Abstract
Aims
Knowledge of consumer perspectives of personalized medicine (PM) is limited. Our study assessed consumer perspectives of PM, with a focus on oncology care, to inform industry, clinician and payer stakeholders' programs and policy.
Materials & Methods
A nationally representative survey of 602 US consumers' ≥30 years old explored familiarity, perspectives and expected value of PM.
Results
Most (73%) respondents have not heard of ‘personalized medicine,’ though after understanding the term most (95%) expect PM to have a positive beneft. Consumer's willingness to pay is associated with products' impact on survival, rather than predicting disease risk. If testing indicates consumers are not candidates for oncology therapies, most (84%) would seek a second opinion or want therapy anyway.
Conclusions
Understanding heterogeneity in consumer perspectives of PM can inform program and policy development.
Keywords: consumers, education, knowledge, oncology, personalized medicine, perspectives, value
While much has been written about the benefts of personalized medicine to determine response to particular pharmacogenomics treatments, limited work has been done to consider the clinical, psychosocial and cost implications to consumers of genetic testing and personalized medicine approaches [1]. Care providers do not yet know how patient care pathways will evolve to include new personalized diagnostic and treatment options. This leads to questions about consumer/patient reactions to being identified as responders versus nonresponders of targeted therapies, information about their risk and future looking prognostics. As with any sea change in clinical practice comes several potential opportunities and challenges for consumers. Today, not enough is known about consumer perspectives and preferences for personalized medicine approaches [2].
Our study objective was to assess US consumer perspectives of personalized medicine, with a specific focus on oncology care, to inform industry, clinician and payer stakeholders' engagement activities and policy development. More representative and generalizable consumer centric information is needed to help manufacturers, clinicians, policy makers and payers understand consumer awareness of personalized medicine and the implications of their familiarity, attitudes and expected behaviors to the roll-out of new care paradigms. Consumer perspectives have key implications for stakeholders attempting to advance the incorporation of personalized medicine into routine practice. Specifically, understanding consumer receptivity to personalized medicine in detail informs how key stakeholders alter and evolve existing programs to meet consumers' actual needs. Examples of this could include: Industry use of patient perspectives to inform value messaging, Clinicians use of patient perspectives to inform patient education, and Payers use of patient perspectives to understand willingness to pay and behavioral outcomes associated with genetic testing. Each application of consumer perspectives on personalized medicine is relevant to the rapidly changing oncology care landscape today.
Materials & methods
A national survey was fielded in the US to gauge familiarity with attitudes and behaviors related to the coming shift from general population-based therapies to personalized medicine approaches, with a focus on the specific application of personalized medicine within oncology (Supplementary Material; Appendix A; see online at: http://www.futuremedicine.com/doi/full/10.2217/PME.14.74). To achieve this, a multiphased research approach was undertaken leading to the deployment of a consumer survey to a representative sample of 602 adults in the US, described below.
Description & definition of personalized medicine
We formulated a description and definition of personalized medicine for consideration during survey development. After evaluating the feedback during the pretesting, final survey respondents were asked a series of questions regarding their familiarity with personalized medicine prior to being provided the following description and definition to complete additional questions on the survey.
Modern medications save millions of lives a year. Yet any one medication might not work for you, even if it works for other people. Your age, lifestyle and health all influence your response to medications. But so do your genes. Scientists are working to match specific gene variations with responses to particular medications. With that information, doctors can:
predict what diseases you may get in the future and attempt to either minimize the impact of that disease or avoid it altogether through the implementation of personalized, preventive medicine;
once diagnosed with a disease, tailor treatments, predicting whether a medication is likely to help or hurt you before you ever take it.
