Abstract
Background
Psychogenic or functional movement disorders (PMDs) pose a challenge in clinical diagnosis. There are several clues, including sudden onset, incongruous symptoms, distractibility, suggestibility, entrainment of symptoms, and lack of response to otherwise effective pharmacological therapies, that help identify the most common psychogenic movements such as tremor, dystonia, and myoclonus.
Methods
In this manuscript, we review the frequency, distinct clinical features, functional imaging, and neurophysiological tests that can help in the diagnosis of uncommon presentations of PMDs, such as psychogenic parkinsonism, tics, and chorea; facial, palatal, and ocular movements are also reviewed. In addition, we discuss PMDs at the extremes of age and mass psychogenic illness.
Results
Psychogenic parkinsonism (PP) is observed in less than 10% of the case series about PMDs, with a female–male ratio of roughly 1:1. Lack of amplitude decrement in repetitive movements and of cogwheel rigidity help to differentiate PP from true parkinsonism. Dopamine transporter imaging with photon emission tomography can also help in the diagnostic process. Psychogenic movements resembling tics are reported in about 5% of PMD patients. Lack of transient suppressibility of abnormal movements helps to differentiate them from organic tics. Psychogenic facial movements can present with hemifacial spasm, blepharospasm, and other movements. Some patients with essential palatal tremor have been shown to be psychogenic. Convergence ocular spasm has demonstrated a high specificity for psychogenic movements. PMDs can also present in the context of mass psychogenic illness or at the extremes of age.
Discussion
Clinical features and ancillary studies are helpful in the diagnosis of patients with uncommon presentations of psychogenic movement disorders.
Keywords: Psychogenic movement disorders, functional movement disorders, parkinsonism, tics, palatal tremor, chorea
Introduction
Movement disorders defined by a lack of organic cause or neurological basis have been given different names throughout the history of modern medicine. Such terms have included “conversion”, “hysterical”, “functional”, or “psychogenic”.1 These movements can adopt the form of any known movement disorder with an organic cause. Tremor, dystonia, myoclonus, and abnormal gait are among the most common presentations of psychogenic movement disorders (PMDs). Details regarding their clinical features, pathophysiology, and treatment have been well described.2–4 However, some presentations of PMDs are less common and though recent studies have defined their clinical features and the utility of ancillary diagnostic tests, a review of this research has not been conducted. In this manuscript, we review the distinct clinical features, functional imaging, and neurophysiological tests that can provide insight into the diagnosis of uncommon presentations of PMDs. There is no consensus regarding the terminology of these movements, with some authors preferring the term “functional” and others preferring “psychogenic”.1–3 Here, we prefer to use the term “psychogenic”, but a discussion of which term is more appropriate is beyond the scope of this review.
Materials and Methods
We performed a systematic search in PubMed using the terms “psychogenic”, “psychogenic movement disorders”, and “functional movement disorders” combined with the terms “parkinsonism”, “tics”, “Tourette syndrome”, “chorea”, “palatal tremor”, “ocular and facial movements”, “children”, “elderly”, “mass hysteria”, and “mass psychogenic illness”. Only papers in English were selected for this review. A total of 789 manuscripts published from 1945 to 2014 were obtained following the PubMed search criteria using all previously mentioned keywords, with overlap among searches. The final reference list was generated after selecting the manuscripts with key information regarding the selected topics.
Psychogenic parkinsonism
Psychogenic parkinsonism (PP) was first characterized in detail in 1995 by Lang and colleagues5 in 14 patients, but manuscripts reporting patients with PP already existed in the literature.6 The frequency of PP among patients with PMDs is variable in different series, but is usually less than 10%.7,8 In a series of 530 patients with PMDs, 17 subjects (3.2%) had parkinsonism as the predominant presentation.9 PP was diagnosed in 6.4% of 64 patients with PMDs from the Toronto Western Hospital series and in 1.9% at the Columbia Presbyterian Medical Center.5 PP represented 0.17% of all cases of parkinsonism diagnosed at the Columbia-Presbyterian Hospital in NY.5 In contrast to more common PMDs where women are overrepresented, the female–male ratio was roughly 1:1 in case series of PP. The majority of these patients presented around the age of 50 years.9 Common precipitating factors include motor vehicle accidents and other forms of physical trauma. Psychological stressors are also important as work-related and personal life stress was reported in 56% of patients in one series, and pending litigation was documented in 22% of cases.9
Patients with PP typically have a sudden onset of symptoms and reach a maximal deficit soon after. This is usually followed by a non-progressive course with fluctuations, and occasionally transient or permanent remissions.8 This clinical evolution contrasts with the insidious onset and progressive evolution of idiopathic Parkinson's disease (PD). Tremor and generalized slowness are the two most common features observed in patients with PP.8 Tremor usually has the same features as psychogenic tremor without parkinsonism, characterized by prominent distractibility, changes in rhythm, direction, and amplitude, with the dominant hand more frequently and severely involved.10–12 Psychogenic tremor may be kinetic and present at rest or with posture, and can be observed in one or both legs. Tremor in PP patients can migrate from one limb to the next, particularly if the limb is actively stopped from trembling.10,12 Bradykinesia in PP patients is usually unaccompanied by amplitude decrement or interruptions in repetitive movements as observed in PD. Generalized slowness with prominent difficulty performing activities of daily living or manual tasks accompanied with grimacing, sighing, or even exhaustion is a common feature observed in these patients.8,9 True rigidity with the “cogwheel” phenomenon is not present in PP. Instead, these patients manifest active resistance to passive movements on examination. Features of psychogenic gait such as buckling of the knees and astasia–abasia may be observed.8,9 Arm swing can be reduced or abolished in PP, sometimes with one arm held stiffly extended and adducted to the side. This feature can persist while running.5 On the pull test, PP patients exhibit minimal or exaggerated responses even with light pulls, but falling is rare.9 Inconsistencies regarding motor abilities are common in PP; for example, patients may show normal writing despite prominent slowness or “bradykinesia”. Other psychogenic manifestations include stuttering, gibberish, whispering, or “baby-like” speech abnormalities, “give-way” weakness, non-anatomical sensory loss, and diffuse muscle pain with tenderness.8 Interestingly, some patients may develop “psychogenic levodopa-related dyskinesias” with hyperkinetic and bizarre movements that are incongruous with the typical levodopa-induced dyskinesias of idiopathic PD.9 Additional features that may help to distinguish PP from PD are presented in Table 1.
