Abstract
Glutamate is the primary excitatory neurotransmitter in the brain, and is implicated in neurodegenerative diseases such as Alzheimer’s disease (AD) and several other tauopathies. The current method for measuring glutamate in vivo is using proton magnetic resonance spectroscopy (1H MRS), although it has poor spatial resolution and weak sensitivity to glutamate changes. In this study, we sought to measure the effect of tau pathology on glutamate levels throughout the brain of a mouse model of tauopathy using a novel magnetic resonance imaging (MRI) technique. We employed glutamate chemical exchange saturation transfer (GluCEST) imaging, which has been previously validated as a complimentary method for measuring glutamate levels with several important advantages over conventional 1H MRS. We hypothesized that the regional changes in glutamate levels would correlate with histological measurements of pathology including pathological tau, synapse and neuron loss. Imaging and spectroscopy were carried out on tau transgenic mice with the P301S mutation (PS19, n=9) and their wild-type littermates (WT, n=8), followed by immunohistochemistry of their brain tissue. GluCEST imaging resolution allowed for sub-hippocampal analysis of glutamate. Glutamate was significantly decreased by 29% in the CA sub-region of the PS19 hippocampus, and by 15% in the thalamus, where synapse loss was also measured. Glutamate levels and synapse density remained high in the dentate gyrus sub-region of the hippocampus, where neurogenesis is known to occur. The further development of GluCEST imaging for preclinical applications will be valuable, as therapies are being tested in mouse models of tauopathy.
Keywords: Glutamate, synapse loss, tauopathy, dentate gyrus, neurogenesis, chemical exchange saturation transfer
1. INTRODUCTION
Tauopathy is a classification of neurodegenerative diseases with more than 20 phenotypes, which include varying symptoms of impaired motor function and dementia (1), (2). The defining pathology of tauopathy is the presence of neurofibrillary tangles (NFTs) comprised of hyper-phosphorylated tau protein (HPtau). Tau is a microtubule binding protein where the extent of phosphorylation at multiple sites regulates its binding to microtubules (3). Pathological tau is found in the form of fibrillar aggregates, which accumulate in the perikarya and dendritic spines of hippocampal neurons and, among other consequences, disrupt the function of neurotransmitters (4). Adequate in vivo diagnoses and therapies for tauopathy are being actively studied, primarily for Alzheimer’s disease (AD), and also for corticobasal degeneration (CBD), progressive supranuclear palsy (PSP), amyotrophic lateral sclerosis/parkinsonism-dementia complex, Down’s syndrome, and several frontotemporal dementias (5).
Alterations in glutamate levels have been observed in several tauopathies. The neurotransmitter pool of glutamate is effected by decreased glutamate transporters and receptors in the hippocampus and cortex of AD patients compared to healthy controls (6). Glutamate transporters were also found to be associated with phosphorylated tau in neurofibrillary tangles in patients with AD, PSP, and CBD (7). The metabolic pool of glutamate is also affected in tauopathies. The TCA-cycle synthesizes glutamate from alpha-ketoglutarate, which is in a state of hypometabolism in early stages of AD (8),(9),(10) and in PSP (11).
Extracellular glutamate can be monitored using microdialysis methods (12), however to measure brain glutamate concentration noninvasively in vivo, the current standard method is proton magnetic resonance spectroscopy (1H MRS). Using 1H MRS, Rupsingh et. al. reported a decrease in glutamate in the hippocampus of AD patients compared with healthy controls (13). Despite the biochemical evidence of reduced glutamate in human tauopathy cases, transgenic mouse models of tauopathy have been only sparsely studied using 1H MRS. Mouse models of tauopathy with the P301L mutation have shown decreased glutamate levels in the hippocampus (14) and in hippocampal extracts (10) by 1H MRS. However, the spatial distribution of glutamate changes is impossible to measure using MRS because of its limited spatial resolution.
We recently described an MRI technique that is sensitive to glutamate levels in the brain: glutamate chemical exchange saturation transfer (GluCEST) imaging (15). GluCEST is complimentary to MRS in that it provides a measure proportional to glutamate concentration ([Glu]), at physiologic pH, but it is superior in several important factors. GluCEST imaging has higher spatial resolution than a spectroscopic method. Also, smaller changes in glutamate levels can be measured by GluCEST. Compared to the glutamate signal at 2.35ppm measured by 1H MRS, the measurable GluCEST effect has two orders of magnitude greater signal. In other words, a higher dynamic range of glutamate levels can be measured using GluCEST imaging compared to single-voxel spectroscopy. This proves to be a valuable tool in diseases where glutamate changes are subtle, as may be the case for early stages of dementia. In a recent publication, our group has demonstrated the feasibility of measuring glutamate changes using GluCEST and validated the measurement with 1H MRS in a mouse model of AD with amyloid-beta pathology (16).
In this study, we have used GluCEST imaging as well as 1H MRS to study the consequence of tau pathology on glutamate in a P301S mouse model. In vivo measures of glutamate are correlated with histological measurements of tau burden, including the severity of pathological tau, neuron loss, and synapse loss. Given the translational opportunities of GluCEST, our findings in a mouse model of tauopathy have immediate potential application to clinical studies.
2. METHODS
2.1 PS19 Transgenic Mouse Model of Tauopathy
The mouse model of tauopathy studied here was the PS19 line of the P301S transgenic mouse, overexpressing the human P301S mutant tau found in FTDP-17 patients, developed by Yoshiyama et. al. (17). The first sign of tau pathology in this mouse model is defective axonal transport, followed by synapse loss and hyper-phosphorylated tau accumulation at presynaptic terminals. As the animals age, pathological tau progresses along the perforant pathway, from the entorhinal cortex into the hippocampus and pre-frontal cortex, while severe neuron loss is apparent at later stages of disease (18). Behavioral studies show decreased ability in spatial learning with the progression of disease (19). In this study, we have imaged aged PS19 mice (n=9, mean age=20.7 months), and their age-matched wild-type (WT) littermates (n=8, mean age=19.0 months). This study was approved by the university’s IACUC. Note that the onset of pathology in this generation of mice is later than originally published (see current generation (20).
2.2 MRS Acquisition
All spectroscopy and imaging studies were performed on a 9.4T using a 30 cm horizontal bore magnet fitted with an 11 cm gradient insert and interfaced to a Varian spectrometer (Agilent Technologies Inc., Santa Clara, CA), with a vendor-supplied, mouse volume coil (M2M Imaging Corp., Cleveland, OH). Mice were anesthetized using isoflurane (1–2% in 1–2 L/min oxygen) for the duration of the scan. During the study animals were kept at 37°C using a heater and air-pump to blow hot air into the bore of the magnet. These methods were approved by the IACUC of the University Pennsylvania.
