Abstract
The cytokine IL-9, derived primarily from T-helper (Th)-9 lymphocytes, promotes expansion of the Th2 subset and is implicated in the mechanisms of allergic asthma. We hypothesize that IL-9 also plays a role in human allergic contact dermatitis (ACD). To investigate this hypothesis, skin biopsy specimens of positive patch test sites from non-atopic patients were assayed using qPCR and immunohistochemistry. Along with Th2 associated cytokines, IFN-γ, IL-4, and IL-17A, expression of IL-9, and PU.1, a Th9-associated transcription factor, were elevated when compared to paired normal skin. Immunohistochemistry on ACD skin biopsies identified PU.1+CD3+, and PU.1+CD4+ cells, consistent with Th9 lymphocytes, in the inflammatory infiltrate. PBMC from nickel-allergic patients, but not non-allergic controls, show significant IL-9 production in response to nickel. Blocking studies with monoclonal antibodies to HLA-DR (but not HLA-A, B, C) or chloroquine significantly reduced this nickel-specific IL-9 production. Additionally, blockade of IL-9 or IL-4 enhanced allergen-specific IFN-γ production. A contact hypersensitivity model using IL-9−/− mice, shows enhanced Th1 lymphocyte immune responses, when compared to WT mice, consistent with our human in vitro data. This study demonstrates that IL-9, through its direct effects on Th1 and ability to promote IL-4 secretion, has a regulatory role for Th1 lymphocytes in ACD.
Introduction
After activation, naïve CD4+ T-helper (Th) cells differentiate along separate lineages into effector T-cell subsets depending on the type of antigen recognized and the selection of cytokines present within the adjacent extracellular milieu. The traditional Th1 or Th2 paradigm has expanded to include Th17, Th9, Treg, and Th22 cells, each with lineage specific transcription factors and production of a distinct array of cytokines (Wan and Flavell, 2009). Our understanding of the interplay between these cells and their involvement in immune pathology is still incomplete. Interleukin-9 (IL-9) is classically considered to be associated with the Th2 subset. However, recently an IL-9 producing T-cell subset, named Th9, has been recognized and is implicated in the pathogenesis of allergic inflammation (Dardalhon et al., 2008; Veldhoen et al., 2008).
IL-9 is shown to have pleiotropic cellular effects involved in promoting allergic inflammation, including inducing mast cell growth and survival, eotaxin production and goblet cell metaplasia (Gounni et al., 2004; Kearley et al., 2011; Vermeer et al., 2003). A significantly elevated level of IL-9 has been found in bronchial biopsies from patients with atopic asthma compared to matched controls and thus IL-9 may have a significant role in the chronic airway inflammation and bronchial hyper-responsiveness seen in asthmatic disease (Ying et al., 2002). Blocking IL-9 inhibits airway remodeling and mastocytosis in a model of chronic lung inflammation (Kearley et al., 2011). A recent study shows that Th9 cells are generated in vivo after epicutaneous sensitization by patch application with ovalbumin in mice (Lin et al., 2012). PU.1, an ETS-family transcription factor, has since been identified as a main regulator of IL-9 production in human Th9 cells. Increased PU.1 expression is associated with a decrease in Th2 cytokines and increased phenotypic characteristics consistent with Th9 cells, including production of IL-9. Additionally, less allergic inflammation was seen in the lungs of mice with PU.1 deficient T-cells compared to their wild-type counterparts (Chang et al., 2010). Despite their known role in allergic lung disease, their participation in allergic contact dermatitis (ACD), has not been established.
In this study we examine IL-9 expression and the frequency of PU.1 positive T lymphocytes (Th9 lymphocytes) infiltrating into positive patch test reactions to a variety of allergens. We identify allergen-specific IL-9 production by PBMC from a group of seven patients with positive patch tests to nickel. Our data shows that IL-9 and related cytokine gene expression as well as PU.1+CD4+ T-cells are significantly increased in positive patch test biopsies from these allergic patients. In vitro experiments show IL-9 synergizing with signature cytokines from Th2 lymphocytes and regulating Th1 lymphocyte allergen specific responses. The role of IL-9 found in our in vitro human data were confirmed using an in vivo mouse model, by studying CHS3 to DNFB4 in IL-9 gene targeted mice (IL-9−/−). This mouse model confirmed the regulatory role of IL-9 in CHS.
Results
The genes encoding IL-9 and Th9 associated transcription factor, PU.1, are elevated in ACD
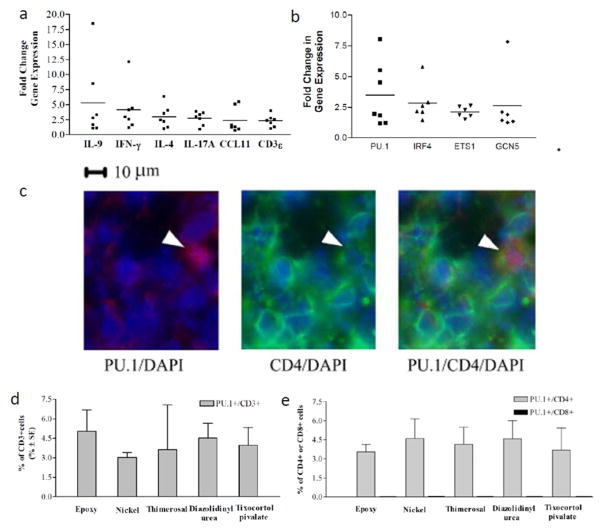
We measured IL-9 and other cytokine genes associated with ACD in positive patch tests, as well as paired normal skin. The mean relative gene expression of IL-9 (Figure 1A) was elevated on average 5 (range 2–18) fold higher than paired normal skin. This was equivalent to the mean change in gene expression for IFN-γ, IL-4 and IL-17A. We also studied Th9-associated transcription factors PU.1, ETS-1, IRF-4 and GcN5 (Oikawa and Yamada 2003, Refaat et al, 2011, Goswami and Kaplan, 2012) which were all similarly elevated 2 to 3 (range 1.5–8)-fold higher than paired control skin (Figure 1B).
Figure 1. Increased expression of IL-9 and associated genes in AC and the detection of Th9 cells in allergic contact dermatitis.
