Abstract
Diet, nutrition, and obesity are important topics of current research. While many insect genome and/or transcriptome models are based on dietary specialists, the lady beetle Coleomegilla maculata, a common New World species, is highly omnivorous. C. maculata feeds on plants, fungi, insects and other arthropods; its diet frequently includes conspecific cannibalism. This study reports and discusses the first nutritionally based C. maculata transcriptomes. These transcriptomes were prepared from highly inbred specimens provided limited diets, after adult eclosion, of either pollen only or eggs of a soft bodied hemipteran insect only. Selected sequences from the transcriptomes were compared to verify basic genetic similarity of the sampled individuals. Differentially expressed genes associated with these diets were identified to aid with studies of omnivore diet and nutrition. Selected transcriptome sequences described herein are filed with the National Center for Biotechnology Information (NCBI), GenBank Bioproject PRJNA236444.
Keywords: insect nutrition, omnivory, digestion, RNA stability, gene expression, Coccinellidae, biological control.
Introduction
North American agroecosystems are highly manipulated, particularly in the United States. Nonetheless, certain key insects persist within these ecosystem, acting as pests (consumers of or damaging to crops), as commensals, and as beneficials. One key beneficial insect found in many U. S. agroecosystems is the lady beetle Coleomegilla maculata (Coccinellidae: Coleoptera). C. maculata is a widely distributed 1 native North American species complex 2 and is generally accepted as an ecological indicator 3 and used in agricultural and ecological research as a representative non-target organism 4, 5. Given its importance in agricultural ecology, the biology of C. maculata has been well studied. Studies of the diet of C. maculata have evaluated nutrition, focusing on pollen 6, 7, prey 8, and aiming at artificial diet development 9. In the present work, gene expression in otherwise identical inbred specimens of this species of lady beetle are compared after restricted dietary intake, during the imaginal (adult) stage, of either pollen or insect eggs. The genetic resources described herein will facilitate further research on diet and digestive function in omnivorous organisms.
Results and Discussion
While C. maculata is accepted as an ecologically important lady beetle, it has not been widely utilized as a biological control agent. Some species of the family Coccinellidae are used as biological control in the US, such as Hippodamia convergens and Adalia bipunctata 10. Harmonia axiridis, an introduced but invasive lady beetle in the US, has recently been modified to produce transgenic research organisms, and will be useful as a genetic model 11. C. maculata has characters that make it a preferred model, including ease of maintenance 12 and visible phenotypic mutant strains (13 and unpublished results). The genome size of C. maculata is relatively small, estimated at 0.19 pg 14; based on standard conversion, assuming a diploid genome:
| Genome size (Mbp) = 978 X DNA content (pg) |
this is roughly equivalent to 186 Mbp 15 so sequencing the full genome of this insect should be achieveable. A reference transcriptome of C. maculata has been published to the internet (http://2ei.univ-perp.fr/?page_id=89, accessed 22 July 2014). The transcripts provided by the study described herein will facilitate further molecular genetic, biochemical, and physiological investigation. Because the insects used for this study were highly inbred and also siblings, the sequences represent a limited number of alleles, and do not represent the wider populations of the species in Mississippi or the broader environment. On the other hand, the sequences obtained may be used for a baseline to determine population genetics and identify variability in the species and between subspecies.
