5767–5773. doi: 10.1182/blood-2011-07-366955. [DOI] [PubMed] [Google Scholar]
- 74.Aviezer D, Almon-Brill E, Shaaltiel Y, Chertkoff R, Hashmueli S, Zimran A. Novel enzyme replacement therapy for Gaucher disease: Phase III pivotal clinical trial with plant cell expressed recombinantglucocerebrosidase (prGCD) – taliglucerase alpha. Mol Genet Metab. 2010;99(2):S9–S10. [Google Scholar]
- 75.Greenovation Bryotechnology – Moss Inherent. www.greenovation.com/moss-inherent.html.
- 76.Greenovation Bryotechnology – Moss Manipulation. www.greenovation.com/moss-manipulation.html.
- 77•.McDonald KA, Hong LM, Trombly DM, Xie Q, Jackman AP. Production of human alpha-1-antitrypsin from transgenic rice cell culture in a membrane bioreactor. Biotechnol Prog. 2005;21(3):728–734. doi: 10.1021/bp0496676. Reports the highest protein yields (247 mg/l) ever produced by plant cell culture, which was achieved with the rice cell culture using a sucrose-inducible RAmy3D promoter that underwent multiple inductions. [DOI] [PubMed] [Google Scholar]
- 78.Kieliszewski MJ, Xu J, Kopchick JJ, Okada S. 20110230404A1. US. 2011
- 79.Kay R, Chan A, Daly M, McPherson J. Duplication of CaMV 35S promoter sequences creates a strong enhancer for plant genes. Science. 1987;236(4806):1299–1302. doi: 10.1126/science.236.4806.1299. [DOI] [PubMed] [Google Scholar]
- 80.Panahi M, Alli Z, Cheng XY, et al. Recombinant protein expression plasmids optimized for industrial E-coli fermentation and plant systems produce biologically active human insulin-like growth factor-1 in transgenic rice and tobacco plants. Transgenic Res. 2004;13(3):245–259. doi: 10.1023/b:trag.0000034619.21613.d0. [DOI] [PubMed] [Google Scholar]
- 81.Lessard PA, Kulaveerasingam H, York GM, Strong A, Sinskey AJ. Manipulating gene expression for the metabolic engineering of plants. Metab Eng. 2002;4(1):67–79. doi: 10.1006/mben.2001.0210. [DOI] [PubMed] [Google Scholar]
- 82.Lam E, Benfey PN, Gilmartin PM, Chua NH. 5023179. US. 1991
- 83.Mishra S, Yadav DK, Tuli R. Ubiquitin fusion enhances cholera toxin B subunit expression in transgenic plants and the plant-expressed protein binds GM1 receptors more efficiently. J Biotechnol. 2006;127(1):95–108. doi: 10.1016/j.jbiotec.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 84.Cowen NM, Smith KA, Armstrong K. 7179902. US. 2007
- 85.Kang TJ, Loc NH, Jang MO, Yang MS. Modification of the cholera toxin B subunit coding sequence to enhance expression in plants. Mol Breed. 2004;13(2):143–153. [Google Scholar]
- 86.Geyer BC, Fletcher SP, Griffin TA, Lopker MJ, Soreq H, Mor TS. Translational control of recombinant human acetylcholinesterase accumulation in plants. BMC Biotechnol. 2007;7:27. doi: 10.1186/1472-6750-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jabeen R, Khan MS, Zafar Y, Anjum T. Codon optimization of cry1Ab gene for hyper expression in plant organelles. Mol Biol Rep. 2010;37(2):1011–1017. doi: 10.1007/s11033-009-9802-1. [DOI] [PubMed] [Google Scholar]
- 88.Wirth S, Calamante G, Mentaberry A, et al. Expression of active human epidermal growth factor (hEGF) in tobacco plants by integrative and non-integrative systems. Mol Breed. 2004;13(1):23–35. [Google Scholar]
- 89.Yasuda H, Hayashi Y, Jomori T, Takaiwa F. The correlation between expression and localization of a foreign gene product in rice endosperm. Plant Cell Physiol. 2006;47(6):756–763. doi: 10.1093/pcp/pcj049. [DOI] [PubMed] [Google Scholar]
