Abstract
Background
Four cases of paroxysmal kinesigenic dyskinesia (PKD) have been reported in individuals with proximal 16p11.2 microdeletions that include PRRT2.
Case Report
We describe a fifth patient with PKD, features of Asperger’s syndrome, and mild language delays. Sanger sequencing of the PRRT2 gene did not identify any mutations implicated in PKD. However, microarray-based comparative genomic hybridization (aCGH) detected a 533.9-kb deletion on chromosome 16, encompassing over 20 genes and transcripts.
Discussion
This case underscores the importance of aCGH testing for individuals with PKD who do not have PRRT2 mutations, particularly when developmental delays, speech problems, intellectual disability, and/or autism spectrum disorder are present.
Keywords: Paroxysmal kinesigenic dyskinesia, 16p11.2 microdeletion, movement disorders
Introduction
Paroxysmal kinesigenic dyskinesia (PKD) is an episodic, brief movement disorder triggered by sudden movements. The phenomenology of the movements may be dystonic and/or choreic, typically lasting less than 1 minute, and occurring as often as 10–100 times per day. Initial linkage studies mapped PKD to the pericentromeric region of chromosome 16,1 which supported the 2011 discovery of heterozygous truncating mutations in the PRRT2 gene (16p11.2) as a highly penetrant cause of familial PKD.2 Since then, multiple mutations in PRRT2 have been reported among patients with PKD and other related phenotypes including benign familial infantile epilepsy (BFIE) and infantile convulsions with choreoathetosis (ICCA) syndrome.3 Most of the reported PRRT2 mutations are nonsense, frameshift, and splice site mutations predicted to result in truncation of the PRRT2 protein and haploinsufficiency, including the most common c.629dupC (p.Arg217Profs*8) mutation.3
Microdeletions of chromosome 16p11.2 that include PRRT2 and over 20 neighboring genes have also been implicated as a rare cause of PKD, with only four patients reported to date.4–7 More commonly, these proximal 16p11.2 microdeletions have been detected among patients with variable clinical features including developmental delays, intellectual disability, minor dysmorphic facial features, and/or autism spectrum disorder (ASD).8–10 These proximal 16p11.2 microdeletions typically occur de novo in affected probands; however, recent studies have identified the aberration in some control subjects and only mildly affected individuals,11–16 suggesting that this deletion may have incomplete penetrance and/or variable expressivity. We report a fifth patient with PKD and a proximal 16p11.2 microdeletion, review the related PKD cases reported to date, and propose phenotypic clues to facilitate a diagnosis of PKD in the context of the 16p11.2 microdeletion syndrome.
Case Report
A 12-year-old boy developed his first paroxysmal episode at age 10.5 years. Episodes were triggered by sudden movement after a period of inactivity such as sitting in a car or watching television on a couch. During attacks, there was dystonia of both arms and trunk with flexion of the left elbow and extension of the right elbow, sometimes back arching, typically lasting 5–10 seconds (Video 1). The frequency of the episodes was initially one or two times per month, but subsequently increased to 4–12 times per day without treatment. He did not lose consciousness during the events, and electroencephalography was unremarkable.
After one of his dystonic episodes, he developed back pain, and spine imaging revealed grade 2 spondylolisthesis involving the lower back. No other vertebral abnormality was identified. His history was also notable for features of Asperger’s syndrome with mild delay in language milestone acquisition, which prompted speech and occupational therapies around age 4–5 years. Motor milestones were normal and there was no history of seizures. He currently attends regular school. He was treated with carbamazepine, up to 250 mg/day, with marked improvement in the frequency and severity of the episodes, but with worsening anxiety. He also had good response to phenytoin but developed swollen gums. Carbamazepine was reintroduced, up to 200 mg/day with improvement, but he then developed leukopenia (white blood cell count of 2,800/µL). He could not tolerate oxcarbazepine, and topiramate was not effective.