Survey development
The first step was a targeted literature review focused on studies published in the past 5 years considering consumer or patient perspectives on personalized medicine and genetic testing. Databases searched include PubMed and Google Scholar. Search terms included ‘personalized medicine +patient,’ ‘personalized medicine+consumer,’ ‘consumer+ targeted treatment,’ ‘patient+ targeted treatment,’ ‘patient preferences+oncology treatment,’ and ‘consumer and/or patient + individualized treatment.’ Once identified, novel research studies or published papers reflecting relevant expert opinion were reviewed. Key issues related to consumer familiarity with and preferences towards personalized medicine were identified, and then validated with expert key informant interviews. Interviewees included three payer Medical Directors, a pathologist, two industry members developing oncology therapeutics and three oncologists. Key informants were selected based on personalized medicine content expertise and cascade sampling technique. Interviews were conducted by phone, lasted approximately 45 minutes and included a structured discussion of key topics expected to be covered in the survey instrument. The survey was then pretested with 15 consumers, randomly selected from the sample frame, to determine participant comprehension of survey questions and functionality. Pretest results were used to optimize the survey design. Questions were asked using Likert scales where possible, leveraging simple language to ensure a high level of participant comprehension. However, due to the respondents being a general population sample, variation in comprehension of scenarios presented is likely.
Survey administration
The survey was administered online to a representative sample of United States health consumers, ages 30 years and older. Participants were recruited by invitation through an internet-based survey panel, GfK KnowledgePanel [3]. This panel has been used extensively in over 400 papers, articles and books, including several studies on genetic testing and is validated by the American Association for Public Opinion Research [4,5,6,7]. The panel uses address-based sampling with a published sample frame of residential addresses that covers approximately 97% of U.S. households. Participants without Internet access were captured by providing them with a netbook and Internet Service Provider. The sample of participants also included cellphone only households. The sample was drawn from the 55,000+ member panel using a probability proportional to size (PPS) weighted sampling approach. KnowledgePanel participants are incented to complete the survey via a points system that is redeemable for rewards credits.
Confidentiality & privacy protections
The KnowledgePanel recruitment and empanelment process is designed to comply with CAN-SPAM [8] and CASRO guidelines [9]. Further, GfK policies conform to participant treatment protocols outlined by the federal Office Management and Budget, following guidelines from the Belmont Report. Survey responses are confidential; personally identifying information is never revealed to clients or other external parties without explicit respondent approval and a client-signed nondisclosure agreement. When surveys are assigned to KnowledgePanel panel members, they are notified in their password-protected email account that a survey is available for completion. Surveys are self-administered and accessible any time of day for a designated period. Participants can complete a password-protected survey only once. Members may withdraw from the panel at any time, and continued provision of the web-enabled device (e.g., laptop or netbook) and Internet service is not contingent on completion of any particular survey.
Participation in research is voluntary at the time that respondents are asked to join the panel, at the time they are asked to participate in any particular survey and at the time they answer any given question in a survey. KnowledgePanel participants are provided detailed privacy disclosure statements and releases prior to participating in the panel and subsequent surveys. More information about KnowledgePanel recruitment and privacy policies can be reviewed in the Supplementary Material: Appendix B.
Data analysis
The survey data were analyzed using descriptive statistical for enumerating responses as proportions and percentages. Differences between subgroups were tested using t-tests and p values less that 0.05 were considered statistically significant (all differences noted below were statistically significant). When calculating percentages, participants who did not answer a particular question were excluded from the denominator for that question. Subgroups analyzed include differentiation by gender, sociodemographic characteristics and those reporting high or low preferences for personalized medicine.
Results
Sample demographics
Between 26 February and 4 March 2013, 602 respondents completed the survey out of 1016 invited, for a response rate of 59%. The survey took an average of 14 minutes to complete. The respondents were generally middle age (mean 53 years), 52% female, non-ethnic (69%), married (61%), variably educated (31% college grads) and employed (53%). The mean household income of participants was $60,550 with a median household size of 2 (Table 1). Respondents were older, more likely to be married and had higher incomes compared with the national average. This is largely due to the inclusion criteria of being aged 30 and over. Overall, respondents were relatively healthy (83% reporting good, very good, or excellent health; 16% reporting very poor, poor, or fair health). Overall, most (67%) reported having one or more medical condition diagnosed. Considering cancer specifically, 8% reported having or having had cancer (3% of which is skin cancer).
Table 1.