(A) Mean relative-gene expression of IL-9, was elevated on average 5 (range 2–18) fold higher than paired control skin. IL-9 increase is similar to increases in IFN-γ, IL-4, IL-17A, CCL11 and CD3ε. (B) Mean relative gene expression of IL-9/Th9-associated transcription factors PU.1, IRF4, ETS1 and GcN5 was 2–3 (range 1.5–8) fold greater than paired control skin. Sections of skin biopsy specimens from a representative (C) Stained slides from an ACD patient were stained with anti-PU.1 (red) and anti-CD4 (green); nuclei are counter-stained with DAPI (blue). Stained T lymphocytes were identified as (D) PU.1+/CD3+ or (E) PU.1+/CD4+. No PU.1+/CD8+ cells were identified. To quantify cell populations, twelve fields from each double-stained section were counted with a mean of 40 infiltrating cells per field. Mean +/− SEM is depicted. For gene expression studies, skin biopsy samples were taken from positive patch tests to nickel or cobalt from seven different patients (ACD 6–12 in Table 1). For the immunochemistry, skin biopsy specimens were taken from five different positive patch tests in five different patients (ACD1–5 in Table 1).
Th9 lymphocytes, but not CD8+/PU.1+ lymphocytes, are detectable in the inflammatory infiltrate of ACD
Immunochemistry was performed for Th9 transcription factor PU.1 and T-cell surface markers (CD3, CD4, or CD8) in positive patch test biopsies from five patients with ACD to different allergens. Either CD3+/PU.1+ or CD4+/PU.1+ double positive cells were readily identifiable in the cellular infiltrates of ACD, present in both the dermis and the epidermis (Figure 1C). Of the total population of infiltrating CD3+/CD4+ T-cells approximately 4% were PU.1+ (Figure 1D–E). There were no CD8+/PU.1+ double cells detected in ACD (Figure 1E). No PU.1 positive T-cells were detectable in the normal skin samples examined (data not shown).
Allergen-specific IL-9 production in vitro by PBMC from nickel allergic patients
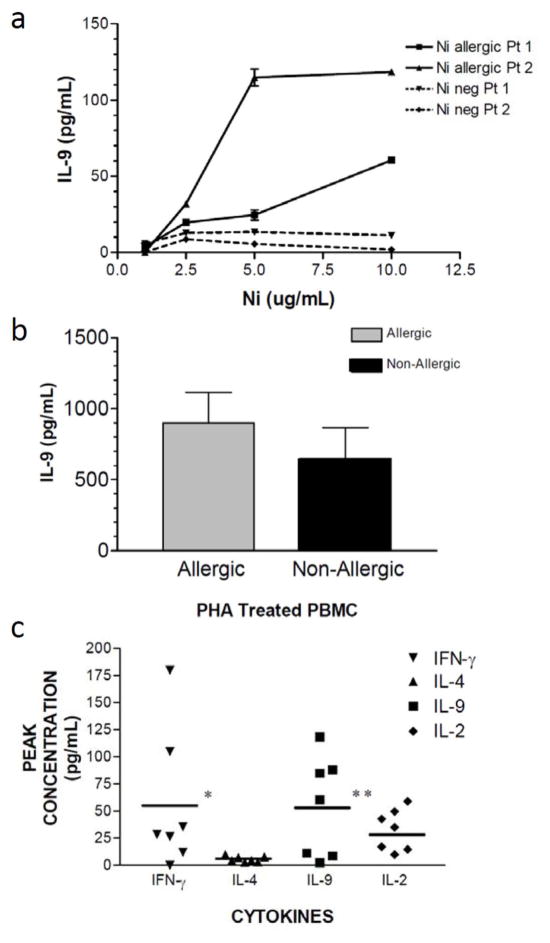
Next, we investigated nickel-specific cytokine production in lymphocytes from seven different nickel allergic patients. We measured levels of IL-9, IFN-γ, IL-2, and IL-4 in culture supernatant in response to in vitro challenge with nickel or cobalt chloride, a non-allergic metal salt as confirmed by patch test. In several patients, we observed a robust dose-dependent production of IL-9 in response to increasing doses of nickel (Figure 2A). In contrast, PBMC derived from two patients that were tolerant of Nickel (negative patch tests), their PBMC did not produce detectable IL-9 in response to Nickel challenge in vitro (Figure 2A). To confirm that PBMC have a similar capacity for the production of IL-9 in the Nickel allergic and tolerant patients, we studied mitogen-induced IL-9 production (Figure 2B). Both groups produced high levels of IL-9 in response to PHA stimulation, which was equivalent between the two groups. In the Nickel allergic patients who had negative patch tests to cobalt chloride, there was no IL-9 or other cytokines produced in response to the irrelevant control metal salt, cobalt chloride (data not shown). From the dose-response curves of nickel-specific cytokine production, we plotted the peak cytokine production from each of the seven Nickel-allergic patients (Figure 2C). The mean peak cytokine production of both IFN-γ and IL-9 were significantly higher than IL-4. These data indicate that allergen specific IL-9 production is at a similar level as IFN-γ in patients with allergic contact dermatitis to nickel.
Figure 2. Allergen-specific IL-9 production by PBMC from nickel-allergic patients.
PBMC were isolated from 7 different patients with ACD to nickel (Table 1), and cultured with nickel chloride (1–10 μg/ml of culture medium) for ninety six hours. Supernatants were harvested, and the following cytokines were assayed in cell-free culture supernatants: IL-9, IFN-γ, IL-4, and IL-2 (data depicted represent mean +/− SD of samples assayed in triplicate). (A) Dose response curve to Nickel titration from two nickel allergic patients and two nickel tolerant patients. (B) IL-9 production from the same two nickel allergic and tolerant patients, demonstrating that both groups can produce IL-9 in an equivalent manner after PHA stimulation. (C) Scatter plot represents the peak cytokine production from the seven allergic patients. The scatter plot of peak cytokine production (horizontal bar represents mean value) for both IFN-γ and IL-9 were significantly higher than of IL-4 (P<0.001), with the average peak IL-9 production is similar to IFN-γ (P>0.05).