1H MRS was performed on WT (n=8) and PS19 (n=7) mice using the PRESS pulse sequence (TR/TE = 3000/14 ms, spectral width = 4 kHz, number of points = 4006). The variable pulse power and optimized relaxation delays (VAPOR) water suppression technique was used to acquire a water-suppressed spectrum (averages = 384), and another spectrum was acquired without water suppression to obtain the water reference signal for normalization (averages = 16). Unsuppressed water spectra had line widths of 20Hz or less after localized shimming. Total acquisition time for spectroscopy was about 20 minutes. Spectra were acquired from a voxel localized in the hippocampus (2×2.5×3mm3).
The integrals of the peaks of glutamate ([Glu]) and other metabolites of interest, including n-acetyl-aspartate (NAA), were calculated from spectra using in-house written software (MATLAB 7.9.0 R2009b). We are reporting the ratio of the integral of the metabolites with that of creatine. Manual baseline correction and phasing were performed. Metabolite peak locations and widths were identified manually, and combined with a pre-determined set of macromolecule peaks. The peak-fitting routine performs a nonlinear, least squares fitting of Lorentzian peaks to the spectra (MATLAB “lsqcurvefit”). The ratio of the integrals of [Glu] and [NAA] peaks to the internal standard of total creatine (tCr) is reported.
2.3 GluCEST Imaging
GluCEST (glutamate chemical exchange saturation transfer) image contrast is based on amine protons of glutamate that are in chemical exchange with protons on bulk water. The amine protons are saturated by application of a frequency-selective radio frequency (RF) pulse. As the saturated magnetization (zero net magnetization) of amine protons is exchanged with bulk water protons, bulk water MRI signal is reduced in proportion to the concentration of glutamate.
In the brain, the signal can be affected by direct water saturation and background magnetization transfer effect. To account for these effects, two images are obtained, one with saturation at the resonance frequency of amine exchanging spins (+3ppm downfield from water for glutamate amine protons) and a second image with equal frequency offset on the other side of the bulk water peak (−3ppm). The CEST effect of the amine spins is given by the difference in the asymmetry ratio (Eqn 1).
| eqn.(1) |
The Msat (±Δω) are the magnetizations obtained with saturation at a ‘+’ and ‘−‘ offset to the water resonance; Δω is equivalent to the resonance offset of the exchanging spins.
There are several important considerations in the pulse sequence design. Amine protons on glutamate resonate at 3ppm offset downfield from water (Δω~7200 rad.s−1 at 9.4T), with an exchange rate (k ~ 3000 s−1) in the slow to intermediate regime (Δω>k). We have implemented a saturation pulse of 1 second duration (four Hanning pulses at 250ms each, with a 4µs inter-pulse-delay), with an amplitude B1rms of 5.9 uT, which has been optimized in order to adequately saturate the amine protons on glutamate (16). A segmented, spoiled GRE readout is used, with 2 shots for each offset frequency. Each shot consists of one saturation pulse and a 64 segment readout (segment TR/TE = 6.7/3.4 ms). The time between shots is set to 8 s to allow for T1 recovery. The final image parameters are: matrix size = 128 × 128, FOV = 20 × 20mm, slice thickness = 1mm, voxel size = 0.16 × 0.16 × 1 mm3. GluCEST maps were acquired from one slice through the mid-hippocampus, with four signal averages for each saturation frequency.
In interpreting the CEST effect, other factors that play a role are B0 inhomogeneities, and the suboptimal amplitude and duration of the saturation pulse (15).
Any local B0 Inhomogeneity will cause the saturation pulse to miss the targeted glutamate protons and results in spurious data. To account for this a B0 map is acquired based on differences in phase accumulation in GRE images acquired at successive TE = 3.5, 4.0, 4.5 ms (2 signal averages each). Additionally, several images are acquired with the saturation pulse applied over a range of offset frequencies (Δω = ± 2.4–3.6ppm, steps of 0.2ppm), which spans the variation in B0 (±0.6ppm) expected from the B0 map. B0 corrected CEST weighted images at ± 3 ppm are calculated from the local B0 value and the acquired CEST weighted images from different offset frequencies using polynomial interpolation.