Participants were drawn from a representative sample and have varying characteristics.
| Demographic | Total n = 602 | US average |
|---|---|---|
| Respondents | ||
| Female | 52% | 49.89% (2013 US Census, over 30 years old) |
| Mean age (years) | 52.99 | 52.3 (median age, US Census, over 30 years old) |
| Nonethnic | 69% | 80.3% (2013 US Census, over 30 years old) |
| Married | 61% | 51% (2011, Pew Research Center, all ages) |
| Completed college or more | 31% | 37% (2013 US Census, over 30 years old) |
| Working | 53% | 59% (Employment–Population ratio, bls.gov, all ages) |
| HH head | 87% | |
|
| ||
| Median household income (in thousands, US$) | $60,550 | $53,046 (2008–2012, US Census, all ages) |
|
| ||
| Region: | ||
| Northeast | 18% | 18% |
| Midwest | 22% | 21% |
| South | 37% | 36% |
| West | 23% | 25% |
|
| ||
| (2010, US Census, all ages) | ||
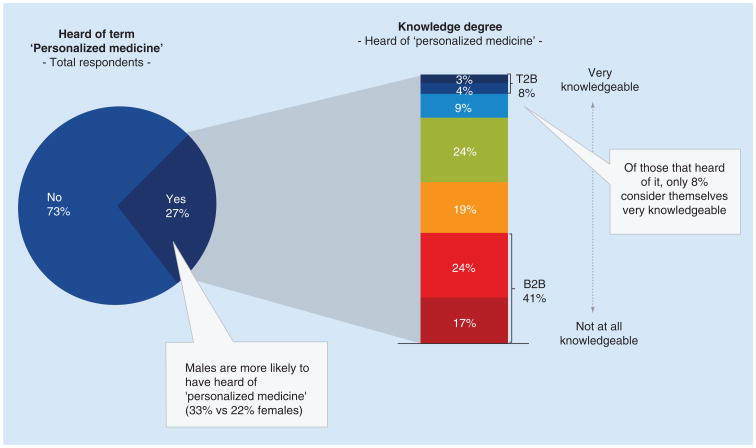
Familiarity with personalized medicine In general, there is low familiarity with the term ‘personalized medicine’ among the respondents, with 73% of individuals indicating they have not heard the term ‘personalized medicine’ (Figure 1). Of those who have heard of personalized medicine (27%, n = 163), males were more likely to have heard of this term than females (33% of males vs 22% of females). Furthermore, of those who heard of personalized medicine, only 8% consider themselves very knowledgeable, compared with 41% indicating not at all or not very knowledgeable (p < 0.05%).
Figure 1. Understanding personalized medicine term and knowledge.
All responses were based on a 1–7 Likert scale, with 1 being ‘not at all knowledgeable’ and 7 being ‘very knowledgeable’.
Once the description and definition of personalized medicine was provided, most respondents (63%) thought personalized medicine would have a very positive or positive impact, while just 5% thought personalized medicine would have a negative or very negative impact. There is variation in expectations of the timing of that impact, be it in the short, i.e., 1–3 years (19%), medium, i.e., 3–5 years (22%), or longer, i.e., more than 5 years (49%) term. At significant levels, (p < 0.05%) respondents who rated their health as very good to excellent report having a higher knowledge of personalized medicine and perceive it more positively. Conversely, respondents who rated their health as poor were less receptive to personalized medicine.
Perceived likely adherence to personalized medicine recommendations
Respondents were asked to predict their likely action upon receiving personalized medicine test results that indicated a more efficacious treatment was not going to work for them, in the hypothetical situation they had been diagnosed with a life threatening cancer. Most (71%) consumers predicted they would get a second opinion. An additional 13% said they would want to get the treatment anyway. In total, most (84%) respondents predict no immediate behavior or treatment change from a personalized medicine approach to treatment selection if it suggests forgoing treatment. This is in contrast to a small subset of respondents (13%) who say they would not get the treatment accepting that it would not work.
Perceived economic impact
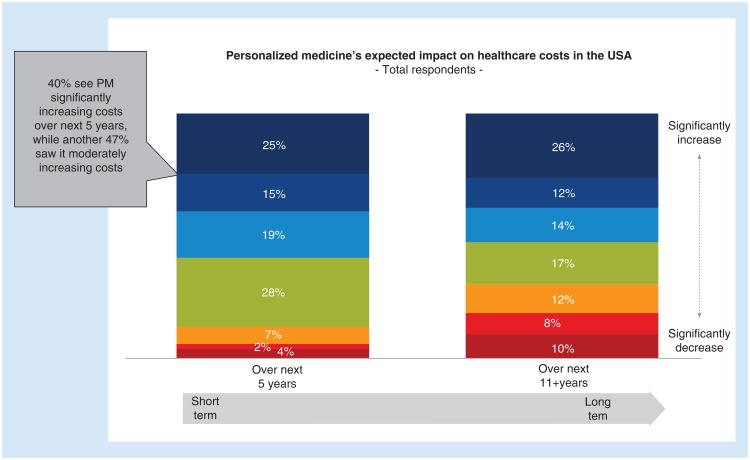
When we examined perceptions of the impact of personalized medicine on healthcare costs, many felt that it would increase overall healthcare costs in both the short (next 5 years) and long term (next 11+ years) (40%- short-term, 38%- longer term). However, some disagreed and saw hope for personalized medicine reducing costs in the long term (18%) (Figure 2).