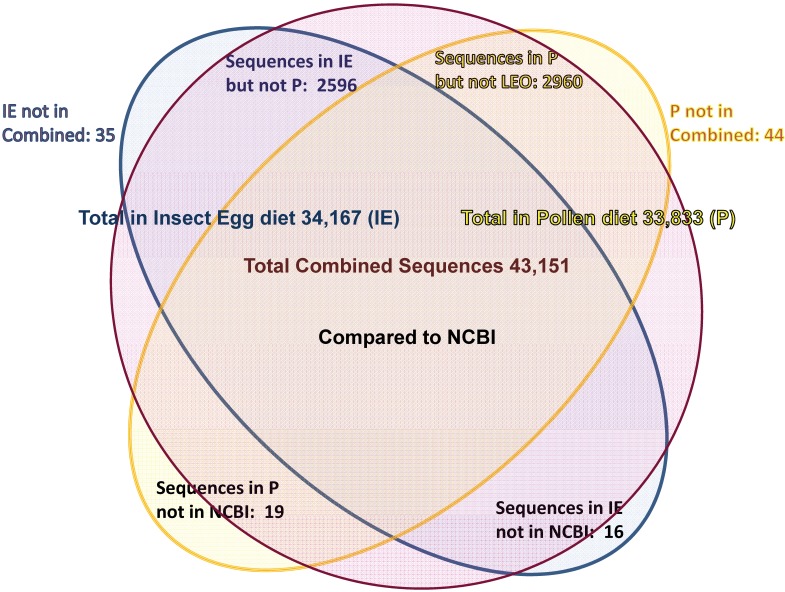
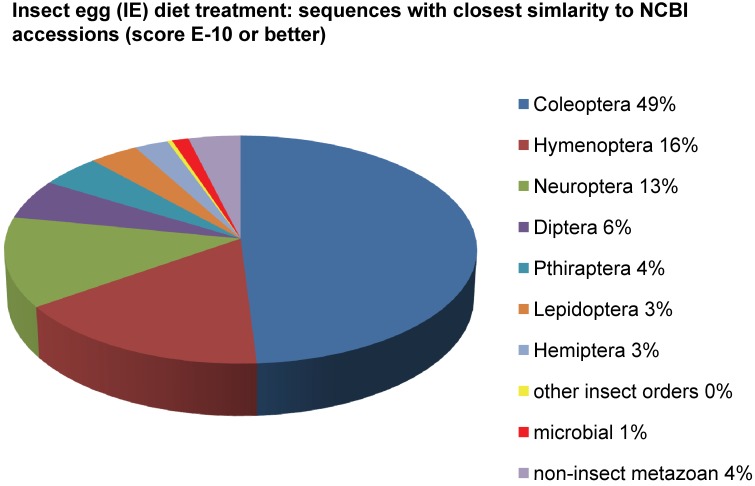
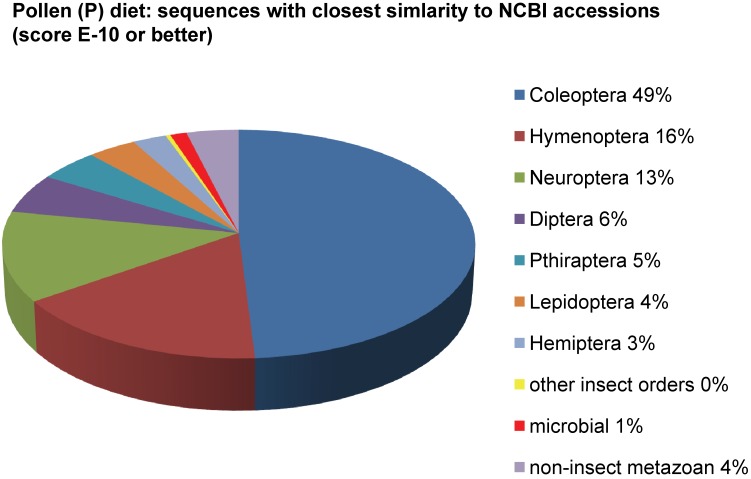
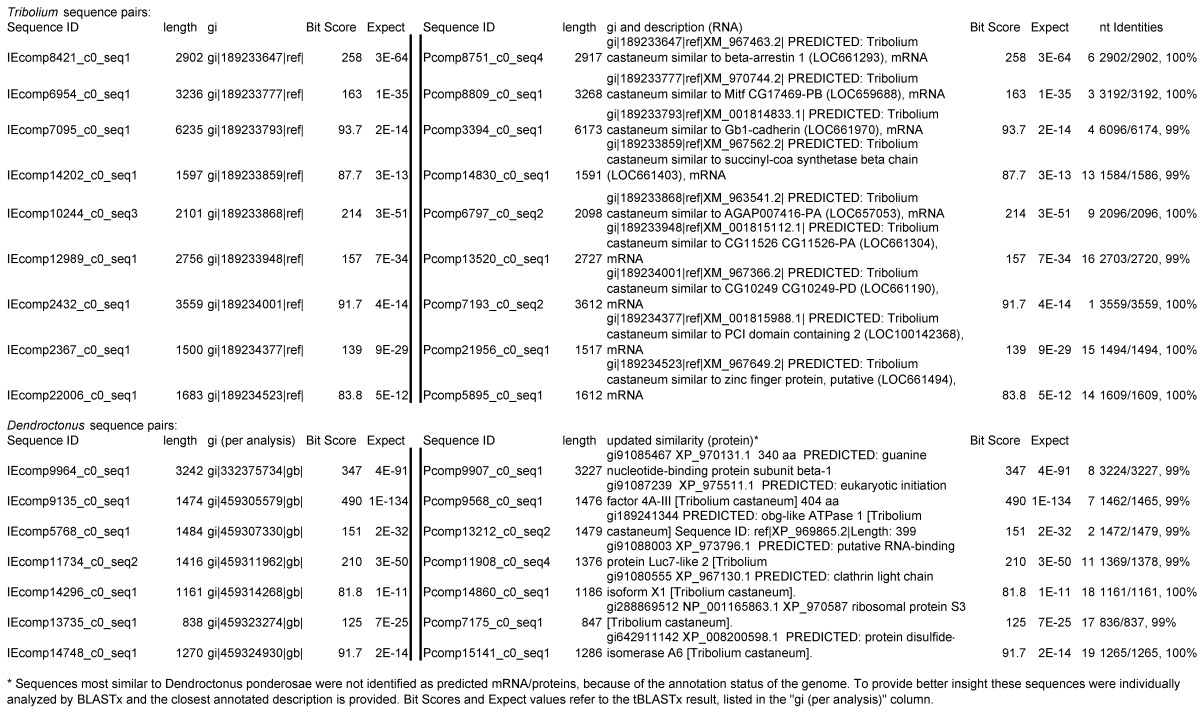
Assemblies of the total RNA yielded 33,833 assembled sequences from the pollen fed treatment and 34,167 assembled sequences from the insect egg fed treatment. The combined sequences assembled into 43,151 sequences. Average sequence lengths were 1403, 1300, and 1456 respectively. While many of the treatment group sequences were identical or nearly so, there were representatives of unique sequences as shown in Figure 1. Because the assemblies were de novo, many unique and unclassified sequences were expected. To gain an insight on the similarities between the assemblies, sequences were analyzed by NCBI BLAST® (US National Library of Medicine) 16 using the tBLASTx “search translated nucleotide database using a translated nucleotide query” (database accessed 22 May 2013). The resulting BLAST spreadsheets were sorted and the longer and more similar (to NCBI accessions) sequences were examined. Sequences shorter than 500 nt and with expect values >1e-10 were discarded. The remaining sequences amounted to around 10% of the total assembled sequences: 3,376 from the pollen fed treatment, and 3,358 from the insect egg fed treatment (Table 1A, Table 1B). The sequences from both treatments were primarily similar to other insect sequences (95%, +/- 0.5%), and to RNA sequences (not specifically analyzed). The largest portion of similarities by insect order was to Coleoptera, as expected (49%), as shown by two pie charts, one for each treatment, in Figure 2. The individual insects used for sequencing were inbred siblings, thus many identical sequences from the two samples were expected. The greatest number of similar sequences in GenBank came from the two beetle species Tribolium castaneum and Dendroctonus ponderosae. To validate the baseline similarity of our sequence sets, sixteen sequences that were most closely related to the same GenBank sequence, and at similar expect values and similar lengths, were chosen from each treatment and compared pairwise in BLAST (BLAST two sequences option). Results are shown in Table 2; all comparisons were at least 99% identical at the nucleotide level. These results support the assumption that the two sets of sequences represent nearly identical specimens, differing primarily in those transcripts of genes responding to diet provided to the adult insects. Also as expected, some sequences that were unique to the treatments were associated with the diet 17. Among the pollen fed sequences were hits similar to plant sequences, and among the egg fed sequences were hits similar to the genus of the insect eggs, Lygus spp. Interestingly, some of the egg diet sequences were nearly identical (e-value 0) to a virus recently described from Lygus lineolaris 18. It appears that the viable Lygus spp. eggs used as diet were carrying the virus, indicating that the virus was present in the laboratory colony and was able to resist degradation by the digestive system of C. maculata. The implications of this finding may be important for future pest control strategies aimed to utilize genetically modified or pathogenic viruses.
