Abstract
The mushroom bodies in the insect brain serve as a central information processing area. Here, focusing mainly on olfaction, we discuss functionally related roles the mushroom bodies play in signal gain control, response sparsening, the separation of similar signals (decorrelation), and learning and memory. In sum, the mushroom bodies assemble and format a context-appropriate representation of the insect’s world.
Keywords: olfaction, sensory, insect, Drosophila, locust, moth, synchrony, oscillation
1. Introduction
The mushroom bodies are striking in appearance, resembling bilaterally arranged cups brimming with tiny neurons, supported by stems that bend and branch in several directions dorsally and laterally. The tiny neurons, Kenyon cells, (KCs) send long thin processes down through the stems, which form distinct lobes. These prominent and complex structures, found in all but the earliest insects, are as interesting as they look – they serve a number of functions important for processing sensory information. In many insects, groups of KCs receive sensory information from visual, gustatory, and mechanosensory areas, and, perhaps most often studied, thick tracts of olfactory input from the antennal lobes[1, 2]. In honeybees and other insects, different populations of KCs appear to receive direct input from different sensory modalities, although some KCs may also be multimodal [3]. The KCs also receive inhibitory and recurrent input, and neuromodulators such as dopamine that provide reward signals[4]. Together, these inputs endow the mushroom bodies with information processing powers that are gradually coming to light. Here, focusing mainly on olfaction, I discuss functionally related roles the mushroom bodies appear to play in signal gain control, response sparsening, the separation of similar signals (decorrelation), and learning and memory.
2. Gain control
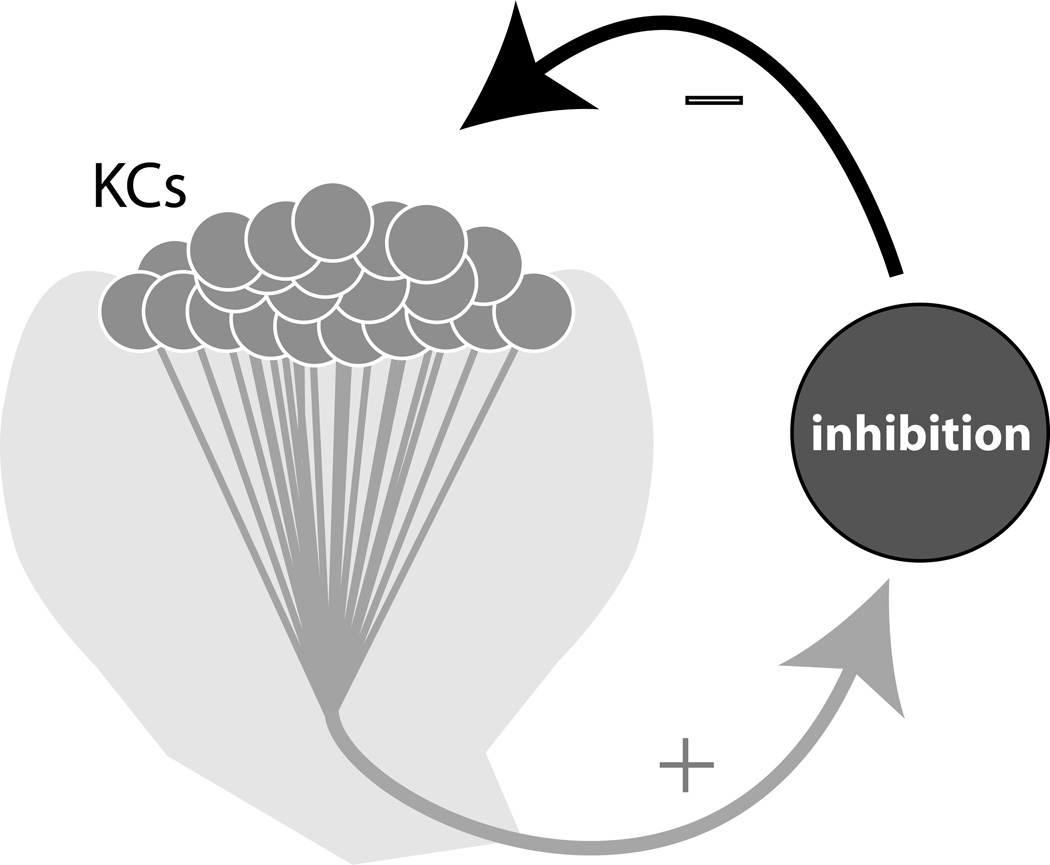
Sensory stimuli can be weak or strong, and sensory systems need to accommodate this dynamic range. In several insect species the mushroom body’s KCs have been found to form feedback connections with powerful inhibitory neurons that may help contain responses to sensory stimulus within limits (Figure 1). The anatomy of feedback connectivity provides a hint that any increase in the output of KCs will be tamped down by inhibition that increases proportionally with the response of the KCs, and is reflected back to them by the inhibitory cells [5]. In fact, in locusts, a singular giant GABAergic neuron (GGN) appears to play precisely this role. GGN is anatomically positioned to receive input from, and provide output to, KCs. Intracellular electrophysiological measurements show GGN depolarizes in response to all tested