Figure 2. Consumers expected impact on healthcare costs in the USA.
All responses were based on a Likert scale of personalized medicine's expected impact on healthcare costs, with 1 being ‘significantly decrease’ and 7 being ‘significantly increase’ PM: Personalized medicine.
The value of personalized medicine, for consumers, does not rest solely with perceived overall health related cost-avoidance. In contrast, personalized medicine is seen as most valuable for tailoring treatments after diagnosis (44% reported as most valuable), minimizing the impact of diseases through preventative medicine (42% reported as most valuable) and predicting what diseases they may get in the future (13%).
To understand how cost-sharing within personalized medicine might impact preferences, consumers were asked whether they would choose a treatment that has a high likelihood of success but higher rates of side effects, versus a lower likelihood of success and lower side effect rates first. Over three quarters (77%) chose the higher efficacy treatment, versus 23% choosing the lower efficacy option. When introduced to the same scenario for the more efficacious drug being ‘high cost,’ represented by a $100,000 cost with a $10,000 co-pay, and the lower efficacy drug having no co-pay only slightly over half (53%) chose the more efficacious high cost drug and 47% chose the lower cost, lower efficacy option. Respondents with multiple health conditions were more likely to choose the lower cost lower efficacy option than those in good health.
Personalized medicine testing
In the second part of the survey, questions specifically dealing with testing as a component of personalized medicine were asked. Overall, 32% of respondents were very interested, 47% moderately interested and 21% not at all interested in testing, described as:
Being able to predict what diseases you may get in the future and attempt to either minimize the impact of that disease or avoid it altogether through the implementation of personalized, preventive medicine;
Once diagnosed with a disease, tailor treatments, predicting whether a medication is likely to help or hurt you before you ever take it.
In contrast, 68% of those who had ever been diagnosed with a life threatening cancer were very interested in the concept (n = 56). Additionally, those interested in personalized medicine testing tended to be more educated, have higher interests in personalized medicine generally, live in more metropolitan areas, have higher incomes and have more access to the internet than those who were not interested in personalized medicine testing.
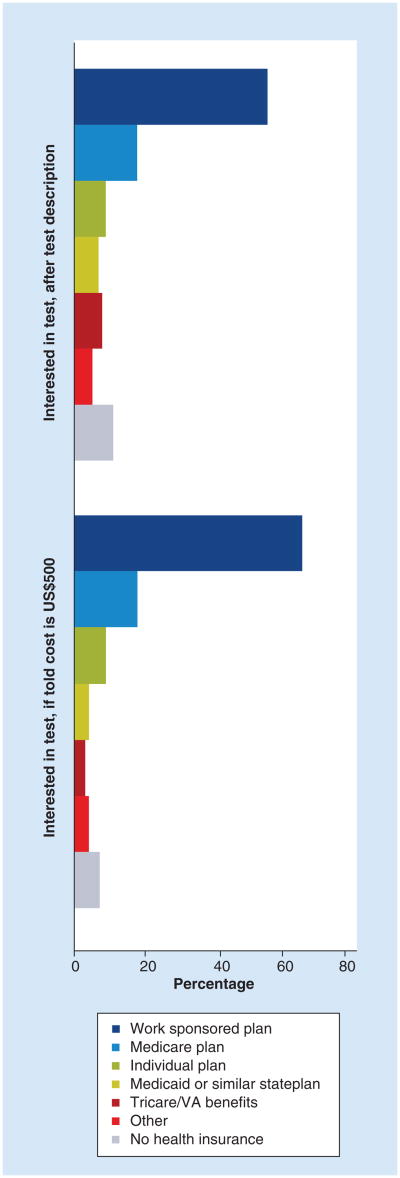
Cost-concerns and disease history in addition to payer type also impact receptivity to personalized medicine related testing. In general, respondents with employer sponsored health plans were most interested in testing when compared with other insured groups. This differential held true when the hypothetical test cost was $500 (Figure 3).
