Abstract
Recently we showed that the fused chorismate-utilizing enzyme from the antibiotic-producing soil bacterium Streptomyces venezuelae is an anthranilate synthase (designated SvAS), not a 2-amino-2-deoxyisochorismate (ADIC) synthase, as was predicted based on its amino acid sequence similarity to the phenazine biosynthetic enzyme PhzE (an ADIC synthase). Here we report the characterization of SvAS using steady-state kinetics, gel filtration chromatography and laser light scattering. The recombinant His-tagged enzyme has Michaelis constants Km with respect to substrates chorismate and glutamine of 8.2 ± 0.2 μM and 0.84 ± 0.05 mM, respectively, and a catalytic rate constant kcat of 0.57 ± 0.02 s−1 at 30°C. Unlike most other anthranilate synthases, SvAS does not utilize ammonia as a substrate. The enzyme is competitively but noncooperatively inhibited by tryptophan (Ki = 11.1 ± 0.1 μM) and is active as a monomer. The finding that SvAS is a monomer jibes with the variety of association modes that have been observed for anthranilate synthases from different microorganisms, and it identifies the enzyme’s minimal functional unit as a single TrpE-TrpG pair.
Keywords: anthranilate synthase, chorismate-utilizing enzyme, fused enzyme, Streptomyces venezuelae
Introduction
The chorismate-utilizing enzyme anthranilate synthase [AS; E.C. 4.1.3.27] catalyzes the first committed step of the tryptophan biosynthetic pathway, i.e., the conversion of chorismate to anthranilate. The enzyme is feedback inhibited by tryptophan in a manner that is competitive with respect to substrate chorismate, and its reaction (Fig. 1) occurs in two steps. The first step involves the transfer of ammonia from glutamine bound to a glutamine amidotransferase (TrpG) subunit (or domain) to chorismate, which is bound to an anthranilate synthase (TrpE) subunit (or domain). Concomitantly, a hydroxyl group is lost from chorismate, producing the intermediate 2-amino-2-deoxyisochorismate (ADIC). The second step utilizes an ADIC lyase activity at the TrpE site to remove a pyruvate group (and a proton) from ADIC, generating the fluorescent compound anthranilate. A magnesium ion (Mg2+) is required for catalysis. All of the AS enzymes characterized so far can use free ammonia (in lieu of glutamine) as substrate.
Fig. 1.
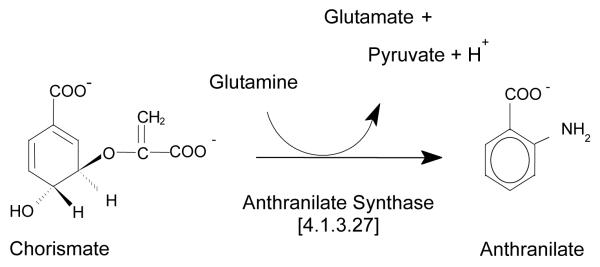
The Anthranilate Synthase-Catalyzed Reaction
In many cases, AS functions as a TrpE2TrpG2 heterotetramer made up of separate (non-fused) TrpE and TrpG subunits. On the other hand, in some bacterial species, including Bacillus subtilis [1] and other members of the genus Bacillus [2], as well as in Acinetobacter calcoaceticus [3] and Pseudomonas putida [4], the enzyme is active as a TrpE-TrpG dimer. For some of these enzymes, intra-generic and even inter-generic hybrid dimeric TrpE-TrpG complexes can be formed [2, 5]. It has been proposed that association and disassociation of the complexes can regulate enzyme activity in Bacillus [6]. Finally, in some bacteria, namely Azospirillum brasilense [7], Rhizobium meliloti [8] and Streptomyces venezuelae [9], putative AS enzymes made of a single type of polypeptide chain having fused TrpE and TrpG domains connected by a linker are present (designated TrpEG). It is the fused TrpEG enzyme from S. venezuelae that is the subject of this study.
Anthranilate synthases from a number of microorganisms, including the mesophilic bacteria Escherichia coli [10], Salmonella enterica (or typhimurium) [11, 12, 13], and Serratia marcescens [14, 15], as well as the hyperthermophilic archaeons Sulfolobus solfataricus [16] and Archaeoglobus fulgidus [17], have been characterized in terms of their kinetic, regulatory and physicochemical properties. X-ray structures of anthanilate synthases from S. enterica [18], S. marcescens [19] and S. solfataricus [20]—all of which are tetrameric—have been reported. These structures reveal different quaternary arrangements of subunits for the first two enzymes (SeAS and SmAS) versus the third (SsAS). Inhibition by tryptophan is cooperative for SeAS and SmAS, but noncooperative for SsAS and A. fulgidus AS. The tryptophan site is located within the TrpE subunit, and tryptophan will inhibit the ammonia-dependent reaction of the isolated subunit, but inhibition is noncooperative [21]. In SmAS and SsAS, the tryptophan site was shown to be approximately 20 Å from the chorismate site [19, 20].