Table 1. Features Distinguishing Parkinson's Disease from Psychogenic Parkinsonism.
| Feature | Parkinson's Disease | Psychogenic Parkinsonism |
|---|---|---|
| Bradykinesia (repetitive movements) | Progressive slowness with amplitude decrement (sequence effect) | Slowness without amplitude decrement |
| Rigidity | Cogwheeling | Paratonia (active resistance) |
| Effect of reinforcement maneuvers | Rigidity increases | Rigidity diminishes |
| Tremor | Rest, postural, and kinetic | Rest, postural, and kinetic |
| Effect of distraction | Increases in amplitude | Decreases in amplitude or disappears |
| Effect of holding weight | Tremor not transmitted | Tremor may be transmitted to other body segments |
| Effect of entrainment | Tremor may entrain to rate and rhythm of repetitive movements | Tremor is frequently entrained to contralateral repetitive movements |
| Finger tremor | Common | Rare |
| Frequency in different body parts1 | Different frequencies | Same frequencies |
| Walking | Slow, stiff, with retropulsion or propulsion | Slow, stiff, may be painful |
| Arm posture while walking | Partially flexed | Extended in adduction, held stiffly at side (this posture may persist while running) |
| Arm swing | Typically decreased | May be decreased |
| Freezing | Common | Absent |
| Pull test | Variable retropulsion, patient may fall | Normal or exaggerated with flailing of the arms during posterior displacements, reeling back, but almost never falling. |
| l-dopa-induced dyskinesias | Dystonia, chorea, athetosis | Hyperkinetic bizarre movements |
Tremor frequency (Hz) measured simultaneously in two different body parts.
It is unclear why some patients develop parkinsonism as a form of PMD. A small proportion of these patients are reported to have family members or friends with PD. Therefore, “modeling” may play a role. Modeling can be defined as the adoption of certain symptoms, behaviors, or motor patterns following the observation of close individuals displaying manifestations. The original description by Lang and colleagues5 described a patient who was actively involved in a PD support group.
PP should be distinguished from other forms of parkinsonism with sudden onset and rapid evolution, such as dopa-responsive dystonia (DRD) or “rapid-onset dystonia-parkinsonism”. DRD (DYT5) is most commonly caused by autosomal-dominant mutations of the GTP cyclohydrolase (GCH1) enzyme gene, located in chromosome 14q22.1-q22.2. Rapid-onset dystonia-parkinsonism is associated with mutations of the ATP1A3 gene (chromosome 19q13.2) and can also present as alternating hemiplegia of childhood.13 These disorders can manifest with prominent asymmetric, mainly dystonic movements triggered by emotional or physical stressors, which can be confused with psychogenic dystonia or parkinsonism.13
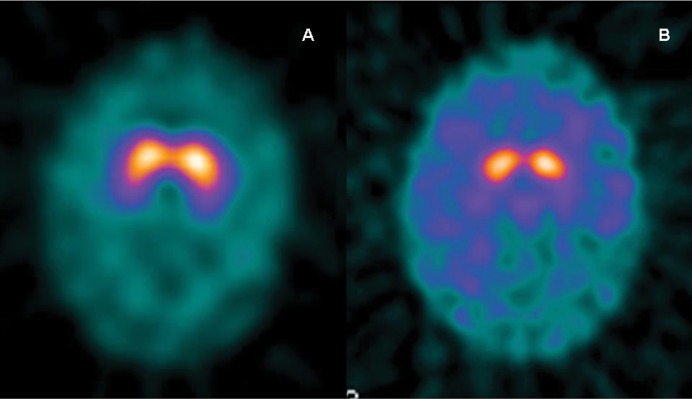
The lack of dopaminergic denervation in the basal ganglia is expected in patients with PP, and can be corroborated using [18F]-fluorodopa positron emission tomography (PET), or photon emission tomography (SPECT) using dopamine transporter (DaT) ligands such as 99mTc-TRODAT-1, 123I-β-CIT (2β-carbomethoxy-3β-(4-iodophenyl)tropane), or 123I-FP-CIT (DaT-SPECT).14–17 The last two are the most widely used, although 123I-FP-CIT (DaTscan, GE Healthcare) requires only a 3–6-hour delay between injection and scan, and this delay is about 24 hours when using 123I-β-CIT. These studies are useful when doubts ensue regarding the organic nature of the parkinsonian syndrome (Figure 1). However, it should be considered that about 10–15% of patients fulfilling the diagnostic criteria for PD have “scans without evidence of dopaminergic deficit”.18 Patients with PP are expected to have normal DaT-SPECT, though this does not necessarily exclude pathology. Therefore, other conditions with normal DaT-SPECT should be considered in the differential diagnosis (Table 2). Diagnosis is further complicated as there are patients with presumed PP who have abnormalities on DaT-SPECT. In those cases, a combined diagnosis of PP and PD should be considered,19 and clinicians should define which symptoms are organic and which are psychogenic. For this purpose, neurophysiology studies with surface electromyography can assess frequency and amplitude as well as the effect of distraction, entrainment, or weight loading on tremor.19 The use of carbidopa alone as a placebo 24 hours after the last dose of levodopa (“off” state) has been advocated as a diagnostic tool in patients with suspected PP.9 This test can also be helpful in patients with suspected combined PD and PP. Nevertheless, a response to placebo does not prove “psychogenicity” but rather suggestibility, as patients with PD may also show some improvement. The use of placebos for diagnostic or therapeutic purposes is a highly controversial topic, but it has been ethically justified when the nature of the placebo is fully disclosed and consent is requested from the patient in order to avoid violating the ethical principle of autonomy.20,21 Treatment of PP is usually a difficult task, but an effort to wean patients off anti-parkinsonian drugs is a reasonable starting approach.9
Figure 1. Patient Scans.
(A) Dopamine transporter imaging with photon emission tomography (DaT-SPECT) in a patient with psychogenic parkinsonism shows normal dopaminergic innervation of the basal ganglia. (B) DaT-SPECT in a patient with Parkinson's disease shows bilateral decreased presynaptic dopamine transporter in the basal ganglia.