In addition, B1 maps are acquired in order to correct for inefficiencies in the saturation pulse, as described previously (15). Specifically, B1 maps were calculated from two images acquired with a rectangular preparation pulse with varying flip angles (30°, 60°), (2 signal averages each). A linear correction for B1 is applied to the CEST map using a ratio of the actual B1 value to the expected value. The total scan time for GluCEST, B0, and B1 maps was under 12 minutes.
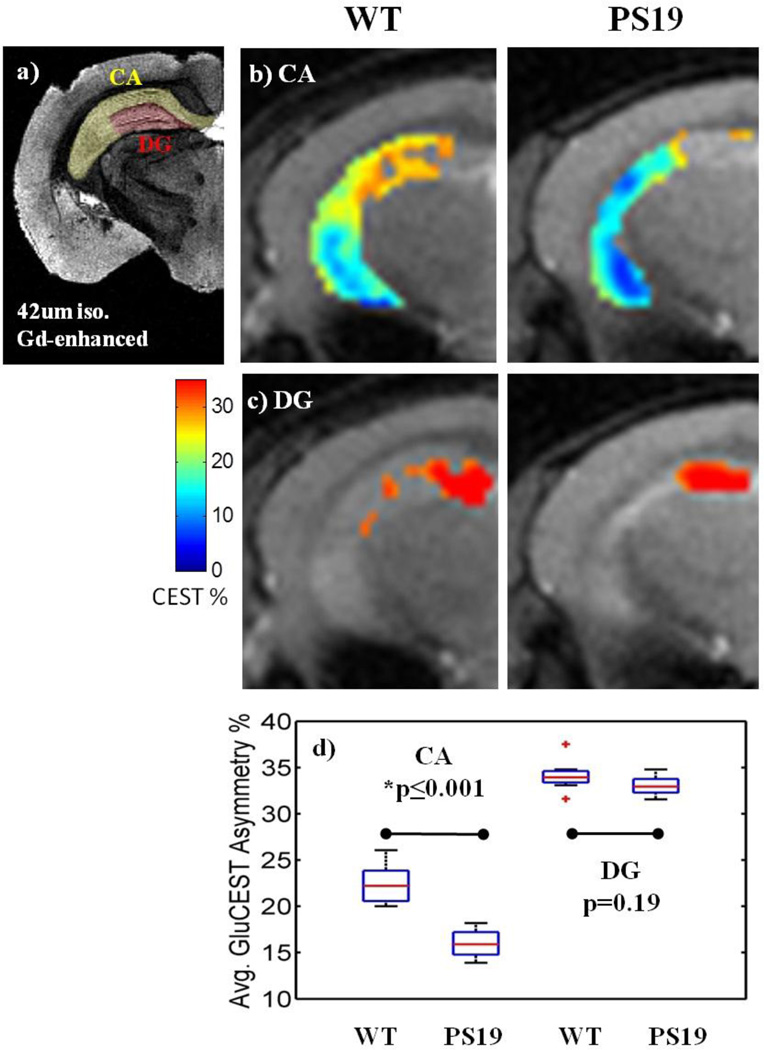
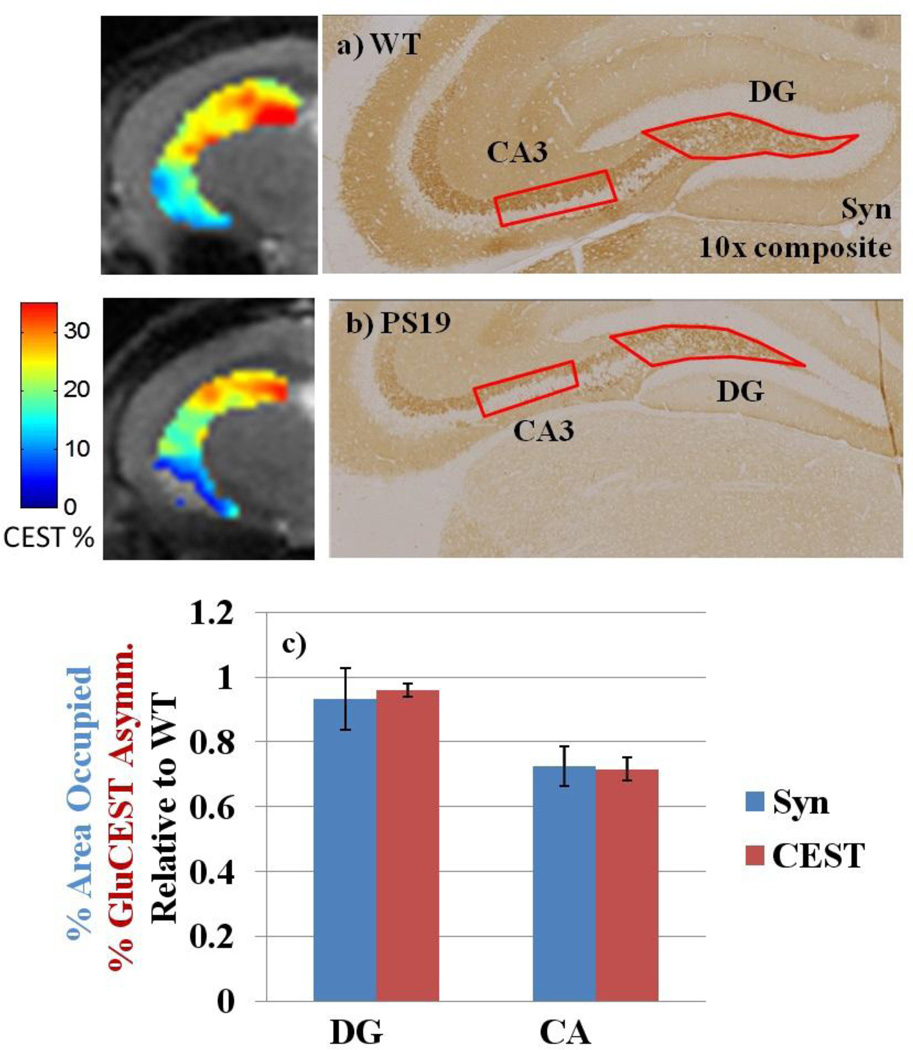
Regions of interest (ROIs) were segmented by hand from T2-weighted images, including the hippocampus, thalamus, and cortex. Sub-regions of the hippocampus cannot be distinguished using the in vivo image, and were segmented based on a threshold applied to the GluCEST maps: above 30% GluCEST asymmetry was considered the dentate gyrus (DG) region, below 5% as CSF and surrounding voxels with partial volume effects, and the mid-range values as the cornu ammonis (CA). For comparison, a high-resolution ex vivo image of the mouse brain shows that this threshold segments the DG from the CA region adequately (Figure 3a). The high-resolution image was acquired based on the gadolinium enhanced protocol developed by Johnson et. al. (21). The thalamus was segmented excluding the cerebral peduncle, and the cortex was segmented excluding the superior sagittal sinus.
Figure 3. Average GluCEST asymmetry in sub-regions of the hippocampus.
a) For anatomical comparison, a high-resolution (42 um isotropic) image of the ex vivo brain was acquired after infusion with gadolinium (Gd) according to the protocol by Johnson et. al. for MR histology (18).
b–c) The hippocampus is a composite of two distinct sub-regions, the CA and the DG. The DG was segmented based on a GluCEST threshold above 30%, which corresponds to the correct anatomical location as compared to the Gd-enhanced image.
d) In the CA sub-region of the PS19 hippocampus, GluCEST is significantly decreased by 29% (p≤0.001). There is no significant difference between groups in the DG sub-region of the hippocampus (p=0.19).
2.4 Immunohistochemistry
After imaging, mice were sacrificed and brains were prepared for immunohistochemical analysis. All mouse brains were prepared using standard methods of transcardial perfusion/fixation as approved by the IACUC. Mice were deeply anesthetized by intraperitoneal injection of a mixture of ketamine hydrochloride (1mg/10g) and xylazine (0.1mg/10g). Animals were perfused with 10ml of phosphate-buffered saline (PBS) followed by 20mL of 4% paraformaldehyde (PFA) (19). The brains were removed from the skull and stored overnight at 4°C in 4% PFA. Paraffin-embedded sections were sliced at 6µm thickness, and stained with an immunohistochemistry (IHC) protocol utilizing peroxide and streptavidin-biotin pre-treatments, and a horseradish peroxidase developing system (BioGenex, Hyderabad, AP India). Three different primary monoclonal antibodies (MAbs) were used in this study. The first MAb is AT8, an antibody specific for phosphorylated tau at residues Ser202 and Thr205 (22) (1:7500 dilution in PBS, Thermo Scientific). This MAb is used to localize pathological tau protein, which is hyperphosphorylated. Adjacent tissue sections were immunostained with NeuN antibody for neuronal nuclei (1:200 dillution, Millipore), and MAb SY38 for synaptophysin localized to the pre-synaptic terminals (1:2000 dillution, Millipore). Quantification of synaptophysin (WT n=4, PS19 n=7) and NeuN (WT n=5, PS19 n=6) immunostaining in the CA3 was performed on 20× images; synaptophysin in the DG on 4× images, in the thalamus on 20× images, and in the entorhinal cortex on 20× images, after setting an intensity threshold for all images. Percentages reported reflect the area occupied by immunostaining, normalized with the area of the ROI (Image J).