Previous work in our laboratory demonstrated that the fused trpEG gene from Streptomyces venezuelae encodes an AS (designated SvAS) [22], not an ADIC synthase as was initially surmised based on its amino acid sequence similarity to the Pseudomonas phenazine biosynthetic enzyme PhzE [23], which is an ADIC synthase (Tin-Wein Yu, personal communication). The goal of the current study was to characterize SvAS in terms of its steady-state kinetic and regulatory properties and its oligomeric state. Our results show that the chorismate-dependent activity of the enzyme is competitively and noncooperatively inhibited by tryptophan. Unexpectedly, SvAS functions as a monomer and is not able to use free ammonia as substrate.
Materials and Methods
Materials
The culture medium components Bacto-Tryptone, Bacto-Agar, Bacto-Yeast Extract were from Dickinson and Co. (Sparks, MD). Streptomycin, ampicillin, chloramphenicol, isopropopyl-β-d-1-thiogalactopyranoside (IPTG), phenylmethylsulfonyl fluoride (PMSF), dithiothreitol (DTT), ethylenediaminetetraacetic acid (EDTA), l-Glutamine, l-Tryptophan, d-Sorbitol, and Betaine were all from Sigma-Aldrich (St. Louis, MO).
Chorismate Preparation
Chorismate was prepared in-house as described [24, 25]. E. coli KA12 cells, which contain a mutation in the chorismate mutase gene and are engineered to overproduce chorismate, which is secreted into the culture medium, were grown in undefined medium for 7-10 h. The cells were then transferred to accumulation medium and grown for an additional 16-17 h in order to overproduce chorismate. The cell suspension was centrifuged (5000 × g for 13 min) to remove the cells. The supernatant was then processed for isolation and purification of chorismate as described [24, 25]. The final chorismic acid preparation, which was >99% pure, was stored at −80°C.
Protein Expression and Purification
To express the recombinant His-tagged SvAS, BL21(DE3)pLysS cells (Novagen) containing the pET21b/SvTrpEG vector [22] were cultured in LBBS (LB containing 2.5 mM Betaine and 1 M d-sorbitol) media containing 100 μg/ml ampicillin and 34 μg/ml chloramphenicol. Cultures were grown at 37°C until OD600 reached 0.6, and were then moved to 20°C, induced with 0.3 mM IPTG, and grown for an additional 16 h at 20°C. Cells were pelleted and stored at −20°C overnight.
The frozen pellets were thawed and resuspended in lysis buffer (100 mM Tris - HCl, pH 8.0, 10% glycerol, 1mM EDTA, 1mM DTT, 1mM PMSF and 15mM imidazole) and lysed by sonication. The lysate was centrifuged at 11,000 rpm for 20 min at 4°C and the clear supernatant loaded onto a Ni-NTA spin column (Qiagen, Valencia, CA) that had been equilibrated with binding buffer (50mM Tris-HCl, pH 7.5, 300 mM NaCl, 5 mM ß-mercaptoethanol, 20 mM imidazole and 5% glycerol). The column was washed sequentially with column buffer (50 mM Tris –HCl, pH 7.5, 300 mM NaCl, 5 mM ß-mercaptoethanol and 5% glycerol) containing 30 mM and 100 mM imidazole to remove the more weakly-bound proteins. Tightly-bound His-tagged SvAS was eluted with column buffer containing 250 mM imidazole. A PD-10 desalting column (GE Healthcare) was used to remove imidazole, and an Amicon ultracentrifugal filter (15 ml volume, 10 kDa molecular weight cut off, Millipore, Billerica, MA) was used to concentrate the purified protein for storing at −20°C in 50% glycerol. Protein was determined using the Bio-Rad assay (Bio-Rad, Hercules, CA) with bovine serum albumin as the standard.