- 90.Stoger E, Sack M, Fischer R, Christou P. Plantibodies: applications, advantages and bottlenecks. Curr Opin Biotechnol. 2002;13(2):161–166. doi: 10.1016/s0958-1669(02)00303-8. [DOI] [PubMed] [Google Scholar]
- 91.Frigerio L, Vitale A, Lord JM, Ceriotti A, Roberts LM. Free ricin a chain, proricin, and native toxin have different cellular fates when expressed in tobacco protoplasts. J Biol Chem. 1998;273(23):14194–14199. doi: 10.1074/jbc.273.23.14194. [DOI] [PubMed] [Google Scholar]
- 92.Xu J, Kieliszewski MJ. A novel plant cell bioproduction platform for high-yield secretion of recombinant proteins. Methods Mol Biol. 2012;824:483–500. doi: 10.1007/978-1-61779-433-9_26. [DOI] [PubMed] [Google Scholar]
- 93.Omar R, Abdullah MA, Hasan MA, Marziah M. Development of growth medium for Centella asiatica cell culture via response surface methodology. Am J Appl Sci. 2004;1(3):215–219. [Google Scholar]
- 94.Prakash G, Srivastava AK. Statistical media optimization for cell growth and azadirachtin production in Azadirachta indica (A. Juss) suspension cultures. Process Biochem. 2005;40(12):3795–3800. [Google Scholar]
- 95.Xu J, Ying PQ, Han AM, Su ZG. Enhanced salidroside production in liquid-cultivated compact callus aggregates of Rhodiola sachalinensis: manipulation of plant growth regulators and sucrose. Plant Cell Tiss Org Cult. 1998;55(1):53–58. [Google Scholar]
- 96.Weathers PJ, Hemmavanh DD, Walcerz DB, Cheetham RD, Smith TC. Interactive effects of nitrate and phosphate salts, sucrose, and inoculum culture age on growth and sesquiterpene production in Artemisia annua hairy root cultures. In Vitro Cell Dev Biol Plant. 1997;33(4):306–312. [Google Scholar]
- 97.Kawana Y, Sasamoto H. Stimulation effects of salts on growth in suspension culture of a mangrove plant, Sonneratia alba, compared with another mangrove, Bruguiera sexangula and non-mangrove tobacco BY-2 cells. Plant Biotechnol. 2008;25(2):151–155. [Google Scholar]
- 98.Doran PM. Foreign protein production in plant tissue cultures. Curr Opin Biotechnol. 2000;11(2):199–204. doi: 10.1016/s0958-1669(00)00086-0. [DOI] [PubMed] [Google Scholar]
- 99.Lee JM, Magnuson NS, An G, Reeves R. 6020169. US. 2000
- 100.Magnuson NS, Linzmaier PM, Gao JW, Reeves R, An GH, Lee JM. Enhanced recovery of a secreted mammalian protein from suspension culture of genetically modified tobacco cells. Protein Expr Purif. 1996;7(2):220–228. doi: 10.1006/prep.1996.0030. [DOI] [PubMed] [Google Scholar]
- 101.Bodeutsch T, James EA, Lee JM. The effect of immobilization on recombinant protein production in plant cell culture. Plant Cell Rep. 2001;20(6):562–566. [Google Scholar]
- 102.Sharp JM, Doran PM. Strategies for enhancing monoclonal antibody accumulation in plant cell and organ cultures. Biotechnol Prog. 2001;17(6):979–992. doi: 10.1021/bp010104t. [DOI] [PubMed] [Google Scholar]
- 103.Cabral JMS. Cell partitioning in aqueous two-phase polymer systems. Cell separation: fundamentals, Analytical and Preparative. Methods. 2007;106:151–171. doi: 10.1007/10_2006_045. [DOI] [PubMed] [Google Scholar]
- 104.Terrier B, Courtois D, Henault N, et al. Two new disposable bioreactors for plant cell culture: the wave and undertow bioreactor and the slug bubble bioreactor. Biotechnol Bioeng. 2007;96(5):914–923. doi: 10.1002/bit.21187. [DOI] [PubMed] [Google Scholar]