Characterization of antigen presenting cell pathways for recognition of nickel-specific IL-9 production
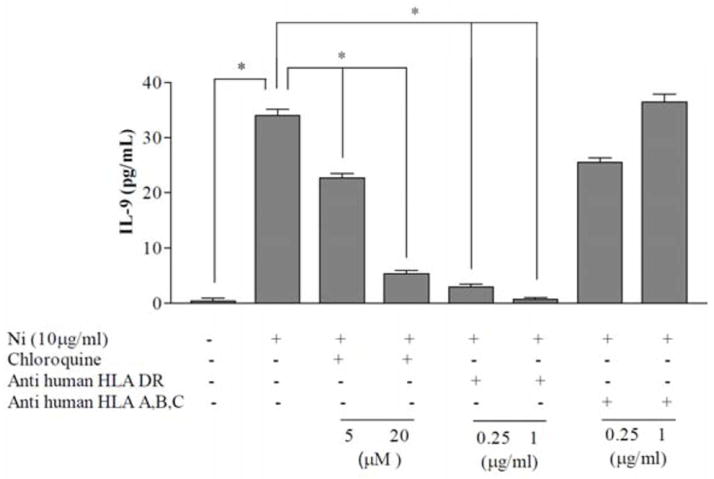
In order to elucidate how Th9 specific antigens are being processed and presented, blocking studies were performed. A monoclonal antibody specific for HLA-DR, associated with the MHC class II pathway, significantly attenuated nickel-specific IL-9 production, while anti-HLA-A, B, or C had no significant effect on IL-9 (Figure 3). Similarly, pre-incubation of PBMC with chloroquine, prior to nickel stimulation, also significantly attenuated nickel-specific IL-9 (Figure 3). These data indicate that allergen-induced IL-9 production is dependent on the MHC class II pathway, and requires processing. This is consistent with the in situ data in which we observed CD4+PU.1+ T-lymphocytes, but not CD8+PU.1+ T-lymphocytes lymphocytes in ACD (Figure 1).
Figure 3. IL-9 production depends on exogenous antigen presentation to Th9-lymphocytes.
IL-9 production was measured 96h after stimulation of PBMC with medium containing nickel chloride (10μg/ml). Samples are incubated with either media, anti-HLA-DR monoclonal antibody (0.25μg/ml and 1μg/ml), anti-HLA-A,B,C monoclonal antibodies (0.25μg/ml and 1μg/ml), or chloroquine (5μM and 20μM) 2 hours prior to stimulation with nickel. Antibodies to HLA-A,B, C had little effect on IL-9 production, while Abs to HLA-DR or incubation with chloroquine resulted in significantly reduced IL-9 production. Mean +/− SD of samples assayed in triplicate is depicted. Our results suggest the processing of nickel via the exogenous pathway of antigen presentation is necessary for PBMC production of IL-9. Experiment was repeated with PBMC from three different patients, and found to be reproducible.
Regulatory role of IL-9 in ACD
We next investigated if IL-9 modulates nickel-specific IFN-γ production. As expected, exogenous IL-4 significantly decreased nickel-specific IFN-γ production by PBMC from a nickel allergic patient, and exogenous IL-9 did not attenuate nickel-specific IFN-γ production (Figure 4A). When exogenous IL-9 and IL-4 were added simultaneously to lymphocyte cultures, the inhibitory effects of IL-4 on IFN-γ production were dominant, and were not influenced by the presence of IL-9. We also studied the cross-regulation of IL-9 and IL-4 during allergen-specific responses (Figures 4B and 4C). Exogenous IL-9 augmented nickel-specific IL-4 (Figure 4B) and exogenous IL-4 augmented nickel-specific IL-9 (Figure 4C). These data suggest that there is synergy between Th2 and Th9 lymphocytes via their cytokines, and also suggests an indirect regulatory role for IL-9 in ACD. Whereas IL-9 itself does not attenuate IFN-γ production by Th1-lymphocytes, this cytokine augments the production of IL-4, which is a known inhibitory cytokine for Th1-lymphocytes (Cher and Mosmann, 1987; Mosmann et al., 1986; Yip et al., 1999). The addition of anti-sera to block either IL-9 or IL-4 in cultures of nickel-stimulated PBMC augmented IFN-γ production, again indicating a regulatory function for IL-9 (Figure 4D).
Figure 4. IL-4 but not IL-9 directly regulates IFN-γ during allergen-specific Th1 activation in vitro.
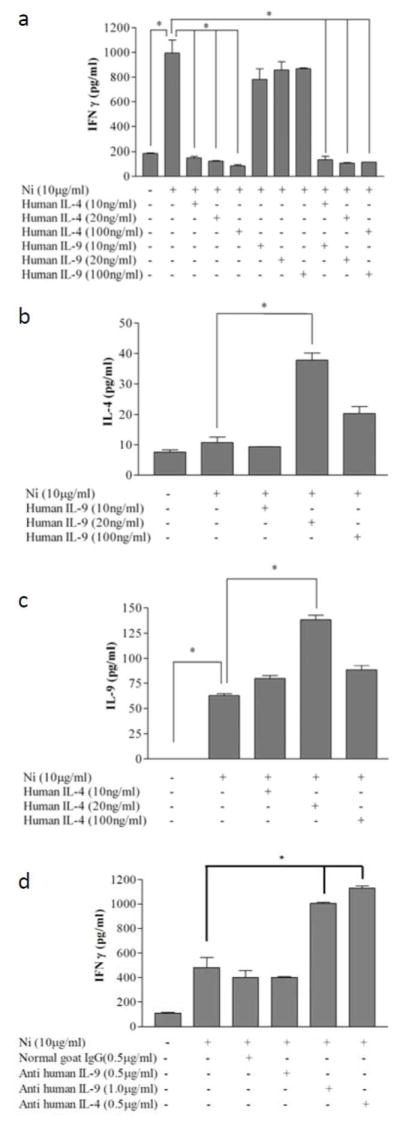
(A) PBMC from a nickel allergic patient were cultured in medium alone or in medium containing nickel-chloride, the indicated doses of IL-9 alone, IL-4 alone, or IL-9 and IL-4 together were added to the nickel-chloride stimulated PBMC. Supernatants were collected 96 h after nickel-stimulation. (B) IL-4 secretion was also measured after addition of IL-9. (C) IL-9 was measured in those culture conditions which had added IL-4. (D) Specific blocking anti-sera were added to the nickel culture, and IFN-γ was assayed using multiplex ELISA. Data depicted represent mean +/− SD of samples assayed in triplicate. This experiment was repeated with PBMC three times, and found to be reproducible. The data in (A–D) represent PBMC from distinct nickel-allergic patients.
