Abstract
Centimeter-long electron conduction through marine sediments, in which electrons derived from sulfide in anoxic sediments are transported to oxygen in surficial sediments, may have an important influence on sediment geochemistry. Filamentous bacteria have been proposed to mediate the electron transport, but the filament conductivity could not be verified and other mechanisms are possible. Surprisingly, previous investigations have never actually measured the sediment conductivity or its basic physical properties. Here we report direct measurements that demonstrate centimeter-long electron flow through marine sediments, with conductivities sufficient to account for previously estimated electron fluxes. Conductivity was lost for oxidized sediments, which contrasts with the previously described increase in the conductivity of microbial biofilms upon oxidation. Adding pyrite to the sediments significantly enhanced the conductivity. These results suggest that the role of conductive minerals, which are more commonly found in sediments than centimeter-long microbial filaments, need to be considered when modeling marine sediment biogeochemistry.
To evaluate the conductivity of coastal anaerobic marine sediments, gold electrodes separated by a 50-μm nonconductive gap were inserted at different depths in intact sediment cores collected from Nantucket Bay, Massachusetts (Figure 1a). Conductivity of the sediments was measured with techniques comparable to those previously used to document the conductivity of microbial pili networks and biofilms (Malvankar et al., 2011, 2012a). This approach to measure in situ dc conductivity is substantially different from previous attempts to probe the conductivity of soils and sediments that either used self-potential monitoring (Ntarlagiannis et al., 2007) or measurements over small timescales (<1 s) (Regberg et al., 2011), which primarily measure the ionic contribution and not electron conductivity (Du et al., 2009; Patra et al., 2010). Direct conductivity measurements revealed values that were low in oxidized surficial sediments (<1 μS cm−1); however, conductivities were significantly higher (P<0.05, t-test) in deeper, highly reduced sediments (Figure 1b). Along with intact sediment cores, experiments were also performed with mixed sediment subsamples. Comparable values of 7±0.15 μS cm−1 (mean±s.e.; n=3) were obtained using this alternative approach, in which reduced sediments were placed on a four-probe electrode array under anaerobic conditions (Figure 1c) and conductivities measured over the 1-cm span of the electrodes.
Figure 1.
Strategy to measure in situ sediment conductivity. (a) Schematic of setup to place intact cores of marine sediment. Gold electrodes with a 50-μm nonconductive spacing were inserted at different depths with respect to the overlying water. (b) Conductivity data of three independent sediment cores as a function of depth from the overlying water. Control setup was comprised of garden sand. Error bars represent s.d. These measurements were performed at 15 °C to mimic the physiological temperature of marine sediments. (c) Schematic of four-probe used to measure sediment conductivity. Current was injected using outer two gold electrodes and the voltage was measured using inner two gold electrodes using a high-impedance voltmeter.
In previous studies (Nielsen et al., 2010) that led to the concept of long-range electron transport via conductive microbial filaments (Pfeffer et al., 2012), electric currents in the sediment were not actually measured, but rather inferred from rates of sediment oxygen consumption that were estimated from oxygen concentration profiles (Nielsen et al., 2010). In Aarhus Bay, estimated rates of oxygen consumption were 9.7 mmol O2 per m2 per day with 31% of this oxygen consumption attributed to electric current from deeper sediments (that is, 3 mmol O2 per m2 per day) and in Aarhus Harbor sediments 42% of the estimated 46 mmol O2 per m2 consumed per day (that is, 19.3 mmol O2 per m2 per day) was attributed to the inferred electric currents (Nielsen et al., 2010). Thus, given that four moles of electrons are required for each mole of oxygen reduced to water (O2+4H++4e−→2H2O), the estimated electron flux through the sediments was 12–77 mmoles of electrons per m2 per day. This electron flux through each m2 of sediment can be converted to electric current as follows (calculations shown for maximum estimated flux): ((7.7 × 10−2 moles of electrons per day) × (1.16 × 10−5 days s−1) × (Amp/1.036 × 10−5 moles of electrons per second)=0.086 Amps or 86 mA for Aarhus Harbor sediments and 14 mA for the Aarhus Bay sediments). The proposed (Nielsen et al., 2010) reaction driving the electron flux is the oxidation of sulfide coupled to the reduction of oxygen, that is, a potential difference of ca. 1.1 V (HS−/S couple, −0.27 V; O2/H2O, +0.82 V; (Thauer et al., 1977)). The current (I) expected through 1 m2 of sediment with the conductivity (σ) of 7 μS cm−1 that was measured over 1 cm (approximate length of proposed conductive microbial filaments) of the Nantucket Bay sediments can be calculated from the relationship σ=G × l/A (l=length=1 cm; A=area=1 m2 or 104 cm2), where G is the conductance (G=I/V) and thus I=(V × A × σ)/l; (I=(1.1 V × 104 cm2 × 7 × 10−6 S cm−1)/1 cm)=7.7 × 10−2 V-S=77 mA. This compares well with the currents of 14–86 mA estimated from oxygen consumption in the sediments (Nielsen et al., 2010) in which it was proposed that microbial filaments were responsible for long-range electron transport (Pfeffer et al., 2012).