Table 2. Differential Diagnosis of Conditions with Normal Dopamine Transporter Imaging with Photon Emission Tomography.
| Psychogenic Parkinsonism |
|---|
| Vascular parkinsonism |
| Drug-induced parkinsonism |
| Dystonic tremor |
| Essential tremor |
| Orthostatic tremor |
| Dopa-responsive dystonia |
| Healthy subjects |
Psychogenic parkinsonism in Parkinson's disease
Some evidence suggests that PP may not be so uncommon in patients with PD.19,22 Somatizations have been reported frequently prior to the onset of some neurodegenerative disorders.23 In one study, somatoform disorders were observed in 18% of patients with Lewy body dementia and in 7.5% of patients with PD, whereas the frequency of somatizations was between 0 and 2% in other neurodegenerative disorders such as Alzheimer's disease, frontotemporal dementia, atypical parkinsonism, and psychiatric disorders.23,24 Catatonic signs with stereotypies and negativism were common psychogenic features, with onset between 6 months and 5 years prior to the onset of the neurodegenerative disorder.24 In another study of 14 patients with PD, somatizations and psychogenic tremor were the most common manifestations, followed by gait disorder and fixed dystonia.25 Psychogenic manifestations usually coincide with the most affected side.25 It has been hypothesized that the increased self-attention to perform movements in patients with PD may increase the vulnerability to develop psychogenic movements, although this does not account for cases with premotor manifestations.25 A careful diagnosis is important in order to avoid unnecessary and invasive therapies such as harmful escalation of anti-parkinsonian medication, surgical ablative therapies, and deep brain stimulation.26
Psychogenic tics
Tourette syndrome (TS) is attributed to the French neurologist George Gilles de la Tourette, after his seminal paper published in 1885.27 Although Tourette suggested a degenerative and hereditary nature of the disorder implying a possible organic cause, in the following decades, Freudian psychodynamic and psychological theories regarding the nature of tics became predominant.28,29 Recent evidence based on detailed characterizations of movements, genetics, functional and morphometric brain imaging studies supports the idea of TS as a disorder with neurobiological basis.30 However, a number of patients with these movements may have an underlying psychogenic cause. The psychogenic myoclonus and psychogenic movements resembling tics (PMRTs) are classified by some authors under the umbrella term “psychogenic jerks”.31
Patients with PMRTs represented 3.2% of individuals presenting for an evaluation of tics in a tertiary-care referral center, whereas PMRTs were observed in 4.9% of 184 patients with PMDs in the same series.32 All patients were adults, with a mean age of onset of 34 years (range 16–66 years).32 PMRTs have also been observed in children.33,34 The combination of PMRTs and tics has also been observed in the same patient.35–37
The characterization of PMRTs has represented a challenge because tics have features that are commonly observed in PMDs, such as sudden onset, fluctuations over time, distractibility, exacerbation with fatigue or emotional stress, etc. Certain features in the clinical history can help distinguish PMRTs from tics. Patients with PMRTs are typically older at the time of presentation. A female predominance is observed in PMRT patients; also, there is usually a lack of family history of tics or TS in those with PMRTs.32 Other distinctive clinical features are that in contrast to patients with tics, most patients with PMRTs are not able to suppress the movements, at least transiently, sudden onset of the movement disorder is more frequent, and there is usually a lack of response to dopamine receptor antagonist or other drugs used for tic suppression.32 Premonitory sensation is present in about 90% of patients with tics but appears to be less common in patients with PMRTs, as it was observed in only two out of nine patients with PMRTs. Such sensations were variable and inconsistent with those classically reported in TS patients. Suggestibility and distractibility are also more prominent in PMRTs than in patients with TS.32 For example, patients claiming an inability to suppress the movement may completely stop moving when distracted. Furthermore, most patients with PMRTs can also have psychogenic seizures or other PMDs, which are usually evident in the clinical evaluation. Video demonstrations of PMRTs can be found elsewhere.32 Patients with psychogenic jerks or PMRTs may have diverse cognitive complaints, but detailed neuropsychological assessments have not found differences when they are compared with those with TS; however, those with psychogenic movements displayed worse performance in verbal memory tasks.38
It is unclear how PMRTs differ in pathophysiology from their organic counterpart. The Bereitschaftspotential (BP) is an early cortical activation potential preceding volitional movements.39,40 The BP has been used to differentiate psychogenic jerks and tics from their organic counterparts. In a study of six patients with TS, the BP was not observed preceding tics but was present when subjects were asked to mimic their tics voluntarily;41 this contrasts with the report of other authors who observed the BP preceding tics in some patients.42 In a larger study, the BP was recorded in 86% of 29 patients with psychogenic jerks or tics, compared with 43% of 14 patients with TS and 0% of patients with organic myoclonus.43 Interestingly, the BP was significantly less frequent in patients with psychogenic jerks than in patients with TS and myoclonus during voluntary wrist extension, supporting the concept of altered access to neural circuitry used for intended movements in psychogenic patients. The BP prior to psychogenic jerks had a sensitivity and specificity of 0.86, whereas the sensitivity was only 0.43 and specificity was 0.26 prior to organic tics.43 In summary, clinical features are useful to differentiate between tics and PMRTs and the presence of the BP preceding the movements supports a psychogenic origin.
Psychogenic chorea
In modern series of PMD, psychogenic chorea and athetosis have relatively low prevalence of 0–12%.44 In a large series of 530 patients with PMDs, chorea was observed in 0.6% of patients.4 Modeling is a possible mechanism for development of psychogenic chorea in subjects with a family history of Huntington's disease (HD).45 In these patients, the lack of motor impersistence in conjunction with normal saccadic movements are clues for psychogenic etiology in the absence of metabolic causes of chorea such as hyperthyroidism or hyperglycemia.45 A suspected diagnosis of HD should be ruled out with genetic testing.