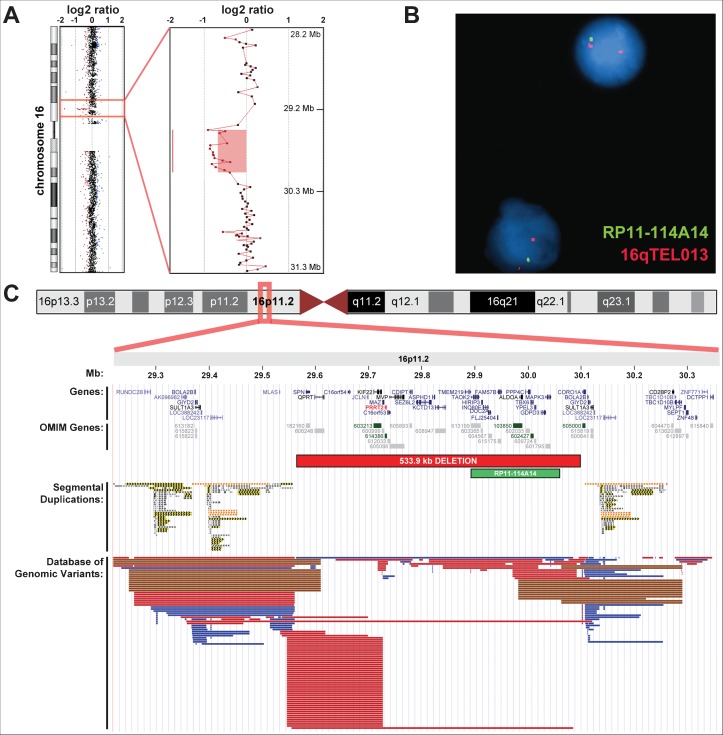
Genetic testing included Sanger sequencing of the PRRT2 coding regions and flanking splice sites, and whole-genome microarray-based comparative genomic hybridization (aCGH; 180K oligonucleotide + SNP microarray, Agilent Technologies). Although no pathogenic PRRT2 mutations were detected by sequencing, aCGH identified a 533.9-kb deletion on chromosome 16 [arr 16p11.2(29564185–30098069)x1; hg18], encompassing over 20 genes and transcripts and including four Mendelian disease genes (KIFF22, PRRT2, ALDOA, and TBX6) (Figure 1). This proximal 16p11.2 deletion was confirmed by fluorescence in situ hybridization (FISH) using a bacterial artificial chromosome (BAC) probe that hybridized to 16p11.2 and a control probe that hybridized to the subtelomere of chromosome 16q (Figure 1). Both parents subsequently were tested by aCGH and were negative for the aberration, indicating that the 16p11.2 microdeletion occurred de novo in the proband.
Figure 1. Identification of the Proximal 16p11.2 Microdeletion.
(A) Microarray-based comparative genomic hybridization (aCGH) results of chromosome 16 in the proband. The identified 16p11.2 deletion is highlighted in the chromosome view and enlarged. Red, black, and blue dots represent aCGH probes with log2 ratios less than −0.25, in between −0.25 and 0.25, and greater than 0.25, respectively. (B) Confirmation of the deletion by fluorescence in situ hybridization (FISH) using a BAC probe that hybridized to 16p11.2 (RP11-114A14) and a control probe that hybridized to the subtelomere of chromosome 16q (16qTEL013). (C) Illustration of the chromosome 16 ideogram with enlarged views from ∼29.2 to 30.3 Mb within cytoband 16p11.2. The location of the 533.9-kb deletion and FISH probe are noted in relation to the location of known human genes and transcripts from the University of California Santa Cruz (UCSC) Genes track (including gene predictions from RefSeq, Genbank, Consensus Coding Sequence [CCDS], and UniProt), Online Mendelian Inheritance in Man (OMIM) disease genes (with OMIM identification [ID] numbers), segmental duplications [color was used to distinguish level of similarity (gray: 90–98% similarity; yellow: 98–99% similarity; orange: >99%], and structural variants from the Database of Genomic Variants34 (blue bars representing copy number gain copy number variants [CNVs], red bars representing copy number loss CNVs, and brown bars representing both copy number gain and loss CNVs).
In addition to the 16p11.2 deletion, aCGH analysis detected a 170.5-kb duplication on chromosome 21 [21q22.11q22.12(34656524–34827038)x3; hg18], including four genes, two of which are Mendelian disease genes (KCNE2 and KCNE1). Parental aCGH analysis indicated that this duplication was paternally inherited, suggesting that it is likely a benign familial copy number variant (CNV).