- 105.Eibl R, Werner S, Eibl D. Disposable bioreactors for plant liquid cultures at litre-scale. Eng Life Sci. 2009;9(3):156–164. [Google Scholar]
- 106.Huang TK, Plesha MA, Falk BW, Dandekar AM, McDonald KA. Bioreactor strategies for improving production yield and functionality of a recombinant human protein in transgenic tobacco cell cultures. Biotechnol Bioeng. 2009;102(2):508–520. doi: 10.1002/bit.22061. [DOI] [PubMed] [Google Scholar]
- 107.Wang GR, Qi NM, Wang ZM. Application of a stir-tank bioreactor for perfusion culture and continuous harvest of Glycyrrhiza inflata suspension cells. Afr J Biotechnol. 2010;9(3):347–351. [Google Scholar]
- 108.Park CI, Lee SJ, Kang SH, Jung HS, Kim DI, Lim SM. Fed-batch cultivation of transgenic rice cells for the production of hCTLA4Ig using concentrated amino acids. Process Biochem. 2010;45(1):67–74. [Google Scholar]
- 109.Su WW, Arias R. Continuous plant cell perfusion culture: bioreactor characterization and secreted enzyme production. J Biosci Bioeng. 2003;95(1):13–20. doi: 10.1016/S1389-1723(03)80142-1. [DOI] [PubMed] [Google Scholar]
- 110.Huang TK, Plesha MA, McDonald KA. Semicontinuous bioreactor production of a recombinant human therapeutic protein using a chemically inducible viral amplicon expression system in transgenic plant cell suspension cultures. Biotechnol Bioeng. 2010;106(3):408–421. doi: 10.1002/bit.22713. [DOI] [PubMed] [Google Scholar]
- 111.Streatfield SJ. Approaches to achieve high-level heterologous protein production in plants. Plant Biotechnol J. 2007;5(1):2–15. doi: 10.1111/j.1467-7652.2006.00216.x. [DOI] [PubMed] [Google Scholar]
- 112.Sharma AK, Sharma MK. Plants as bioreactors: recent developments and emerging opportunities. Biotechnol Adv. 2009;27(6):811–832. doi: 10.1016/j.biotechadv.2009.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Desai P, Shrivastava N, Padh H. Production of heterologous protein in plants: strategies for optimal expression. Biotechnol Adv. 2010;28(4):427–435. doi: 10.1016/j.biotechadv.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 114.Ponstein AS, Verwoerd TC, Pen J. Production of enzymes for industrial use. Ann NY Acad Sci. 1996;792:91–98. [Google Scholar]
- 115.Doran PM. Foreign protein degradation and instability in plants and plant tissue cultures. Trends Biotechnol. 2006;24(9):426–432. doi: 10.1016/j.tibtech.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 116.Nuttall J, Vine N, Hadlington JL, Drake P, Frigerio L, Ma JKC. ER-resident chaperone interactions with recombinant antibodies in transgenic plants. Eur J Biochem. 2002;269(24):6042–6051. doi: 10.1046/j.1432-1033.2002.03302.x. [DOI] [PubMed] [Google Scholar]
- 117.Faye L, Boulaflous A, Benchabane M, Gomord W, Michaud D. Protein modifications in the plant secretory pathway: current status and practical implications in molecular pharming. Vaccine. 2005;23(15):1770–1778. doi: 10.1016/j.vaccine.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 118.Mandal MK, Fischer R, Schillberg S, Schiermeyer A. Inhibition of protease activity by antisense RNA improves recombinant protein production in Nicotiana tabacum cv Bright Yellow 2 (BY-2) suspension cells. Biotechnol J. 2014;9(8):1065–1073. doi: 10.1002/biot.201300424. [DOI] [PubMed] [Google Scholar]