Psychogenic facial and ocular movements
Psychogenic facial movements are not rare; however, isolated psychogenic facial movements are not common and few studies have specifically focused on these movements. In a case series of 61 patients with psychogenic facial movements, the average age of onset was 37 years with a strong female predominance (91.8%).46 The most common pattern of movements was lateral or downward lip protrusion with ipsilateral deviation of the jaw. In contrast to organic oromandibular dystonia, patients in this series had unilateral involvement, and no sensory tricks or speech involvement.46 Psychogenic hemifacial spasm (HFS) is another presentation of psychogenic facial movements. In a large study of 215 patients with HFS, 16 (7.5%) were diagnosed as “psychogenic”. These patients had an earlier age of onset (37.4±19.5 years) than those with idiopathic HFS (55.4±15.0 years).47 In non-psychogenic HFS, there are simultaneous compensatory contractions of the frontalis muscle, leading to elevation of the eyebrow ipsilateral to the contractions of orbicularis oculi.48 This so-called “other Babinski sign” has been found to occur contralateral to the eye closure in psychogenic HFS.46 Blepharospasm is another presentation of psychogenic facial movements; studies analyzing the R2 component of the blink reflex have demonstrated marked disinhibition in patients with organic or “essential” blepharospasm but normal R2 in presumed psychogenic patients and healthy controls.49
Altered ocular movements have also been identified in patients with PMDs. Convergence spasm (CS) consists of episodes of lens accommodation with ocular convergence usually causing diplopia, evoked mainly by horizontal ocular pursuit.50,51 In a study with blinded raters, CS was observed in 69% of patients with PMDs, but in only 36% and 33% of patients with non-psychogenic movement disorders and healthy controls, respectively (p = 0.049), with good agreement between raters.50 While CS may be due to brainstem pathology, it is often associated with other psychogenic manifestations leading to inappropriate workup.50 A case of functional upward gaze paralysis with CS on attempts to follow the examiner's finger upwards has also been reported.52 The clue for a psychogenic cause in this case was the absence of volitional compensation demonstrated by lack of frontal corrugation and eyebrow elevation in attempted upward gaze.52 CS has also been reported in psychogenic patients mimicking positional vertigo.53 It should be noted that CS can occur with upward gaze attempts in organic pathology as well, for example, in attempts to overcome vertical gaze paresis due to Sylvian aqueduct obstruction.54
Psychogenic palatal tremor
Palatal tremor (also known as palatal myoclonus) is a group of disorders characterized by abnormal rhythmic contractions of muscles involved in palatal movements. Palatal tremor is divided into two categories termed “symptomatic,” affecting the levator veli palatini muscle and usually secondary to brainstem lesions and “essential” affecting the tensor veli palatini muscle, and with typically no evidence of lesions on imaging studies.55 Researchers have attempted to delineate the etiology of essential palatal tremor for years.56 Currently, it has been proposed that at least a proportion of these patients may have “psychogenic palatal tremor”.57–59 Voluntary control of palatal muscles seems necessary for the development of psychogenic palatal tremor.60,61 Activation of the inferior olive and dentate nucleus has been recorded in a subject with voluntary contractions of palatal muscles using functional magnetic resonance imaging.62 In a retrospective study of 10 patients with a clinical diagnosis of essential palatal tremor, the authors identified a psychogenic cause in seven of them.63 Patients with psychogenic palatal tremor were younger than patients with symptomatic palatal tremor, more frequently female and had a history of a precipitating event. Psychogenic patients frequently complain of ear clicking and multiple somatizations. Entrainment and distractibility of palatal movements in psychogenic patients are key features that should be sought during clinical examination.61,63 Treatment with oral medications usually fails, but improvement with botulinum toxin is possible and has been observed in selected cases.64,65
Psychogenic movement disorders at the extremes of age
Psychogenic movement disorders are also observed in populations younger than 18 years of age. Conversion or psychogenic disorders among children have been estimated to have a prevalence of two to four per 100,000.66 PMDs represent 2–4% of children presenting to a movement disorders clinic for a first evaluation,67 whereas children represented 5.7% of all patients with PMDs; 3.1% of 1,722 children evaluated for a movement disorder had a psychogenic cause in the largest reported case series.68 The mean age of onset varies between 11.5 and 14.2 years in published case series focused on this population (Table 3). A precipitating factor has been identified in the majority of cases. Psychiatric comorbidity such as anxiety, irritability, and depression are not uncommon.68 After pooling the reported case series (120 patients) tremor was the most common presentation (n = 63, 52.5%), followed by dystonia (n = 52, 43.3%), and myoclonus (n = 34, 28.3%) (Table 3).69 Psychogenic gait disorders have also been reported in children70 and represented 13.3% (n = 16) of patients in the pooled analysis. Modeling is expected to occur more frequently in children than adults, but this phenomenon was relatively uncommon, with 0–11% in different series.68,71 Of interest, a recent case–control study demonstrated a significant association between PMDs and exposure to phenotypically congruent models of movement disorders.72 The diagnostic process of PMDs in children does not differ from that used for adults. Electromyography can be used to support the diagnosis, although some features are not easily detected and studies can be technically more difficult in children than adults.71 For psychogenic gait disorders, the “chair test”, initially described by Paul Blocq in a 15-year-old patient, can be helpful for diagnosis. Patients are asked to walk 20–30 feet and then propel a swivel chair with wheels while seated.73 Patients with psychogenic gait are observed to have improved ability to propel the wheeled chair compared with subjects with non-psychogenic gait disorders.73 Studies suggest that a multidisciplinary approach and family involvement is important for the effective treatment of these patients.36 The prognosis in children with PMDs seems more favorable than in adults, although chronic disability has been reported to occur in between 8% and 20% of these patients.74 A short duration of the symptoms with a lack of psychopathology and the presence of an identifiable stressor are related to a better prognosis, similar to what has been reported in adults.67,75
Table 3. Case Series Studies of Children with Psychogenic Movement Disorders.
| Reference | No. of Patients | Female–Male | Mean Age or Range of Onset (years) | Most Common PMDs1 |
|---|---|---|---|---|
| Ferrara and Jankovic68 | 54 | 42:12 | 14.2 | Tremor n = 35, dystonia n = 29, myoclonus n = 20, gait disorders n = 12, other n = 12 |
| Ahmed et al.34 | 11 | 4:7 | 6.11–15.11 | Tics n = 6, tremor n = 4, clonus n = 1 |
| Schwingenschuh et al.74 | 15 | 12:3 | 12.3 | Dystonia n = 7, tremor n = 6, gait disorder n = 2 |
| Dale et al.69 | 12 | 10:2 | 12.7 | Tremor n = 10, myoclonus n = 5, dystonia n = 4, tics n = 1 |
| Faust and Soman36 | 14 | 11:3 | 13.1 | Dystonia n = 6, myoclonus n = 3, tremor n = 3, chorea n = 2 |
| Canavese et al.71 | 14 | 8:6 | 11.5 | Tremor n = 5, myoclonus n = 6, dystonia n = 6, gait disorder n = 2 |
Some patients present with more than one movement disorder; therefore, frequencies may exceed the total number of studied subjects.
