Abstract
Fungal viruses (mycoviruses) with RNA genomes are believed to lack extracellular infective particles. These viruses are transmitted laterally among fungal strains through mycelial anastomoses or vertically via their infected spores, but little is known regarding their prevalence and patterns of dispersal under natural conditions. Here, we examined, in detail, the spatial and temporal changes in a mycovirus community and its host fungus Heterobasidion parviporum, the most devastating fungal pathogen of conifers in the Boreal forest region. During the 7-year sampling period, viruses accumulated in clonal host individuals as a result of indigenous viruses spreading within and between clones as well as novel strains arriving via airborne spores. Viral community changes produced pockets of heterogeneity within large H. parviporum clones. The appearance of novel viral infections in aging clones indicated that transient cell-to-cell contacts between Heterobasidion strains are likely to occur more frequently than what was inferred from genotypic analyses. Intraspecific variation was low among the three partitivirus species at the study site, whereas the unassigned viral species HetRV6 was highly polymorphic. The accumulation of point mutations during persistent infections resulted in viral diversification, that is, the presence of nearly identical viral sequence variants within single clones. Our results also suggest that co-infections by distantly related viral species are more stable than those between conspecific strains, and mutual exclusion may play a role in determining mycoviral communities.
Introduction
Fungal viruses (mycoviruses) occur commonly in all major fungal taxa (Pearson et al., 2009; Ghabrial, 2013). The wide phylogenetic diversity of mycoviruses is reflected by their current classification into seven dsRNA families and five ssRNA families (www.ictvonline.org), and many novel taxa are awaiting description (e.g., Preisig et al., 2000; Ahn and Lee, 2001; Márquez et al., 2007; Hammond et al., 2008; Liu et al., 2009; Yu et al., 2010). Mycoviruses with RNA genomes propagate within the cytoplasm or mitochondria of their hosts and are transmitted laterally between fungal strains through anastomoses. These viruses typically form cryptic (latent), persistent infections, although detrimental as well as mutualistic effects are known (Anagnostakis and Day, 1979; Huang and Ghabrial, 1996; Lakshman et al., 1998; Preisig et al., 2000; Ahn and Lee, 2001; Márquez et al., 2007; Yu et al., 2010). Species of Hypoviridae infect the Chestnut blight fungus (Cryphonectria parasitica) and reduce its pathogenicity. Consequently, hypoviruses are used as biocontrol agents in Europe (MacDonald and Fulbright, 1991).
Heterobasidion parviporum Niemelä and Korhonen causes white-rot wood decay in Norway spruce (Picea abies) and related species (Niemelä and Korhonen, 1998; Dai et al., 2003). This pathogen spreads efficiently via basidiospores that infect fresh stump surfaces or butt wounds of living trees and may travel hundreds of kilometers in favorable conditions (Stenlid and Redfern, 1998). A single germinating basidiospore produces a homokaryotic, effectively haploid, primary mycelium; and a compatible pairing between two such mycelia results in the formation of a secondary heterokaryotic mycelium with two different nuclear haplotypes (Korhonen, 1978; Hansen et al., 1993). The mycelium spreads vegetatively via root contacts, and single genotypes (clones) may infect dozens of Norway spruce trees and survive for decades. In the case of recent spore-mediated infections, a single conifer stump may harbor several distinct Heterobasidion genotypes (Johannesson and Stenlid, 2004). On average, every seventh spruce tree is infected by H. parviporum in Southern Finland (Kaarna-Vuorinen, 2000). The disease is currently controlled by tree species selection, chemical or biological stump treatments and stump removal.
Approximately 15–17% of Heterobasidion strains are infected by dsRNA viruses (Ihrmark, 2001; Vainio et al., 2011b). The unassigned species Heterobasidion RNA virus 6 (HetRV6) is responsible for approximately 70% of dsRNA infections in European strains of Heterobasidion (Vainio et al., 2012) and shares a relatively high polymerase similarity with the Curvularia thermal tolerance virus (Márquez et al., 2007). All other Heterobasidion viruses described to date are members of Partitiviridae (Ihrmark, 2001; Vainio et al., 2010, 2011a, 2011b, 2013b). Many Heterobasidion viruses are readily transmitted between incompatible host strains and even distantly related species of Heterobasidion in the laboratory and in nature (Ihrmark, 2001; Vainio et al., 2010, 2011a, 2011b). These viruses also spread vertically via basidiospores and conidia (Ihrmark et al., 2002, 2004). Most Heterobasidion viruses do not cause drastic phenotypic changes in their host, but some may reduce the germination of basidiospores (Ihrmark et al., 2004) or affect the growth of the host fungus (Vainio et al., 2010, 2012). Notably, a single virus strain may have beneficial, commensal or detrimental effects on a single host isolate, depending on environmental and ecological conditions (Hyder et al., 2013). The ecological effects of such complex host–virus relationships can only be understood via long-term field studies.