2.5 Statistical analyses
Statistical analyses were performed using MATLAB (7.9.0 R2009b). Boxplots were generated in MATLAB and represent the median in red, upper and lower quartiles within the box, and the extremes at the whiskers. Outliers were considered those data points beyond one times the interquartile range. Mean and standard deviations of all measures are reported in mean ± std format throughout. Student’s t-test was performed to determine significance of differences between measures obtained in PS19 brains to that from WT animals.
3. RESULTS
3.1 1H MRS
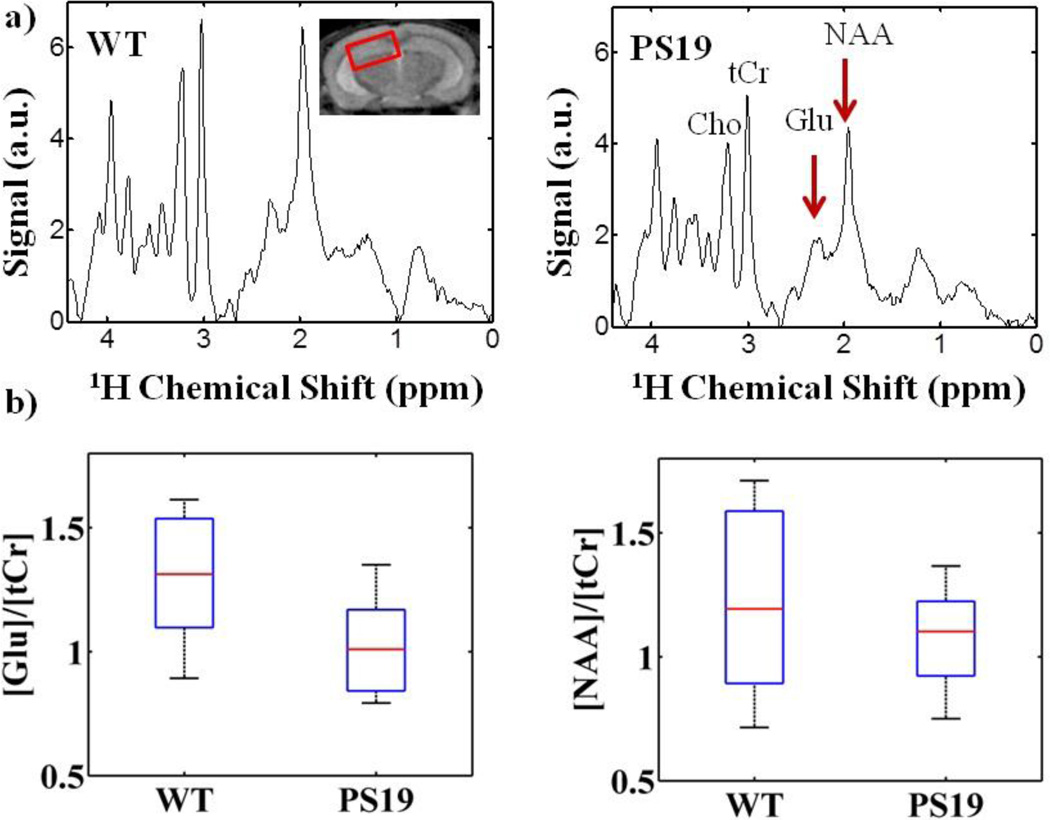
Representative metabolite spectra from the hippocampus of a WT and PS19 mouse are shown in Figure 1a. Differences in metabolite profiles were evident between WT and PS19 cohorts. The greatest difference was measured in glutamate at 2.35ppm, where the average ratio of glutamate to total creatine [Glu]/[tCr] in PS19 mice was 1.0 ± 0.10 (mean ± std) compared to 1.3 ± 0.13 in the WT cohort (p=0.11). The average concentration of tCr was not different between PS19 and WT mice. The metabolite N-acetyl-aspartate (NAA, 2.01ppm), as a ratio to [tCr], was also decreased in the PS19 mice (1.1 ± 0.10 vs. 1.2 ± 0.19, p=0.34).
Figure 1.
In the hippocampus, PS19 mice have decreased glutamate compared to WT according to 1H MRS. a) Example spectra from a PS19 mouse compared to WT shows diminished peaks of N-acetyl-aspartate (NAA, 2.01ppm) and glutamate (Glu, 2.35ppm). b) The average ratio of glutamate to total creatine [Glu]/[tCr] was decreased in the majority of PS19 mice compared to the WT cohort (n=7, 1.0 ± 0.10, mean ± std/2, vs. 1.3 ± 0.13, p=0.11). There was also a slightly lower group average of NAA in PS19 mice (1.1 ± 0.10 vs. 1.2 ± 0.19, p=0.34).
3.2 GluCEST
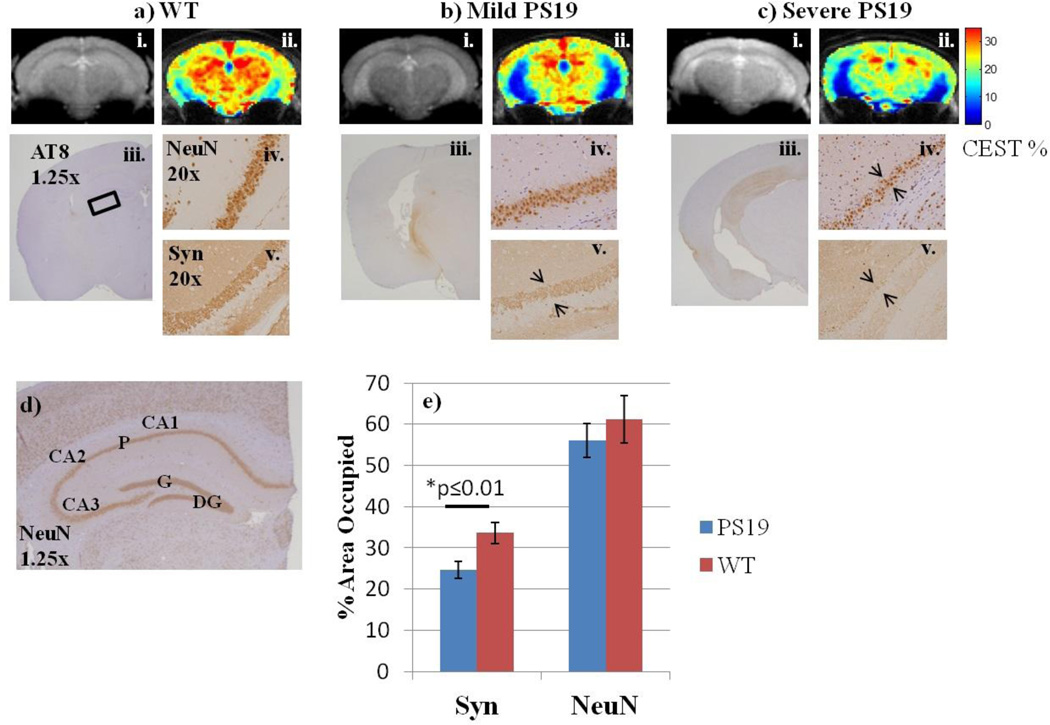
GluCEST asymmetry in WT mice was higher in grey matter as compared to white matter with very low glutamate levels in the CSF (Figure 2ii). The GluCEST maps from PS19 mice showed distinctively decreased glutamate across the majority of the brain slice (Figure 2bii, 2cii). These results are consistent with those previously observed from APP+PS1 mice (16).