odors; artificially depolarizing it reduces the responsiveness of every tested KC and effectively silences lobe neurons that receive inputs from KCs [6]. Thus, GGN appears to receive input from all KCs, and, in turn, provide inhibitory output to all KCs. GGN itself appears to be regulated by another inhibitory neuron, IG. Other insects also have GABAergic neurons that seem similar to GGN; for example, in Drosophila, genetic manipulations of activity and calcium recordings have shown that a neuron called APL similarly regulates KCs [7]. The mushroom body circuitry comprising these inhibitory neurons and KCs together regulates the excitability of the KCs, allowing them to respond with appropriate amounts of spiking to a wide dynamic range of sensory signals arriving from the antennal lobe and perhaps elsewhere.
Figure 1.
Gain control in the Mushroom bodies. In several insect species, singular giant inhibitory neurons, or groups of smaller inhibitory neurons, have been shown to receive output from all Kenyon cells (KCs) and then feed it back as inhibition to all KCs. This mechanism maintains the activity of KCs within a narrow range.
3. Sparsening and decorrelation
Among the inputs received by KCs are olfactory signals carried by projection neurons from the antennal lobe. Anatomical studies show that each olfactory KC receives input from multiple presynaptic projection neurons [8, 9], and electrophysiological recordings show that the PNs, which are spontaneously active in the absence of stimuli [10], respond to odors with voluble bursts of spikes. Given the sheer number of action potentials arriving at KCs, one might predict these neurons would roil with activity before, during, and after any given olfactory stimulation. Yet, KCs are nearly silent at rest [11];[9];[12]. Further, any given KC responds only to a narrow range of odors or even particular concentrations of those odors [13, 14], and the responses of each KC consist of very few spikes, often only one or two. Thus, the mushroom bodies transform the flood of odor-elicited spikes arriving from PNs into very sparse representations of the odor (Figure 2, top).
Figure 2.
Sparsening and decorrelation. Left: PNs (4 examples shown here) are spontaneously active and respond to odors with bursts of temporally-patterned spikes. Different odors (light gray bar at left, dark gray bar at right) elicit different patterns of activity. The responses of the PN population can be visualized as clouds of points (here, in a 2-dimensional space). Right: KCs, by contrast, are nearly silent at rest and respond to odors with great specificity, and with only a few spikes. Sparsening and decorrelation mechanisms separate the responses of KCs elicited by different odors, making them easy to distinguish.