Enzyme Activity Assay and Steady-State Kinetics Analysis
The standard 2-ml activity assay contained chorismate (200 μM), glutamine (20 mM), MgCl2 (12.5 mM) and SvTrpEG enzyme (10 μg) in 50 mM Tricine buffered at pH 7.5. To measure ammonia-dependent activity, the pH was increased to pH 8.5. A Fluoromax-3 photon-counting fluorescence spectrometer (Jobin-Yvonne) was used for recording production of anthranilate over time. Excitation and emission wavelengths were 315 nm and 395 nm, respectively (slit widths 2 nm). A standard curve ([anthranilate] vs. fluorescence intensity) was constructed using standards of known concentration; this allowed conversion of fluorescence intensity to anthranilate concentration. Initial velocity experiments were performed by measuring activity at increasing concentrations of one substrate while keeping the other constant at a saturating level. Steady-state kinetic constants (Km, Vmax) were obtained by fitting initial velocity data to the Michaelis-Menten equation using SigmaPlot (Systat, Inc.). The kcat value (s−1) was calculated from the average of the Vmax values (nmol/min/mg) obtained from chorismate and glutamine saturation curves; a molecular mass of 67 kDa for SvAS was used. To determine the optimal pH, activity was measured at 30°C in Tricine solutions buffered at pH-values ranging from 6 to 9.0 (0.5-unit increments). To determine the effect of temperature on activity, standard assays were carried out at temperatures ranging from 15°C to 55°C.
Tryptophan Inhibition
For determination of the tryptophan inhibition constant (Ki), initial velocity experiments were carried out as described above, but in the presence of one of four different tryptophan concentrations: 2.5, 5, 10, or 20 μM. Chorismate concentrations ranging from 0.39 to 100 μM were used, and glutamine was kept constant at a saturating level (20 mM). Lineweaver-Burk analysis (1/Vi versus 1/[chorismate]) of the data allowed a determination of inhibition type (competitive, noncompetitive, mixed). Fitting the data to the equation for type of inhibition (competitive in this case) allowed determination of the Ki-value. The SigmaPlot enzyme kinetics module was used.
Gel Filtration Chromatography and Laser Light Scattering
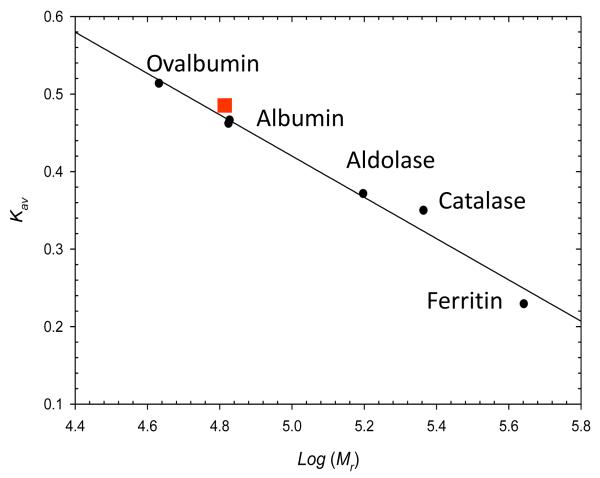
A Sehpacryl S-300 gel filtration column (2.5 × 100 cm) was equilibrated overnight at 4 °C in a solution containing 50 mM Tricine (pH 8.0), 100 mM NaCl, 1 mM EDTA, and 1 mM DTT. The protein sample was concentrated to 1 mg/ml and loaded onto the column at a flow rate of 1.0 mL/min. Elution was carried out at the same flow rate. Fractions (1 ml) were collected, and both absorbance at 280 nm and anthranilate synthase activity were measured. The column was calibrated using the following molecular mass markers: chymotrypsinogen A (25 kDa), ovalbumin (43 kDa), albumin (67 kDa), aldolase (158 kDa), catalase (232 kDa), and ferritin (440 kDa). A standard curve for determining the size of the intact, active SvAS enzyme was constructed by plotting Kav vs. log Mr for the five standard proteins. Here, Kav = (Ve – Vo) / (Vt – Vo), where Vo is the void volume of the column, Vt is the total volume, and Ve is the elution volume; Mr is the molecular mass.
For laser light scattering determination of the solution molecular mass of SvAS, a KW-804 Shodex HPLC size exclusion column (8.0 × 300 mm) connected in-line to light scattering (mini DAWN TREOS, Wyatt Corp., Santa Barbara, CA), refractive index (Opti-Lab rEx; Wyatt Tech Corp., Santa Barbara, CA) and UV detectors was used. The column was equilibrated with (10 mM phosphate buffer, pH 6.5, 100 mM ammonium sulfate, 0.10 mM EDTA, 0.01% sodium azide) and was run isocratically at a flow rate of 0.5 ml/min. Twenty microliters of a 5 mg/ml purified SvAS protein sample were injected for analysis. A refractive index increment of 0.185 mL/g was used to estimate concentrations for molecular weight determination, and bovine serum albumin was used as an isotropic scatterer for detector normalization. Data from the detectors was integrated and analyzed using ASTRA software (Wyatt Corp., Santa Barbara, CA).