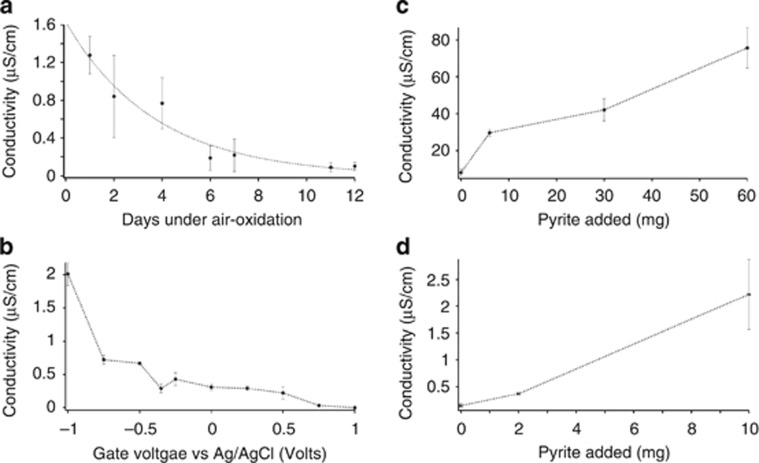
To evaluate the contrast in conductivity between surficial oxidized sediments and deeper reduced sediments, reduced sediments were oxidized under air at 4 °C to preserve the biological components in the marine sediments such as bacterial c-type cytochromes and pili nanowires that have been hypothesized to confer conductivity to marine sediments (Nielsen et al., 2010; Reguera, 2012). Upon air-oxidation, conductivity of marine sediments declined ca. 90% (Figure 2a). Electrochemical oxidation of the sediments resulted in a similar conductivity loss (Figure 2b).
Figure 2.
Effect of oxidation and mineral addition on sediment conductivity. (a) Effect of air-oxidation over days on the conductivity of marine sediments. Error bars represent s.d. (n=3 biological replicates). (b) Effect of electrochemical oxidation on the conductivity of marine sediments as a function of gate voltage using electrolyte-gated field-effect transistor geometry. (c) Effect of the addition of pyrite mineral to the conductivity of marine sediments. (d) Effect of the addition of pyrite mineral to the conductivity of freshwater sediments. All error bars represent s.d.
The decrease in sediment conductivity upon oxidation contrasts with the two orders of magnitude increase in conductivity following oxidation of Geobacter sulfurreducens biofilms (Malvankar et al., 2011, 2012b), which are the only microbial system in which centimeter-long electron conduction has been directly documented. It is likely that oxidation increases conduction in G. sulfurreducens biofilms because the networks of pili that are thought to mediate long-range electron transport exhibit p-type conduction in which holes are majority carriers (Malvankar et al., 2011). The conductivity of p-type materials increases upon oxidation because the oxidation process increases the density of hole carriers (Heeger et al., 2010; Malvankar and Lovley, 2012). The suppression of sediment conductivity with oxidation indicated that long-range electron transport through the sediments is significantly different than that through G. sulfurreducens biofilms. It is unknown how oxidizing conditions might have an impact on the previously proposed conductivity through filamentous bacteria (Pfeffer et al., 2012; Malkin et al., 2014) because the hypothesized conductivity and the mechanisms for conduction have not been documented (Reguera, 2012).