Several mechanisms contribute to this sparsening function. One is the gain control effect exerted by giant inhibitory neurons like GGN and APL, which tamps down the excitability of KCs (see Figure 1). A second, demonstrated in the cockroach, is GABAergic inhibition that tonically hyperpolarizes the membrane potential, [15]; the source of this tonic inhibition is uncertain. A third is the oscillatory structure of the spikes arriving from PNs. Owing to reciprocal excitatory and inhibitory circuitry in the antennal lobe, PNs are excited by repeatedly-encountered odors to oscillatory synchronization of their spiking (locusts: [16]; bees: [17]; moths: [18]; flies: [19]); each cycle consists of a burst of spikes alternating with a period of relative quiescence. Thus, KCs receive an extra measure of excitatory input from PNs during a small cyclic interval of each odor-elicited response. The contribution of feedback inhibition to sparsening is magnified by the oscillatory responses as each pulse of excitation arising from KCs is reflected back after a brief delay as a pulse of inhibition. This leaves KCs free to spike only during the brief depolarized “integration window” occurring between consecutive waves of inhibition [20]. Evidence from physiology experiments in locusts and computational models suggests that the duration of the integration window can vary with the intensity of the input from PNs: more intense input causes GGN to respond earlier in each oscillatory cycle, thus shortening the integration window [21, 22]. This mechanism helps maintain the sparseness of responses in KCs regardless of input intensity.
In addition to these circuit mechanisms, intrinsic properties of KCs also favor sparse responses. In cockroaches, whole-cell electrophysiological recordings from KCs have revealed two unusual conductances that promote sparseness: an inward calcium conductance with a very low activation threshold; and an outward potassium conductance with an unusually depolarized response threshold. Together, these conductances appear to support sparseness by amplifying only the strongest inputs, and by causing the neuron to rapidly adapt to spiking [15]. The effects of intrinsic properties like these had been characterized earlier with sharp-electrode recordings from locusts, where KCs were shown to have highly non-linear voltage dependent responses to gradually increasing electrical activation of the antennal lobe [23, 24].
Combining the contributions in the Mushroom body of inhibitory gain control, tonic inhibition, oscillatory activation, and the intrinsic properties of KCs, one arrives at a circuit ideal for detecting coincident inputs from the population of olfactory projection neurons. A given KC receives input from a subset of the full PN population. Because of their coincidence detection properties, KCs will only spike when this subset of PNs fires simultaneously (that is, within the same integration window). Since these subsets of simultaneously spiking PNs change with the eliciting odor and the concentration of the odor, different KCs will respond to each stimulus. In locusts, this arrangement has been shown to be ideal for the efficient representation of high dimensional stimuli like odors [8]. More generally, sparse coding has been proposed to offer a number of computational benefits. These include decreasing the number of synapses that need to be altered or accessed during memory tasks, and increasing the separation between responses evoked by similar stimuli. This later effect increases the coding capacity of the neural population and makes it easier to distinguish one stimulus from another [25].
Similar stimuli (for example, odorants with similar molecular structures) often evoke similar responses from front-line sensory structures like populations of olfactory receptor neurons; these similar population responses are said to be highly correlated with one another. In both insects [25] and vertebrates [26], olfactory circuitry in the antennal lobe and olfactory bulb, critically including inhibition, begins the process of creating differences between the neural responses of similar stimuli. In the insect, this decorrelation process continues and perhaps reaches its apex in the mushroom bodies, where the sparse responses of KCs to different odors are widely separated (Figure 2, bottom). In Drosophila, decorrelating contributions of the inhibitory feedback neuron APL has been shown, through behavioral tasks, to be essential for maintaining the specificity of olfactory memories [7].
4. Learning and memory
The mushroom bodies have long been associated with learning and memory. In numerous insect species, the volume of the mushroom body calyx has been shown to increase with sensory experience, not just with age (see [27] for several examples). Retrograde amnesia following olfactory training was induced in honeybees by specifically cooling the mushroom bodies [28], and honeybees treated early in life to develop without full mushroom bodies behaved quite normally as adults, but were deficient in learning a classical conditioning olfactory task [29]. In Drosophila, mutants lacking normal mushroom body structures showed visual and olfactory learning deficits [30, 31, 32].