Figure 1.
Diagram of comparison of total contiguous sequences generated from adult insect samples fed either a diet of only insect eggs (IE) or only pollen (P).
Table 1A.
Closest homologous sequences; quantities by taxonomy. Treatment: pollen diet, 3376 non-redundant sequences with expect value of <1.00e-10. Right hand columns are insect genera, left hand columns are non-insect. Sequences that differ from the insect egg diet treatment in quantity of hits are indicated by asterisk (*).
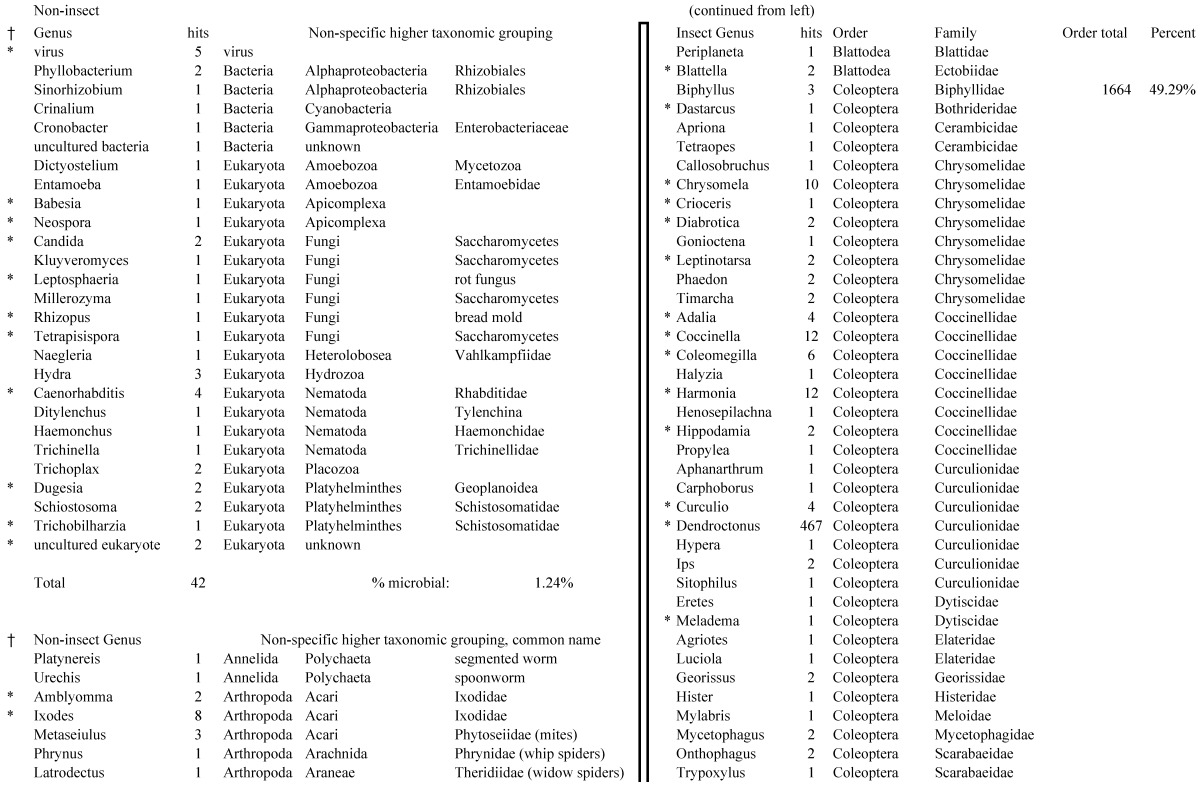
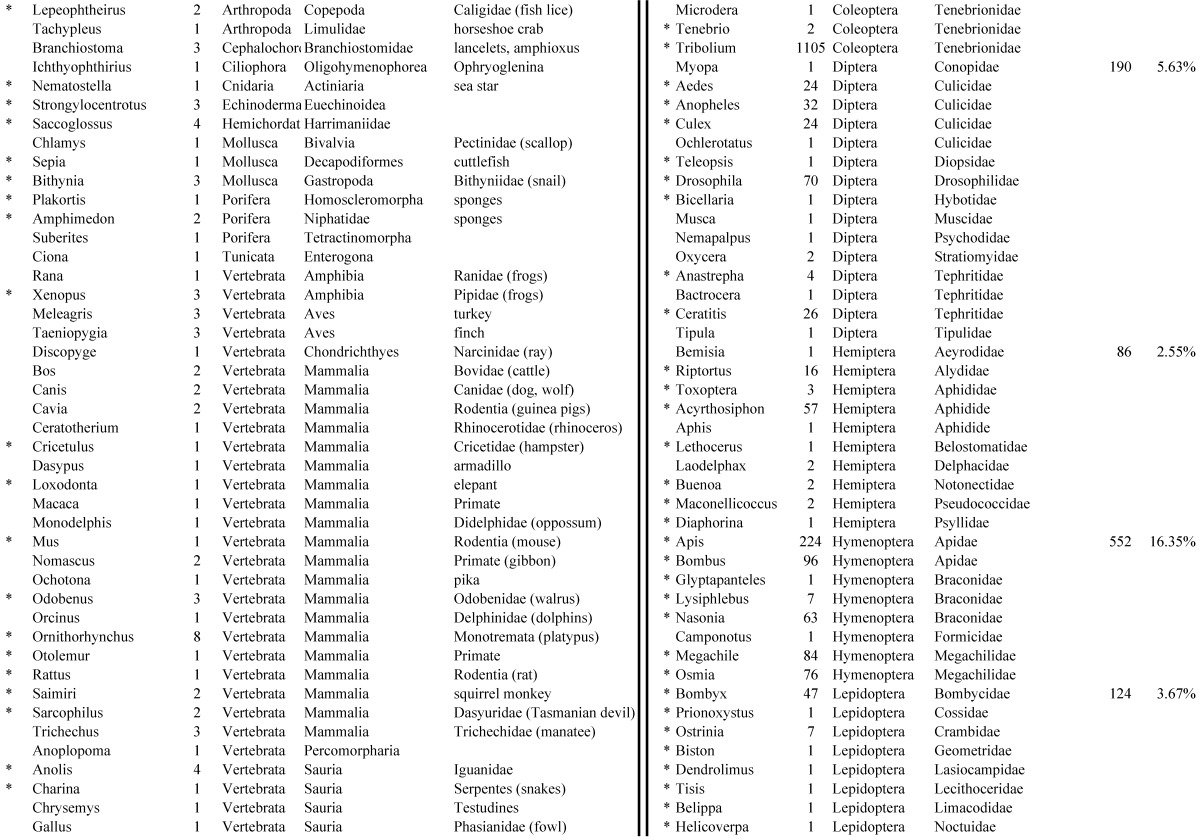
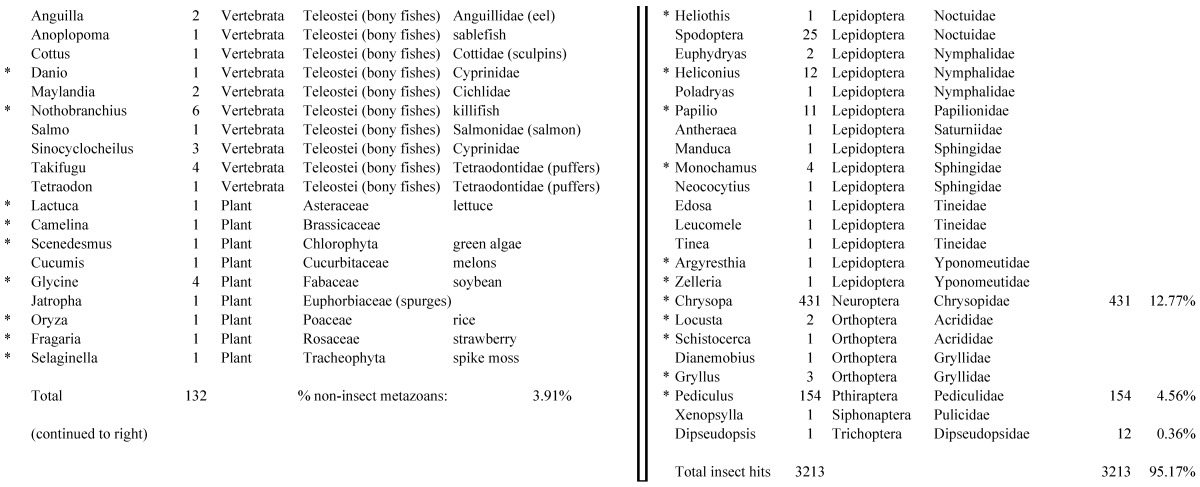
Table 1B.
Closest homologous sequences; quantities by taxonomy. Treatment: insect egg diet, 3358 non-redundant sequences with expect value of <1.00e-10. Right hand columns are insect genera, left hand columns are non-insect. Sequences that differ from the pollen diet treatment in quantity of hits are indicated by asterisk (*).