Results
Steady State Kinetic Properties and Tryptophan Inhibition
The pH optimum for SvAS (Table 1) was found to be 7.5, a value consistent with those for other anthranilate synthases. With the exception of activity assays that attempted to use ammonia as substrate, for which pH was set at 8.5, all assays were carried out at pH 7.5 (and 30°C). Initial velocity plots with respect to substrates chorismate and glutamine yielded values of Kmchr, Kmgln and kcat (Table 1) that were consistent with those of other anthranilate synthases. The Kmchr obtained was within the range of those for other AS enzymes studied to date (0.9 to 18 μM) [10-17]; the Kmgln value was likewise within the range for other AS enzymes (19 μM to 7.0 mM) [10-17] and was close in value to those for S. enterica and S. marcescens AS, both of which are 0.5 mM [11-15]. Unlike all other anthranilate synthase studied to date, however, free ammonia was found to not be a substrate for SvAS; concentrations higher than 1 M gave no activity.
Table 1.
Steady State Kinetic Properties of Recombinant SvAS
| Kinetic Constanta | Value |
|---|---|
| Kmchorismate (μM) | 8.2 ± 0.2 |
| Kmglutamine (mM) | 0.84 ± 0.05 |
| Kmammonia | not applicable |
| kcat (avg.) at 30°C (s−1) | 0.57 ± 0.02 |
| pH optimum | 7.5 ± 0.1 |
| Temperature of maximal activity | ~45°C |
| Kitryptophan (μM) | 11.1 ± 0.1 (competitive) |
All assays were performed at 30°C unless otherwise indicated. The Km and Vmax values were determined by non-linear regression analysis of initial velocity data using the Michaelis-Menten equation. The kcat value is the average obtained from the saturation curves with respect to both substrates.
The catalytic rate constant kcat of SvAS (Table 1) was closer in value to those for the noncooperative enzymes from the hyperthermophiles S. solfataricus and A. fulgidus (0.14 and 0.25 s−1, respectively; T = 60°C [16-17]) than for the cooperative ones from mesophiles S. enterica and S. marcescens (5 and 10 s−1 at 25°C and 35°C, respectively [11-15]). The temperature dependence of the activity of SvAS was as one would expect for an enzyme from a soil-dwelling mesophile: maximum activity was at 45°C (Table 1), with activity dropping dramatically at temperatures above this value (not shown).
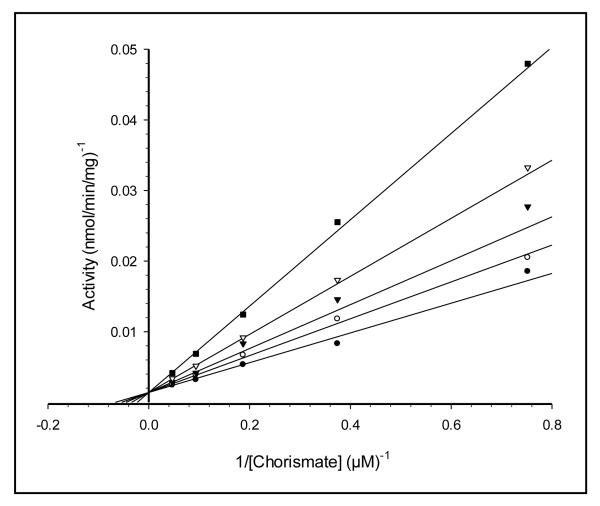
Tryptophan competitively inhibited the enzyme (Fig. 2). The lack of upward curvature in the double-reciprocal lines in Fig. 2 indicates that the inhibition is non-cooperative. The value of Ki that was obtained (Table 1) is marginally higher than those of other anthranilate synthases, which are in the low-micromolar range (0.3 to 5 μM) [10-17].
Fig. 2. Tryptophan Inhibition of SvAS Activity.