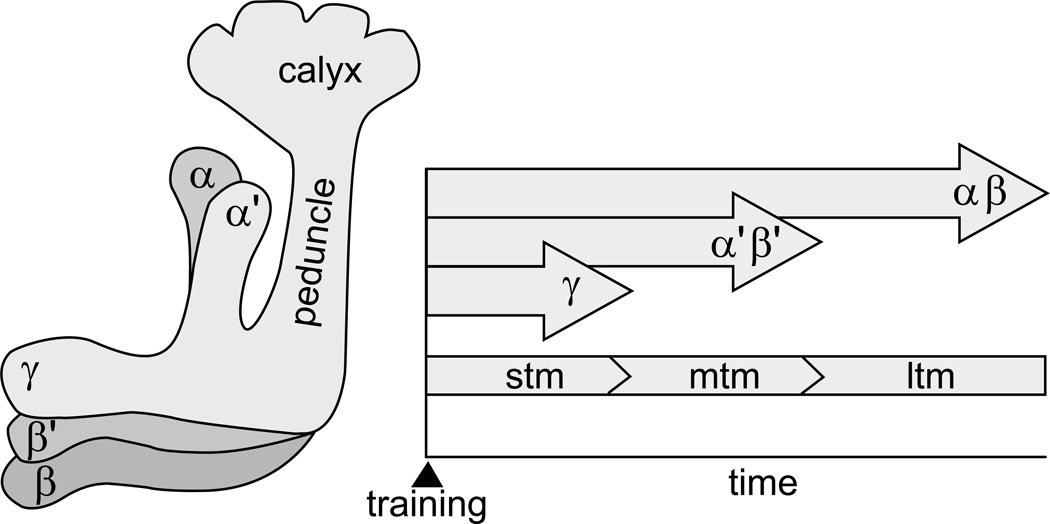
The organization of the mushroom bodies has been a topic of great interest [33, 34, 35, 36]. Recently, highly specific genetic manipulations in Drosophila have given rise to a more nuanced view of the mushroom bodies, assigning specific roles to different populations of neurons and to specific neurons, and to the molecular cascades operating within them. Functional analyses show that different anatomical areas each process different aspects of learning and memory [37]. In the fly, three groups of KCs, γ, α’/β’, and α/β, have been distinguished based upon the mushroom body lobes innervated by their output fibers. The γ KCs appear to process short-term memory, perhaps detecting the coincidence of conditioned and unconditioned stimuli; the α’/β’ KCs are needed for consolidating memories; and the α/β KCs for the retrieval, or expression of memory after training is complete [38]. Thus, different lobes contribute at different times to learning and memory tasks. Surprisingly, but consistent with this analysis, genetic dissections of the plasticity process, focusing specifically on the roles of the APL neuron, show that, training can produce long-term memories can emerge even when the formation of shorter-term memories are prevented [39].
In addition to receiving sensory information, specific regions of each mushroom body lobe receive separate streams of dopaminergic innervation that appear establish different sub-areas specialized for appetitive or aversive processing. All of these KCs respond by spiking when odors are presented, raising the interesting possibility that these separate areas may perform their information processing tasks in parallel (Figure 3). Thus, different populations of KCs may simultaneously assign different meanings to a given odor, with the animal’s behavioral choices determined by plasticity-dependent adjustments to the balance of activity [40].
Figure 3.
Learning and memory. Left: In Drosophila, the processes of KCs extend from their bodies in the calyx down through the peduncle into the α, α’, β, β’, and γ lobes. These lobes appear to play different roles in the formation, storage, and recall of memories. Right: Short-term memory (stm), medium-term memory (mtm) and long-term memory (ltm) are processed in the γ, α’/β’, and α/β lobes, respectively. An interesting possibility is that these three forms of memory are processed in parallel (see text).