- 119.Huang TK, McDonald KA. Molecular farming using bioreactor-based plant cell suspension cultures for recombinant protein production. In: Wang A, Ma S, editors. Molecular Farming in Plants: Recent Advances and Future Prospects. Springer; Dordrecht The Netherlands: 2012. pp. 37–67. [Google Scholar]
- 120.Lee SY, Kim YH, Roh YS, Myoung HJ, Lee KY, Kim DI. Bioreactor operation for transgenic Nicotiana tabacum cell cultures and continuous production of recombinant human granulocyte-macrophage colony-stimulating factor by perfusion culture. Enzyme Microb Technol. 2004;35(6–7):663–671. [Google Scholar]
- 121.van Gulik WM, ten Hoopen HJG, Heijnen JJ. The application of continuous culture for plant cell suspensions. Enzyme Microb Technol. 2001;28(9–10):796–805. doi: 10.1016/s0141-0229(01)00331-3. [DOI] [PubMed] [Google Scholar]
- 122.Kieliszewski MJ. The latest hype on Hyp-O-glycosylation codes. Phytochemistry. 2001;57(3):319–323. doi: 10.1016/s0031-9422(01)00029-2. [DOI] [PubMed] [Google Scholar]
- 123.Xu J, Tan L, Lamport DTA, Showalter AM, Kieliszewski MJ. The O-Hyp glycosylation code in tobacco and Arabidopsis and a proposed role of Hypglycans in secretion. Phytochemistry. 2008;69(8):1631–1640. doi: 10.1016/j.phytochem.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 124.James E, Lee JM. Loss and recovery of protein productivity in genetically modified plant cell lines. Plant Cell Rep. 2006;25(7):723–727. doi: 10.1007/s00299-005-0096-z. [DOI] [PubMed] [Google Scholar]
- 125.Garcia-Casado G, Sanchez-Monge R, Chrispeels MJ, Armentia A, Salcedo G, Gomez L. Role of complex asparagine-linked glycans in the allergenicity of plant glycoproteins. Glycobiolology. 1996;6(4):471–477. doi: 10.1093/glycob/6.4.471. [DOI] [PubMed] [Google Scholar]
- 126.Li HJ, d’Anjou M. Pharmacological significance of glycosylation in therapeutic proteins. Curr Opin Biotechnol. 2009;20(6):678–684. doi: 10.1016/j.copbio.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 127.Frey AD, Karg SR, Kallio PT. Expression of rat beta(1,4)-N-acetylglucosaminyltransferase III in Nicotiana tabacum remodels the plant-specific N-glycosylation. Plant Biotechnol J. 2009;7(1):33–48. doi: 10.1111/j.1467-7652.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- 128.Wilson IBH, Harthill JE, Mullin NP, Ashford DA, Altmann F. Core alpha 1,3-fucose is a key part of the epitope recognized by antibodies reacting against plant N-linked oligosaccharides and is present in a wide variety of plant extracts. Glycobiology. 1998;8(7):651–661. doi: 10.1093/glycob/8.7.651. [DOI] [PubMed] [Google Scholar]
- 129.Bardor M, Faveeuw C, Fitchette AC, et al. Immunoreactivity in mammals of two typical plant glycoepitopes, core alpha(1,3)-fucose and core xylose. Glycobiology. 2003;13(6):427–434. doi: 10.1093/glycob/cwg024. [DOI] [PubMed] [Google Scholar]
- 130.Lerouge P, Cabanes-Macheteau M, Rayon C, Fischette-Laine AC, Gomord V, Faye L. N-glycoprotein biosynthesis in plants: recent developments and future trends. Plant Mol Biol. 1998;38(1–2):31–48. [PubMed] [Google Scholar]