Figure 3. Interest in genetic testing with and without cost prompt, by respondent's insurance type.
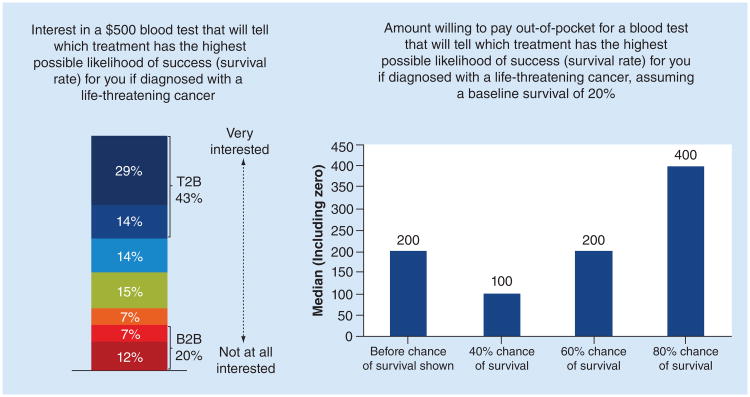
A proportion of respondents (43%) indicated they are willing to pay $500 for a predictive or prognostic test. The dollar amount respondents are willing to pay out-of-pocket increases along with predicted rates of cancer survival (Figure 4). Of those who have ever been diagnosed with cancer, more (62%) were very interested in testing at that cost than the general respondent pool. Many respondents (45%) did not think cost would impact their willingness to recommend testing to family members who had been diagnosed with a life-threatening cancer. Though near equal numbers of others felt it would (29%) or they were not sure (26%).
Figure 4. Interest in and amount willing to pay out-of-pocket for blood test if diagnosed with cancer with varying survival rates.
All responses were based on a 1–7 Likert scale with 1 being ‘not at all Interested’ and 7 being ‘very interested’
However, when consumers were asked what price they would be willing to pay for testing, without information about the relationship between the test and survival improvement, the median price reported was $200. When told that survival improvement was 40%, 60%, or 80%- compared with an expected average survival without testing of 20%, median willingness to pay was $100, $200 and $400, respectively. These results suggest that respondents are willing to bear a cost for testing that positively impacts survival, and that willingness to pay increases with the expected clinical impact of the test.
Discussion
Overall, among US consumers there was a lack of familiarity with personalized medicine. However, when provided definitions, respondents were optimistic about the prospect of personalized medicine providing safe and effective treatment options in oncology in the near future. Within that positivity there is significant variation across consumers in how they would embrace personalized medicine, their willingness to pay for it (both on the diagnostic and therapeutic side) and what they would do with test results that indicated they should forgo treatment.
Previous qualitative research by Bombard et al. has found that breast cancer patients value gene expression profiling (a form of personalized medicine testing for early stage breast cancer); however their understanding of the test was variable [10]. Furthermore, factors relating to access to personalized medicine heightened the value of gene expression profiling to breast cancer patients [11]. Similarly, we found that the understanding of personalized medicine was variable, however when explained further respondents were optimistic about the value of personalized medicine.
Our results are consistent with the available literature and recent consumer studies that speak to consumer familiarity and knowledge gaps, personalized medicine education challenges and preference variability. For example, a recent unpublished survey conducted by the Personalized Medicine Coalition found that a ‘large majority of people have not heard of personalized medicine but react positively when it is described to them; most feel excited about the potential benefits of personalized medicine, including choosing a treatment that is most likely to work for them and the potential to prevent illness; and a large majority also recognize the value of these technologies and believe that they should be covered by insurance’ [12]. Another recent analysis of patients receiving genetic counseling associated with personalized medicine care found that participants had difficulty with basic genetic concepts and education to understand the complexities of genomic risk information was often needed [13]. In another recently published study, authors found that ‘a complex interplay of philosophical, professional and cultural issues can create impediments to genomic education of the public’ [14]. Other studies point out that levels of awareness related to genetics role in treatment selection were variable [15] and that consumers are more willing to learn their risk for developing deadly diseases versus nondeadly ones [16].