5. Reading the output of KCs
The processing taking place within KCs exerts its effects upon follower cells. In Drosophila, most of the neurons following from KCs have been identified and mapped [41, 42], and their contributions to mushroom body function no doubt will soon be revealed. To date, though, work in other insects has provided interesting clues about how the output of KCs is processed by follower neurons. Recent work in the locust shows that the precise timing of the very sparse spikes elicited by odors in KCs carries information about the odor, and indeed shapes the responses of follower neurons in the β-lobe [43]. Experiments performed in moths show that, during training trials that lead to effective conditioning, the extremely sparse spiking in KCs elicited by odors can end seconds before reinforcement stimuli are delivered [18], raising the question of how odor and reinforcement stimuli are associated. Experiments performed in the locust suggest a mechanism to bridge the temporal gap: spike-timing dependent plasticity (STDP) occurring at synapses connecting KCs with follower neurons in the β-lobe serves to “tag” activated synapses, and a subsequent pulse of reinforcement-elicited octopamine can then act specifically at tagged synapses to modify their firing [44, 45]. These results show that central processing in the mushroom bodies only begins with stimulus-elicited activity in KCs.
6. Conclusions: What the mushroom body is for
The mushroom body serves as a central processing unit within the insect brain. It receives sensory input from multiple modalities as well as modulatory signals reflecting internal state and external reward conditions; these modulators alter the properties and responses of the mushroom body’s KCs and other neurons. The mushroom body combines and reformats the information it receives, projecting its volubly spiking input into sparse and well-separated representations. It excels at detecting coincidence: the synchronized spiking of groups of olfactory projection neurons; the meaningful coactivity of conditioned and unconditioned stimulus pathways. It divides its input into multiple streams, each processed to efficiently compare and store information for a moment or for the duration of the animal’s life, and sends its output onward through its lobes and beyond to generate behaviors. Altogether, it assembles and formats a representation of the insect’s world.
Highlights.
The mushroom bodies integrate and format sensory information.
Mushroom body information processing functions include gain control, response sparsening, decorrelation, and learning and memory
The mushroom bodies assemble and format a representation of the insect’s world
Acknowledgement
Many thanks to Dr. Kazumichi Shimizu for providing helpful comments on the manuscript.
Abbreviations
- APL
anterior paired lateral neuron
- GABA
γ-aminobutyric acid
- GGN
Giant GABAergic Neuron
- IG
inhibitor of GGN
- KC
Kenyon cell
- PN
projection neuron
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zars T, et al. Localization of a short-term memory in Drosophila. Science. 2000;288(5466):672–675. doi: 10.1126/science.288.5466.672. [DOI] [PubMed] [Google Scholar]
- 2.Masek P, Scott K. Limited taste discrimination in Drosophila. Proc Natl Acad Sci U S A. 2010;107(33):14833–14838. doi: 10.1073/pnas.1009318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kirkhart K, Scott K. Gustatory learning and processing in the Drosophila mushroom bodies. Journal of Neuroscience. doi: 10.1523/JNEUROSCI.3930-14.2015. in review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Claridge-Chang A, et al. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139(2):405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kee T, Sanda P, Gupta N, Stopfer M, Bazhenov M. Feed-forward versus feedback inhibition in basic olfactory circuit. doi: 10.1371/journal.pcbi.1004531. in preparation. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Papadopoulou M, et al. Normalization for sparse encoding of odors by a wide-field interneuron. Science. 2011;332(6030):721–725. doi: 10.1126/science.1201835. Characterizes a giant GABAergic neuron (GGN) in locusts that contributes to gain control and sparsening in the mushroom bodies.
- 7. Lin AC, et al. Sparse, decorrelated odor coding in the mushroom body enhances learned odor discrimination. Nat Neurosci. 2014;17(4):559–568. doi: 10.1038/nn.3660. Demonstrates that APL, a giant GABAergic neuron in Drosophila, sparsens the olfactory responses of KCs and helps to decorrelate their responses.