Figure 2. GluCEST maps and corresponding IHC.
a–c i) Anatomical images corresponding to the GluCEST slice through the mid-hippocampus.
a–c ii) GluCEST maps in a WT and two PS19 mice reveal lower glutamate levels throughout the brain of tauopathy mice.
a–c iii) Corresponding slices of brain tissue stained for hyper-phosphorylated tau (AT8) reveal varying severity of pathological tau protein within the PS19 cohort.
a–c iv. & v.) Neuron (NeuN) and synapse (Syn) immunostaining in the CA3 pyramidal cell layer of the hippocampus. While neuron loss in this region is inconsistent, synapse loss is apparent in PS19 mice, where immunostaining is fainter and reveals a thinning band of synapses.
d) Detailed hippocampal anatomy for reference. CA1-3: cornu ammonis, P: pyramidal cell layer of CA, DG: dentate gyrus, G: granule cell layer of DG.
e) Quantified NeuN and Syn staining in the CA3 of the hippocampus. Synapse density is significantly reduced in PS19 mice (24.6 ± 2.1 vs. 33.6 ± 2.6, p≤0.01). Neuron density is less-severely reduced in PS19 mice (55.4 ± 4.4 vs. 61.2 ± 5.7, p=0.18).
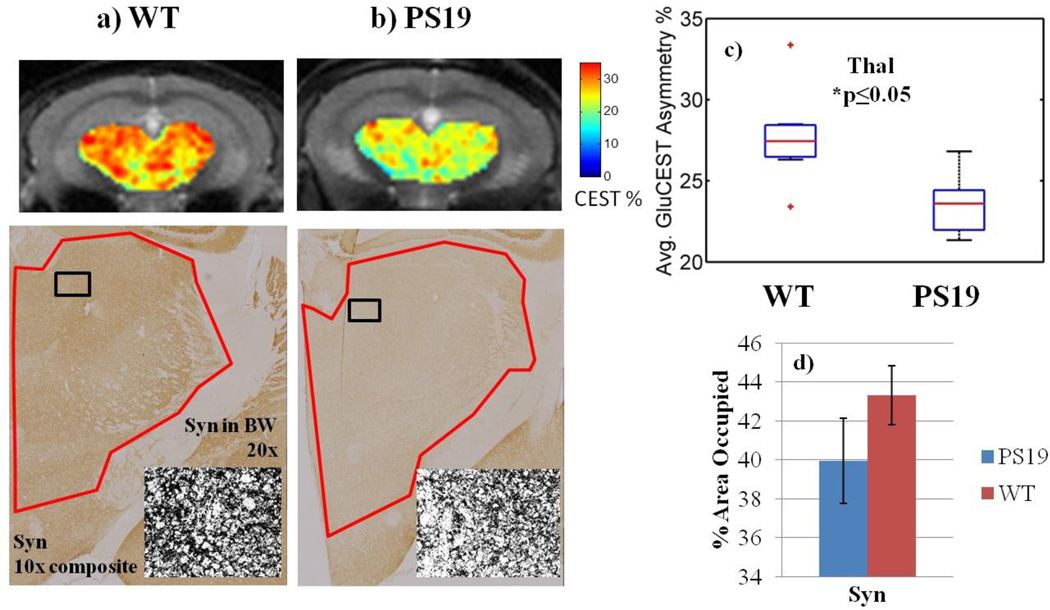
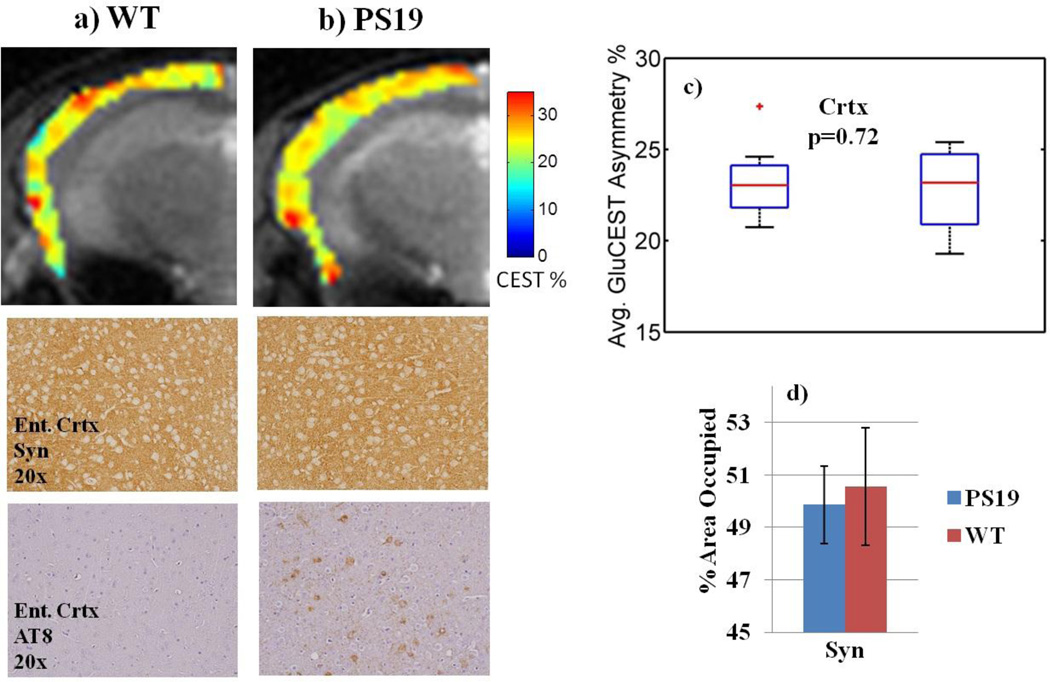
Within the hippocampus, two sub-regions of GluCEST contrast are distinguishable (Figure 3b, 3c). The first region includes the majority of the hippocampus, which has lower GluCEST signal in PS19 mice (15.9% ± 0.76, mean GluCEST asymmetry% ± std); compared to 22.4% ± 1.1 for WT, p≤0.001). This region corresponds to the anatomical sub-region of the hippocampus called the cornu ammonis (CA) and the surrounding axonal layers. The second region corresponds to the anatomical region of the dentate gyrus (DG, Figure 3c) and has consistently high GluCEST signal in both WT (34.1% ± 0.84) and PS19 (33.0% ± 0.55, p=0.19). Significantly reduced GluCEST levels were also measured in the thalamus region of PS19 mice (23.5% ± 0.89 vs. 27.7% ± 1.4, p≤0.05, Figure 5c). Average GluCEST asymmetry was not statistically different in the cortex of PS19 mice compared to WT (22.8% ± 1.2 vs. 23.3% ± 1.1, p=0.72, Figure 6c).