The elderly may also have PMDs. In a series of 151 patients with PMDs, those older than 60 years of age represented 21% of cases.76 When compared with younger patients, older patients had a similar frequency of tremor and dystonia, but had a higher frequency of psychogenic gait abnormalities and psychogenic non-epileptic seizures, while fixed dystonia was observed only in younger patients.76 This study suggests that some clinical features may differ depending on the age of onset of PMDs; however, these findings require further research. In another study analyzing psychogenic gait patterns in patients with or without other PMDS, a slightly older age of onset was observed in patients with psychogenic gait disturbances.77
Mass psychogenic movement disorders
Psychogenic movement disorders may affect cohesive social groups or families. “Mass hysteria” has also been termed “mass psychogenic illness” or “mass sociogenic illness”.78 Registers of mass psychogenic illness date back to the Middle Ages, with the outbreaks of “dancing mania” in central Europe associated with the Black Plague.29 The manifestation was named “Chorea Sancti Viti” or “Saint Vitus dance” after the early Christian martyr Saint Vitus, who was invoked to intercede for those affected.29 Several other examples of mass psychogenic illness have been reported, mostly in recent years, some of them with abnormal movements.66 A recent outbreak of mass psychogenic illness in Le Roy, NY, received extensive media coverage when 18 individuals, mostly female, presented with psychogenic jerks, PMRTs, psychogenic seizures, speech disorders, and other PMDs. Two conditions are considered necessary to induce mass psychogenic illness: 1) a perceived exposure to an illness-causing agent, and 2) observation of other persons developing symptoms, particularly females observing other females.79,80
Features such as high female to male ratio, atypical manifestations with inconsistencies in anatomy and physiology, increased anxiety, and spread of symptoms via visual or oral communication are common in such patients.67 However, studies on a specific vulnerable personality type are inconclusive.78 Mass psychogenic illness can present along with real biological threats and, in some cases, may cause overwhelming consumption of health resources. For example, more than 85% of the 5,500 people searching for medical attention after the release of sarin gas in the metro of Tokyo were deemed psychogenic.81 Controlled experiments have demonstrated that induction of psychogenic symptoms is possible with suggestion regarding the noxious effect of an inert exposure.79,82 Furthermore, an experiment demonstrated that exposure to media reports can play a role in the induction of psychogenic symptoms in healthy volunteers.79 PMDs have also been reported within particular families; in such cases the phenomenology of the movements tend to be similar among members of the same family.83 Familial PMDs may represent a restrictive form of mass psychogenic illness.
Conclusions
PMDs can resemble practically the whole spectrum of organic movement disorders. The diagnosis of uncommon PMDs should no longer be of exclusion, but rather one based on positive clinical signs, many of them examined in this review. However, clinicians should be mindful that combined organic and psychogenic movement disorders can coexist in the same patient. Readers should also be aware that some of the presentations reviewed here, although uncommon among patients with PMDs, may actually be common in other contexts. For example, psychogenic palatal tremor seems to be common among patients with essential palatal tremor and PMDs are not infrequently reported in series of children with movement disorders. Treatment of PMDs is usually difficult; an effort to wean patients off dopamine receptor blockers and anti-parkinsonian drugs is a reasonable starting approach in patients with PMRTs and PP, respectively. Other therapies such as psychotherapy, physical therapy, biofeedback, transcranial magnetic stimulation, transcutaneous electrical stimulation, anxiolytics, and antidepressants may be appropriate in some patients but further investigation into treatments is warranted.
Acknowledgment
The authors wish to thank Dr. Jennie Valles for assistance with preparation of the manuscript.
Footnotes
Funding: None.
Financial Disclosures: J.F.B.C. has received grants from Merz Pharmaceuticals and Medtronic Inc.; he is a contributor to Medlink Neurology. R.F. served as a consultant for Teva Neuroscience, Inc., Lundbeck, LLC., and US World Meds as well as on the advisory board for General Electric. In addition, he is a contributor to Medlink, Neurology.
Conflict of Interests: The authors report no conflict of interest.
References
- 1.Fahn S, Olanow CW. Psychogenic movement disorders: They are what they are. Mov Disord. 2014;29:853–856. doi: 10.1002/mds.25899. [DOI] [PubMed] [Google Scholar]
- 2.Factor SA, Podskalny GD, Molho ES. Psychogenic movement disorders: Frequency, clinical profile, and characteristics. J Neurol Neurosurg Psychiatry. 1995;59:406–412. doi: 10.1136/jnnp.59.4.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards MJ, Bhatia KP. Functional (psychogenic) movement disorders: Merging mind and brain. Lancet Neurol. 2012;50:250–260. doi: 10.1016/S1474-4422(11)70310-6. [DOI] [PubMed] [Google Scholar]
- 4.Thomas M, Jankovic J. Psychogenic movement disorders: Diagnosis and management. CNS Drugs. 2004;18:437–452. doi: 10.2165/00023210-200418070-00003. [DOI] [PubMed] [Google Scholar]