- 8.Jortner RA, Farivar SS, Laurent G. A simple connectivity scheme for sparse coding in an olfactory system. J Neurosci. 2007;27(7):1659–1669. doi: 10.1523/JNEUROSCI.4171-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Turner GC, Bazhenov M, Laurent G. Olfactory representations by Drosophila mushroom body neurons. J Neurophysiol. 2008;99(2):734–746. doi: 10.1152/jn.01283.2007. [DOI] [PubMed] [Google Scholar]
- 10.Joseph J, Dunn FA, Stopfer M. Spontaneous olfactory receptor neuron activity determines follower cell response properties. J Neurosci. 2012;32(8):2900–2910. doi: 10.1523/JNEUROSCI.4207-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Laurent G, Naraghi M. Odorant-induced oscillations in the mushroom bodies of the locust. J Neurosci. 1994;14(5 Pt 2):2993–3004. doi: 10.1523/JNEUROSCI.14-05-02993.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Campbell RA, et al. Imaging a population code for odor identity in the Drosophila mushroom body. J Neurosci. 2013;33(25):10568–10581. doi: 10.1523/JNEUROSCI.0682-12.2013. With wide-field calcium imaging techniques, demonstrates that information about odors is distributed across Kenyon cells in Drosophila.
- 13.Stopfer M, Jayaraman V, Laurent G. Intensity versus identity coding in an olfactory system. Neuron. 2003;39(6):991–1004. doi: 10.1016/j.neuron.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Gruntman E, Turner GC. Integration of the olfactory code across dendritic claws of single mushroom body neurons. Nat Neurosci. 2013;16(12):1821–1829. doi: 10.1038/nn.3547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demmer H, Kloppenburg P. Intrinsic membrane properties and inhibitory synaptic input of kenyon cells as mechanisms for sparse coding? J Neurophysiol. 2009;102(3):1538–1550. doi: 10.1152/jn.00183.2009. [DOI] [PubMed] [Google Scholar]
- 16.Laurent G, Davidowitz H. Encoding of olfactory information with oscillating neural assemblies. Science. 1994;265(5180):1872–1875. doi: 10.1126/science.265.5180.1872. [DOI] [PubMed] [Google Scholar]
- 17.Stopfer M, et al. Impaired odour discrimination on desynchronization of odour-encoding neural assemblies. Nature. 1997;390(6655):70–74. doi: 10.1038/36335. [DOI] [PubMed] [Google Scholar]
- 18.Ito I, et al. Frequency transitions in odor-evoked neural oscillations. Neuron. 2009;64(5):692–706. doi: 10.1016/j.neuron.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka NK, Ito K, Stopfer M. Odor-evoked neural oscillations in Drosophila are mediated by widely branching interneurons. J Neurosci. 2009;29(26):8595–8603. doi: 10.1523/JNEUROSCI.1455-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta N, Stopfer M. Oscillatory integration windows in olfactory neurons. doi: 10.1038/ncomms13808. in revision. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Assisi C, et al. Adaptive regulation of sparseness by feedforward inhibition. Nat Neurosci. 2007;10(9):1176–1184. doi: 10.1038/nn1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta N, Stopfer M. Functional analysis of a higher olfactory center, the lateral horn. J Neurosci. 2012;32(24):8138–8148. doi: 10.1523/JNEUROSCI.1066-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perez-Orive J, et al. Oscillations and sparsening of odor representations in the mushroom body. Science. 2002;297(5580):359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- 24.Perez-Orive J, Bazhenov M, Laurent G. Intrinsic and circuit properties favor coincidence detection for decoding oscillatory input. J Neurosci. 2004;24(26):6037–6047. doi: 10.1523/JNEUROSCI.1084-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laurent G. Olfactory network dynamics and the coding of multidimensional signals. Nat Rev Neurosci. 2002;3(11):884–895. doi: 10.1038/nrn964. [DOI] [PubMed] [Google Scholar]
- 26.Friedrich RW, Laurent G. Dynamic optimization of odor representations by slow temporal patterning of mitral cell activity. Science. 2001;291(5505):889–894. doi: 10.1126/science.291.5505.889. [DOI] [PubMed] [Google Scholar]