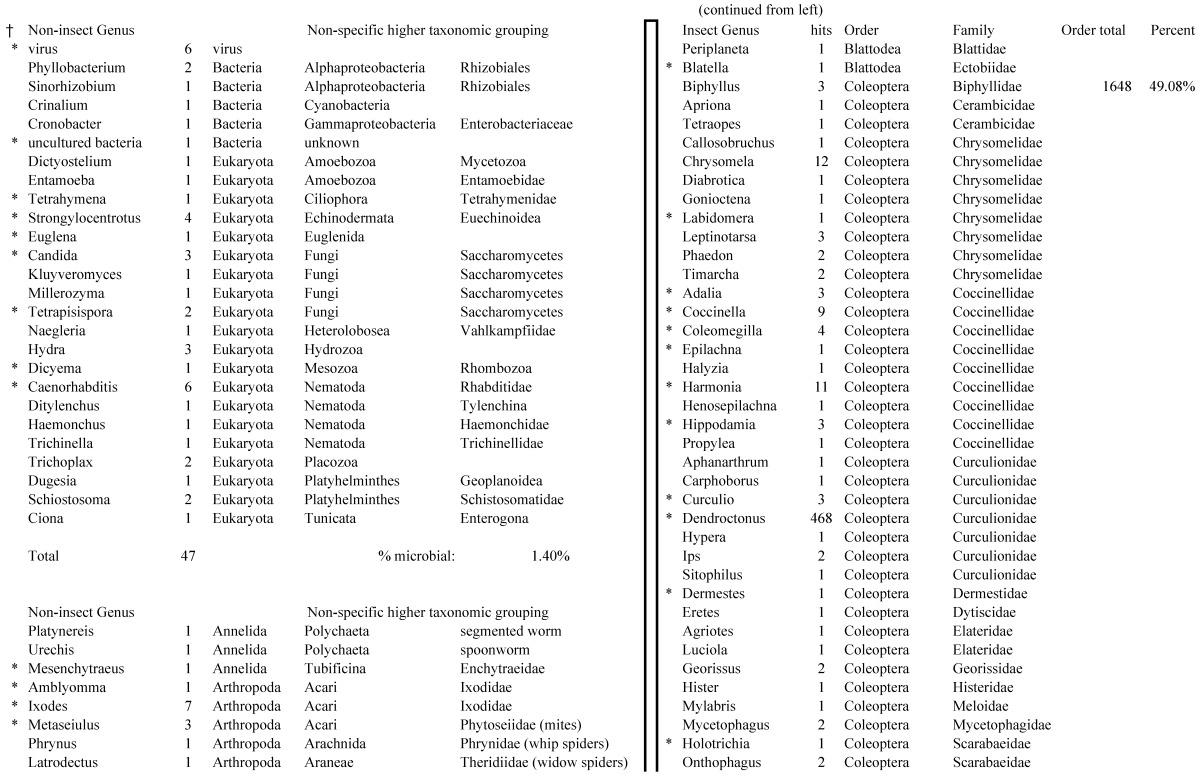
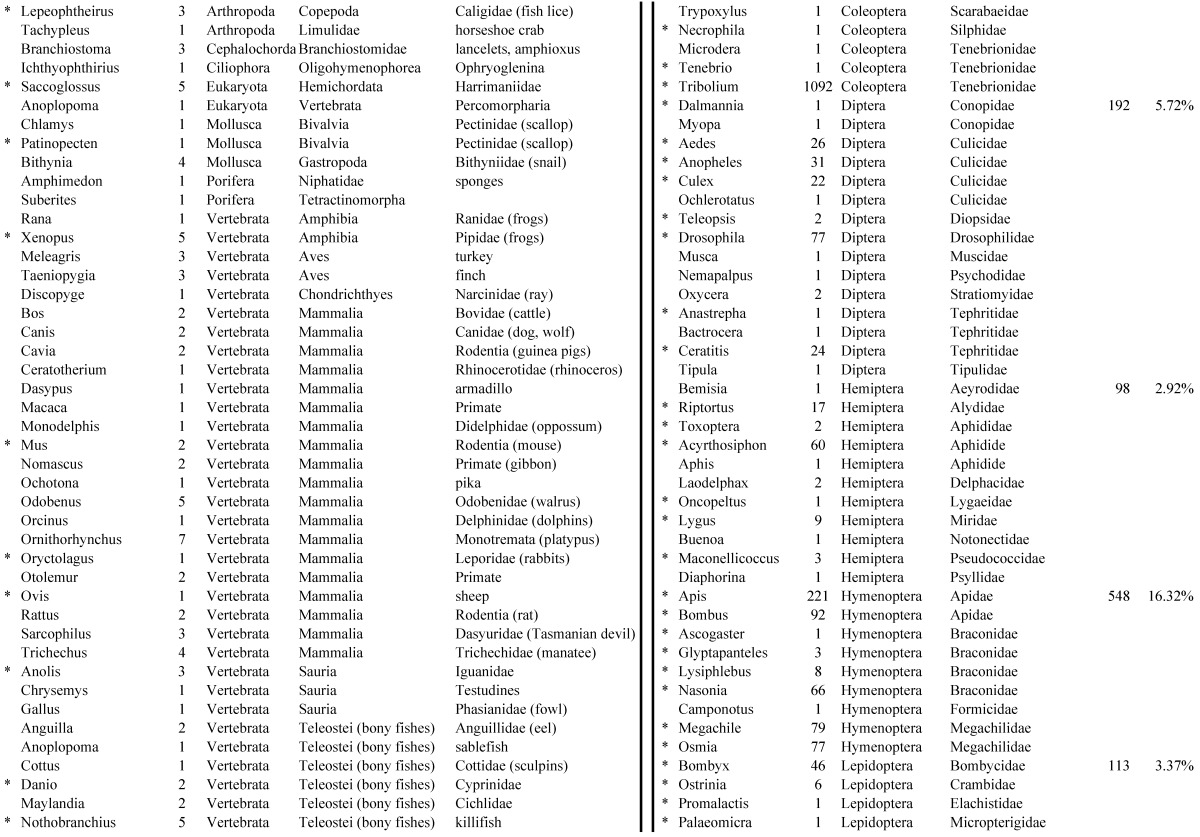
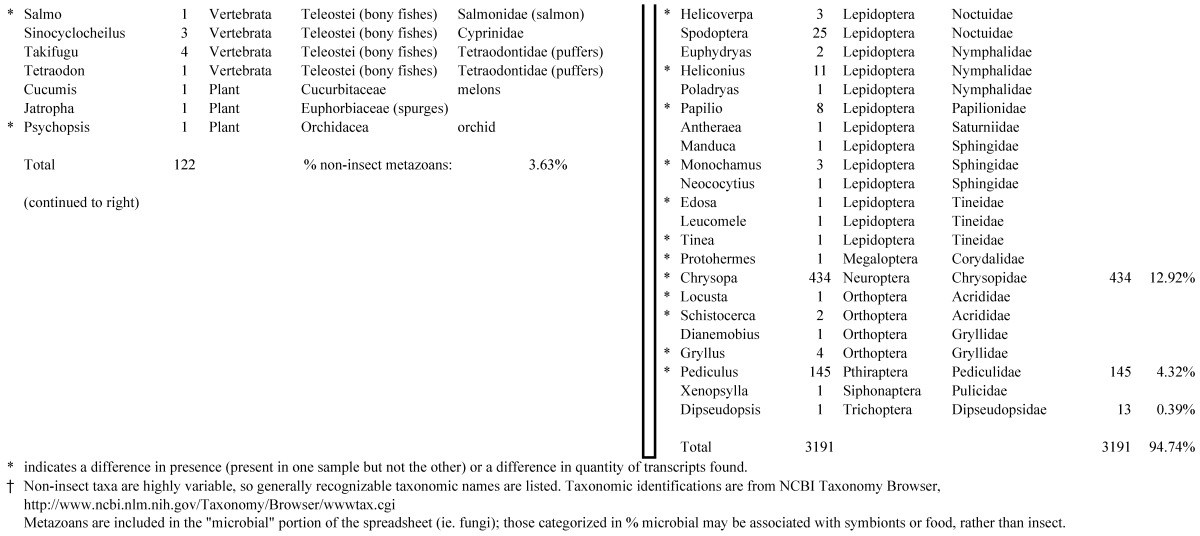
Figure 2.
Individual transcriptomes of samples are very similar in overall characteristics, as expected for inbred sibling samples. Pie chart comparison of most similar (expect <e-10) and longest (>500 nt) contiguous sequences generated from adult insect samples fed either a diet of only insect eggs (IE) or only pollen (P). Roughly 10% of the total sequences are represented.
Table 2.
Selected sequences (16) from treatment samples that appear identical, and their actual nt identities. Abbreviations incorporated into sequence IDs: Insect egg (IE); pollen.
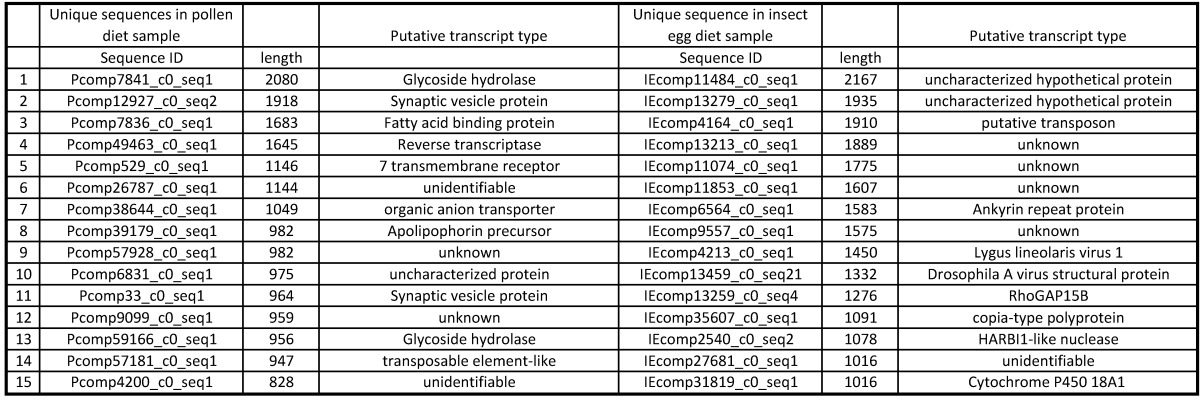
The fifteen longest unique sequences from each diet treatment are listed in Table 3. While the unique sequences in pollen-fed transcripts appear to be related to carbohydrate cleavage, which seems logical for animals digesting plant materials, the unique sequences from the egg-fed did not appear to match any obvious category of transcript, other than the insect virus 18 mentioned earlier.
Table 3.
Longest 15 sequences unique in treatments. P: pollen diet, IE: insect egg diet.
Further analyses of the transcripts reported here will provide insight on genes that are linked to differential metabolism of plant and animal based diets. The sequences identified here, after validation among further representatives of this species, will be used to measure quantitative changes in gene expression between insects utilizing multiple sources of foods, and those that are deprived of specific dietary components. Potential projects include varying diet at different stages of insect development; this species is known to utilize different diet components at different stages of development 19-21. Another possibility is to evaluate genetic responses to specific prey, as C. maculata has been shown to utilize prey of specific sizes and species 22. This will help us understand how to produce high quality generalist predator biological control agents, and assure conservation of beneficial insects in our constantly changing world environment. The details of the genes expressed by this omnivorous insect, and their correlation with diet and nutrition, will provide insights into nutritional health of other omnivores including humans.