Chorismate concentration was varied in the presence of the following fixed concentrations of tryptophan: none (closed circles), 2.5 μM (open circles), 5 μM (closed triangles), 10 μM (open triangles) and 20 μM (closed squares). See Materials and Methods for further details
SvAS is a Monomer
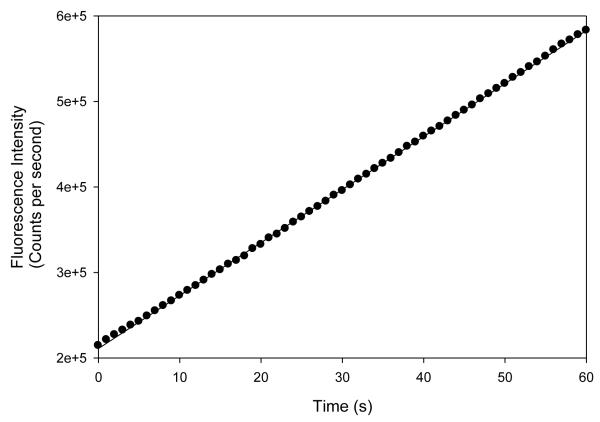
The gel filtration (Fig. 3) and laser light scattering (not included) experiments gave molecular masses of 66 ± 2 and 66.6 ± 1.0 kDa, respectively, for the active SvAS enzyme. Since the molecular mass of the single polypeptide chain determined by SDS-PAGE [22] and calculated from the amino acid sequence is 67 kDa, these results indicate that SvAS functions as a monomer. In order to rule out the possibility that SvAS moved through the gel filtration column as an (inactive) monomer, but then associated to form an (active) dimer in the subsequent activity assay, we performed an experiment in which fractions taken from the column were directly assayed for activity, and the build-up of anthranilate (measured by fluorescence intensity) was recorded in short 1-second time intervals. We wanted to test this possibility because the isolated TrpE and G subunits of some Bacillus AS enzymes are known to undergo rapid association under assay conditions, thereby forming an active dimeric complex. In those other cases, hysteresis, caused by an initial lag in activity, was observed when the reaction was followed over time [6]. Our results (Fig 3, inset) show that there is no hysteretic effect, i.e., no lag in activity, suggesting that a transition from an inactive monomer to an active oligomer is not taking place, and that the active form is a monomer.
Fig. 3. Determination of the Size of SvAS by Gel Filtration Chromatography.
The position of the SvAS protein is indicated with a solid square. Albumin was run twice for this experiment. Inset (bottom panel), the results were obtained by removing an aliquot of the active fraction corresponding to a molecular mass of 67 kDa from the gel filtration column, adding it to the assay cuvette, and immediately measuring the change in fluorescence intensity (in counts per second) over time (in sec). The fact that no lag is observed at the beginning of the assay suggests that assembly of the monomers into higher-order structures (dimers) is not required for activity. See Materials and Methods for details
Discussion
Our results show that the fused anthranilate synthase from Streptomyces venezuelae (SvAS) utilizes chorismate and glutamine in a manner similar to other anthranilate synthases, but cannot use ammonia as substrate. The enzyme is end-product inhibited by tryptophan, the inhibition is non-cooperative, and it is competitive with respect to chorismate. Surprisingly, SvAS functions as a monomer.
The finding that SvAS, unlike other AS enzymes, cannot utilize ammonia as substrate suggests that the association between TrpG and E domains is tight enough to create a structural constraint at the chorismate site that prevents exogenous ammonia from entering, binding and being utilized in the reaction. It is possible that the 50-amino acid long linker between TrpE and G domains, which is present in SvAS but not other AS enzymes characterized, may play a role in helping to form a less open or accessible chorismate site. But its structural role is currently unknown.
The tryptophan site in SvAS has not yet been elucidated, but in SsAS and SmAS it has been identified to be present within the TrpE subunit, where its location is approximately 20 Å away from the chorismate site [19, 20]. Amino acid sequence alignment shows that many of the residues involved in tryptophan inhibition in other anthranilate synthases (e.g., from Salmonella enterica and Serratia marcescens) are not conserved or are completely absent in SvAS (see [22]). Notably, although tryptophan binds to a site other than the active site, its inhibition is clearly competitive with respect to chorismate. This indicates that the inhibitory signal of tryptophan binding is relayed across the short distance from the tryptophan site to the chorismate site through localized conformational changes.