Our study results add to the literature by exploring consumer preferences in greater detail among a representative sample where others use nonprobability-based samples like convenience, random dial, or voluntary sampling. Our study also adds by taking a specific focus on consumer perceptions related to genetic testing and oncology applications of personalized medicine. We also explore the differences in responses between both demographic subpopulations (i.e., education levels, gender) and between those who have had cancer and those who have not.
In concert with other studies in the literature, our study demonstrates a need for consumer education related to several aspects of PM's value proposition. For example, one critical need highlighted by the research is that consumers may not be willing to forgo treatment based solely on genetic testing. Compliance to testing and treatment algorithms, including forgoing treatments that are not expected to be effective, is required for personalized medicine to realize optimal value. If patients see genetic testing results as something to be ignored or challenged via second opinion when they suggest forgoing treatment, the paradigm loses significant value and reduces the potential for cost-effective care solutions. From a payer perspective, cost savings from personalized medicine depend on differentiated treatment pathways based on genetic profiling and associated response rates. As levels of awareness of and comfort with PM grow, it is expected that ‘second opinion’ redundancy would decrease and efficiencies would be realized.
Consumers' perspectives about personalized medicine and willingness-to-pay can provide useful insights for manufacturers as to the perceived value of different treatments in development. Today, patient cost sharing is routine and costs to the patient do play a significant role. The 2013 Employer Health Benefits Survey found that co-insurance rates of 16–38% of drug costs are typical within many health insurance plans, with higher rates associated with branded and/or higher tiered products [17]. As patients are increasingly responsible for cost-sharing, their role as both patient and payer further supports the need to understand their perspectives on PM value. Consumer and payer preferences together will help align test and therapeutic product development programs with purchasing decision-makers. Additionally, the varying perspectives toward different attributes of personalized medicine captured in this study can inform development of value-based evaluations by industry, payers and clinicians.
Ultimately, to reach the true potential of a personalized medicine care paradigm, the perspectives of consumers must be understood and addressed within education and outreach initiatives. From familiarity and knowledge gaps of diagnostic and treatment options, to concerns about cost, there remain several unanswered questions for consumers related to personalized medicine.
While our research reviewed consumer perspectives from the US, consumer perspectives from other geographic contexts are likely to vary based on practice, cultural, and healthcare financing differences. For example, one recent study noted patient preferences for personalized approaches to breast cancer management, but systemic factors (payer and clinical gatekeepers within the Canadian health system) rather than treatment preferences prevented access. This example demonstrates the need to further assess patient preferences within markets rather than taking key findings and generalizing them across all settings [10]. Future research focused on exploring the heterogeneity among consumer and patient perspectives within markets would be beneficial.
Furthermore, measures of patient preferences examining risk–benefit trade-offs in genetic testing need to be examined. Although previous research and our current research provide valuable qualitative information regarding the value of personalized medicine, further research needs to be done to quantitatively estimate the value of personalized medicine. Conjoint analysis is an accepted method in healthcare used to quantitatively measure stated patient preferences by forcing respondents to make benefit-risk trade-offs when making choices [18,19].
Lastly, the differences between a healthy consumer, a current patient and a consumer who has experienced a life threatening disease must be considered. This is an area requiring further research to determine the nuances between these three groups, only briefly touched on within this research. Future studies should investigate how consumer trade-offs among the various attributes (i.e., costs, utility expectations) impact preferences [18]. Measuring the risk–benefit trade-offs in this manner will affect estimates of willingness to pay, expectations of utility and other consumer preferences.
Strength & limitations
In order to put study findings in context, the authors note several strengths and limitations. First, the large representative sample of consumers in the US provides an opportunity for generalizability of results not possible with smaller samples. Additionally, due to the design of the consumer panel, significant demographic data were known about each survey participant, including gender, sociodemographics, payer and relevant disease history. This enabled greater granularity in data analysis, without consumers having to complete an extremely long survey – which can lead to survey fatigue and drop-out. Additionally, knowing this information a priori decreases reporting bias of each of these variable points. There are also certain limitations of the study, including how financial incentive for participation may introduce incentive bias and the definition of personalized medicine used in this study may not account for all complexities associated with the discipline or regional variations in how the term is applied. Additionally, the survey instrument used was a nonvalidated tool, though tested with key stakeholders prior to use. Lastly, while the survey was cross-sectional in design and thus does not compare the evolution of thought or experience prospectively.