In this study, we deciphered changes in a mycovirus community and its host fungus over a 7-year period in a Norway spruce forest stand suffering from serious Heterobasidion infection. We tested the following hypotheses: (i) viruses and fungal host clones are co-distributed, (ii) lateral transmission of viruses occurs between adjacent Heterobasidion clones, (iii) viruses accumulate in long-lived clones, (iv) pre-existing viruses influence the probability of other viruses infecting a given host, and (v) accumulated point mutations lead to in situ diversification of viruses within a given host.
Materials and methods
Forest site characteristics
The investigated forest site was a naturally regenerated, unthinned, Oxalis-Myrtillus (herb-rich)-type Norway spruce stand in southern Finland (Ruotsinkylä Research Area, 60°22'05” N, 24°59'53” E; 875 m2 area; stand age 43 years). In 2005, 34% of the previous-generation spruce stumps and 32% of standing spruce trees were infected by Heterobasidion (for more detailed site characteristics, see Piri and Korhonen, 2008).
Fungal strains and culture conditions
In 2005, Piri and Korhonen surveyed 290 stumps or standing trees for Heterobasidion infections and isolated mycelial cultures from 67 of them (Piri and Korhonen, 2008; Figure 1). In 2012, the same 18 stumps and 49 trees (hereafter referred to as ‘isolation sources') were resampled by collecting splinters from the stumps or increment cores from the standing trees. Sampling was repeated when no visible decay (i.e., brownish discoloration) was observed at the first sample point. Fungal isolates were assigned individual codes according to the isolation source and year (e.g., isolates retrieved from isolation source 5 in 2005 and 2012 are designated 5-05 and 5-12, respectively). Each of the 290 sample points was assigned a consecutive number by Piri and Korhonen (2008), and we maintained their scheme in this study (small cleaning stumps without Heterobasidion infections were omitted from Figure 1).
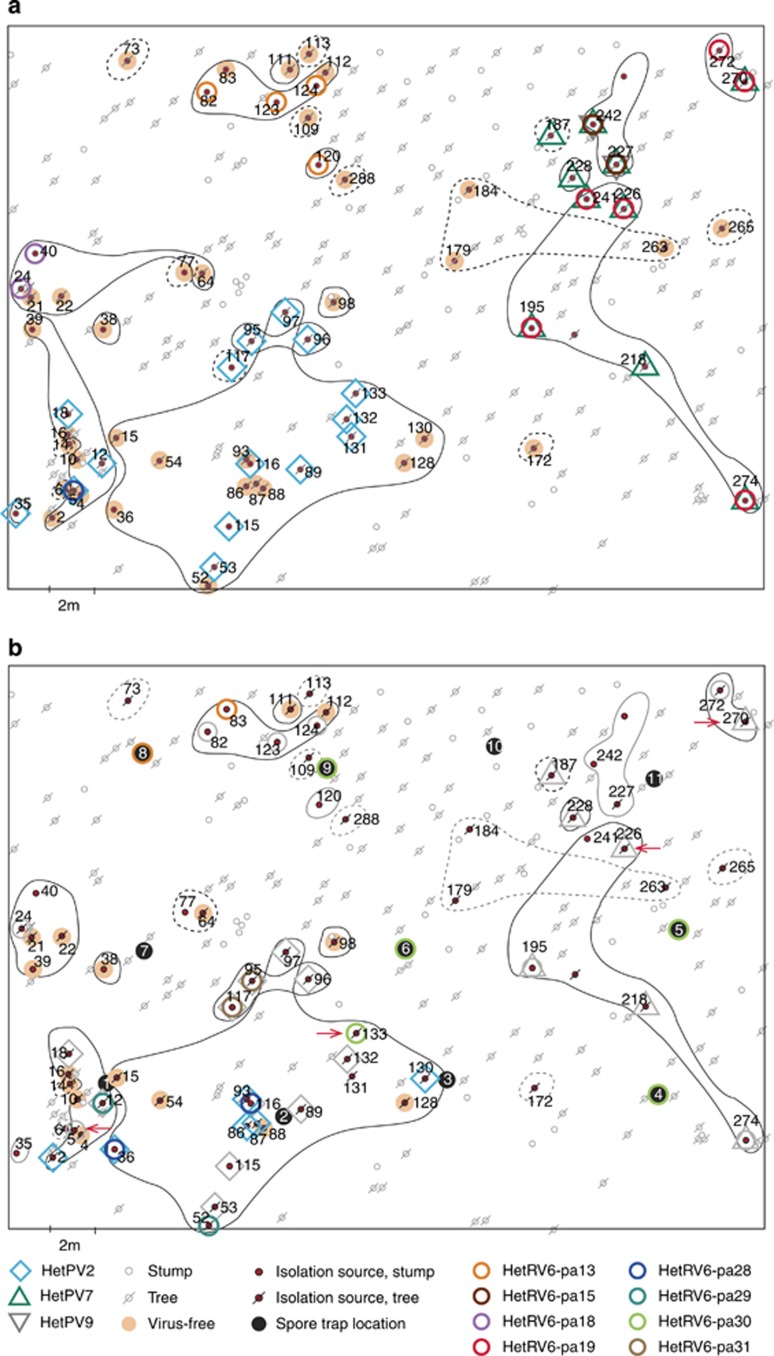
Figure 1.
Spatial distribution of viruses and Heterobasidion clones at the study site in 2005 (a) and 2012 (b). The isolation sources refer to individual stumps or trees found to be infected by H. parviporum in 2005 and resampled in 2012. Isolation sources infected by the same Heterobasidion clone are circled (solid line=heterokaryon; dashed line=homokaryon; black line=viable clone; grey line=clone was not found in 2012). Isolation sources not indicated as virus-infected or virus-free (see symbols) did not yield viable Heterobasidion cultures. In 2012 (b), viral infections that were already present in 2005 are shown in greyscale, new infections are shown in color, whereas viral losses, when compared to year 2005, are indicated by red arrows.