- 131.Petruccelli S, Otegui MS, Lareu F, et al. A KDEL-tagged monoclonal antibody is efficiently retained in the endoplasmic reticulum in leaves, but is both partially secreted and sorted to protein storage vacuoles in seeds. Plant Biotechnol J. 2006;4(5):511–527. doi: 10.1111/j.1467-7652.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- 132.Lai HF, Engle M, Fuchs A, et al. Monoclonal antibody produced in plants efficiently treats West Nile virus infection in mice. Proc Natl Acad Sci USA. 2010;107(6):2419–2424. doi: 10.1073/pnas.0914503107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sourrouille C, Marquet-Blouin E, D’Aoust MA, et al. Down-regulated expression of plant-specific glycoepitopes in alfalfa. Plant Biotechnol J. 2008;6(7):702–721. doi: 10.1111/j.1467-7652.2008.00353.x. [DOI] [PubMed] [Google Scholar]
- 134.Strasser R, Stadlmann J, Schahs M, et al. Generation of glyco-engineered Nicotiana benthamiana for the production of monoclonal antibodies with a homogeneous human-like N-glycan structure. Plant Biotechnol J. 2008;6(4):392–402. doi: 10.1111/j.1467-7652.2008.00330.x. [DOI] [PubMed] [Google Scholar]
- 135••.Koprivova A, Stemmer C, Altmann F, et al. Targeted knockouts of physcomitrella lacking plantspecific immunogenic N-glycans. Plant Biotechnol J. 2004;2(6):517–523. doi: 10.1111/j.1467-7652.2004.00100.x. Genetic glycoengineering was emploled toward the generation of moss lines suitable for the production of plant-made glycosylated biopharmaceuticals with nonallergenic N-glycans. [DOI] [PubMed] [Google Scholar]
- 136.Cox KM, Sterling JD, Regan JT, et al. Glycan optimization of a human monoclonal antibody in the aquatic plant Lemna minor. Nat Biotechnol. 2006;24(12):1591–1597. doi: 10.1038/nbt1260. [DOI] [PubMed] [Google Scholar]
- 137.Bakker H, Bardor M, Molthoff JW, et al. Galactose-extended glycans of antibodies produced by transgenic plants. Proc Natl Acad Sci USA. 2001;98(5):2899–2904. doi: 10.1073/pnas.031419998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Bosch D, Castilho A, Loos A, Schots A, Steinkellner H. N-glycosylation of plant-produced recombinant proteins. Curr Pharm Des. 2013;19(31):5503–5512. doi: 10.2174/1381612811319310006. [DOI] [PubMed] [Google Scholar]
- 139.Kittur FS, Bah M, Archer-Hartmann S, et al. Cytoprotective effect of recombinant human erythropoietin produced in transgenic tobacco plants. PLoS ONE. 2013;8(10):e76468. doi: 10.1371/journal.pone.0076468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kittur FS, Hung CY, Darlington DE, Sane DC, Xie JH. N-glycosylation engineering of tobacco plants to produce asialoerythropoietin. Plant Cell Rep. 2012;31(7):1233–1243. doi: 10.1007/s00299-012-1244-x. [DOI] [PubMed] [Google Scholar]
- 141.Willats WGT, Marcus SE, Knox JP. Generation of a monoclonal antibody specific to (1 –> 5)-alpha-L-arabinan. Carbohydr Res. 1998;308(1–2):149–152. doi: 10.1016/s0008-6215(98)00070-6. [DOI] [PubMed] [Google Scholar]
- 142.Yates EA, Valdor JF, Haslam SM, et al. Characterization of carbohydrate structural features recognized by anti-arabinogalactan-protein monoclonal antibodies. Glycobiology. 1996;6(2):131–139. doi: 10.1093/glycob/6.2.131. [DOI] [PubMed] [Google Scholar]
- 143.Leonard R, Petersen BO, Himly M, et al. Two novel types of O-glycans on the mugwort pollen allergen Art v 1 and their role in antibody binding. J Biol Chem. 2005;280(9):7932–7940. doi: 10.1074/jbc.M410407200. [DOI] [PubMed] [Google Scholar]
- 144.Karnoup AS, Turkelson V, Anderson WHK. O-Linked glycosylation in maize-expressed human IgA1. Glycobiology. 2005;15(10):965–981. doi: 10.1093/glycob/cwi077. [DOI] [PubMed] [Google Scholar]