The regulatory role of IL-9 in an in vivo model of contact hypersensitivity
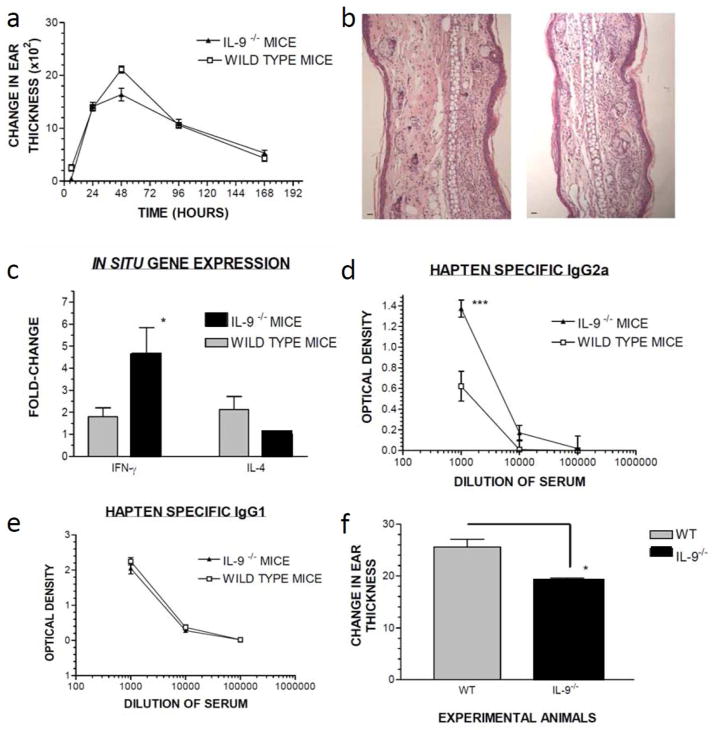
To further confirm our human in vitro data, we studied contact hypersensitivity in IL-9 gene targeted mice (IL-9−/−), and compared their ear swelling responses to wild type mice of the same H-2d background. When compared to wild type mice, IL-9−/− mice exhibited slightly diminished ear swelling responses at 48 hours (Figure 5A). Histologic analysis of ear sections taken 24 hours after hapten challenge from either wild type or IL-9−/− mice exhibited qualitatively similar pathologic findings (Figure 5B). However, analysis of in situ cytokine gene expression revealed that during CHS reaction IL-9−/− mice showed enhanced Th1 and diminished Th2 gene expression, while WT have a balanced Th1-Th2 profile (Figure 5C). The mean fold change in IFN-γ gene expression was significantly greater in IL-9−/− mice compared to the wild type mice (p<0.05, ANOVA). To better understand the systemic effects of the role of IL-9 in contact hypersensitivity, we studied DNP specific IgG1, and IgG2a in the sera of sensitized mice 3 weeks after sensitization with topical DNFB. DNP-specific IgG1 (Th2 driven) was similar in WT and IL-9−/− mice, and there was significant enhancement of IgG2a (Th1 driven) in IL-9−/− mice compared to wild type mice (Figures 5D and 5E). These data indicate that IL-9 exhibits a regulatory role in ACD. Also, that compensatory inflammatory mechanisms operate in the absence of IL-9, resulting in a relatively preserved ear swelling response in which Th1-lymphocytes play a more important role both locally and systemically. To further study the response to inflammatory agents, we challenged naïve WT and IL-9−/− with the topically applied irritant, TPA. Twenty-four hours after application, IL-9−/− mice exhibited significantly less ear swelling compared to WT control mice (t= 4.069, unpaired t-test, p < 0.0023).
Figure 5. The regulatory role of IL-9 in mouse contact hypersensitivity.
(A) Ear swelling response of WT or IL-9−/− mice (n=5/group) was measured over time. (B) Ear punches taken 24 hours after elicitation are stained with H&E; depicted ears from WT (left) and IL-9−/− mice (right). (C) Gene expression was measured by qPCR from ear skin 24 hours after elicitation. Mean +/− SD fold-change in gene expression is shown (n=4 mice/group, *,p<0.05). (D) Sera (n=5 mice/group) from WT or IL-9−/− mice 21 days after sensitization, was measured for DNP-specific IgG1 or (E) IgG2a by ELISA. Levels of DNP specific Ab from pre-bleed sera (prior to sensitization) was subtracted to calculate the net DNP-specific response. Data depicted represent mean +/− SD (***p<0.001). (E) Irritant contact dermatitis to croton oil in WT and IL-9−/− mice; data indicate mean +/− SD (p<0.0023).
Discussion
Allergic contact dermatitis (ACD) is a result of a complex network of APC-T-cell interactions, and represents a balance between effector subsets and regulatory subsets (Gober and Gaspari, 2008). Whereas CD8+ T lymphocytes are effector cells that mediate tissue damage during the elicitation phase of ACD, the CD4+ T-lymphocyte subset plays a regulatory role in ACD by secreting an immune suppressive milieu of cytokines (Bour et al., 1995; Gocinski and Tigelaar, 1990; Martin et al., 2000; Vocanson et al., 2006). In human positive patch test reactions, a multitude of cytokines can be identified (Zhao et al., 2009), suggesting that a variety of Th subsets may be involved in ACD. Our data investigating human skin biopsies from ACD to nickel and other clinically relevant allergens (Table I) are consistent with this. We found that genes encoding several cytokines were increased in human ACD (Figure 1). However, we also identified increased levels of IL-9 and the Th9-associated transcription factors, PU.1, ETS-1, IRF4 and GcN5 at similar levels as these well-known ACD associated cytokines. These results suggest that Th9-derived IL-9, as well as other classically associated cytokines, play a role in human ACD.
Table I.
Allergen and patient data
| Patient no.a | Age(years)/gender/raceb | Allergen/Scoringc/duration | Studiesd |
|---|---|---|---|
| ACD1 | 59/M/W | Epoxy resin/+/48h | ICC |
| ACD2 | 68/M/W | Thimerosal/++/96h | ICC |
| ACD 3 | 60/M/A | Diazolidinyl urea/++/48h | ICC |
| ACD 4 | 46/F/AA | Tixocortolpivalate/+++/96h | ICC |
| ACD 5 | 72/F/W | Nickel sulfate/+/72h | ICC |
| ACD 6 | 54/F/W | Nickel sulfate/+++/72h | PCR |
| ACD 7 | 38/F/W | Nickel sulfate/+++/72h | PCR |
| ACD 8 | 29/F/W | Nickel sulfate/+++/72h | PCR |
| ACD 9 | 41/F//W | Nickel sulfate/+/72h | PCR |
| ACD 10 | 54/F/W | Nickel sulfate/+++/72h | PCR |
| ACD 11 | 43/M/W | Cobalt chloride/+/72h | PCR |
| ACD 12 | 64/F/W | Nickel sulfate/++/72h | PCR |
| ACD 13 | 39/F/A | Nickel sulfate/+/48h | ELISA |
| ACD 14 | 62/F/AA | Nickel sulfate/++/48h, Cobalt chloride/++/48h | ELISA |
| ACD 15 | 58/F/W | Nickel sulfate/+++/72h, Neomycin/+/72h, Thimerosal/+/72h | ELISA |
| ACD 16 | 29/F/W | Nickel sulfate/++/48h | ELISA |
| ACD 17 | 58/F/W | Nickel sulfate/++/72h, Cobalt chloride/++/72h | ELISA |
| ACD 18 | 25/F/W | Nickel sulfate/++/72h, Cobalt chloride/++/72h | ELISA |
| ACD 19 | 58/F/AA | Nickel sulfate/+/72h, Cobalt chloride/++/72h | ELISA |
Patient no. refers to allergic contact dermatitis patients no. ACD1–19
Race: A, Asian; AA, African–American; W, white (caucasian).