Discussion
The PRRT2 gene, located on the short arm of chromosome 16 (16p11.2), encodes the proline-rich transmembrane protein 2. Although its biological function is presently unknown, murine studies indicate that it is predominantly expressed in the brain and spinal cord.2 Heterozygous mutations in PRRT2 were discovered by exome sequencing in 2011 to be an autosomal dominant cause of familial PKD.2 The prevalence of PRRT2 mutations in PKD ranges from 20–65%,17–21 suggesting that other genes and mutations are likely responsible for PKD in patients without a PRRT2 mutation or deletion. Since the initial discovery of PRRT2 mutations in PKD, the phenotypes related to PRRT2 have expanded. For example, PRRT2 mutations have also been identified among patients with BFIE and ICCA,22,23 indicating that disruption of normal PRRT2 function can have pleiotropic effects. The majority of PRRT2 mutations associated with PKD result in protein truncation and haploinsufficiency;3 however, deletion of PRRT2 and neighboring genes at proximal 16p11.2 has also been reported in four cases of PKD.
Patients with the 16p11.2 microdeletion syndrome typically present with developmental delays, intellectual disability, speech delay, and/or ASD.8–10 These features are most likely due to haploinsufficiency of neighboring 16p11.2 genes since PKD patients with truncating PRRT2 mutations have not been reported with these developmental issues.2,22,23 The recurrent proximal 16p11.2 microdeletion has an estimated prevalence of two or three in 10,0008,24 and is flanked by segmental duplications and common CNVs (Figure 1), suggesting that the rearrangement is most likely mediated by non-allelic homologous recombination. The reciprocal ∼600-kb duplication of proximal 16p11.2 has also been associated with developmental delays, ASD, and other neurological phenotypes;8,9,25,26 however, this aberration has significantly reduced penetrance compared to the deletion, given its frequent inheritance from clinically unaffected parents. Of note, smaller deletions (∼200 kb) at distal 16p11.2, also flanked by segmental duplications, have been implicated in developmental delay, childhood obesity, and susceptibility to schizophrenia,27–29 underscoring the clinical significance of the 16p11.2 region and the complex variability in phenotypic expression.
In addition to PRRT2, the identified 533.9-kb deletion also included the Mendelian disease genes TBX6, KIF22, and ALDOA. These genes have been implicated in autosomal dominant spondylocostal dysostosis,30 autosomal dominant spondyloepimetaphyseal dysplasia with joint laxity,31,32 and autosomal recessive glycogen storage disease and hemolytic anemia,33 respectively. Of note, the autosomal dominant conditions associated with TBX6 and KIF22 were due to disruption of the stop codon and missense mutations, respectively, and not from gene deletions. Our patient does not have any features of hemolytic anemia or spondylocostal dysostosis. KIF22 missense mutations have been described in spondyloepimetaphyseal dysplasia patients with skeletal issues including vertebral endplate irregularities, joint laxity, scoliosis, and at risk for joint dislocation.31,32 Our patient had grade 2 spondylolisthesis, but there were no other skeletal abnormalities or joint laxity. As such, it is currently unclear if the heterozygous deletion of KIF22 in our patient contributed to his spine issue.
A paternally inherited 170.5-kb duplication on chromosome 21q22.11-q22.12 was also detected in the proband. This region has minimal overlap with CNVs reported among normal individuals in the Database of Genomic Variants34 and includes the Mendelian disease genes KCNE1 and KCNE2. Heterozygous missense mutations in KCNE2 and homozygous mutations in KCNE1 have been associated with long QT syndrome, and may be pathogenic. Because triplosensitivity to these genes has not been established, this variant was initially considered to be of uncertain clinical significance; however, detection of the CNV in a clinically unaffected parent suggested that the duplication was likely benign.
This case and the previously reported cases of PKD with 16p11.2 microdeletions are summarized in Table 1. All cases were assessed by genome-wide copy number analyses, including aCGH and/or single nucleotide polymorphism (SNP) arrays, and the size of the overlapping deletions ranged from 432.0 to 895.0 kb. Of the five cases, the age at onset varied from infancy up to 10.5 years. In addition to PKD, one patient also had dopa-responsive parkinsonism. Three patients had a history of infantile convulsions, which is likely attributable to PRRT2 haploinsufficiency since this phenotype is also present in PKD cases with pathogenic mutations in PRRT2. Consistent with the 16p11.2 microdeletion syndrome, all patients had some developmental delay, the most common being language delay. Treatment data were available in two cases, both of which had a very good response to carbamazepine. Parkinsonism in the first case responded well to low dose levodopa (200 mg/day). Notably, the paucity of PKD cases reported with this aberration supports the incomplete penetrance and variable expressivity previously associated with the relatively common proximal 16p11.2 microdeletion. The infrequent finding of PKD among 16p11.2 cases also suggests that deletion of PRRT2 alone may not always result in a PKD phenotype, and that unknown modifier genes or other important variables may exist.