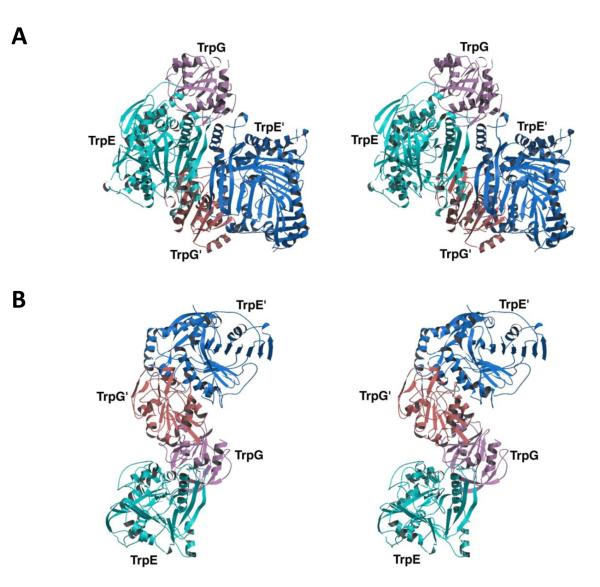
The observation that SvAS is a monomer means that the conformational changes associated with tryptophan inhibition are transmitted within the monomeric unit and are not relayed across subunit-subunit interfaces, which are not present in SvAS. The absence of cooperativity is consistent with a lack of oligomeric structure. Moreover, while it is true that this appears to be the first report of a monomeric anthranilate synthase, there have been reports of dimeric TrpE-TrpG AS enzymes from various Bacillus species, as well as from Acinetobacter and Pseudomonas [1-4]. Thus, there is a precedent for anthranilate synthases having only one TrpE and one TrpG unit, albeit on separate polypeptide chains that associate together. Moreover, two different association states have been reported for anthranilate synthases whose X-ray structures have been determined. In one association state, exemplified by the (TrpE)2(TrpG)2 tetrameric enzymes from Salmonella enterica and Serratia marcescens [18,19], the TrpE-TrpG dimer-dimer interface is formed from central TrpE subunits, with TrpG subunits on the outside (Fig. 4A). In this case, the functional unit is the entire tetramer since all four subunits participate in the inhibitory allosteric transition [26] and there is a viable pathway through which conformational changes can be transmitted across the TrpE-TrpE interface in the center of the molecule [18,19].
Fig. 4. Variable Association Modes for Tetrameric Anthranilate Synthases.
Stereo ribbon diagrams of: A, the S. enterica (typhimurium) heterotetramer showing that the TrpE-TrpG and TrpE’-TrpG’ dimers associate via TrpE subunits; B, the S. solfataricus heterotetramer showing that the TrpE-TrpG and TrpE’-TrpG’ dimers associate via TrpG subunits. Courtesy of Michael J. Eck. Adapted by permission from Macmillan Publishers Ltd.: Nature Structural Biology [18], copyright 2001
However, in a second association state, exemplified by the tetramer from Sulfolobus solfataricus (SsAS) [20], the TrpE-TrpG dimer-dimer interface involves central TrpG subunits with TrpE subunits on the outside (Fig. 4B). This mode of association is consistent with the fact that tryptophan inhibition of SsAS is non-cooperative, and it is also consistent with there being a single TrpE-TrpG pair as the functional unit. Presumably, conformational changes associated with tryptophan inhibition in SsAS are propagated within the TrpE-TrpG dimer and do not cross the central dimer-dimer interface, a phenomenon that jibes with what is seen in our study with SvAS, where inhibition is within the TrpEG monomer. Our results thus highlight the conclusion that a variety of association modes is possible for anthranilate synthases, and that the only absolute requirement is the presence of an intact TrpE-TrpG unit.
We note that the function of the ~50 amino acid long polypeptide linker, and whether or not it plays a role, either directly or indirectly, in catalysis or tryptophan inhibition, is unknown. In the fused PhzE from Burkholderia lata, which is active as an intertwined dimer in which the functional TrpE-TrpG unit is contributed from two different subunits, the linker does contribute to conformational changes associated with catalysis (but the enzyme is, as expected, not inhibited by tryptophan) [27]. Since our results show that SvAS is a monomer and not a dimer, these insights regarding the role of the linker may not be relevant despite the fact that SvAS shares high sequence similarity with PhzE enzymes from Pseudomonas. Insights about the precise location of the tryptophan site and the linker’s role may be revealed by future structural and structure-function studies.
The most significant conclusion from our work is that, unlike other AS enzymes studied to date, the fused AS from S. venezuelae functions as a monomer. This finding reconciles earlier results showing two very different subunit association modes for anthranilate synthases [18, 19, 20]. It highlights the notion that a variety of modes is possible, and that the only absolute requirement is an intact TrpE-TrpG functional unit.