An abiological mechanism for electron transport through sediments that could potentially be eliminated with oxidation is conduction through iron–sulfur minerals. Dense assemblages of conductive iron–sulfur minerals in ore bodies (Sato and Mooney, 1960) or hydrothermal vents (Nakamura et al., 2010) may be capable of conducting electrons over distances of centimeters (Nakamura et al., 2010) to meters (Sato and Mooney, 1960). To determine whether lower abundances of an iron–sulfur mineral, comparable to those that might be found in reduced marine sediments, could contribute to conductivity, finely ground pyrite (10–100 μm diameters) was added to the reduced sediment. There was a significant increase in conductivity at higher pyrite concentrations (Figure 2c). The conductivity of freshwater sediment also increased upon addition of pyrite (Figure 2d).
Although it has been suggested that filaments of cells closely related to Desulfobulbus species accounted for conductivity to reduced marine sediments from Aarhus Bay, it is, in fact, unknown whether this is possible because the conductivity of the filaments was not demonstrated (Pfeffer et al., 2012; Reguera, 2012; Malkin et al., 2014). No long bacterial filaments were observed in the Nantucket sediments used in these studies. The findings reported here, based on direct measurements of sediment conductivity, demonstrate that conductive minerals can confer substantial conductivity to anaerobic marine sediments and could potentially have an important role in sediment biogeochemistry. The simple method described here for assessing sediment conductivity is expected to be a useful tool for future studies of long-range electron conduction in a diversity of soils and sediments.
Materials and methods
Sediment collection and maintenance
Near-shore sediments were collected from the previously described (Holmes et al., 2005) study site in Nantucket, Massachusetts, USA in PVC cores (15.24 cm diameter; 53.34 cm long). Both ends of the cores were sealed with rubber stoppers for transport. The sediment cores were incubated at 15 °C to mimic in situ temperatures. For pyrite addition experiments, 6–60 mg (dry weight) of finely ground pyrite (10–100 μm particle size) was added to 3 g of reduced sediments, which were then homogenized under anaerobic conditions. The sediment cores were collected from upper 50 cm depth zone. For other sediment experiments, reduced sediment was collected from the top 2 cm. Sediments were homogenized only for the experiments that involved the addition of pyrite. All other experiments were performed on intact sediment cores or undisturbed sediments.
Electrode fabrication
Gold electrode fabrication was performed as described previously (Malvankar et al., 2011, 2012c). Glass slides (2.54 cm × 2.54 cm) were cleaned ultrasonically using successive rinses of trichloroethylene, acetone and methanol, and then blown dry with nitrogen. To achieve an insulating gap in the anode, a 50-μm diameter tungsten wire was placed on the glass substrate as a deposition mask. For four-probe measurements, electrodes were fabricated using standard photolithography processing. A 40-nm Au film atop a 10-nm Cr adhesion layer was thermally evaporated on these substrates, at 10−6 mbar, at a deposition rate 0.1 nm s−1, thus producing gold split electrodes with a 50-μm nonconductive spacing. Optical microscopy was used to confirm that the gap was uniform, and resistance measurements were employed to assure that the electrodes were well insulated from each other with Ggap<10−10 S.
DC conductivity measurements
Sediment conductivity was measured using an approach similar to that described previously for measuring the conductivity of microbial biofilms and pili nanowires (Malvankar et al., 2011). A voltage ramp of −0.05 to 0.05 V was applied across gold electrodes in steps of 0.025 V for two-probe measurements using a source meter (Keithley Instruments, Cleveland, OH, USA; Model 2400). For each measurement, after allowing the exponential decay of the transient ionic current, the steady-state electronic current for each voltage was measured every second over a minimum period of 100 s using a Labview data acquisition program (National Instruments, Austin, TX, USA). Time-averaged current for each applied voltage was calculated to create the current–voltage (I–V) characteristics. For four-probe measurements, a source meter (Keithley Instruments, Model 2400) was used to apply a fixed current between outer of the four electrodes and to measure the potential drop between two inner electrodes (Lange and Mirsky, 2008), by measuring the voltage for each current every second over a period of 100 s, after reaching the steady state (Figure 1c). An additional high-impedance voltmeter (Keithley Instruments, Model 2000) was used to record the output voltage of the current source to calculate conductance (Lange and Mirsky, 2008) as described previously. For both two- and four-probe measurements, linearity of I–V characteristics was maintained by applying appropriate low voltage/current. The dissipative power was kept under 10−6 W to eliminate self-heating effects.