Table 1. Case Reports of PKD with Proximal 16p11.2 Microdeletions.
| Patient | Reference | Year | Age at Onset (Years) | Movement Disorders | History of Seizure | Developmental Disability | Chromosomal Microarray Analysis | Microdeletion Size (kb) | Deletion Coordinates (hg18) | Treatment (response) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Lipton and Rivkin4 | 2009 | Infancy | PKD and dopa-responsive parkinsonism | Yes, infantile convulsions | Verbal learning disabilities | SNP array | 544.3 | Chr16:29560500–30104842 | CBZ (very good to PKD); levodopa 200 mg/day (resolution of parkinsonism) |
| 2 | Dale et al.6 | 2012 | 6 | PKD | No | Speech delay, mild orobuccal dyspraxia | aCGH | 432.0 | Chr16:29581455–30013488 | No information |
| 3 | Dale et al.5,6 | 2011,2012 | 10 | PKD | Yes, benign infantile seizures | Mild intellectual disability | aCGH | 597.8 | Chr16:29500284–30098069 | No information |
| 4 | Weber et al.7 | 2013 | Infancy | PKD | Yes, infantile convulsions | Mainly speech delay, and impaired fine motor skills and balance | SNP array | 895.0 | Chr16: 29210745–30105652 | No information |
| 5 | Current case | 2014 | 10.5 | PKD | No | Asperger’s syndrome (language delay) | CGH + SNP array | 533.9 | Chr16:34656524–34827038 | CBZ (very good) |
Abbreviations: aCGH, Microarray-based Comparative Genomic Hybridization; CBZ, Carbamazepine; PKD, Paroxysmal Kinesigenic Dyskinesia; SNP, Single Nucleotide Polymorphism.
The American College of Medical Genetics and Genomics has recommended chromosomal microarray (CMA) as a first tier diagnostic test for any patient with developmental delay.35 It has also been suggested by Dale et al. that CMAs be used as first tier testing in patients with suspected genetic movement disorders, especially for those with developmental or intellectual issues.6 Even mild presentations of developmental delays, speech delays, and/or co-existing ASD should prompt clinicians to suspect the 16p11.2 microdeletion syndrome for patients with PKD, as evidenced by our case and those previously reported. Importantly, sequencing of PRRT2 will not detect gene deletions, including the recurrent 16p11.2 deletion, and a negative sequencing result should not dissuade clinicians from the potential diagnosis of this syndrome for patients with PKD.
Video 1.
Segment 1: This home video demonstrated an episode with truncal dystonia, seen as twisting of his trunk, while the patient is standing and holding a chair. The duration of the episode in the video lasted less than 5 seconds, but the episode was not captured from the outset. Segment 2: This home video demonstrated dystonia of bilateral upper extremities and trunk, seen as truncal flexion. The episode lasted less than 5 seconds but it was not captured from the outset.
Footnotes
Funding: None.
Financial Disclosures: None.
Conflict of Interests: The authors report no conflict of interest.