Finally, this study adds to a growing body of knowledge about the function and structure of bacterial anthranilate synthases and other chorismate-utilizing enzymes, which are, by virtue of their central roles in primary and secondary metabolism and their absence in humans, excellent antimicrobial drug targets (see [28]).
Acknowledgements
We acknowledge support from the U. S. National Institutes of Health (NIH) RCMI (grant number 2G12RR003048-18) and MBRS-SCORE programs (grants number 3S06GM0816-33S1 and 1SC3GM083752) to WMB. We thank Tin-Wein Yu, formerly of the University of Washington, for assistance with the development of protocols for expression of SvAS, Santiago Ramon-Maiques of the Centro Nacional de Investigaciones Oncológicas in Madrid for helpful discussions about the structure of the enzyme, and Michael J. Eck of Harvard University for providing us with the high resolution images used in Fig. 4. Disclaimer: certain commercial equipment, instruments and materials are identified in this paper in order to specify the experimental procedure as completely as possible. In no case does such identification imply a recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the material, instrument, or equipment identified is necessarily the best available for the purpose.
References
- 1.Kane JF, Jensen RA. The molecular aggregation of anthranilate synthase in Bacillus subtilis. Biochem Biophys Res Commun. 1970;41:328–333. doi: 10.1016/0006-291x(70)90507-3. [DOI] [PubMed] [Google Scholar]
- 2.Patel N, Holmes WM, Kane JF. Homologous and hybrid complexes of anthranilate synthase from Bacillus species. J Bacteriol. 1974;119:220–227. doi: 10.1128/jb.119.1.220-227.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sawula RV, Crawford IP. Anthranilate synthetase of Acinetobacter calcoaceticus: separation and partial characterization of subunits. J Biol Chem. 1973;248:3573–3581. [PubMed] [Google Scholar]
- 4.Queener SW, Queener SF, Meeks JR, Gunsalus IC. Anthranilate synthase from Pseudomonas putida: purification and properties of a two-component enzyme. J Biol Chem. 1973;248:151–161. [PubMed] [Google Scholar]
- 5.Patel N, Holmes WM, Kane JF. Intergeneric complementation of anthranilate synthase subunits. J Bacteriol. 1973;114:600–602. doi: 10.1128/jb.114.2.600-602.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kane JF, Holmes WM, Smiley KL, Jensen RA. Rapid regulation of an anthranilate synthase aggregate by hysteresis. J Bacteriol. 1973;113:224–232. doi: 10.1128/jb.113.1.224-232.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Troch P, Dosselaere F, Keijers V, de Wilde P, Vanderleyden J. Isolation and characterization of the Azospirillum brasilense trpE(G) gene, encoding anthranilate synthase. Curr Microbiol. 1997;34:27–32. doi: 10.1007/s002849900139. [DOI] [PubMed] [Google Scholar]
- 8.Bae YM, Holmgren E, Crawford IP. Rhizobium meliloti anthranilate synthase gene: cloning, sequence, and expression in Escherichia coli. J Bacteriol. 1989;171:3471–3478. doi: 10.1128/jb.171.6.3471-3478.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paradkar AS, Stuttard C, Vining LC. Molecular cloning of the genes for anthranilate synthetase from Streptomyces venezuelae ISP 5230. FEMS Microbiol Lett. 1991;62:177–181. doi: 10.1016/0378-1097(91)90154-3. [DOI] [PubMed] [Google Scholar]
- 10.Baker TI, Crawford IP. Anthranilate synthase: partial purification and some kinetic studies on the enzyme from Escherichia coli. J Biol Chem. 1969;241:5577–5584. [PubMed] [Google Scholar]
- 11.Tamir H, Srinivasan PR. Purification and properties of anthranilate synthase from Salmonella typhimurium. J Biol Chem. 1969;244:6507–6513. [PubMed] [Google Scholar]
- 12.Henderson EJ, Nagano H, Zalkin H, Hwang LH. The anthranilate synthetase-anthranilate 5-phosphoribosyltransferase complex of Salmonella typhimurium. Implications concerning the mode of assembly of the complex. Biochemistry. 1970;13:1416–1423. [Google Scholar]