Conductivity calculation
Conductivity was calculated using the methods described previously (Malvankar et al., 2011). Conformal mapping (the Schwarz–Christoffel transformation) (Kankare and Kupila, 1992) was employed to calculate sediment conductivity from measured conductance (G) according to the following formula
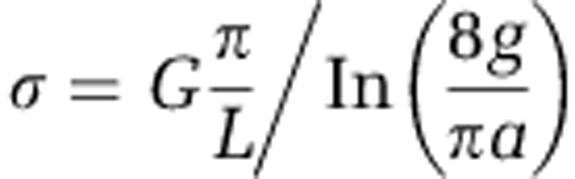 |
where L is the length of the electrodes (L=2.54 cm); a is the half-spacing between the electrodes (2a=50 μm); and g is the sediment thickness. This formula is valid for the case a<g≪b where b is the half-width of the electrodes (2b=2.54 cm). Sediment thicknesses were individually measured for each experiment except for sediment cores where a sediment thickness of 1 mm was used for all comparisons.
Sediment oxidation using air and electrochemical gating
For oxidation of sediments, 1 g of sediments was placed on the electrode arrays. For electrochemical gating, a source meter (Keithley Instruments, Model 2400) was used to apply voltage (Vg) between gate (Ag/AgCl, 3 M KCl reference electrode, BAS, West Lafayette, IN, USA) and the source–drain electrodes to create the electrolyte-gated field effect as described previously (Malvankar et al., 2011, 2012b). Another source meter (Keithley Instruments, Model 2400) was used to apply voltage between source and drain to measure conductance. IGOR Pro software (WaveMetrics Inc., OR, USA) was used for data fitting and analysis. Experiments involving air-oxidation of sediments were performed in an incubator maintained at 4 °C and sediments of 3 mm thickness were exposed to air. Conductivity was measured periodically to evaluate the effect of air-oxidation (Figure 2a).
Acknowledgments
We thank Prof. Kelly Nevin and Prof. Mark Tuominen for helpful discussions and experimental assistance. This research was supported by the Office of Naval Research (grant no. N00014-12-1-0229 and N00014-13-1-0550), the Office of Science (BER), US Department of Energy (award no. DE-SC0006790) as well as the NSF Center for Hierarchical Manufacturing (grant no. CMMI-1025020). Nikhil S. Malvankar holds a Career Award at the Scientific Interface from the Burroughs Wellcome Fund.
The authors declare no conflict of interest.