Patch test scoring system: 0 = negative; + = erythema, some induration (low-level reaction); ++ = erythema, spreading and more marked induration (moderate reaction); +++ = erythema, marked induration, and blisters (extreme reaction).
Studies refers to PCR(real-time polymerase chainreaction), ICC (immunocytochemistry), ELISA (studies on culture supernatants from allergen-stimulated lymphocytes).
We demonstrate using biopsies from positive patch tests, that allergen-specific CD4+/PU.1+ T cells, but not CD8+/PU.1+ T cells, are detectable in the efferent phase of ACD (Figure 1). This is consistent with a regulatory, rather than pathogenic, role for IL-9 producing T-cells in ACD. It has been shown that lymphocytes from patients with positive patch tests (i.e., ACD) to metal salts produce both Th-1 associated IFN-γ, as well as Th2 associated IL-4 and IL-13 upon culture with the appropriate allergen (Minang et al., 2006). When measuring allergen specific cytokine production of ex vivo cultured PBMC from ACD patients, we find allergen specific IL-9 production by ex vivo PBMC is augmented in allergic patients, but not in non-allergic control patients (Figures 2–4). We were able to detect nickel specific IL-4 at low levels, while IFN-γ and IL-9 showed a robust response to nickel (Figure 2). We were not able to identify CD4+/PU.1+ T-cells in normal human skin (data not shown). However, this cell type constituted approximately 4% of the inflammatory infiltrate of ACD and we noted elevated IL-9 transcripts in ACD compared to paired normal skin (Figure 1). These data suggest that the IL-9 producing CD4+/PU.1+ T-cells migrate into the skin of ACD.
Xenobiotic chemicals are thought to be chemically reactive with host proteins, by forming haptens which are presented by migratory cutaneous antigen presenting cells in the local lymph node (Kaplan et al., 2012). Haptens can be recognized by class-I MHC restricted CD8+ T-lymphocytes, or class-II MHC restricted CD4+ T-lymphocytes. Studies of processing/presentation of nickel to reactive T-lymphocytes suggested two pathways for nickel recognition: one involves a processing independent pathway in which nickel binds to peptides already loaded into MHC molecules, and the other pathway involves a processing dependent pathway in which nickel binds to membrane or exogenous proteins which are then taken up by an APC for processing and presentation to reactive T-lymphocytes (Budinger and Hertl, 2000). We find that production of nickel-specific IL-9 is blocked by anti-class II MHC monoclonal antibodies but not anti-class I MHC antibodies supporting the notion that Th9 lymphocytes, but not CD8+/PU.1+ lymphocytes, are the predominant source of IL-9 (Figure 3). We also find that chloroquine, an agent that interferes with the acidification of the endosome and hence antigen processing (Lombard-Platlet et al., 1993), interferes with nickel-specific IL-9 production, which supports the processing-dependent pathway of nickel presentation.
In some model systems, neutralization of IL-9 enhances IFN-γ production in PBMC from patients with latent tuberculosis infections, suggesting that this cytokine blocks Th1 cytokine secretion (Wu et al., 2008). IL-9 can also enhance IFN-γ production and suppress Th2 responses during active tuberculosis infections (Finiasz et al., 2007). In our in vitro assays, exogenous IL-9 did not inhibit nickel-specific IFN-γ production (Figure 4); however, blockade of endogenous IL-9 by using a specific anti-serum enhanced IFN-γ production. Our data indicate that IL-9 acts to regulate allergen-specific Th1-lymphocyte responses directly (Figure 4D) and indirectly by enhancing IL-4 production (Figure 4B) by Th2-lymphocytes. IL-9 gene expression has been implicated in ACD to the hair dye paraphenylenediamine. PBMC from allergic, but not tolerant individuals, increased IL-9 gene expression upon exposure to this chemical in vitro, suggesting this cytokine plays are role in ACD (Coulter et al., 2010).
Further experiments in this study demonstrate a regulatory role of IL-9 in a mouse model of CHS to the strong Th1-polarizing hapten DNFB. In mouse models of ACD using strong lipophilic haptens, Th1 lymphocytes positively regulate ACD, and play a role in promoting an inflammatory response via IFN-γ production and other pro-inflammatory cytokines (Saulnier et al., 1995; Xu et al., 1996). In cytokine gene targeted mice, IFN-γ as well as IL-4 are necessary for sensitization and elicitation, suggesting both Th1 and Th2 lymphocytes play a role in murine ACD (Campos et al., 2003; Saulnier et al., 1995; Xu et al., 1996). Previous studies of protein contact dermatitis in a mouse model demonstrated that Th9 lymphocytes are detectable in the afferent phase of sensitization to topical polypeptides in BALB/c mice, a phenomenon associated with Th2 dominant immune response (Lin et al., 2012). Whereas past work has focused on the effector functions of IL-9 in allergic inflammation and the role of IL-9 in contact dermatitis to polypeptides, our study of CHS in IL-9−/− mice addresses its role in the sensitization/elicitation phases of this allergic reaction.
Our data indicate that CHS to the Th1 polarizing hapten DNFB can occur in the absence of IL-9, but its inflammatory characteristics are altered. We find that there is an enhanced Th1 response locally, as well as enhanced hapten-specific IgG2a found in the sera of IL-9−/− mice (Figures 5D and 5E). It has been established that IgG2a (enhanced in IL-9−/− mice) is dependent on Th1-derived cytokines (Jurado et al., 1989), whereas IgG1 (not enhanced in IL-9−/− mice) is dependent on Th2-derived cytokines such as IL-4 (Coffman et al., 1989). Our histologic analysis of skin challenge sites of DNFB sensitized mice did not reveal quantitatively significant differences in the quality of the inflammatory infiltrate in wild type or IL-9−/− mice (Figure 5B). We noted T lymphocytes, neutrophils and mast cells in both groups of experimental animals. For the hapten DNFB, it is likely that the loss of the pro-inflammatory effects of IL-9 may have been mitigated by enhanced Th1-lymphocyte derived help in the form of IFN-γ, and possibly other cytokines, hence a relatively preserved ear swelling response in the IL-9−/− mice (Figure 5A). In contrast, under induction of irritant contact dermatitis by TPA, ear swelling inflammatory responses were significantly decreased in IL-9−/− mice compared to WT mice. We hypothesize this occurs because (in the absence of inflammatory effects of IL-9 in knockout mice), there the T cell compensatory mechanisms observed in ACD (Figure 5A) are not present in irritant contact dermatitis (Figure 5F).