Consent statement: Written informed consent was obtained from the parent of the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
References
- 1.Szepetowski P, Rochette J, Berquin P, Piussan C, Lathrop GM, Monaco AP. Familial infantile convulsions and paroxysmal choreoathetosis: A new neurological syndrome linked to the pericentromeric region of human chromosome 16. Am J Hum Genet. 1997;61:889–898. doi: 10.1086/514877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen WJ, Lin Y, Xiong ZQ, et al. Exome sequencing identifies truncating mutations in PRRT2 that cause paroxysmal kinesigenic dyskinesia. Nat Genet. 2011;43:1252–1255. doi: 10.1038/ng.1008. [DOI] [PubMed] [Google Scholar]
- 3.Heron SE, Dibbens LM. Role of PRRT2 in common paroxysmal neurological disorders: A gene with remarkable pleiotropy. J Med Genet. 2013;50:133–139. doi: 10.1136/jmedgenet-2012-101406. [DOI] [PubMed] [Google Scholar]
- 4.Lipton J, Rivkin MJ. 16p11.2-related paroxysmal kinesigenic dyskinesia and dopa-responsive parkinsonism in a child. Neurology. 2009;73:479–480. doi: 10.1212/WNL.0b013e3181b16393. [DOI] [PubMed] [Google Scholar]
- 5.Dale RC, Grattan-Smith P, Fung VS, Peters GB. Infantile convulsions and paroxysmal kinesigenic dyskinesia with 16p11.2 microdeletion. Neurology. 2011;77:1401–1402. doi: 10.1212/WNL.0b013e31823152d7. [DOI] [PubMed] [Google Scholar]
- 6.Dale RC, Grattan-Smith P, Nicholson M, Peters GB. Microdeletions detected using chromosome microarray in children with suspected genetic movement disorders: A single-centre study. Dev Med Child Neurol. 2012;54:618–623. doi: 10.1111/j.1469-8749.2012.04287.x. [DOI] [PubMed] [Google Scholar]
- 7.Weber A, Kohler A, Hahn A, Neubauer B, Muller U. Benign infantile convulsions (IC) and subsequent paroxysmal kinesigenic dyskinesia (PKD) in a patient with 16p11.2 microdeletion syndrome. Neurogenetics. 2013;14:251–253. doi: 10.1007/s10048-013-0376-7. [DOI] [PubMed] [Google Scholar]
- 8.Weiss LA, Shen Y, Korn JM, et al. Association between microdeletion and microduplication at 16p11.2 and autism. N Engl J Med. 2008;358:667–675. doi: 10.1056/NEJMoa075974. [DOI] [PubMed] [Google Scholar]
- 9.Fernandez BA, Roberts W, Chung B, et al. Phenotypic spectrum associated with de novo and inherited deletions and duplications at 16p11.2 in individuals ascertained for diagnosis of autism spectrum disorder. J Med Genet. 2010;47:195–203. doi: 10.1136/jmg.2009.069369. [DOI] [PubMed] [Google Scholar]
- 10.Shinawi M, Liu P, Kang SH, et al. Recurrent reciprocal 16p11.2 rearrangements associated with global developmental delay, behavioural problems, dysmorphism, epilepsy, and abnormal head size. J Med Genet. 2010;47:332–341. doi: 10.1136/jmg.2009.073015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bijlsma EK, Gijsbers AC, Schuurs-Hoeijmakers JH, et al. Extending the phenotype of recurrent rearrangements of 16p11.2: Deletions in mentally retarded patients without autism and in normal individuals. Eur J Med Genet. 2009;52:77–87. doi: 10.1016/j.ejmg.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Glessner JT, Wang K, Cai G, et al. Autism genome-wide copy number variation reveals ubiquitin and neuronal genes. Nature. 2009;459:569–573. doi: 10.1038/nature07953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanson E, Nasir RH, Fong A, et al. Cognitive and behavioral characterization of 16p11.2 deletion syndrome. J Dev Behav Pediatr. 2010;31:649–657. doi: 10.1097/DBP.0b013e3181ea50ed. [DOI] [PubMed] [Google Scholar]
- 14.Cooper GM, Coe BP, Girirajan S, et al. A copy number variation morbidity map of developmental delay. Nat Genet. 2011;43:838–846. doi: 10.1038/ng.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanson E, Bernier R, Porche K, et al. The cognitive and behavioral phenotype of the 16p11.2 deletion in a clinically ascertained population. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zufferey F, Sherr EH, Beckmann ND, et al. A 600 kb deletion syndrome at 16p11.2 leads to energy imbalance and neuropsychiatric disorders. J Med Genet. 2012;49:660–668. doi: 10.1136/jmedgenet-2012-101203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YC, Lee MJ, Yu HY, et al. PRRT2 mutations in paroxysmal kinesigenic dyskinesia with infantile convulsions in a Taiwanese cohort. PLoS One. 2012;7:e38543. doi: 10.1371/journal.pone.0038543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meneret A, Grabli D, Depienne C, et al. PRRT2 mutations: A major cause of paroxysmal kinesigenic dyskinesia in the European population. Neurology. 2012;79:170–174. doi: 10.1212/WNL.0b013e31825f06c3. [DOI] [PubMed] [Google Scholar]