Methods
Insect culture
Lady beetles used to establish laboratory colonies were collected from fields surrounding the USDA-ARS and Mississippi State University Delta Research and Extension Center in Stoneville, MS, 38776. Insect cultures were maintained in the National Biological Control Laboratory at Stoneville, MS without wild specimen introgression from August 2010 through the time of RNA sample collection in March 2013. Insects were reared as larvae in Petri dishes of sizes ranging from 35 to 250mm with mesh glued into one side of the dish for ventilation, and as adults in 5.25 x 5.25 inch cages 12. Temperature was generally maintained at 24o C for 16 lighted hours and 19o C for 8 dark hours in Percival (Perry, IA) E30B growth chambers. Larvae and adults were fed ad libitum a combination of pollen, Daphnia, Brewer's yeast, honey, and eggs of laboratory cultures of Lygus spp. 12. Water was provided in 1.5 ml microcentrifuge tubes with caps removed and plugged with cotton.
Inbreeding for homozygosity
Inbreeding of beetle stock was performed by isolating individual gravid females from the primary wild type colony, collecting and rearing all eggs from individual females, and continuing culture using only offspring of the most fertile and fecund female. From the resulting culture the inbreeding step (female selection) was repeated for a total of six isofemale (I6) selection steps.
Sample preparation
Insect specimens from a single egg mass collected from the I6 colony were fed standard diet as larvae. Surviving individuals were isolated after pupation and provided either pollen alone or Lygus eggs alone upon adult eclosion. Total RNA was isolated from whole individual adults six days after adult eclosion. Specimens were spray washed with reverse osmosis (RO) water to remove any food particles. After one hour isolation (resting from wash and away from food) specimens were briefly anaesthetized with carbon dioxide. Insects were transferred to sample tubes and crushed whole in RNA extraction buffer using blue Kontes® (Kimble Chase, Thermo Fisher Scientific, Waltham, MA) pestle. Total RNA was extracted using USB PrepEase total RNA kit following manufacturer instructions (Affymetrix, Santa Clara, CA). Samples were measured using a NanoDrop 1000 (Thermo Fisher Scientific) spectrophotometer, and the samples with highest final concentration and highest 260/230 ratio were chosen for sequencing. RNA samples were kept in ultralow freezer set for -75oC until shipping.
Transcript Sequencing
Total RNA samples were shipped on dry ice to the University of Washington High-Throughput Genomics Center, WTC East, Suite 600, 2211 Elliott Ave., Seattle, WA 98121-1692. Illumina (Illumina, Inc., San Diego, CA, USA) RNA-seq library construction, 36 bp single end multiplex quality control library testing, and 76 bp paired end multiplex 4x sequencing was followed by Trinity (Broad Institute, MA) contiguous sequence assembly 23. Sequences were assembled by diet treatment (eggs only or pollen only) and as a combined group assembly. Assembled sequences were limited by request to >200nt. Assembled sequences were provided to the USDA ARS Genomics and Bioinformatics Research Unit (GBRU) in Stoneville, MS for further analysis.
Sequence Analyses
Assembled sequences were analyzed by NCBI BLAST® (US National Library of Medicine) 16 using the tBLASTx “search translated nucleotide database using a translated nucleotide query” (database accessed 22 May 2013). BLAST results were analyzed by diet treatment (eggs only or pollen only) and as a combined group. Spreadsheet results were sorted by Hit Score and only those sequences in the two treatment groups meeting the combined criteria of >500 nucleotide (nt) and E (expect) score <E-10 were used for further analysis.
Because the sequenced samples were derived from two individual insects that differed only in their adult diet, the transcriptomes were expected to be nearly identical for those sequences representing genes that were not associated with diet. To verify that assemblies represented genetically similar individuals as expected, sixteen sequences were chosen based on apparent identity by length of assembled sequence and closest tBLASTx hit. Sequences were chosen from the group most similar to Tribolium castaneum sequences and from the group most similar to Dendroctonus ponderosae sequences, with the assumption that sequences from other beetles would represent conserved transcripts. Sequences from pollen-fed (P) and insect egg-fed (IE) were compared at the nucleotide level using BLAST.
Acknowledgments
I acknowledge the many Biological Science Technicians who supported this work: Catherine Louise Smith, Fannie Mae Byrd, Ebony Williams, Mary Elizabeth Huddleston, and Joseph Grey Ballenger. Many thanks go to Dr. Jeffrey Gore of Mississippi State University for collecting and donating live lady beetle specimens, and to Drs. Don Weber and Jonathan Lundgren of USDA ARS for helpful advice on raising beetles in the laboratory. Insect eggs (Lygus spp.) were kindly provided by the NBCL Insect Production team, Dr. M. Guadalupe Rojas, Orswald (Archie) Clark, Derrick T. Jones, Matthew D. McDaniel, and Terry E. Johnson. Assistance in ordering sequencing from University of Washington High-Throughput Genomics Center was provided by Dr. Peter Sabo and other helpful support staff. Batch BLAST analyses were performed by Linda Ballard, Bioinformaticist of the ARS Genomics and Bioinformatics Research Unit led by Dr. Brian Scheffler. Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the US Department of Agriculture (USDA). USDA Agricultural Research Service is an equal opportunity employer. I thank Mark Weaver and Jeremy Marshall for reviewing an earlier version the manuscript prior to submission.