References
- Du K, Knutson CR, Glogowski E, McCarthy KD, Shenhar R, Rotello VM, et al. Self-assembled electrical contact to nanoparticles using metallic droplets. Small. 2009;5:1974–1977. doi: 10.1002/smll.200900203. [DOI] [PubMed] [Google Scholar]
- Heeger AJ, Sariciftci NS, Namdas EB. Semiconducting and Metallic Polymers. Oxford University Press: New York, NY, USA; 2010. [Google Scholar]
- Holmes DE, Nevin KP, O'Neil RA, Ward JE, Adams LA, Woodard TL, et al. Potential for quantifying expression of the Geobacteraceae citrate synthase gene to assess the activity of Geobacteraceae in the subsurface and on current-harvesting electrodes. Appl Environ Microbiol. 2005;71:6870–6877. doi: 10.1128/AEM.71.11.6870-6877.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kankare J, Kupila EL. In situ conductance measurement during electropolymerization. J Electroanal Chem. 1992;322:167–181. [Google Scholar]
- Lange U, Mirsky VM. Separated analysis of bulk and contact resistance of conducting polymers: comparison of simultaneous two-and four-point measurements with impedance measurements. J Electroanal Chem. 2008;622:246–251. [Google Scholar]
- Malkin SY, Rao AM, Seitaj D, Vasquez-Cardenas D, Zetsche E-M, Hidalgo-Martinez S, et al. 2014Natural occurrence of microbial sulphur oxidation by long-range electron transport in the seafloor ISME Je-pub ahead of print 27 March 2014;doi: 10.1038/ismej.2014.41 [DOI] [PMC free article] [PubMed]
- Malvankar NS, Vargas M, Nevin KP, Franks AE, Leang C, Kim B-C, et al. Tunable metallic-like conductivity in microbial nanowire networks. Nat Nanotechnol. 2011;6:573–579. doi: 10.1038/nnano.2011.119. [DOI] [PubMed] [Google Scholar]
- Malvankar NS, Lou J, Nevin K, Franks AE, Tuominen MT, Lovley DR. Electrical conductivity in a mixed-species biofilm. Appl Environ Microbiol. 2012;78:5967–5971. doi: 10.1128/AEM.01803-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvankar NS, Lovley DR. Microbial nanowires: a new paradigm for biological electron transfer and bioelectronics. ChemSusChem. 2012;5:1039–1046. doi: 10.1002/cssc.201100733. [DOI] [PubMed] [Google Scholar]
- Malvankar NS, Tuominen MT, Lovley DR. Lack of cytochrome involvement in long-range electron transport through conductive biofilms and nanowires of Geobacter sulfurreducens. Energy Environ Sci. 2012;5:8651–8659. [Google Scholar]
- Malvankar NS, Tuominen MT, Lovley DR. Biofilm conductivity is a decisive variable for high-current-density microbial fuel cells. Energy Environ Sci. 2012;5:5790–5797. [Google Scholar]
- Nakamura R, Takashima T, Kato S, Takai K, Yamamoto M, Hashimoto K. Electrical current generation across a black smoker chimney. Angew Chem Int Ed. 2010;49:7692–7694. doi: 10.1002/anie.201003311. [DOI] [PubMed] [Google Scholar]
- Nielsen LP, Risgaard-Petersen N, Fossing H, Christensen PB, Sayama M. Electric currents couple spatially separated biogeochemical processes in marine sediment. Nature. 2010;463:1071–1074. doi: 10.1038/nature08790. [DOI] [PubMed] [Google Scholar]
- Ntarlagiannis D, Atekwana EA, Hill EA, Gorby Y. Microbial nanowires: Is the subsurface ‘‘hardwired''. Geophys Res Lett. 2007;34:L17305. [Google Scholar]
- Patra D, Malvankar N, Chin E, Tuominen M, Gu Z, Rotello VM. Fabrication of conductive microcapsules via self-assembly and crosslinking of gold nanowires at liquid-liquid interfaces. Small. 2010;6:1402–1405. doi: 10.1002/smll.200902380. [DOI] [PubMed] [Google Scholar]
- Pfeffer C, Larsen S, Song J, Dong M, Besenbacher F, Meyer RL, et al. Filamentous bacteria transport electrons over centimetre distances. Nature. 2012;491:218–221. doi: 10.1038/nature11586. [DOI] [PubMed] [Google Scholar]
- Regberg A, Singha K, Tien M, Picardal F, Zheng Q, Schieber J, et al. Electrical conductivity as an indicator of iron reduction rates in abiotic and biotic systems. Water Resour Res. 2011;47:W04509. [Google Scholar]
- Reguera G. Microbiology: bacterial power cords. Nature. 2012;491:201–202. doi: 10.1038/nature11638. [DOI] [PubMed] [Google Scholar]
- Sato M, Mooney HM. The electrochemical mechanism of sulfide self-potentials. Geophysics. 1960;25:226. [Google Scholar]
- Thauer RK, Jungermann K, Decker K. Energy conservation in chemotrophic anaerobic bacteria. Bacteriol Rev. 1977;41:100–180. doi: 10.1128/br.41.1.100-180.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]