- 19.Groffen AJ, Klapwijk T, van Rootselaar AF, Groen JL, Tijssen MA. Genetic and phenotypic heterogeneity in sporadic and familial forms of paroxysmal dyskinesia. J Neurol. 2013;260:93–99. doi: 10.1007/s00415-012-6592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen YP, Song W, Yang J, et al. PRRT2 mutation screening in patients with paroxysmal kinesigenic dyskinesia from southwest China. Eur J Neurol. 2014;21:174–176. doi: 10.1111/ene.12122. [DOI] [PubMed] [Google Scholar]
- 21.Erro R, Sheerin UM, Bhatia KP. Paroxysmal dyskinesias revisited: a review of 500 genetically proven cases and a new classification. Mov Disord. 2014;29:1108–1116. doi: 10.1002/mds.25933. [DOI] [PubMed] [Google Scholar]
- 22.Wang JL, Cao L, Li XH, et al. Identification of PRRT2 as the causative gene of paroxysmal kinesigenic dyskinesias. Brain. 2011;134:3493–3501. doi: 10.1093/brain/awr289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Heron SE, Grinton BE, Kivity S, et al. PRRT2 mutations cause benign familial infantile epilepsy and infantile convulsions with choreoathetosis syndrome. Am J Hum Genet. 2012;90:152–160. doi: 10.1016/j.ajhg.2011.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenfeld JA, Coppinger J, Bejjani BA, et al. Speech delays and behavioral problems are the predominant features in individuals with developmental delays and 16p11.2 microdeletions and microduplications. J Neurodev Disord. 2010;2:26–38. doi: 10.1007/s11689-009-9037-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McCarthy SE, Makarov V, Kirov G, et al. Microduplications of 16p11.2 are associated with schizophrenia. Nat Genet. 2009;41:1223–1227. doi: 10.1038/ng.474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall CR, Noor A, Vincent JB, et al. Structural variation of chromosomes in autism spectrum disorder. Am J Hum Genet. 2008;82:477–488. doi: 10.1016/j.ajhg.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bachmann-Gagescu R, Mefford HC, Cowan C, et al. Recurrent 200-kb deletions of 16p11.2 that include the SH2B1 gene are associated with developmental delay and obesity. Genet Med. 2010;12:641–647. doi: 10.1097/GIM.0b013e3181ef4286. [DOI] [PubMed] [Google Scholar]
- 28.Guha S, Rees E, Darvasi A, et al. Implication of a rare deletion at distal 16p11.2 in schizophrenia. JAMA Psychiatry. 2013;70:253–260. doi: 10.1001/2013.jamapsychiatry.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Walters RG, Jacquemont S, Valsesia A, et al. A new highly penetrant form of obesity due to deletions on chromosome 16p11.2. Nature. 2010;463:671–675. doi: 10.1038/nature08727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sparrow DB, McInerney-Leo A, Gucev ZS, et al. Autosomal dominant spondylocostal dysostosis is caused by mutation in TBX6. Hum Mol Genet. 2013;22:1625–1631. doi: 10.1093/hmg/ddt012. [DOI] [PubMed] [Google Scholar]
- 31.Boyden ED, Campos-Xavier AB, Kalamajski S, et al. Recurrent dominant mutations affecting two adjacent residues in the motor domain of the monomeric kinesin KIF22 result in skeletal dysplasia and joint laxity. Am J Hum Genet. 2011;89:767–772. doi: 10.1016/j.ajhg.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Min BJ, Kim N, Chung T, et al. Whole-exome sequencing identifies mutations of KIF22 in spondyloepimetaphyseal dysplasia with joint laxity, leptodactylic type. Am J Hum Genet. 2011;89:760–766. doi: 10.1016/j.ajhg.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreuder J, Borkhardt A, Repp R, et al. Brief report: Inherited metabolic myopathy and hemolysis due to a mutation in aldolase A. N Engl J Med. 1996;334:1100–1104. doi: 10.1056/NEJM199604253341705. [DOI] [PubMed] [Google Scholar]
- 34.MacDonald JR, Ziman R, Yuen RK, Feuk L, Scherer SW. The Database of Genomic Variants: A curated collection of structural variation in the human genome. Nucleic Acids Res. 2014;42:D986–992. doi: 10.1093/nar/gkt958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shaffer LG, American College of Medical Genetics Professional P, Guidelines C American College of Medical Genetics guideline on the cytogenetic evaluation of the individual with developmental delay or mental retardation. Genet Med. 2005;7:650–654. doi: 10.1097/01.gim.0000186545.83160.1e. [DOI] [PMC free article] [PubMed] [Google Scholar]