Abstract
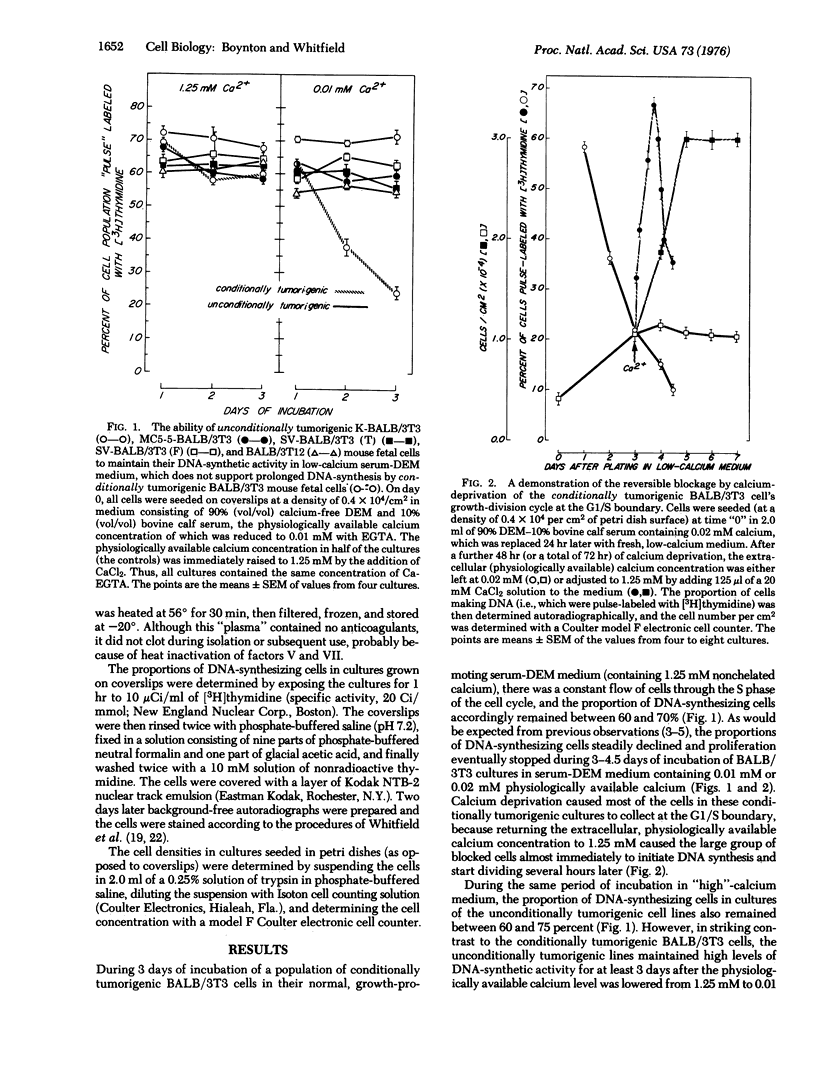
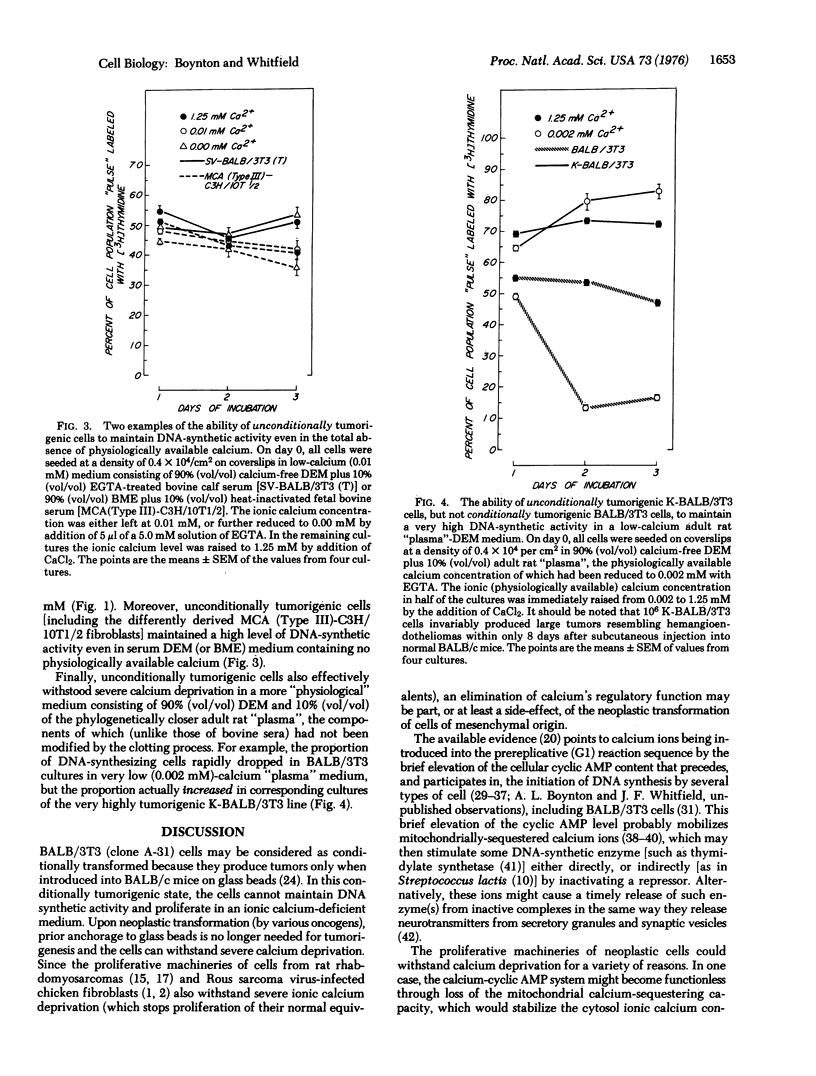
Conditionally tumorigenic BALB/3T3 mouse cells (which produce tumors in BALB/c mice only under special conditions) cannot sustain DNA synthesis and consequently stop proliferating in media containing low concentrations (0-0.02 mM) of physiologically available calcium. By contrast, cells that have been neoplastically transformed (tumorigenic in mice without special assistance) in vitro by different oncogens, can sustain DNA synthesis and proliferate in such calcium-deficient media. The possible importance for tumor growth of an ability to withstand calcium deprivation is examined. It is suggested that this property may prove to be a reliable indicator of neoplastic transformation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A., Todaro G. J. Basis for the acquisition of malignant potential by mouse cells cultivated in vitro. Science. 1968 Nov 29;162(3857):1024–1026. doi: 10.1126/science.162.3857.1024. [DOI] [PubMed] [Google Scholar]
- Armato U., Andreis P. G., Draghi E. Effect of adenosine 3',5'-cyclic monophosphate on the RNA and DNA synthesis and cell proliferation of rat hepatocytes in primary culture: a radioautographic study. Chem Biol Interact. 1975 Aug;11(2):67–90. doi: 10.1016/0009-2797(75)90015-0. [DOI] [PubMed] [Google Scholar]
- Balk S. D. Calcium as a regulator of the proliferation of normal, but not of transformed, chicken fibroblasts in a plasma-containing medium. Proc Natl Acad Sci U S A. 1971 Feb;68(2):271–275. doi: 10.1073/pnas.68.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balk S. D., Whitfield J. F., Youdale T., Braun A. C. Roles of calcium, serum, plasma, and folic acid in the control of proliferation of normal and Rous sarcoma virus-infected chicken fibroblasts. Proc Natl Acad Sci U S A. 1973 Mar;70(3):675–679. doi: 10.1073/pnas.70.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boone C. W. Malignant hemangioendotheliomas produced by subcutaneous inoculation of Balb/3T3 cells attached to glass beads. Science. 1975 Apr 4;188(4183):68–70. doi: 10.1126/science.1114343. [DOI] [PubMed] [Google Scholar]
- Borle A. B., Briggs F. N. Microdetermination of calcium in biological material by automatic fluorometric titration. Anal Chem. 1968 Feb;40(2):339–344. doi: 10.1021/ac60258a056. [DOI] [PubMed] [Google Scholar]