References
- 1.Gordon RD. The Coccinellidae (Coleoptera) of America north of Mexico. J New York Entomol S. 1985;93:1–912. [Google Scholar]
- 2.Pérez OG, Hoy MA. Reproductive incompatibility between two subspecies of Coleomegilla maculata (Coleoptera: Coccinellidae) Fla Entomol. 2002;85(1):203–207. [Google Scholar]
- 3.Halloy SRP, Barratt BIP. Patterns of abundance and morphology as indicators of ecosystem status: A meta-analysis. Ecol Complex. 2007;4(3):128–147. [Google Scholar]
- 4.Lundgren JG, Weber DC. Changes in digestive rate of a predatory beetle over its larval stage: implications for dietary breadth. J Insect Physiol. 2010;56(4):431–437. doi: 10.1016/j.jinsphys.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Pilorget L, Buckner J, Lundgren JG. Sterol limitation in a pollen-fed omnivorous lady beetle (Coleoptera: Coccinellidae) J Insect Physiol. 2010;56(1):81–87. doi: 10.1016/j.jinsphys.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Lundgren JG, Wiedenmann RN. Nutritional suitability of corn pollen for the predator Coleomegilla maculata (Coleoptera: Coccinellidae) J Insect Physiol. 2004;50(6):567–575. doi: 10.1016/j.jinsphys.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Michaud JP, Grant AK. Suitability of pollen sources for the development and reproduction of Coleomegilla maculata (Coleoptera: Coccinellidae) under simulated drought conditions. Biol Control. 2005;32(3):363–370. [Google Scholar]
- 8.Phoofolo MW, Obrycki JJ. Potential for intraguild predation and competition among predatory Coccinellidae and Chrysopidae. Entomol Exp Appl. 1998;89(1):47–55. [Google Scholar]
- 9.Vargas G, Michaud JP, Nechols JR. Maternal effects shape dynamic trajectories of reproductive allocation in the ladybird Coleomegilla maculata. Bull Entomol Res. 2012;102(5):558–565. doi: 10.1017/S000748531200020X. [DOI] [PubMed] [Google Scholar]
- 10.Michaud JP. Coccinellids in Biological Control. In: Hodek I, van Emden HF, Honek A, editors. Ecology and behaviour of the ladybird beetles (Coccinellidae) West Sussex: Wiley-Blackwell; 2012. pp. 488–519. [Google Scholar]
- 11.Kuwayama H, Gotoh H, Konishi Y, Nishikawa H, Yaginuma T, Niimi T. Establishment of Transgenic Lines for Jumpstarter Method Using a Composite Transposon Vector in the Ladybird Beetle, Harmonia axyridis. PLoS One. 2014;9(6):e10080. doi: 10.1371/journal.pone.0100804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Allen ML, Riddick EW. A system for harvesting eggs from the pink-spotted lady beetle. Psyche. 2012. Article923653.
- 13.Allen ML, Ballenger JG. Genetics and Characteristics of a Pigmentation Defective Laboratory Strain of the Lady Beetle, Coleomegilla maculata. Adv in Entomol. 2014;2:161–166. [Google Scholar]
- 14.Gregory TR, Nedved O, Adamowicz SJ. C-value estimates for 31 species of ladybird beetles (Coleoptera: Coccinellidae) Hereditas. 2003;139(2):121–7. doi: 10.1111/j.1601-5223.2003.01766.x. [DOI] [PubMed] [Google Scholar]
- 15.Dolezel J, Bartos J, Voglmayr H, Greilhuber J. Nuclear DNA content and genome size of trout and human. Cytom Part A. 2003;51(2):127–128. doi: 10.1002/cyto.a.10013. [DOI] [PubMed] [Google Scholar]
- 16.Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25(17):3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hoy MA, Yu F, Meyer JM, Tarazona OA, Jeyaprakash A, Wu K. Transcriptome sequencing and annotation of the predatory mite Metaseiulus occidentalis (Acari: Phytoseiidae): a cautionary tale about possible contamination by prey sequences. Exp Appl Acarol. 2013;59(3):283–296. doi: 10.1007/s10493-012-9603-4. [DOI] [PubMed] [Google Scholar]
- 18.Perera OP, Snodgrass GL, Allen KC, Jackson RE, Becnel JJ, O'Leary PF, Luttrell RG. The complete genome sequence of a single-stranded RNA virus from the tarnished plant bug, Lygus lineolaris (Palisot de Beauvois) J Invertebr Pathol. 2012;109(1):11–19. doi: 10.1016/j.jip.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 19.Matos B, Obrycki JJ. Prey suitability of Galerucella calmariensis L. (Coleoptera: Chrysomelidae) and Myzus lythri (Schrank) (Homoptera: Aphididae) for development of three predatory species. Environ Entomol. 2006;35(2):345–350. [Google Scholar]
- 20.Wiebe AP, Obrycki JJ. Prey suitability of Galerucella pusilla eggs for two generalist predators, Coleomegilla maculata and Chrysoperla carnea. Biol Control. 2002;23(2):143–148. [Google Scholar]
- 21.Riddick EW, Wu Z, Rojas MG. Is Tetranychus urticae suitable prey for development and reproduction of naïve Coleomegilla maculata? Insect Sci. 2014;21(1):99–104. doi: 10.1111/1744-7917.12033. [DOI] [PubMed] [Google Scholar]
- 22.Roger C, Coderre D, Boivin G. Differential prey utilization by the generalist predator Coleomegilla maculata lengi according to prey size and species. Entomol Exp Appl. 2000;94(1):3–13. [Google Scholar]
- 23.Grabherr MG, Haas BJ, Yassour M, Levin JZ, Thompson DA, Amit I, Adiconis X, Fan L, Raychowdhury R, Zeng Q. et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat Biotech. 2011;29(7):644–652. doi: 10.1038/nbt.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]