In total, these in vivo data are consistent with our in vitro data in humans, showing that Th9-lymphocytes modulate allergen-specific IFN-γ production by Th1-lymphocytes. Our study contributes to the emerging evidence that Th9 play an important role in allergic skin reactions, and provides evidence that Th9 and its associated cytokine, IL-9, represent a potential target for immunotherapy in the treatment of non-atopic allergic skin diseases.
Materials and Methods
Patients
Patients being evaluated for ACD were recruited from University of Rome and the University of Maryland, Baltimore (Table I). Written informed consent was obtain from each patient. The study was approved by the local institutional review committee and followed the Declaration of Helsinki Principles for research involving human subjects. The standard North American Series (Dormer Laboratories, Inc., Toronto, Ontario, Canada) were applied in 8 mm Finn Chambers (Epitest Ltd Oy, Tuusula, Finland) to upper back of each patient using standard methods Results were interpreted with the standard grading system (0–3+) at 48 and 72 hours indicated in Table I. Paired biopsy samples of positive patch test sites and normal control skin were collected and snap-frozen then stored at −80°C before RNA extraction. Patch test-positive biopsies were obtained from additional patients and frozen in OCT compound for immunohistochemistry studies. Tonsil sections are used as negative and positive controls for immunohistochemistry.
Real-time polymerase chain reaction
RNA extraction, cDNA synthesis, and qPCR were performed as previously described (Zhao et al., 2009). Primer reference sequence accession number and positions (SABiosciences, Valencia, CA, U.S.A.) are as follows: IL-9: NM_000590.1 (Reference position: 353); CD3ε: NM_007648.4 (Reference position: 480), PU.1: NM_003120.2 (Reference position: 805); ETS1: NM_005238.2 (Reference position 1561), IRF4: NM_002460.3 (Reference position 1303), GcN5 (KAT2A): NM_021078.2 (Reference position 1664); eotaxin gene (CCL11): NM_002986.2 (Reference position: 285); IL-4: NM_000589.2 (Reference position:122); IFN-γ: NM_000619.2 (617); IL-17A: NM_010552.3 (Reference position:111). For mouse cytokine genes: IL-4: NM_021283 (Reference position: 46); IFN-γ: NM_008337 (Reference position: 309). Levels of 18S mRNA are used to normalize cytokine gene expression levels before calculating fold changes among patch test-positive and normal skin. For mouse tissue, the CHS skin was normalized to normal mouse ear skin.
Immunohistochemistry
ACD skin biopsies were embedded in OCT, then immediately frozen in liquid nitrogen followed by sectioning and fixation in acetone. Slides were incubated with rabbit anti-human PU.1 polyclonal antibody (Cell Signaling Technology, Danvers, MA. U.S.A.), followed by AlexaFluor594 goat anti-rabbit IgG (Invitrogen, Carlsbad, CA. U.S.A.). For double-labeling of T-cells and T-cell subsets, the slides were then incubated with fluorescein isothicyanate (FITC) labeled monoclonal mouse anti-human CD3, CD4, or CD8 antibodies (BD Pharmingen, San Diego, CA. U.S.A.). The slides were examined by a fluorescence microscope and two color digital images recorded. On average, twelve fields from each double-stained section were counted with a mean of 40 infiltrating cells per field.
In vitro Cytokine Secretion and Hapten-Specific Antibody ELISA
PBMCs were isolated from allergic patients and non-allergic control subjects using Accuprep (Accurate Chemical & Scientific Corporation, Westbury, New York, U.S.A.). PBMCs were cultured at concentration of 2×105/ml in AIM-V® medium with 10% FBS in triplicate. Cells were cultured with the pan T-cell mitogen phytohemagglutinin (PHA) 20 μg/ml, nickel (1.25, 2.50, 5.00, or 10.00 μg/ml), cobalt (1.25,2.50, 5.00, or 10.00μg/ml), or culture medium alone, and then incubated at 37°C for 96 hours. Then supernatants were collected and in vitro cytokine secretion for IFN-γ, IL-2 and IL-4 was measured by Luminex® assay with MILLIPLEX® MAP Kit (Millipore, Billerica, MA U.S.A.) and were assessed for IL-9 production with LEGEND MAX human IL-9 ELISA kit (Biolegend, San Diego, CA U.S.A.) (limit of detection for IL-9 is 1pg/mL) according to the manufacturer’s instructions (Pickering et al., 2002). ELISA to detect hapten-specific antibody responses was performed as previously described (Thatcher et al., 2006).
Blocking IL-9 production in vitro
PBMC were pre-treated with either anti-HLA DR (Mouse IgG2α, κ, mAb, L243, Biolegend, San Diego, CA) or HLA-A,B,C (Mouse IgG2α, κ, mAb, W6/32, Biolegend, San Diego, CA) at a concentration of 0.25μg/ml, or 1μg/ml. In parallel, they were pre-incubated with chloroquine (Sigma, St. Louis, MO) at a concentration of 5 μM or 20 μM for 2 hours, washed extensively, and then incubated with medium containing nickel chloride or medium alone at 37°C for 96 hours. Supernatants were collected for in vitro cytokine IL-9 detection.
Regulation of IFN-γ production in vitro by IL-9 or IL-4
PBMCs were incubated with recombinant human IL-9 (R&D systems, Minneapolis, MN); human IL-4 (E. coli derived, R&D systems, Minneapolis, MN) at a concentration of 10, 20, or 100 ng/ml; or combinations of IL-4 and IL-9 during the stimulation with metal nickel chloride (10 μg/ml) or medium, and then incubated at 37°C for 96 hours. Supernatants were collected for in vitro cytokine analysis of IFN-γ, IL-4 and IL-9.
Blocking IL-4 or IL-9 in vitro
PBMCs were incubated with anti-human IL-9 (polyclonal goat IgG, R&D systems, Minneapolis, MN) at a concentration of 0.5μg/ml or 1.0 μg/ml; anti-human IL-4 (polyclonal goat IgG, R&D systems, Minneapolis, MN) at a concentration of 0.5 μg/ml; or normal goat IgG (polyclonal, R&D systems, Minneapolis, MN) at a concentration of 0.5μg/ml during the stimulation with metal nickel chloride (10μg/ml) or medium and then incubated at 37°C for 96 hours. Supernatants were collected for in vitro cytokine analysis of IFN-γ.