Airborne Heterobasidion spores were trapped using freshly cut, peeled disks of Norway spruce wood that were placed at the study site and exposed in open air for ∼24 h. The sampling was repeated twice using 11 disks for each experiment. Cultures were established by collecting multiple conidia from Heterobasidion colonies growing on the disks (see Supplementary information for detailed sampling procedure). Sixty Heterobasidion isolates were obtained using spore trapping. A schematic of the disk locations is shown in Figure 1b.
The spatial distribution of each Heterobasidion clone was determined based on vegetative incompatibility reactions between isolates obtained from different isolation sources, as described earlier (Stenlid, 1985). This method was also used to confirm the identity of a recipient host strain after virus transmission experiments.
To examine whether the congeneric HetPV2 and HetPV7 are able to exist within the same mycelium, we conducted transmission experiments as described by Ihrmark et al. (2002) and Vainio et al. (2013a). Isolate 18-05 was used as a donor for HetPV2-pa1, and 228-05 was a donor for HetPV7-pa1 in four replicate, dual cultures on 2% malt extract agar plates.
Extraction of dsRNA by CF11 chromatography
The presence of dsRNA in the H. parviporum isolates was examined using CF11 cellulose affinity chromatography, as described earlier (Morris and Dodds 1979; Vainio et al., 2010), using approximately 1.5–3.0 g (fresh weight) of fungal mycelia. The CF11 chromatography was repeated if no dsRNA was observed in isolates that yielded viral amplification products via RT-PCR.
Molecular cloning and sequencing
Genomic sequences of the new viral species HetPV7 and HetPV9 were determined from isolate 242-05 using the single-primer amplification method of Lambden et al. (1992) as modified by Vainio et al. (2011a). PCR-amplified cDNA products were cloned into the pCR2.1-TOPO cloning vector (Invitrogen, Carlsbad, CA, USA) and sequenced at Macrogen Inc. (www.macrogen.com) with an Applied Biosystems 96-capillary ABI 3730xl DNA analyzer (Applied Biosystems, Thermo Fischer Scientific, Waltham, MA, USA). Sequence positions with insufficient coverage were determined using specific primers (Supplementary Table S1). At least three cloned inserts or two overlapping PCR products were analyzed to confirm the identity of each nucleotide.