Figure 5. Comparison of GluCEST with synapse immunostaining in the thalamus.
a–b) Synapse immunostaining and corresponding GluCEST ROIs in the thalamus. The black and white inset shows less punctate staining in the PS19 thalamus.
c) Average GluCEST asymmetry of the thalamus (Thal) region is significantly decreased by 15% (p≤0.05) in PS19 mice compared to WT.
d) Synapse density shows a similar trend, decreasing 8%, although significance was not reached.
Figure 6. Comparison of GluCEST with synapse immunostaining in the cortex.
a–b) GluCEST ROIs in the cortex and corresponding immunostaining in the entorhinal cortex showing no difference in synapse density even though pathological tau is present.
c) Average GluCEST asymmetry was not statistically different in the cortex of PS19 mice (22.8% ± 1.2) compared to WT (23.3% ± 1.1), p=0.72.
d) Quantified synapse density in the entorhinal cortex was not different between the two cohorts.
3.3 Histological Measures of Tau Burden
We sought a neuropathological correlation to glutamate changes in the tauopathy mouse brain. Brain tissue was processed by IHC for hyper-phosphorylated tau (AT8, Figure 2iii), for neuron density (NeuN, Figure 2iv), and for synapse density (Syn38, Figure 2v). The severity of tau pathology varied among PS19 mice. An example of a PS19 mouse with less pathology is shown in Figure 2biii. The majority of PS19 mice demonstrated tau immunostaining in the entire hippocampus and in several layers of entorhinal cortex, as in Figure 2ciii.
Neuron density in the CA3 region of the PS19 hippocampus appears to be decreased in some PS19 mice (Figure 2civ), but not in others (Figure 2biv.), compared to the consistently dense band of neurons that are seen in WT mice (Figure 2aiv). Quantified neuron density reflects this inconsistency, where no significant difference was measured between WT and PS19 NeuN immunostaining (Figure 2e).
Synapse density is diminished in the CA3 of PS19 mice as evidenced by fainter staining and a thinning band of the pyramidal cell layer compared to WT mice (Figure 2bcv vs. 2av). Quantification reveals significantly lower synapse density in the CA3 of PS19 mice compared to WT (24.6 ± 2.1 vs. 33.6 ± 2.6, p≤0.01, Figure 2e). In the DG, there is no significant difference in synapse immunostaining in PS19 mice relative to WT (74.2 ± 7.5 vs. 79.6 ± 1.2, p=0.25, Figure 4c). The thalamus region has a lower density of punctuate immunostaining under high magnification, as depicted in the black and white (BW) insets (Figure 5a–b). Quantification of the intensity in this region shows an 8% lower area occupied in the PS19 thalamus (39.9 ± 2.2 vs. 43.3 ± 1.5, p=0.10, Figure 5d). There was no difference in synapse density in the entorhinal cortex of PS19 mice (49.9 ± 1.5 vs. 50.5 ± 2.2, p=0.37, Figure 6d).
Figure 4. Comparison of GluCEST with synapse immunostaining in sub-regions of the hippocampus.
a–b) Synapse immunostaining and corresponding GluCEST images of the hippocampus region.
c) Quantification of IHC and GluCEST in the DG and CA show similar trends. Synapses are maintained in the PS19 DG, where GluCEST was also high. Both synapse density and GluCEST asymmetry are significantly reduced by 27 and 29%, respectively, in PS19 mice relative to WT.
4. DISCUSSION
Here we have investigated the effect of tau pathology on glutamate levels in the brain of a transgenic mouse model, using both in vivo MR techniques and immunohistochemistry. We are reporting, for the first time, evidence that glutamate is decreased in the CA sub-region of the hippocampus, and in the thalamus, where synapse loss also occurs.
Using spectroscopic methods, we have measured a decrease in the [Glu]/[tCr] ratio in the hippocampus (Figure 1b). Glutamate differences were not significant according to 1H MRS likely due to the all-encompassing voxel over distinct sub-regions of the hippocampus. The hippocampus can be divided into two sub-regions, the CA and the DG, which have unique connectivity and function, and which have distinct structural changes in AD patients (23).
The GluCEST imaging technique was able to distinguish these two sub-regions, as a result of higher spatial resolution compared to conventional 1H MRS, as well as increased sensitivity to glutamate. Specifically, using the GluCEST method, the amine protons of glutamate are saturated over a period of one second. During this time, the saturated amine proton magnetization exchanges ~103 times and accumulates on the water pool. In theory, this amplifies 1 mM glutamate signal to 1M signal. But in practice, other experimental parameters such as suboptimal saturation and back exchange preclude the theoretical maximum amplification. As has been shown previously (15), in practice, GluCEST has at least two orders of magnitude sensitivity advantage over 1H MRS.
Note that the absolute measures are not comparable between 1H MRS and GluCEST in this study; due to spectral quality, it was necessary to acquire spectra from a thicker voxel (3mm) than GluCEST images (1mm). Previously, we have validated the direct correlation of the GluCEST signal to the concentration of glutamate measured by 1H MRS (see Haris et. al. 2013).
The GluCEST signal in the CA of the hippocampus was significantly reduced by 29%, which is associated with a significant reduction in synapse density by 27% (Figure 4c). This is in contrast to the DG sub-region, where glutamate was maintained even in the PS19 brain where neurodegeneration occurs in the rest of the hippocampus (Figure 3d). Synapse integrity was also intact in the DG of PS19 mice, with no significant differences measured from immunostaining (Figure 4c). This is consistent with the fact that there is a continuous turnover of neurons in the DG region compared to the other regions of the mouse brain (24), (25), (26). In the DG of AD patients (27) and a mouse model of AD (26), there is evidence that neurogenesis is increased. Therefore, it is important to distinguish the DG from the rest of the hippocampus of tauopathy brains. To the best of our knowledge, these are the first MRI results reporting neurogenesis in vivo.
Tau tangles are most abundant in the hippocampus of PS19 mice, which is where we expected to find reduced glutamate. However, we found more wide-spread glutamate loss in regions where pathological tau was not present, as in the case of the thalamus (Figure 5a–b). Vice versa, pathological tau was present in the entorhinal cortex of most PS19 mice, yet GluCEST was not decreased in this region (Figure 6a–b). Therefore, lower glutamate cannot only be explained by the occurrence of tau pathology.