- 5.Lang AE, Koller WC, Fahn S. Psychogenic parkinsonism. Arch Neurol. 1995;52:802–810. doi: 10.1001/archneur.1995.00540320078015. [DOI] [PubMed] [Google Scholar]
- 6.Walters AS, Boudwin J, Wright D, Jones K. Three hysterical movement disorders. Psychol Rep. 1988;62:979–985. doi: 10.2466/pr0.1988.62.3.979. [DOI] [PubMed] [Google Scholar]
- 7.Ertan S, Uluduz D, Ozekmekçi S, et al. Clinical characteristics of 49 patients with psychogenic movement disorders in a tertiary clinic in Turkey. Mov Disord. 2009;24:759–762. doi: 10.1002/mds.22114. [DOI] [PubMed] [Google Scholar]
- 8.Hallett M. Psychogenic parkinsonism. J Neurol Sci. 2011;310:163–165. doi: 10.1016/j.jns.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jankovic J. Diagnosis and treatment of psychogenic parkinsonism. J Neurol Neurosurg Psychiatry. 2011;82:1300–1303. doi: 10.1136/jnnp-2011-300876. [DOI] [PubMed] [Google Scholar]
- 10.Kenney C, Diamond A, Mejia N, Davidson A, Hunter C, Jankovic J. Distinguishing psychogenic and essential tremor. J Neurol Sci. 2007;263:94–99. doi: 10.1016/j.jns.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Thenganatt MA, Jankovic J. Psychogenic tremor: A video guide to its distinguishing features. Tremor Other Hyperkinet Mov (NY) 2014;4:253. doi: 10.7916/D8FJ2F0Q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hellmann MA, Melamed E, Steinmetz AP, Djaldetti R. Unilateral lower limb rest tremor is not necessarily a presenting symptom of Parkinson's disease. Mov Disord. 2010;25:924–927. doi: 10.1002/mds.23030. [DOI] [PubMed] [Google Scholar]
- 13.Rosewich H, Ohlenbusch A, Huppke P, et al. The expanding clinical and genetic spectrum of ATP1A3-related disorders. Neurology. 2014;82:945–955. doi: 10.1212/WNL.0000000000000212. [DOI] [PubMed] [Google Scholar]
- 14.Factor SA, Seibyl J, Innis R, Marek K. Psychogenic parkinsonism: Confirmation of diagnosis with -CIT SPECT scans. Mov Disord. 1998;13:860. [Google Scholar]
- 15.Tolosa E, Coelho M, Gallardo M. DAT imaging in drug-induced and psychogenic parkinsonism. Mov Disord. 2003;18:S28–S33. doi: 10.1002/mds.10575. [DOI] [PubMed] [Google Scholar]
- 16.Gaig C, Martí MJ, Tolosa E, et al. 123I-Ioflupane SPECT in the diagnosis of suspected psychogenic parkinsonism. Mov Disord. 2006;21:1994–1998. doi: 10.1002/mds.21062. [DOI] [PubMed] [Google Scholar]
- 17.Booij J, Speelman JD, Horstink MW, Wolters EC. The clinical benefit of imaging striatal dopamine transporters with [123I]FP-CIT SPET in differentiating patients with presynaptic parkinsonism from those with other forms of parkinsonism. Eur J Nucl Med. 2001;28:266–272. doi: 10.1007/s002590000460. [DOI] [PubMed] [Google Scholar]
- 18.Schwingenschuh P, Ruge D, Edwards MJ, et al. Distinguishing SWEDDs patients with asymmetric resting tremor from Parkinson's disease: A clinical and electrophysiological study. Mov Disord. 2010;25:560–569. doi: 10.1002/mds.23019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benaderette S, Zanotti Fregonara P, Apartis E, et al. Psychogenic parkinsonism: A combination of clinical, electrophysiological, and [(123)I]-FP-CIT SPECT scan explorations improves diagnostic accuracy. Mov Disord. 2006;21:310–317. doi: 10.1002/mds.20720. [DOI] [PubMed] [Google Scholar]
- 20.Rommelfanger KS. A role for placebo therapy in psychogenic movement disorders. Nat Rev Neurol. 2013;9:351–356. doi: 10.1038/nrneurol.2013.65. [DOI] [PubMed] [Google Scholar]
- 21.Lim EC, Ong BK, Seet RC. Is there a place for placebo in management of psychogenic movement disorders? Ann Acad Med Singapore. 2007;36:208–210. [PubMed] [Google Scholar]
- 22.Felicio AC, Godeiro-Junior C, Moriyama TS, et al. Degenerative parkinsonism in patients with psychogenic parkinsonism: A dopamine transporter imaging study. Clin Neurol Neurosurg. 2010;112:282–285. doi: 10.1016/j.clineuro.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 23.Onofrj M, Bonanni L, Manzoli L, Thomas A. Cohort study on somatoform disorders in Parkinson disease and dementia with Lewy bodies. Neurology. 2010;74:1598–1606. doi: 10.1212/WNL.0b013e3181df09dd. [DOI] [PubMed] [Google Scholar]
- 24.Onofrj M, Thomas A, Tiraboschi P, et al. Updates on somatoform disorders (SFMD) in Parkinson's disease and dementia with Lewy bodies and discussion of phenomenology. J Neurol Sci. 2011;310:166–171. doi: 10.1016/j.jns.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Pareés I, Saifee TA, Kojovic M, et al. Functional (psychogenic) symptoms in Parkinson's disease. Mov Disord. 2013;28:1622–1627. doi: 10.1002/mds.25544. [DOI] [PubMed] [Google Scholar]
- 26.Pourfar MH, Tang CC, Mogilner AY, Dhawan V, Eidelberg D. Using imaging to identify psychogenic parkinsonism before deep brain stimulation surgery. Report of 2 cases. J Neurosurg. 2012;116:114–118. doi: 10.3171/2011.10.JNS11554. [DOI] [PubMed] [Google Scholar]
- 27.Gilles de la Tourette G. Étude sur une affection nerveuse caracterisée par l′incoordination motrice accompagnée d′echolalie et de coprolalie. Arch Neurol (Paris) 1885;9:19–42. 158–200. [Google Scholar]
- 28.McNaught KS, Mink JW. Advances in understanding and treatment of Tourette syndrome. Nat Rev Neurol. 2011;7:667–676. doi: 10.1038/nrneurol.2011.167. [DOI] [PubMed] [Google Scholar]
- 29.Lanska DJ. Chapter 33: The history of movement disorders. Handb Clin Neurol. 2010;95:501–546. doi: 10.1016/S0072-9752(08)02133-7. [DOI] [PubMed] [Google Scholar]
- 30.Jankovic J, Kurlan R. Tourette syndrome: Evolving concepts. Mov Disord. 2011;26:1149–1156. doi: 10.1002/mds.23618. [DOI] [PubMed] [Google Scholar]