- Borle A. B. Cyclic AMP stimulation of calcium efflux from kidney, liver and heart mitochondria. J Membr Biol. 1974;16(3):221–236. doi: 10.1007/BF01872416. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J. Calcium-dependent stimulation of BALB/c 3T3 mouse cell DNA synthesis by a tumor-promoting phorbol ester (PMA). J Cell Physiol. 1976 Jan;87(1):25–32. doi: 10.1002/jcp.1040870105. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J., Morton H. J. Control of 3T3 cell proliferation by calcium. In Vitro. 1974 Jul-Aug;10:12–17. doi: 10.1007/BF02615333. [DOI] [PubMed] [Google Scholar]
- Boynton A. L., Whitfield J. F., Isaacs R. J. The different roles of serum and calcium in the control of proliferation of BALB/c 3T3 mouse cells. In Vitro. 1976 Feb;12(2):120–123. doi: 10.1007/BF02796358. [DOI] [PubMed] [Google Scholar]
- DANIEL J. W., RUSCH H. P. The pure culture of Physarum polycephalum on a partially defined soluble medium. J Gen Microbiol. 1961 May;25:47–59. doi: 10.1099/00221287-25-1-47. [DOI] [PubMed] [Google Scholar]
- Daniel J. W., Järlfors U. Light-induced changes in the ultrastructure of a plasmodial myxomycete. Tissue Cell. 1972;4(3):405–426. doi: 10.1016/s0040-8166(72)80018-1. [DOI] [PubMed] [Google Scholar]
- Diamantstein T., Ulmer A. The control of immune response in vitro by Ca2+. II. The Ca2+-dependent period during mitogenic stimulation. Immunology. 1975 Jan;28(1):121–125. [PMC free article] [PubMed] [Google Scholar]
- Ebina Y., Iwai H., Fukui N., Otsuka H., Miura Y. Prereplicative enzymic changes in regenerating rat liver. J Biochem. 1975 Mar;77(3):641–645. doi: 10.1093/oxfordjournals.jbchem.a130766. [DOI] [PubMed] [Google Scholar]
- Gail M. H., Boone C. W., Thompson C. S. A calcium requirement for fibroblast motility and prolifertion. Exp Cell Res. 1973 Jun;79(2):386–390. doi: 10.1016/0014-4827(73)90458-8. [DOI] [PubMed] [Google Scholar]
- Hurst A., Lazarus W. Calcium uptake during growth of Streptococcus lactis. Nature. 1968 Jul 27;219(5152):404–405. doi: 10.1038/219404a0. [DOI] [PubMed] [Google Scholar]
- LEBLOND C. P. THE TIME DIMENSION IN HISTOLOGY. Am J Anat. 1965 Jan;116:1–27. doi: 10.1002/aja.1001160102. [DOI] [PubMed] [Google Scholar]