Rather, synapse loss appears to be the closest neuropathological correlate to GluCEST imaging in this study. A thinning band of synapses were consistently observed in the CA3 region of the hippocampus of PS19 mice, unlike neuron loss which occured in only a few cases (Figure 2e). Similarly, neuronal dysfunction was only slightly indicated by a decrease in [NAA]/[tCr] in the PS19 hippocampus (Figure 1b). Also, synapse loss was measured in the thalamus of PS19 mice, where GluCEST decreases were significant, and yet pathological tau was not yet present (Figure 5). Therefore, synapse loss, and not neuron loss or pathological tau, correlates most closely with the location of glutamate loss measured by GluCEST maps in both the CA and thalamus.
Specifically, the mouse in Figure 2b has mild tau pathology and intact neurons, however the synapse band is weak. This case supports the hypothesis that decreased glutamate reflects early stages of neurodegeneration, before neuron loss. In a more severe case in Figure 2c, the GluCEST map is greatly decreased from WT, and associated with severe pathology, thinning neurons, and virtually no synapses in the CA3. This example supports the hypothesis that GluCEST will be decreased further as the severity of tau burden progresses beyond synapse loss.
Synapses are an important location of early neurologic dysfunction (28). Synapse loss is also the closest correlate to cognitive deficits in AD patients, rather than the amount of tangles (29). Glutametergic synapse loss is among the earliest symptoms of disease found in AD brain tissue (28). GluCEST imaging, therefore, has the potential to monitor synapse loss in vivo as an early marker of dementia symptoms.
In conclusion, relevant regions of glutamate loss in the P301S mouse model of tauopathy include the CA sub-region of the hippocampus and the thalamus. Reduced synapse density was also associated with these regions. Therefore, the further development of our novel imaging method, GluCEST MRI, for preclinical applications will be valuable as microtubule-stabilizing therapies are being tested in the PS19 mouse model of tauopathy (20). The DG sub-region maintains glutamate levels in tauopathy mice. Future studies should consider using GluCEST imaging to monitor the health of the DG as a potential region for neurogenic therapy (30).
Highlights.
GluCEST is sigificantly decreased in the CA of the hippocampus and thalamus.
Synapse loss is the closest histological correlate to GluCEST loss.
Glutamate is maintained in the dentate gyrus, neurogenic region of the hippocampus.
ACKNOWLEDGEMENTS
This work was supported by NIH grants from NIBIB (P41EB015893S1, P41EB015893) NIDA (R21DA0332256-01) and NIA (P01AG17586). Support from CTSA UL1 prior to July 1, 2012 “The project described was supported by the Penn CTSA National Center for Research Resources, Grant UL1RR024134, and is now at the National Center for Advancing Translational Sciences, Grant UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.” Support from the CTSA UL1 after July 1, 2012 “The project described was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR000003. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.”
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Hutton M, Lendon CL, Rizzu P, Baker M, Froelich S, Houlden H, Pickering-Brown S, Chakraverty S, Isaacs A, Grover A, Hackett J, Adamson J, Lincoln S, Dickson D, Davies P, Petersen RC, Stevens M, de Graaff E, Wauters E, van Baren J, Hillebrand M, Joosse M, Kwon JM, Nowotny P, Che LK, Norton J, Morris JC, Reed LA, Trojanowski J, Basun H, Lannfelt L, Neystat M, Fahn S, Dark F, Tannenberg T, Dodd PR, Hayward N, Kwok JBJ, Schofield PR, Andreadis A, Snowden J, Craufurd D, Neary D, Owen F, Oostra BA, Hardy J, Goate A, van Swieten J, Mann D, Lynch T, Heutink P. Association of missense and 5 '-splice-site mutations in tau with the inherited dementia FTDP-17. Nature. 1998;393(6686):702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- 2.Ballatore C, Lee VMY, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer's disease and related disorders. Nature Reviews Neuroscience. 2007;8(9):663–672. doi: 10.1038/nrn2194. [DOI] [PubMed] [Google Scholar]
- 3.Alonso AD, Zaidi T, Novak M, Grundke-Iqbal I, Iqbal K. Hyperphosphorylation induces self-assembly of tau into tangles of paired helical filaments/straight filaments. Proceedings of the National Academy of Sciences of the United States of America. 2001;98(12):6923–6928. doi: 10.1073/pnas.121119298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, Delocourte A, Galasko D, Gauthier S, Jicha G, Meguro K, O'Brien J, Pasquier F, Robert P, Rossor M, Solloway S, Stern Y, Visser PJ, Scheltens P. Research criteria for the diagnosis of Alzheimer"s disease: revising the NINCDS-ADRDA criteria. Lancet Neurology. 2007;6(8):734–746. doi: 10.1016/S1474-4422(07)70178-3. [DOI] [PubMed] [Google Scholar]
- 5.Lee VMY, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annual Review of Neuroscience. 2001;24:1121–1159. doi: 10.1146/annurev.neuro.24.1.1121. [DOI] [PubMed] [Google Scholar]
- 6.Greenamyre JT, Maragos WF. NEUROTRANSMITTER RECEPTORS IN ALZHEIMER-DISEASE. Cerebrovascular and Brain Metabolism Reviews. 1993;5(2):61–94. [PubMed] [Google Scholar]
- 7.Sasaki K, Shimura H, Itaya M, Tanaka R, Mori H, Mizuno Y, Kosik KS, Tanaka S, Hattori N. Excitatory amino acid transporter 2 associates with phosphorylated tau and is localized in neurofibrillary tangles of tauopathic brains. Febs Letters. 2009;583(13):2194–2200. doi: 10.1016/j.febslet.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 8.Mosconi L. Brain glucose metabolism in the early and specific diagnosis of Alzheimer's disease - FDG-PET studies in MCI and AD. European Journal of Nuclear Medicine and Molecular Imaging. 2005;32(4):486–510. doi: 10.1007/s00259-005-1762-7. [DOI] [PubMed] [Google Scholar]