- 31.van der Salm SM, Koelman JH, Henneke S, van Rootselaar AF, Tijssen MA. Axial jerks: A clinical spectrum ranging from propriospinal to psychogenic myoclonus. J Neurol. 2010;257:1349–1355. doi: 10.1007/s00415-010-5531-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baizabal-Carvallo JF, Jankovic J. The clinical features of psychogenic movement disorders resembling tics. J Neurol Neurosurg Psychiatry. 2014;85:573–575. doi: 10.1136/jnnp-2013-305594. [DOI] [PubMed] [Google Scholar]
- 33.Mejia NI, Jankovic J. Secondary tics and tourettism. Rev Bras Psiquiatr. 2005;27:11–17. doi: 10.1590/S1516-44462005000100006. [DOI] [PubMed] [Google Scholar]
- 34.Ahmed MA, Martinez A, Yee A, Cahill D, Besag FM. Psychogenic and organic movement disorders in children. Dev Med Child Neurol. 2008;50:300–304. doi: 10.1111/j.1469-8749.2008.02043.x. [DOI] [PubMed] [Google Scholar]
- 35.Dooley JM, Stokes A, Gordon KE. Pseudo-tics in Tourette syndrome. J Child Neurol. 1994;9:50–51. doi: 10.1177/088307389400900112. [DOI] [PubMed] [Google Scholar]
- 36.Faust J, Soman TB. Psychogenic movement disorders in children: Characteristics and predictors of outcome. J Child Neurol. 2012;27:610–614. doi: 10.1177/0883073811422753. [DOI] [PubMed] [Google Scholar]
- 37.Tan EK. Psychogenic tics: Diagnostic value of the placebo test. J Child Neurol. 2004;19:976–977. doi: 10.1177/088307380401900813. [DOI] [PubMed] [Google Scholar]
- 38.Heintz CE, van Tricht MJ, van der Salm SM, et al. Neuropsychological profile of psychogenic jerky movement disorders: Importance of evaluating non-credible cognitive performance and psychopathology. J Neurol Neurosurg Psychiatry. 2013;84:862–867. doi: 10.1136/jnnp-2012-304397. [DOI] [PubMed] [Google Scholar]
- 39.Shibasaki H, Hallett M. What is the Bereitschaftspotential? Clin Neurophysiol. 2006;117:2341–2356. doi: 10.1016/j.clinph.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 40.Hallett M. Physiology of psychogenic movement disorders. J Clin Neurosci. 2010;17:959–965. doi: 10.1016/j.jocn.2009.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obeso JA, Rothwell JC, Marsden CD. Simple tics in Gilles de la Tourette's syndrome are not prefaced by a normal premovement EEG potential. J Neurol Neurosurg Psychiatry. 1981;44:735–738. doi: 10.1136/jnnp.44.8.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karp BI, Porter S, Toro C, et al. Simple motor tics may be preceded by a premotor potential. J Neurol Neurosurg Psychiatry. 1996;61:103–106. doi: 10.1136/jnnp.61.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Van der Salm SM, Tijssen MA, Koelman JH, van Rootselaar AF. The bereitschaftspotential in jerky movement disorders. J Neurol Neurosurg Psychiatry. 2012;83:1162–1167. doi: 10.1136/jnnp-2012-303081. [DOI] [PubMed] [Google Scholar]
- 44.Giugni JC, Martinez-Ramirez D, Rodriguez-Cruz RL. Psychogenic chorea. In: Michelli FE, LeWitt PA, editors. Chorea causes and management. London: Springer; 2014. pp. 356–361. p. [Google Scholar]
- 45.Fekete R, Jankovic J. Psychogenic chorea associated with family history of Huntington disease. Mov Disord. 2010;25:503–504. doi: 10.1002/mds.22925. [DOI] [PubMed] [Google Scholar]
- 46.Fasano A, Valadas A, Bhatia KP, et al. Psychogenic facial movement disorders: Clinical features and associated conditions. Mov Disord. 2012;27:1544–1551. doi: 10.1002/mds.25190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yaltho TC, Jankovic J. The many faces of hemifacial spasm: Differential diagnosis of unilateral facial spasms. Mov Disord. 2011;26:1582–1592. doi: 10.1002/mds.23692. [DOI] [PubMed] [Google Scholar]
- 48.Stamey W, Jankovic J. The other Babinski sign in hemifacial spasm. Neurology. 2007;69:402–404. doi: 10.1212/01.wnl.0000266389.52843.3b. [DOI] [PubMed] [Google Scholar]
- 49.Schwingenschuh P, Katschnig P, Edwards MJ, et al. The blink reflex recovery cycle differs between essential and presumed psychogenic blepharospasm. Neurology. 2011;76:610–614. doi: 10.1212/WNL.0b013e31820c3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fekete R, Baizabal-Carvallo JF, Ha AD, Davidson A, Jankovic J. Convergence spasm in conversion disorders: Prevalence in psychogenic and other movement disorders compared with controls. J Neurol Neurosurg Psychiatry. 2012;83:202–204. doi: 10.1136/jnnp-2011-300733. [DOI] [PubMed] [Google Scholar]
- 51.Ghosh A, Padhy SK, Gupta G, Goyal MK. Functional convergence spasm. Indian J Psychol Med. 2014;36:332–334. doi: 10.4103/0253-7176.127262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bruno E, Mostile G, Dibilio V, Raciti L, Nicoletti A, Zappia M. Clinical diagnostic tricks for detecting psychogenic gaze paralysis. Eur J Neurol. 2013;20:e107–8. doi: 10.1111/ene.12181. [DOI] [PubMed] [Google Scholar]
- 53.Gordon CR, Almog Y. Positional convergence spasm mimicking benign paroxysmal positional vertigo. Neurology. 2012;78:681–682. doi: 10.1212/WNL.0b013e318248df04. [DOI] [PubMed] [Google Scholar]
- 54.Smith JL, Zieper I, Gay AJ, Cogan DG. Nystagmus retractorius. AMA Archives of Ophthalmology. 1959;62:864–867. doi: 10.1001/archopht.1959.04220050124020. [DOI] [PubMed] [Google Scholar]
- 55.Deuschl G, Toro C, Hallett M. Symptomatic and essential palatal tremor. 2. Differences of palatal movements. Mov Disord. 1994;9:676–678. doi: 10.1002/mds.870090615. [DOI] [PubMed] [Google Scholar]
- 56.Zadikoff C, Lang AE, Klein C. The ‘essentials’ of essential palatal tremor: A reappraisal of the nosology. Brain. 2006;129:832–840. doi: 10.1093/brain/awh684. [DOI] [PubMed] [Google Scholar]
- 57.Pirio Richardson S, Mari Z, Matsuhashi M, Hallett M. Psychogenic palatal tremor. Mov Disord. 2006;21:274–276. doi: 10.1002/mds.20731. [DOI] [PubMed] [Google Scholar]