RT-PCR and partial sequence determination
Reverse transcription was conducted by random priming using total nucleic acid samples, as described earlier (Vainio et al., 2013a). The presence of viruses was examined using selected PCR primers (Supplementary Table S1), and a set of 3–5 primer pairs was used to amplify overlapping fragments covering the entire RdRp coding regions for each viral infection. Any putative mutations were verified by analyzing two independent PCR products from two independent cDNAs. Sequencing PCRs were carried out as described previously (Vainio et al., 2013a) using Dynazyme II DNA polymerase (Thermo Scientific, Vantaa, Finland) and primer-specific amplification conditions (Supplementary Table S1). Sequences determined in this study have been deposited into the NCBI GenBank under the accession numbers listed in Table 1.
Table 1. Viral sequence characteristics.
| Virus namea | Gene | GenBank accession | Sequence length | Host isolates harboring viral sequence variantb |
|---|---|---|---|---|
| HetPV2-pa1-a | RdRp | HM565953 | 2290c | [18-05, 18-12] |
| HetPV2-pa1-a | Capsid | HM565954 | 2238c | — |
| HetPV2-pa1-b | RdRp | KF551880 | 2151d | [2–12, 5-05, 12-05, 12-12] |
| HetPV2-pa1-c | RdRp | KF551881 | 2151d | [35-05] [53-05, 86-12, 87-12, 97-05, 97-12, 115-05, 115-12, 116-05, 116-12, 131-05, 132-05, 133-05] [95-05, 95-12, 96-05, 96-12, 117-12] [117-05] |
| HetPV2-pa1-d | RdRp | KF551884 | 2151d | [89-05, 89-12e, 93-12, 130-12] |
| HetPV2-pa1-e | RdRp | KF551882 | 2151d | 36-12 |
| HetPV2-pa1-f | RdRp | KF551883 | 2151d | 53-12 |
| HetPV2-pa1-g | RdRp | KF551885 | 2151d | 132-12 |
| HetPV7-pa1-a | RdRp | JN606091 | 2297c 2157d | [187-05] [228-05, 228-12] [227-05, 242-05] [195-05, 195-12, 218-05, 218-12, 226-12, 241-05, 270-05, 270-12, 274-05, 274-12] |
| HetPV7-pa1-a | Capsid | JN606090 | 2231c | — |
| HetPV7-pa1-b | RdRp | KF551886 | 2157d | 187-12 |
| HetPV7-pa1-c | RdRp | KF551887 | 2157d | 226-05 |
| HetPV9-pa1 | RdRp | JN606085 | 2030c 1895f | [227-05, 242-05] |
| HetRV6-pa13-a | RdRp | HQ189463 | 1906f | [120-05] [82-05, 82-12, 83-12, 123-05, 123-12, 124-05, 124-12] |
| HetRV6-pa13-b | RdRp | KF551888 | 1930f | D8III/1 |
| HetRV6-pa15-a | RdRp | KF551889 | 2031f | 242-05 |
| HetRV6-pa15-b | RdRp | HQ189464 | 1922f | 227-05 |
| HetRV6-pa18-a | RdRp | JN606086 | 1881f | [24-05, 24-12] |
| HetRV6-pa18-b | RdRp | KF551890 | 1846f | 40-05 |
| HetRV6-pa19-a | RdRp | JN606087 | 1880f | 270-05 |
| HetRV6-pa19-b | RdRp | KF551891 | 1885f | [272-05, 272-12] |
| HetRV6-pa19-c | RdRp | KF551892 | 1957f | 226-05 |
| HetRV6-pa19-d | RdRp | JN606089 | 1946f | 241-05 |
| HetRV6-pa19-e | RdRp | KF551893 | 1904f | 274-05 |
| HetRV6-pa19-f | RdRp | KF551894 | 1892f | 274-12 |
| HetRV6-pa19-g | RdRp | JN606088 | 1913f | 195-05 |
| HetRV6-pa19-h | RdRp | KF551895 | 1893f | 195-12 |
| HetRV6-pa28-a | RdRp | KF551896 | 1912f | [5-05, 5–12] |
| HetRV6-pa28-b | RdRp | KF551897 | 1912f | [36-12, 116-12] |
| HetRV6-pa29 | RdRp | KF551898 | 1926f | [12-12] [52-12] |
| HetRV6-pa30 | RdRp | KF551899 | 1884f | [133-12], D4I/5, D5I/4, D6I/4, D9I/3 |
| HetRV6-pa31 | RdRp | KF551900 | 1914f | [95-12, 117-12] |
Sequence names indicate the viral species (HetPV2, HetPV7, HetPV9 and HetRV6), while suffixes indicate strains (e.g., pa1, pa31), followed by sequence variants (a, b, c etc.).