Conclusion
Manufacturers, clinicians and payers should begin to prepare patients and consumers to ask the right questions and educate them about how to best understand how the rapidly evolving field of personalized medicine can impact their healthcare. Incorporating the multidimensional consumer perspectives identified in this and others' research into practice planning will help stakeholders streamline current inefficiencies and ultimately realize the clinical and economic promise of personalized medicine.
Future perspective
There are several NIH funded studies underway that intend to better understand the consumer's and patient's knowledge, perspectives and risk–benefit trade-off preferences of Personalized Medicine. This research addresses both currently available PM tests as well as emerging tests focusing on next generation sequencing technologies. Furthermore, our study population was selected to provide generalizable results for the US consumer and which may not be applicable to a population of patients in a clinic/hospital setting. As follow-on research, surveying patients would add a complementary perspective to these results to examine how patient perspectives compare to the perspectives of the general consumer population. Additionally, multivariate statistical analysis would be informative regarding the key factors that contribute to knowledge about PM after controlling for the characteristics of the study population.
Supplementary Material
Executive Summary.
Understanding of personalized medicine
Low familiarity with the term ‘personalized medicine’ among the general population with 73% of individuals not having heard the term.
Once definitions for personalized medicine were provided, most respondents thought it would have a very positive impact, though there was variability in expectations of timing of impact.
Perceived likely adherence to personalized medicine recommendations
Respondents are willing to pay $500 for a predictive or prognostic test. However, respondents have high likelihood of seeking a second opinion associated with directed care if it implies nonresponse.
84% of respondents may not have an immediate behavior or treatment change from a personalized medicine approach if it suggests forgoing treatment.
Perceived economic impact
Most respondents saw the primary value of personalized medicine to be tailoring treatments and predicting whether a medication is likely to help or hurt before taking it (44%), or minimizing the impact of diseases through preventive medicine (42%), rather than predicting what disease they would get in the future (13%).
Consumers previously diagnosed with a potentially fatal disease have different perceptions about the value of various aspects of PM than the general public.
The type and prognosis of disease affected consumers' familiarity with and willingness-to-pay for PM treatments
Personalized medicine testing
Consumers with private insurance or Medicare coverage displayed a greater interest in genetic tests
Footnotes
Financial & competing interests disclosure: This research was partly funded by a National Institutes of Health grant (Grant No. R01HG007063) to Kathryn Phillips The authors are grateful for contributions from collaborators at the University of California, San Francisco, Center for Translational and Policy Research on Personalized Medicine. Deborah Marshall receives salary support via her positions as a Canada Research Chair in Health Systems and Services Research, and Arthur JE Child Chair in Rheumatology. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed
No writing assistance was utilized in the production of this manuscript
Ethical conduct of research: The authors state that they have obtained appropriate institutional review board approval or have followed the principles outlined in the Declaration of Helsinki for all human or animal experimental investigations. In addition, for investigations involving human subjects, informed consent has been obtained from the participants involved
References
Papers of special note have been highlighted as:
• of interest; •• of considerable interest
- 1.Hamburg MA, Collins FS. The path to personalized medicine. N Engl J Med. 2010;363(4):301–304. doi: 10.1056/NEJMp1006304. [DOI] [PubMed] [Google Scholar]
- 2•.Ginsburg GS, Willard HF. Genomic and personalized medicine: foundations and applications. Transl Res. 2009;154(6):277–287. doi: 10.1016/j.trsl.2009.09.005. A review of genomic information tools with the focus on enabling a paradigm shift to a comprehensive approach that will identify individual risks and guide clinical management and decision making. The article outlines the challenges from both a scientific and a policy perspective to personalized healthcare. [DOI] [PubMed] [Google Scholar]
- 3.Chen S, Parmigiani G. Meta-analysis of BRCA1 and BRCA2 penetrance. J Clin Oncol. 2007;25(11):1329–1333. doi: 10.1200/JCO.2006.09.1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baker L, Bundorf M, Singer S, Wagner T. Networks' Panel and Sampling. Stanford University Press; Stanford, CA: 2003. Validity of the Survey of Health and the Internet, and Knowledge. [Google Scholar]
- 5.Baker L, Wagner TH, Singer S, Bundorf MK. Use of the Internet and e-mail for healthcare information: results from a national survey. JAMA. 2003;289(18):2400–2406. doi: 10.1001/jama.289.18.2400. [DOI] [PubMed] [Google Scholar]
- 6.Baker R, Blumberg S, Brick J, et al. Research synthesis: AAPOR Report on Online Panels. Public Opin Q. 2010;74(4):711–781. [Google Scholar]
- 7.Smith T. An experimental comparison of Knowledge Networks and the GSS. Int J Public Opin Res. 2003;15(2):167. [Google Scholar]
- 8.Food and Drug Administration: Companion Diagnostic Devices. www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/InVitroDiagnostics/ucm301431.htm.