- 58.Williams DR. Psychogenic palatal tremor. Mov Disord. 2004;19:333–335. doi: 10.1002/mds.10632. [DOI] [PubMed] [Google Scholar]
- 59.Margari F, Giannella G, Lecce PA, Fanizzi P, Toto M, Margari L. A childhood case of symptomatic essential and psychogenic palatal tremor. Neuropsychiatr Dis Treat. 2011;7:223–227. doi: 10.2147/NDT.S15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klein C, Gehrking E, Vieregge P. Voluntary palatal tremor in two siblings. Mov Disord. 1998;13:545–548. doi: 10.1002/mds.870130328. [DOI] [PubMed] [Google Scholar]
- 61.Samuel M, Kleiner-Fisman G, Lang AE. Voluntary control and a wider clinical spectrum of essential palatal tremor. Mov Disord. 2004;19:717–719. doi: 10.1002/mds.20034. [DOI] [PubMed] [Google Scholar]
- 62.Nitschke MF, Krüger G, Bruhn H, Klein C, et al. Voluntary palatal tremor is associated with hyperactivation of the inferior olive: A functional magnetic resonance imaging study. Mov Disord. 2001;16:1193–1195. doi: 10.1002/mds.1202. [DOI] [PubMed] [Google Scholar]
- 63.Stamelou M, Saifee TA, Edwards MJ, Bhatia KP. Psychogenic palatal tremor may be underrecognized: Reappraisal of a large series of cases. Mov Disord. 2012;27:1164–1168. doi: 10.1002/mds.24948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cho JW, Chu K, Jeon BS. Case of essential palatal tremor: Atypical features and remarkable benefit from botulinum toxin injection. Mov Disord. 2001;16:779–782. doi: 10.1002/mds.1132. [DOI] [PubMed] [Google Scholar]
- 65.Penney SE, Bruce IA, Saeed SR. Botulinum toxin is effective and safe for palatal tremor: A report of five cases and a review of the literature. J Neurol. 2006;253:857–860. doi: 10.1007/s00415-006-0039-9. [DOI] [PubMed] [Google Scholar]
- 66.Kozlowska K, Nunn KP, Rose D, Morris A, Ouvrier RA, Varghese J. Conversion disorder in Australian pediatric practice. J Am Acad Child Adolesc Psychiatry. 2007;46:68–75. doi: 10.1097/01.chi.0000242235.83140.1f. [DOI] [PubMed] [Google Scholar]
- 67.Mink JW. Conversion disorder and mass psychogenic illness in child neurology. Ann NY Acad Sci. 2013;1304:40–44. doi: 10.1111/nyas.12298. [DOI] [PubMed] [Google Scholar]
- 68.Ferrara J, Jankovic J. Psychogenic movement disorders in children. Mov Disord. 2008;23:1875–1881. doi: 10.1002/mds.22220. [DOI] [PubMed] [Google Scholar]
- 69.Dale RC, Singh H, Troedson C, Pillai S, Gaikiwari S, Kozlowska K. A prospective study of acute movement disorders in children. Dev Med Child Neurol. 2010;52:739–748. doi: 10.1111/j.1469-8749.2009.03598.x. [DOI] [PubMed] [Google Scholar]
- 70.Ozekmekçi S, Apaydin H, Ekinci B, Yalçinkaya C. Psychogenic movement disorders in two children. Mov Disord. 2003;18:1395–1397. doi: 10.1002/mds.10539. [DOI] [PubMed] [Google Scholar]
- 71.Canavese C, Ciano C, Zibordi F, Zorzi G, Cavallera V, Nardocci N. Phenomenology of psychogenic movement disorders in children. Mov Disord. 2012;27:1153–1157. doi: 10.1002/mds.24947. [DOI] [PubMed] [Google Scholar]
- 72.Pellicciari R, Superbo M, Gigante AF, Livrea P, Defazio G. Disease modeling in functional movement disorders. Parkinsonism Relat Disord. 2014;20:1287–1289. doi: 10.1016/j.parkreldis.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 73.Okun MS, Rodriguez RL, Foote KD, Fernandez HH. The “chair test” to aid in the diagnosis of psychogenic gait disorders. Neurologist. 2007;13:87–91. doi: 10.1097/01.nrl.0000256358.52613.cc. [DOI] [PubMed] [Google Scholar]
- 74.Schwingenschuh P, Pont Sunyer C, Surtees R, Edwards MJ, Bhatia KP. Psychogenic movement disorders in children a report of 15 cases and a review of the literature. Mov Disord. 2008;23:1882–1888. doi: 10.1002/mds.22280. [DOI] [PubMed] [Google Scholar]
- 75.Gelauff J, Stone J, Edwards M, Carson A. The prognosis of functional (psychogenic) motor symptoms: A systematic review. J Neurol Neurosurg Psychiatry. 2014;85:220–226. doi: 10.1136/jnnp-2013-305321. [DOI] [PubMed] [Google Scholar]
- 76.Batla A, Stamelou M, Edwards MJ, et al. Functional movement disorders are not uncommon in the elderly. Mov Disord. 2013;28:540–543. doi: 10.1002/mds.25350. [DOI] [PubMed] [Google Scholar]
- 77.Baik JS, Lang AE. Gait abnormalities in psychogenic movement disorders. Mov Disord. 2007;22:395–399. doi: 10.1002/mds.21283. [DOI] [PubMed] [Google Scholar]
- 78.Balaratnasingam S, Janca A. Mass hysteria revisited. Curr Opin Psychiatry. 2006;19:171–174. doi: 10.1097/01.yco.0000214343.59872.7a. [DOI] [PubMed] [Google Scholar]
- 79.Broderick JE, Kaplan-Liss E, Bass E. Experimental induction of psychogenic illness in the context of a medical event and media exposure. Am J Disaster Med. 2011;6:163–172. [PMC free article] [PubMed] [Google Scholar]
- 80.Jones TF, Craig AS, Hoy D, et al. Mass psychogenic illness attributed to toxic exposure at a high school. New Engl J Med. 2000;342:96–100. doi: 10.1056/NEJM200001133420206. [DOI] [PubMed] [Google Scholar]
- 81.Smithson A. Rethinking the lessons of Tokyo. In: Smithson A, Levy L, editors. Ataxia: The chemical and biological terrorism threat and the US response. Washington, DC: The Henry Stimson Center; 2000. pp. 71–111. p. [Google Scholar]
- 82.Lorber W, Mazzoni G, Kirsch I. Illness by suggestion: Expectancy, modeling, and gender in the production of psychosomatic symptoms. Ann Behav Med. 2007;33:112–116. doi: 10.1207/s15324796abm3301_13. [DOI] [PubMed] [Google Scholar]
- 83.Stamelou M, Cossu G, Edwards MJ, et al. Familial psychogenic movement disorders. Mov Disord. 2013;28:1295–1298. doi: 10.1002/mds.25463. [DOI] [PubMed] [Google Scholar]