Isolates of single genets shown in brackets.
Complete sequence including 5′ and 3′ UTRs.
Complete open reading frame (ORF) sequence excluding the forward primer (NPFor) region of 18 bp.
This isolate harbors two sequence variants: HetPV2-pa1-d and HetPV2-pa1-c.
Complete ORF region included.
DNA extraction and microsatellite markers
We used virus-specific primers and genomic DNA of the host fungus as a template for PCR to investigate whether endogenization of viral sequences into the host genome was possible (Liu et al., 2010; Chiba et al., 2011, see Supplementary Table S2).
Microsatellite markers were determined for representative isolates of each Heterobasidion clone to identify putative siblings (Supplementary Table S3). Initially, microsatellite sizes were identified using homokaryotized cultures (i.e., strains obtained by picking individual homokaryotic conidia from heterokaryotic mycelia). Total DNA was isolated from a hyphal mass using a modification of the method described by Vainio et al. (1998). Four microsatellite markers were used for the analysis: Ha-ms1, Ha-ms2, Hams6 and Ha-ms10 (Johannesson and Stenlid, 2004). Detailed procedures for homokaryozation, DNA extraction and microsatellite analysis are provided in the supplementary information.
Bioinformatics
Phylogenetic analyses were conducted using Geneious Pro 5.5.8 (Biomatters Ltd., Auckland, New Zealand) and MEGA 5 (Tamura et al., 2011), and Median-Joining Networks were constructed using Network 4.6.1.1. (Fluxus Technology Ltd, Clare, UK). Analyses were conducted with equal weight of characters (10) and a ɛ-value of zero. Putative recombination events were identified using RDP4 (v.4.16; Martin et al., 2010) with the recombination detection methods RDP, GENECONV, MaxChi, Chimaera, SiScan and 3Seq using default settings for linear sequences and appropriate window sizes.
Results
Spatial distribution of Heterobasidion clones
On the basis of vegetative incompatibility reactions and microsatellite markers, the 67 H. parviporum isolates retrieved in 2005 represented 26 individual clones, 12 of which were identified as homokaryons (Figure 1a; Supplementary Table S3). In 2012, new heterokaryotic genotypes were retrieved from stumps 39 and 117, and a new homokaryon was present in tree 64, apparently because of vegetative spread from a neighboring clone (Supplementary Table S3). Several previously infected isolation sources did not yield viable Heterobasidion cultures in 2012 (Supplementary Table S2) because of the extensive decay of some stumps or the apparent lack of decayed wood in some standing trees. Most of these standing trees (N=10; 83.3%) had been infected by homokaryons in 2005. In 2012, 49 Heterobasidion isolates, representing 14 clones, were obtained, and only three isolates were homokaryotic (14-12, 64-12 and 187-12). Note that the majority of trees that had been infected by homokaryons were alive, but two had died by October 2013 (trees 6 and 187). As no thinnings had been conducted at the site; most of the Heterobasidion clones are assumed to be long-lived and originate from the previous tree generation and/or stumps generated during the last logging, which took place 43 years ago.