- 9.Sancheti H, Kanamori K, Patil I, Brinton RD, Ross BD, Cadenas E. Reversal of metabolic deficits by lipoic acid in a triple transgenic mouse model of Alzheimer's disease: a C-13 NMR study. Journal of Cerebral Blood Flow and Metabolism. 2014;34(2):288–296. doi: 10.1038/jcbfm.2013.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nilsen LH, Rae C, Ittner LM, Goetz J, Sonnewald U. Glutamate metabolism is impaired in transgenic mice with tau hyperphosphorylation. Journal of Cerebral Blood Flow and Metabolism. 2013;33(5):684–691. doi: 10.1038/jcbfm.2012.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albers DS, Augood SJ, Park LCH, Browne SE, Martin DM, Adamson J, Hutton M, Standaert DG, Vonsattel JPG, Gibson GE, Beal MF. Frontal lobe dysfunction in progressive supranuclear palsy: Evidence for oxidative stress and mitochondrial impairment. Journal of Neurochemistry. 2000;74(2):878–881. doi: 10.1046/j.1471-4159.2000.740878.x. [DOI] [PubMed] [Google Scholar]
- 12.Minkeviciene R, Ihalainen J, Malm T, Matilainen O, Keksa-Goldsteine V, Goldsteins G, Iivonen H, Leguit N, Glennon J, Koistinaho J, Banerjee P, Tanila H. Age-related decrease in stimulated glutamate release and vesicular glutamate transporters in APP/PS1 transgenic and wild-type mice. Journal of Neurochemistry. 2008;105(3):584–594. doi: 10.1111/j.1471-4159.2007.05147.x. [DOI] [PubMed] [Google Scholar]
- 13.Rupsingh R, Borrie M, Smith M, Wells JL, Bartha R. Reduced hippocampal glutamate in Alzheimer disease. Neurobiology of Aging. 2011;32(5):802–810. doi: 10.1016/j.neurobiolaging.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 14.Yang DW, Xie ZY, Stephenson D, Morton D, Hicks CD, Brown TM, Sriram R, O'Neill S, Raunig D, Bocan T. Volumetric MRI and MRS provide sensitive measures of Alzheimer's disease neuropathology in inducible Tau transgenic mice (rTg4510) Neuroimage. 2011;54(4):2652–2658. doi: 10.1016/j.neuroimage.2010.10.067. [DOI] [PubMed] [Google Scholar]
- 15.Cai KJ, Haris M, Singh A, Kogan F, Greenberg JH, Hariharan H, Detre JA, Reddy R. Magnetic resonance imaging of glutamate. Nature Medicine. 2012;18(2):302–306. doi: 10.1038/nm.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haris M, Nath K, Cai K, Singh A, Crescenzi R, Kogan F, Verma G, Reddy S, Hariharan H, Melhem ER, Reddy R. Imaging of glutamate neurotransmitter alterations in Alzheimer's disease. Nmr in Biomedicine. 2013;26(4):386–391. doi: 10.1002/nbm.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshiyama Y, Higuchi M, Zhang B, Huang SM, Iwata N, Saido TC, Maeda J, Suhara T, Trojanowski JQ, Lee VMY. Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53(3):337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 18.Hurtado DE, Molina-Porcel L, Iba M, Aboagye AK, Paul SM, Trojanowski JQ, Lee VMY. A beta Accelerates the Spatiotemporal Progression of Tau Pathology and Augments Tau Amyloidosis in an Alzheimer Mouse Model. American Journal of Pathology. 2010;177(4):1977–1988. doi: 10.2353/ajpath.2010.100346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brunden KR, Zhang B, Carroll J, Yao YM, Potuzak JS, Hogan AML, Iba M, James MJ, Xie SX, Ballatore C, Smith AB, Lee VMY, Trojanowski JQ. Epothilone D Improves Microtubule Density, Axonal Integrity, and Cognition in a Transgenic Mouse Model of Tauopathy. Journal of Neuroscience. 2010;30(41):13861–13866. doi: 10.1523/JNEUROSCI.3059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang B, Carroll J, Trojanowski JQ, Yao YM, Iba M, Potuzak JS, Hogan AML, Xie SX, Ballatore C, Smith AB, Lee VMY, Brunden KR. The Microtubule-Stabilizing Agent, Epothilone D, Reduces Axonal Dysfunction, Neurotoxicity, Cognitive Deficits, and Alzheimer-Like Pathology in an Interventional Study with Aged Tau Transgenic Mice. Journal of Neuroscience. 2012;32(11):3601–3611. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson GA, Ali-Sharief A, Badea A, Brandenburg J, Cofer G, Fubara B, Gewalt S, Hedlund LW, Upchurch L. High-throughput morphologic phenotyping of the mouse brain with magnetic resonance histology. Neuroimage. 2007;37(1):82–89. doi: 10.1016/j.neuroimage.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goedert M, Jakes R, Vanmechelen E. MONOCLONAL-ANTIBODY AT8 RECOGNIZES TAU-PROTEIN PHOSPHORYLATED AT BOTH SERINE-202 AND THREONINE-205. Neuroscience Letters. 1995;189(3):167–170. doi: 10.1016/0304-3940(95)11484-e. [DOI] [PubMed] [Google Scholar]
- 23.Greene SJ, Killiany RJ. Alzheimers Dis N. Hippocampal Subregions are Differentially Affected in the Progression to Alzheimer's Disease. Anatomical Record-Advances in Integrative Anatomy and Evolutionary Biology. 2012;295(1):132–140. doi: 10.1002/ar.21493. [DOI] [PubMed] [Google Scholar]
- 24.Eriksson PS, Perfilieva E, Bjork-Eriksson T, Alborn AM, Nordborg C, Peterson DA, Gage FH. Neurogenesis in the adult human hippocampus. Nature Medicine. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 25.Spampanato J, Sullivan RK, Turpin FR, Bartlett PF, Sah P. Properties of Doublecortin Expressing Neurons in the Adult Mouse Dentate Gyrus. Plos One. 2012;7(9) doi: 10.1371/journal.pone.0041029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin KL, Galvan V, Xie L, Mao XO, Gorostiza OF, Bredesen DE, Greenberg DA. Enhanced neurogenesis in Alzheimer's disease transgenic (PDGF-APP(sw,lnd))mice. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(36):13363–13367. doi: 10.1073/pnas.0403678101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jin KL, Peel AL, Mao XO, Xie L, Cottrell BA, Henshall DC, Greenberg DA. Increased hippocampal neurogenesis in Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(1):343–347. doi: 10.1073/pnas.2634794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298(5594):789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 29.Terry RD, Masliah E, Salmon DP, Butters N, Deteresa R, Hill R, Hansen LA, Katzman R. PHYSICAL BASIS OF COGNITIVE ALTERATIONS IN ALZHEIMERS-DISEASE -SYNAPSE LOSS IS THE MAJOR CORRELATE OF COGNITIVE IMPAIRMENT. Annals of Neurology. 1991;30(4):572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 30.Chohan MO, Li B, Blanchard J, Tung YC, Heaney AT, Rabe A, Iqbal K, Grundke-Iqbal I. Enhancement of dentate gyrus neurogenesis, dendritic and synaptic plasticity and memory by a neurotrophic peptide. Neurobiology of Aging. 2011;32(8):1420–1434. doi: 10.1016/j.neurobiolaging.2009.08.008. [DOI] [PubMed] [Google Scholar]