- 145.Yang Z, Bennett EP, Jorgensen B, et al. Toward stable genetic engineering of human O-glycosylation in plants. Plant Physiol. 2012;160(1):450–463. doi: 10.1104/pp.112.198200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Phillips RL, Kaeppler SM, Olhoft P. Genetic instability of plant-tissue cultures – breakdown of normal controls. Proc Natl Acad Sci USA. 1994;91(12):5222–5226. doi: 10.1073/pnas.91.12.5222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Matzke AJM, Matzke MA. Position effects and epigenetic silencing of plant transgenes. Curr Opin Plant Biol. 1998;1(2):142–148. doi: 10.1016/s1369-5266(98)80016-2. [DOI] [PubMed] [Google Scholar]
- 148.Schmale K, Rademacher T, Fischer R, Hellwig S. Towards industrial usefulness – cryo-cell-banking of transgenic BY-2 cell cultures. J Biotechnol. 2006;124(1):302–311. doi: 10.1016/j.jbiotec.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 149.Su WW, Lee KT. Plant cell and hairy-root cultures-process characteristics, products, and application. In: Yang ST, editor. Bioprocessing for Value-Added Products from Renewable Resources. Elsevier Science; Amsterdam, The Netherlands: 2007. pp. 263–292. [Google Scholar]
- 150.Sajc L, Grubisic D, Vunjak-Novakovic G. Bioreactors for plant engineering: an outlook for further research. Biochem Eng J. 2000;4(2):89–99. [Google Scholar]
- 151.Dunlop DS, Yang XR, Lajtha A. The effect of elevated plasma phenylalanine levels on protein synthesis rates in adult rat brain. Biochem J. 1994;302(Pt 2):601–610. doi: 10.1042/bj3020601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Wang SJ, Zhong JJ. A novel centrifugal impeller bioreactor .1. Fluid circulation, mixing, and liquid velocity profiles. Biotechnol Bioeng. 1996;51(5):511–519. doi: 10.1002/(SICI)1097-0290(19960905)51:5<511::AID-BIT2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 153.Kieran PM, MacLoughlin PF, Malone DM. Plant cell suspension cultures: some engineering considerations. J Biotechnol. 1997;59(1–2):39–52. doi: 10.1016/s0168-1656(97)00163-6. [DOI] [PubMed] [Google Scholar]
- 154.Ananta I, Subroto MA, Doran PM. Oxygen-transfer and culture characteristics of self-immobilized solanum aviculare aggregates. Biotechnol Bioeng. 1995;47(5):541–549. doi: 10.1002/bit.260470506. [DOI] [PubMed] [Google Scholar]
- 155.Eibl R, Eibl D. Design and use of the wave bioreactor for plant cell culture. Plant Tiss Cult Eng. 2006;6:203–227. [Google Scholar]
- 156.Eibl R, Kaiser S, Lombriser R, Eibl D. Disposable bioreactors: the current state-of-the-art and recommended applications in biotechnology. Appl Microbiol Biotechnol. 2010;86(1):41–49. doi: 10.1007/s00253-009-2422-9. [DOI] [PubMed] [Google Scholar]
- 157•.Kwon JY, Yang YS, Cheon SH, Nam HJ, Jin GH, Kim DI. Bioreactor engineering using disposable technology for enhanced production of hCTLA4Ig in transgenic rice cell cultures. Biotechnol Bioeng. 2013;110(9):2412–2424. doi: 10.1002/bit.24916. Two kinds of disposable bioreactors were compared with stirred-tank reactors for scaling up the cultures of transgenic rice cells. Experiments demonstrate pneumatically driven disposable bioreactors are applicable for the production of recombinant proteins in plant cell cultures. [DOI] [PubMed] [Google Scholar]
- 158.Eibl R, Eibl D. Design of bioreactors suitable for plant cell and tissue cultures. Phytochem Rev. 2008;7:593–598. [Google Scholar]