Viral incidence
The analysis of 176 H. parviporum isolates revealed 87 viral infections (82 incidences among 116 isolates cultured from wood samples, and 5 incidences among 60 isolates obtained by spore trapping; Supplementary Tables S2 and S4). A total of 47.8% and 67.3% of Heterobasidion isolates from wood samples were infected in 2005 and 2012, respectively. At the isolate level, more cases of viruses were gained (N=14) than lost (N=4) during the sampling period. Most of the Heterobasidion isolates retrieved from stumps were infected (2005: 72.2%, 2012: 83.3%), while the frequency was lower (2005: 38.8%, 2012: 62.2%) among isolates from standing trees. If we consider only isolation sources that were infected by Heterobasidion in both 2005 and 2012, there were 30 viral incidences in 2005 and 40 in 2012. Despite repeated examination of virus-infected isolates, we did not observe cases of virus loss during storage and subculturing.
Virus diversity
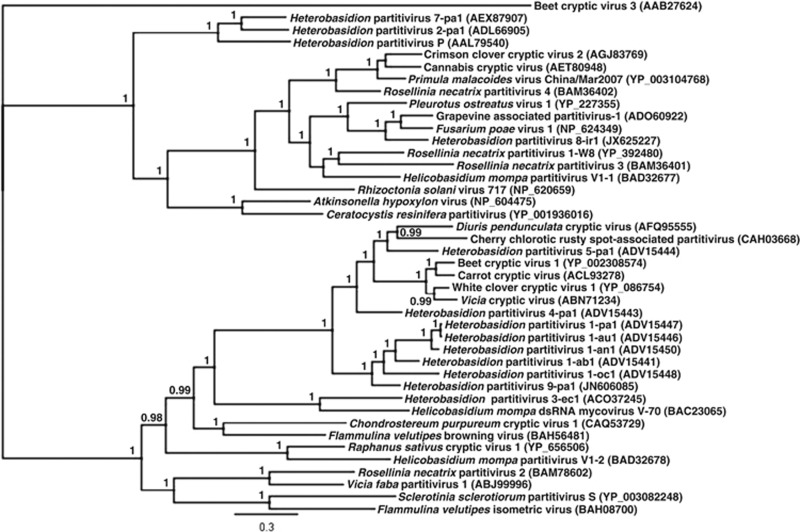
Four distinct viral species occurred at the sample plot: three putative members of Partitiviridae and the yet-to-be-assigned HetRV6 (Vainio et al., 2012). We found no indications of additional viral strains being present at the study plot, as the dsRNA patterns shown by CF11 chromatography agreed with the RT-PCR results and all dsRNA-positive isolates could be associated with one (or more) of these four viral species by RT-PCR. Viral strains designated as HetPV2-pa1 (Heterobasidion partitivirus 2, strain 1 from H. parviporum) and HetPV7-pa1 shared 66% and 60% identity in their RdRp and capsid protein sequences, respectively, and could be regarded as congeneric members of the genus Betapartitivirus (Nibert et al., 2014). The genome of HetPV2-pa1 (formerly HetRV2-pa1) was characterized by Vainio et al. (2011a), and the complete genome sequence of HetPV7-pa1 (Heterobasidion partitivirus 7, strain 1 from H. parviporum) is presented here. The genome of HetPV7-pa1 consists of an RdRp-encoding segment of 2297 bp, which contains an AUG-initiated open reading frame of 724 aa (Mr 85 348; GC-content 45.6%), and a capsid-encoding segment of 2231 bp, which includes an open reading frame of 654 aa (Mr 73 158; GC-content 50.4%).
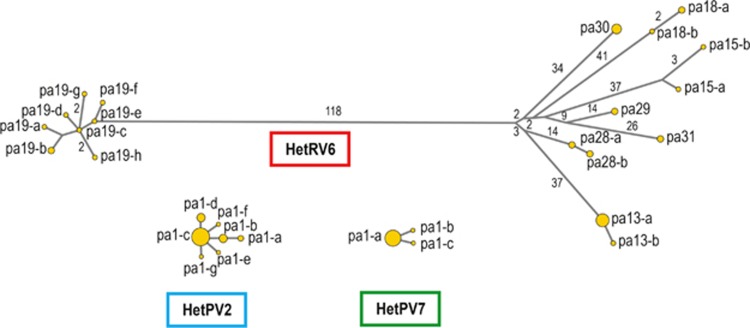
HetPV2-pa1 was nearly identical among 32 host isolates (Table 1), with only 0–2 point mutations over 2151 bp (99.9% sequence identity; 9.3 × 10−4 mutations per nt). Notably, one specific mutation (T to A at nt site 691) was characteristic of all HetPV2 infections in the clone residing in isolation source 5, while another viral sequence variant (with an A to G substitution at nt site 59) was present in isolate 89-05 and two newly infected isolates of the same clone in 2012 (93-12 and 130-12). There were two contradicting cDNAs for isolate 89-12 (A or G at nt site 59), suggesting the presence of two HetPV2 sequence variants. HetPV2 infections in isolates 36, 53 and 132 showed novel point mutations in 2012. All observed mutations resulted in aa substitutions. A Median-Joining Network for HetPV2 sequence variants is presented in Figure 2.