Contact hypersensitivity assay
DNFB CHS experiments with WT and IL-9−/− mice were conducted as previously described (Thatcher et al., 2006). At challenge, both sides of the ears were treated with DNFB. Ear swelling was calculated by subtracting the baseline ear thickness from thickness 24 hours after DNFB challenge (Minang et al., 2006). The CHS assay was repeated twice, and found to be reproducible. For irritant contact dermatitis, 1% croton oil in acetone applied to mouse ears as previously described (Schwarz et al, 1993), and ear swelling was measured at 24 hours. Skin biopsy specimens were taken from the pinna of the ear in selected animals at 24 hours after hapten challenge. These biopsies were paraffin-embedded, sectioned onto glass slides, stained with hematoxylin–eosin, and examined and photographed using a E600 Nikon microscope equipped with a Spot Digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI).
Experimental mice
The experimental mice were either wild type BALB/c mice (H-2d) (Jackson Labs, Bar Harbor, ME) or IL-9 knockout mice (IL-9−/−) generated on the BALB/c (H-2d) background in the laboratory of Dr. Flavell. The experimental animals were kept in standard housing conditions, fed mouse chow ad libatum, and exposed to a 12 hours light/dark cycle. All experimental procedures were reviewed and approved by the Yale University Institutional Animal Care and Use Committee.
Statistical Analysis
The data were expressed as mean ± SD of indicated numbers of experiments with GraphPad Prism3.03. The Pearson’s r correlation analysis test was performed with GraphPadInStat3.06. Unpaired t-test is performed for cytokine release; p values less than 0.05 were considered significant. ANOVA was used to compare the wild type and IL-9−/− mice for DNP5-specific immunoglobulin production.
Acknowledgments
Funding source: AAG: VA Merit Award 1 I01 BX000405-01A2
Footnotes
ACD = Allergic contact dermatitis
CHS = Contact hypersensitivity
DNFB = Dinitrofluorobenzene
DNP= Dinitrophenyl
PPD= paraphenylenediamine
The authors have no conflict of interest to declare.
References
- Bour H, Peyron E, Gaucherand M, Garrigue JL, Desvignes C, Kaiserlian D, et al. Major histocompatibility complex class I-restricted CD8+ T cells and class II-restricted CD4+ T cells, respectively, mediate and regulate contact sensitivity to dinitrofluorobenzene. Eur J Immunol. 1995;25:3006–10. doi: 10.1002/eji.1830251103. [DOI] [PubMed] [Google Scholar]
- Budinger L, Hertl M. Immunologic mechanisms in hypersensitivity reactions to metal ions: an overview. Allergy. 2000;55:108–15. doi: 10.1034/j.1398-9995.2000.00107.x. [DOI] [PubMed] [Google Scholar]
- Campos RA, Szczepanik M, Itakura A, Akahira-Azuma M, Sidobre S, Kronenberg M, et al. Cutaneous immunization rapidly activates liver invariant Valpha14 NKT cells stimulating B-1 B cells to initiate T cell recruitment for elicitation of contact sensitivity. J Exp Med. 2003;198:1785–96. doi: 10.1084/jem.20021562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang HC, Sehra S, Goswami R, Yao W, Yu Q, Stritesky GL, et al. The transcription factor PU.1 is required for the development of IL-9-producing T cells and allergic inflammation. Nat Immunol. 2010;11:527–34. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cher DJ, Mosmann TR. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987;138:3688–94. [PubMed] [Google Scholar]
- Coffman RL, Savelkoul HF, Lebman DA. Cytokine regulation of immunoglobulin isotype switching and expression. Seminars in immunology. 1989;1:55–63. [PubMed] [Google Scholar]
- Coulter EM, Jenkinson C, Farrell J, Lavergne SN, Pease C, White A, Aleksic M, Basketter D, Williams DP, King C, Pirmohamed M, Park BK, Naisbitt DJ. Measurement of CD4+ and CD8+ T-lymphocyte cytokine secretion and gene expression changes in p-phenylenediamine allergic patients and tolerant individuals. J Invest Dermatol. 2010;130:161–174. doi: 10.1038/jid.2009.187. [DOI] [PubMed] [Google Scholar]
- Dardalhon V, Awasthi A, Kwon H, Galileos G, Gao W, Sobel RA, et al. IL-4 inhibits TGF-beta-induced Foxp3+ T cells and, together with TGF-beta, generates IL-9+ IL-10+ Foxp3(-) effector T cells. Nat Immunol. 2008;9:1347–55. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finiasz MR, Franco MC, de la Barrera S, Rutitzky L, Pizzariello G, del Carmen Sasiain M, et al. IL-9 promotes anti-Mycobacterium leprae cytotoxicity: involvement of IFNgamma. Clin Exp Immunol. 2007;147:139–47. doi: 10.1111/j.1365-2249.2006.03241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gober MD, Gaspari AA. Allergic contact dermatitis. Curr Dir Autoimmun. 2008;10:1–26. doi: 10.1159/000131410. [DOI] [PubMed] [Google Scholar]
- Gocinski BL, Tigelaar RE. Roles of CD4+ and CD8+ T cells in murine contact sensitivity revealed by in vivo monoclonal antibody depletion. J Immunol. 1990;144:4121–8. [PubMed] [Google Scholar]
- Goswami R, Kaplan MH. Gcn5 is required for PU.1-dependent IL-9 induction in Th9 cells. Journal of Immunology. 2012;189(6):3026–33. doi: 10.4049/jimmunol.1201496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gounni AS, Hamid Q, Rahman SM, Hoeck J, Yang J, Shan L. IL-9-mediated induction of eotaxin1/CCL11 in human airway smooth muscle cells. J Immunol. 2004;173:2771–9. doi: 10.4049/jimmunol.173.4.2771. [DOI] [PubMed] [Google Scholar]
- Jurado A, Carballido J, Griffel H, Hochkeppel HK, Wetzel GD. The immunomodulatory effects of interferon-gamma on mature B-lymphocyte responses. Experientia. 1989;45:521–6. doi: 10.1007/BF01990501. [DOI] [PubMed] [Google Scholar]