- 27.Menzel R. The insect mushroom body, an experience-dependent recoding device. J Physiol Paris. 2014 doi: 10.1016/j.jphysparis.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 28.Menzel R, Erber J, Masuhr T. Learning and memory in the honeybee. In: Barton-Browne L, editor. Experimental Analysis of Insect Behavior. Berlin: Springer; 1974. pp. 195–217. [Google Scholar]
- 29.Malun D, et al. Hydroxyurea-induced partial mushroom body ablation in the honeybee Apis mellifera: volumetric analysis and quantitative protein determination. J Neurobiol. 2002;50(1):31–44. doi: 10.1002/neu.10015. [DOI] [PubMed] [Google Scholar]
- 30.Liu L, et al. Context generalization in Drosophila visual learning requires the mushroom bodies. Nature. 1999;400(6746):753–756. doi: 10.1038/23456. [DOI] [PubMed] [Google Scholar]
- 31.Heisenberg M, et al. Drosophila mushroom body mutants are deficient in olfactory learning. J Neurogenet. 1985;2(1):1–30. doi: 10.3109/01677068509100140. [DOI] [PubMed] [Google Scholar]
- 32.de Belle JS, Heisenberg M. Associative odor learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263(5147):692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- 33.Strausfeld NJ, Sinakevitch I, Vilinsky I. The mushroom bodies of Drosophila melanogaster: an immunocytological and golgi study of Kenyon cell organization in the calyces and lobes. Microsc Res Tech. 2003;62(2):151–169. doi: 10.1002/jemt.10368. [DOI] [PubMed] [Google Scholar]
- 34.Strausfeld NJ. Organization of the honey bee mushroom body: representation of the calyx within the vertical and gamma lobes. J Comp Neurol. 2002;450(1):4–33. doi: 10.1002/cne.10285. [DOI] [PubMed] [Google Scholar]
- 35.Fahrbach SE. Structure of the mushroom bodies of the insect brain. Annu Rev Entomol. 2006;51:209–232. doi: 10.1146/annurev.ento.51.110104.150954. [DOI] [PubMed] [Google Scholar]
- 36.Murthy M, Fiete I, Laurent G. Testing odor response stereotypy in the Drosophila mushroom body. Neuron. 2008;59(6):1009–1023. doi: 10.1016/j.neuron.2008.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Krashes MJ, et al. Sequential use of mushroom body neuron subsets during Drosophila odor memory processing. Neuron. 2007;53(1):103–115. doi: 10.1016/j.neuron.2006.11.021. Provides an elegant demonstration of how mnemonic information transits through the mushroom body in Drosophila.
- 38.Guven-Ozkan T, Davis RL. Functional neuroanatomy of Drosophila olfactory memory formation. Learn Mem. 2014;21(10):519–526. doi: 10.1101/lm.034363.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pitman JL, et al. A pair of inhibitory neurons are required to sustain labile memory in the Drosophila mushroom body. Curr Biol. 2011;21(10):855–861. doi: 10.1016/j.cub.2011.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perisse E, et al. Different Kenyon cell populations drive learned approach and avoidance in Drosophila. Neuron. 2013;79(5):945–956. doi: 10.1016/j.neuron.2013.07.045. Describes a variety of groups of Kenyon cells, suggesting that different groups serve different functions.
- 41.Tanaka NK, Tanimoto H, Ito K. Neuronal assemblies of the Drosophila mushroom body. J Comp Neurol. 2008;508(5):711–755. doi: 10.1002/cne.21692. [DOI] [PubMed] [Google Scholar]
- 42.Aso submitted. [Google Scholar]
- 43.Gupta N, Stopfer M. A temporal channel for information in sparse sensory coding. Curr Biol. 2014;24(19):2247–2256. doi: 10.1016/j.cub.2014.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cassenaer S, Laurent G. Hebbian STDP in mushroom bodies facilitates the synchronous flow of olfactory information in locusts. Nature. 2007;448(7154):709–713. doi: 10.1038/nature05973. [DOI] [PubMed] [Google Scholar]
- 45.Cassenaer S, Laurent G. Conditional modulation of spike-timing-dependent plasticity for olfactory learning. Nature. 2012;482(7383):47–52. doi: 10.1038/nature10776. [DOI] [PubMed] [Google Scholar]