- 13.Bauerle R, Hess J, French S. Anthranilate synthase-anthranilate phosphoribosyltransferase complex and subunits of Salmonella typhimurium. Methods Enzymol. 1987;142:366–386. doi: 10.1016/s0076-6879(87)42049-1. [DOI] [PubMed] [Google Scholar]
- 14.Zalkin H, Hwang LH. Anthranilate synthetase from Serratia marcescens: on the properties and relationship to the enzyme from Salmonella typhimurium. J Biol Chem. 1971;246:6899–6907. [PubMed] [Google Scholar]
- 15.Robb F, Hutchinson MA, Belser WL. Anthranilate synthetase: some physical and kinetic properties of the enzyme from Serratia marcescens. J Biol Chem. 1971;246:6908–6912. [PubMed] [Google Scholar]
- 16.Tutino ML, Tosco A, Marino G, Sannia G. Expression of Sulfolobus solfataricus trpE and trpG genes in E. coli. Biochem Biophys Res Commun. 1997;230:306–310. doi: 10.1006/bbrc.1996.5951. [DOI] [PubMed] [Google Scholar]
- 17.Byrnes WM, Vilker VL. Extrinsic factors potassium chloride and glycerol induce thermostability in recombinant anthranilate synthase from Archaeoglobus fulgidus. Extremophiles. 2004;8:455–462. doi: 10.1007/s00792-004-0406-3. [DOI] [PubMed] [Google Scholar]
- 18.Morollo AA, Eck MJ. Structure of the cooperative allosteric anthranilate synthase from Salmonella typhimurium. Nature Struct Biol. 2001;8:243–247. doi: 10.1038/84988. [DOI] [PubMed] [Google Scholar]
- 19.Spraggon G, Kim C, Nguyen-Huu X, Yee M-C, Yanofsky C, Mills SE. The structures of anthranilate synthase of Serratia marcescens crystallized in the presence of (i) its substrates, chorismate and glutamine, and a product, glutamate, and (ii) its end-product inhibitor, L-tryptophan. Proc Natl Acad Sci USA. 2001;98:6021–6026. doi: 10.1073/pnas.111150298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knöchel T, Ivens A, Hester G, Gonzalez A, Bauerle R, Wilmanns M, Kirschner K, Jansonius JN. The crystal structure of anthranilate synthase from Sulfolobus solfataricus: functional implications. Proc Natl Acad Sci USA. 1999;96:9479–9484. doi: 10.1073/pnas.96.17.9479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zalkin H, Kling D. Anthranilate synthetase. Purification and properties of component I from Salmonella typhimurium. Biochemistry. 1968;7:3566–3573. doi: 10.1021/bi00850a034. [DOI] [PubMed] [Google Scholar]
- 22.Ashenafi M, Carrington R, Collins AC, Byrnes WM. The fused TrpEG from Streptomyces venezuelae is an anthranilate synthase, not a 2-amino-2-deoxyisochorismate (ADIC) synthase. Ethn Dis. 2008;18:S2, 9–13. [PMC free article] [PubMed] [Google Scholar]
- 23.Lin C, Paradkar AS, Vining LC. Regulation of an anthranilate synthase gene in Streptomyces venezuelae by a trp attenuator. Microbiol. 1998;144:1971–1980. doi: 10.1099/00221287-144-7-1971. [DOI] [PubMed] [Google Scholar]
- 24.Grisostomi C, Kast P, Pulido R, Huynh J, Hilvert D. Efficient in vivo synthesis and rapid purification of chorismic acid using an engineered Escherichia coli strain. Bioorg Chem. 1997;25:297–305. [Google Scholar]
- 25.Addadi L, Jaffe EK, Knowles JR. Secondary tritium isotope effects as probes of the enzymic and nonenzymic conversion of chorismate to prephenate. Biochemistry. 1983;22:4494–4501. doi: 10.1021/bi00288a022. [DOI] [PubMed] [Google Scholar]
- 26.Caligiuri MG, Bauerle R. Subunit communication in the anthranilate synthase complex from Salmonella typhimurium. Science. 1991;252:1845–1848. doi: 10.1126/science.2063197. [DOI] [PubMed] [Google Scholar]
- 27.Li QA, Mavrodi DV, Thomashow LS, Roessle M, Blankenfeldt W. Ligand binding induces an ammonia channel in 2-amino-2-desoxyisochorismate (ADIC) synthase PhzE. J Biol Chem. 2011;286:18213–21. doi: 10.1074/jbc.M110.183418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ziebart KT, Dixon SM, Avila B, El-Badri MH, Guggenheim KG, Kurth MJ, Toney MD. Targeting multiple chorismate-utilizing enzymes with a single inhibitor: validation of a three-stage design. J Med Chem. 2010;53:3718–3729. doi: 10.1021/jm100158v. [DOI] [PubMed] [Google Scholar]