- Lehninger A. L. Mitochondria and calcium ion transport. Biochem J. 1970 Sep;119(2):129–138. doi: 10.1042/bj1190129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacManus J. P., Braceland B. M., Youdale T., Whitfield J. F. Adrenergic antagonists, and a possible link between the increase in cyclic adenosine 3',5'-monophosphate and DNA synthesis during liver regeneration. J Cell Physiol. 1973 Oct;82(2):157–164. doi: 10.1002/jcp.1040820204. [DOI] [PubMed] [Google Scholar]
- Millis A. J., Forrest G. A., Pious D. A. Cyclic AMP dependent regulation of mitosis in human lymphoid cells. Exp Cell Res. 1974 Feb;83(2):335–343. doi: 10.1016/0014-4827(74)90347-4. [DOI] [PubMed] [Google Scholar]
- Perris A. D., Whitfield J. F. Calcium homeostasis and erythropoietic control in the rat. Can J Physiol Pharmacol. 1971 Jan;49(1):22–35. doi: 10.1139/y71-004. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B., Tenenhouse A. The role of cyclic AMP and calcium in cell activation. CRC Crit Rev Biochem. 1972 Feb;1(1):95–148. doi: 10.3109/10409237209102545. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Rixon R. H., Whitfield J. F. Hypoplasia of the bone marrow in rats following removal of the parathyroid glands. J Cell Physiol. 1972 Jun;79(3):343–352. doi: 10.1002/jcp.1040790304. [DOI] [PubMed] [Google Scholar]
- Rixon R. H., Whitfield J. F. Parathyroid hormone and liver regeneration. Proc Soc Exp Biol Med. 1974 Jul;146(3):926–930. doi: 10.3181/00379727-146-38221. [DOI] [PubMed] [Google Scholar]
- Rixon R. H., Whitfield J. F. The control of liver regeneration by parathyroid hormone and calcium. J Cell Physiol. 1975 Dec;87(2):147–155. doi: 10.1002/jcp.1040870203. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Stambrook P. J. Cell cycle specific fluctuations in adenosine 3':5'-cyclic monophosphate and polyamines of Chinese hamster cells. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1482–1486. doi: 10.1073/pnas.72.4.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert W., Rudland P. S. Cyclic nucleotides and growth control in cultured mouse cells: correlation of changes in intracellular 3':5' cGMP concentration with a specific phase of the cell cycle. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4920–4924. doi: 10.1073/pnas.71.12.4920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J., Tsukada K., Rudert W. A., Lieberman I. Cyclic adenosine 3':5'-monophosphate and the induction of deoxyribonucleic acid synthesis in liver. J Biol Chem. 1975 May 25;250(10):3602–3606. [PubMed] [Google Scholar]