- Kaplan DH, Igyarto BZ, Gaspari AA. Early immune events in the induction of allergic contact dermatitis. Nat Rev Immunol. 2012;12:114–24. doi: 10.1038/nri3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, et al. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am J Respir Crit Care Med. 2011;183:865–75. doi: 10.1164/rccm.200909-1462OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JY, Chen JS, Hsu CJ, Miaw SC, Liu CY, Lee SJ, et al. Epicutaneous sensitization with protein antigen induces Th9 cells. The Journal of investigative dermatology. 2012;132:739–41. doi: 10.1038/jid.2011.382. [DOI] [PubMed] [Google Scholar]
- Lombard-Platlet S, Bertolino P, Deng H, Gerlier D, Rabourdin-Combe C. Inhibition by chloroquine of the class II major histocompatibility complex-restricted presentation of endogenous antigens varies according to the cellular origin of the antigen-presenting cells, the nature of the T-cell epitope, and the responding T cell. Immunology. 1993;80:566–73. [PMC free article] [PubMed] [Google Scholar]
- Martin S, Lappin MB, Kohler J, Delattre V, Leicht C, Preckel T, et al. Peptide immunization indicates that CD8+ T cells are the dominant effector cells in trinitrophenyl-specific contact hypersensitivity. J Invest Dermatol. 2000;115:260–6. doi: 10.1046/j.1523-1747.2000.00038.x. [DOI] [PubMed] [Google Scholar]
- Minang JT, Arestrom I, Troye-Blomberg M, Lundeberg L, Ahlborg N. Nickel, cobalt, chromium, palladium and gold induce a mixed Th1- and Th2-type cytokine response in vitro in subjects with contact allergy to the respective metals. Clinical and experimental immunology. 2006;146:417–26. doi: 10.1111/j.1365-2249.2006.03226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136:2348–57. [PubMed] [Google Scholar]
- Oikawa T, Yamad T. Molecular biology of the Ets family of transcription factors. Gene. 2003;303:11–34. doi: 10.1016/s0378-1119(02)01156-3. [DOI] [PubMed] [Google Scholar]
- Pickering JW, Martins TB, Greer RW, Schroder MC, Astill ME, Litwin CM, et al. A multiplexed fluorescent microsphere immunoassay for antibodies to pneumococcal capsular polysaccharides. Am J Clin Pathol. 2002;117:589–96. doi: 10.1309/lmch-c4q2-vfl9-3t1a. [DOI] [PubMed] [Google Scholar]
- Refaat A, Zhou Y, Suzuki S, Takasaki I, Koizumi K, Yamaoka S, Tabuchi Y, Saiki I, Sakurai H. Distinct roles of transforming growth factor-beta-activated kinase 1 (TAK1)-c-Rel and interferon regulatory factor 4 (IRF4) pathways in human T cell lymphotropic virus 1-transformed T helper 17 cells producing interleukin-9. Journal of Biological Chemistry. 2011;286(24):21092–9. doi: 10.1074/jbc.M110.200907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saulnier M, Huang S, Aguet M, Ryffel B. Role of interferon-gamma in contact hypersensitivity assessed in interferon-gamma receptor-deficient mice. Toxicology. 1995;102:301–12. doi: 10.1016/0300-483x(95)03101-k. [DOI] [PubMed] [Google Scholar]
- Schwarz A, Krone C, Trautinger F, Aragane Y, Neuner P, Luger TA, Schwarz T. Pentoxifylline suppresses irritant and contact hypersensitivity reactions. J Investig Dermatol. 1993;101:549–552. doi: 10.1111/1523-1747.ep12365951. [DOI] [PubMed] [Google Scholar]
- Thatcher TH, Luzina I, Fishelevich R, Tomai MA, Miller RL, Gaspari AA. Topical imiquimod treatment prevents UV-light induced loss of contact hypersensitivity and immune tolerance. The Journal of investigative dermatology. 2006;126:821–31. doi: 10.1038/sj.jid.5700167. [DOI] [PubMed] [Google Scholar]
- Veldhoen M, Uyttenhove C, van Snick J, Helmby H, Westendorf A, Buer J, et al. Transforming growth factor-beta ‘reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat Immunol. 2008;9:1341–6. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- Vermeer PD, Harson R, Einwalter LA, Moninger T, Zabner J. Interleukin-9 induces goblet cell hyperplasia during repair of human airway epithelia. Am J Respir Cell Mol Biol. 2003;28:286–95. doi: 10.1165/rcmb.4887. [DOI] [PubMed] [Google Scholar]
- Vocanson M, Hennino A, Cluzel-Tailhardat M, Saint-Mezard P, Benetiere J, Chavagnac C, et al. CD8+ T cells are effector cells of contact dermatitis to common skin allergens in mice. J Invest Dermatol. 2006;126:815–20. doi: 10.1038/sj.jid.5700174. [DOI] [PubMed] [Google Scholar]
- Wan YY, Flavell RA. How diverse--CD4 effector T cells and their functions. J Mol Cell Biol. 2009;1:20–36. doi: 10.1093/jmcb/mjp001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B, Huang C, Kato-Maeda M, Hopewell PC, Daley CL, Krensky AM, et al. IL-9 is associated with an impaired Th1 immune response in patients with tuberculosis. Clin Immunol. 2008;126:202–10. doi: 10.1016/j.clim.2007.09.009. [DOI] [PubMed] [Google Scholar]
- Xu H, DiIulio NA, Fairchild RL. T cell populations primed by hapten sensitization in contact sensitivity are distinguished by polarized patterns of cytokine production: interferon gamma-producing (Tc1) effector CD8+ T cells and interleukin (Il) 4/Il-10-producing (Th2) negative regulatory CD4+ T cells. J Exp Med. 1996;183:1001–12. doi: 10.1084/jem.183.3.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying S, Meng Q, Kay AB, Robinson DS. Elevated expression of interleukin-9 mRNA in the bronchial mucosa of atopic asthmatics and allergen-induced cutaneous late-phase reaction: relationships to eosinophils, mast cells and T lymphocytes. Clin Exp Allergy. 2002;32:866–71. doi: 10.1046/j.1365-2222.2002.01376.x. [DOI] [PubMed] [Google Scholar]
- Yip HC, Karulin AY, Tary-Lehmann M, Hesse MD, Radeke H, Heeger PS, et al. Adjuvant-guided type-1 and type-2 immunity: infectious/noninfectious dichotomy defines the class of response. J Immunol. 1999;162:3942–9. [PubMed] [Google Scholar]
- Zhao Y, Balato A, Fishelevich R, Chapoval A, Mann DL, Gaspari AA. Th17/Tc17 infiltration and associated cytokine gene expression in elicitation phase of allergic contact dermatitis. The British journal of dermatology. 2009;161:1301–6. doi: 10.1111/j.1365-2133.2009.09400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]