- 9.Food and Drug Administration: Table of Pharmacogenomic Biomarkers in Drug Labeling. www.fda.gov/drugs/scienceresearch/researchareas/pharmacogenetics/ucm083378.htm.
- 10.Bombard Y, Rozmovits L, Trudeau ME, Leighl NB, Deal K, Marshall DA. Patients' perceptions of gene expression profiling in breast cancer treatment decisions. Curr Oncol. 2014;21(2):e203–e211. doi: 10.3747/co.21.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bombard Y, Rozmovits L, Trudeau M, Leighl NB, Deal K, Marshall DA. Access to personalized medicine: factors influencing the use and value of gene expression profiling in breast cancer treatment. Curr Oncol. 2014;21(3):e426–e433. doi: 10.3747/co.21.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.U.S. Public Opinion about Personalized Medicine. www.personalizedmedicinecoalition.org/Resources/PMC__Public_Opinion_Survey.
- 13•.Schmidlen TJ, Wawak L, Kasper R, Garcia-Espana JF, Christman MF, Gordon ES. Personalized genomic results: analysis of informational needs. J Genet Counsel. 2014;23(4):578–587. doi: 10.1007/s10897-014-9693-8. The article provides results from a retrospective qualitative study of notes from 157 genetic counseling inquiries. Participants appeared to recognize the multifactorial nature of the diseases for which results were provided; however education to understand the complexities of genomic risk information was often needed. [DOI] [PubMed] [Google Scholar]
- 14••.Dressler LG, Jones SS, Markey JM, Byerly KW, Roberts MC. Genomics education for the public: perspectives of genomic researchers and ELSI advisors. Genet Test Mol Biomarkers. 2014;18(3):131–140. doi: 10.1089/gtmb.2013.0366. The article presents results from a semi-structured telephone interview study conducted with researchers and advisors associated with the SNP/HAPMAP studies and the Cancer Genome Atlas Study. Respondents described approach(es) associated with educating the public about their study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldsmith L, Jackson L, O'connor A, Skirton H. Direct-to-consumer genomic testing: systematic review of the literature on user perspectives. Eur J Hum Genet. 2012;20(8):811–816. doi: 10.1038/ejhg.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16•.Boeldt DL, Schork NJ, Topol EJ, Bloss CS. Influence of individual differences in disease perception on consumer response to direct-to-consumer genomic testing. Clin Genet. 2014 doi: 10.1111/cge.12419. (Epub ahead of print). A study on a total of 2037 participants whom received DTC genomic testing and completed baseline and follow-up surveys assessing disease perceptions and health behaviors. Findings may inform development of educational and counseling services. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Claxton G, Rae M, Panchal N, et al. 2013 Employer Health Benefits Survey. Health Aff (Millwood) 2013;32(9):1667–1676. doi: 10.1377/hlthaff.2013.0644. [DOI] [PubMed] [Google Scholar]
- 18.Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health--a checklist: a report of the ISPOR Good Research Practices for Conjoint Analysis Task Force. Value Health. 2011;14(4):403–413. doi: 10.1016/j.jval.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 19.Bridges JF. Stated preference methods in healthcare evaluation: an emerging methodological paradigm in health economics. Appl Health Econ Health Policy. 2003;2(4):213–224. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.