- Swierenga S. H., MacManus J. P., Whitfield J. F. Regulation by calcium of the proliferation of heart cells from young adult rats. In Vitro. 1976 Jan;12(1):31–36. doi: 10.1007/BF02832790. [DOI] [PubMed] [Google Scholar]
- Thrower S., Ord M. G. Hormonal control of liver regeneration. Biochem J. 1974 Nov;144(2):361–369. doi: 10.1042/bj1440361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Boynton A. L., Gillan D. J., Isaacs R. J. Concanavalin A and the initiation of thymic lymphoblast DNA synthesis and proliferation by a calcium-dependent increase in cyclic GMP level. J Cell Physiol. 1974 Dec;84(3):445–458. doi: 10.1002/jcp.1040840312. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Gillan D. J. The ability of calcium to change cyclic AMP from a stimulator to an inhibitor to thymic lymphoblast proliferation. J Cell Physiol. 1973 Apr;81(2):241–250. doi: 10.1002/jcp.1040810212. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., MacManus J. P., Rixon R. H., Boynton A. L., Youdale T., Swierenga S. The positive control of cell proliferation by the interplay on calcium ions and cyclic nucleotides. A review. In Vitro. 1976 Jan;12(1):1–18. doi: 10.1007/BF02832787. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., Rixon R. H., MacManus J. P., Balk S. D. Calcium, cyclic adenosine 3',5'-monophosphate, and the control of cell proliferation: a review. In Vitro. 1973 Jan-Feb;8(4):257–278. doi: 10.1007/BF02615905. [DOI] [PubMed] [Google Scholar]
- Whitfield J. F., Rixon R. H., Perris A. D., Youdale T. Stimulation by calcium of the entry of thymic lymphocytes into the deoxyribonucleic acid-synthetic (S) phase of the cell cycle. Exp Cell Res. 1969 Sep;57(1):8–12. doi: 10.1016/0014-4827(69)90360-7. [DOI] [PubMed] [Google Scholar]
- Whitney R. B., Sutherland R. M. Requirement for calcium ions in lymphocyte transformation stimulated by phytohemagglutinin. J Cell Physiol. 1972 Dec;80(3):329–337. doi: 10.1002/jcp.1040800303. [DOI] [PubMed] [Google Scholar]
- Youdale T., MacManus J. P. Failure of tritiated thymidine incorporation into DNA to reflect the autoradiographically demonstrable calcium-induced increase in thymic lymphoblast DNA synthesis. J Cell Physiol. 1975 Dec;86(3 Pt 1):495–502. doi: 10.1002/jcp.1040860306. [DOI] [PubMed] [Google Scholar]