Figure 2.
Median-Joining Networks for HetRV6, HetPV2 and HetPV7. Node areas are proportional to the frequency of each detected viral sequence variant. Branch labels indicate the number of base substitutions between sequence variants (only values different from 1 are shown). The lengths of the RdRp sequences were 2151 bp for HetPV2, 2157 for HetPV7 and 1821 for HetRV6.
HetPV7-pa1 was highly conserved (Table 1), and only two point mutations were detected among the 17 HetPV7-pa1 sequences of 2157 bp from isolates 187-12 and 226-05 (4.6 × 10−4 mutations per nt; Figure 2). Both substitutions were silent. We have currently found HetPV7 in only one other location, ∼40 km from the present study site, and this strain (HetPV7-an1) differed from HetPV7-pa1 by 62 point mutations (Hyder et al., unpublished), which suggests that the single nucleotide microvariations found among variants of HetPV7-pa1 were in situ mutations.
The third partitivirus species was designated as HetPV9 (Heterobasidion RNA virus 9; Figure 3; Nibert et al., 2014). HetPV9 was found to be identical among isolates 227-05 and 242-05 of the same clone (Figure 1a; Table 1), but no Heterobasidion cultures were obtained from these isolation sources in 2012. HetPV9 clusters within the genus Alphapartitivirus and shares 68–76% RdRp protein sequence identity with strains of HetPV1 (previously HetRV1; Vainio et al., 2011b; Nibert et al., 2014). The RdRp segment of HetPV9-pa1 (pa1=strain 1 from H. parviporum) was 2030 bp in length and contained an AUG-initiated open reading frame of 620 aa (Mr 72 362; GC-content 47.6%).
Figure 3.
Bayesian dendrogram including HetPV2, HetPV7 and HetPV9 and related species of Partitiviridae. The scale bar shows 0.3 aa substitutions per site, and percentages of posterior probabilities over 90% are shown at the branch nodes. The aa sequence alignment was based on complete RdRp sequences, generated using MAFFT version 7.017 and edited manually according to conserved motifs. Phylogenetic clustering was conducted using MrBayes with gamma among-site rate variation and 1.1 × 106 cycles for the MCMC algorithm, with sampling one tree per 200 cycles and discarding 105 samples as burn-in. The Blosum matrix was used as the protein evolution model, and Beet cryptic virus 3 was used as an outgroup.
HetRV6 was considerably more polymorphic compared with the three partitiviruses, and there were 242 variable sites within an open reading frame region of 1821 bp (the strains shared at least 86.7% identity). To distinguish specific HetRV6 strains from minor sequence variants, we performed a phylogenetic analysis that included published sequences of HetRV6 from distant locations in its global distribution (Vainio et al., 2012). Based on Neighbor-Joining (NJ) analysis (Supplementary Figure S1), eight distinct clusters of HetRV6 sequences (corresponding to viral strains) were recovered from the study site. Notably, HetRV6-pa19 seemed to be more closely related to HetRV6 strains from H. annosum than those from H. parviporum, suggesting a lateral transfer (i.e., host shift). No recombination events were detected among the HetRV6 sequences.
Most of the eight HetRV6 strains showed minor sequence polymorphisms (1–4 point mutations when compared with the dominant variant; 5.5 × 10−4-2.2 × 10−3 mutations per nt), yielding 19 HetRV6 sequence variants (Figure 2; Table 1). Spatial and temporal microvariations were observed among HetRV6 infections from single Heterobasidion clones. Four ambiguous nucleotide sites were found among the 36 HetRV6 sequences analyzed, which corresponds to a mutation frequency of 6.1 × 10−5. Specifically, the sequences from isolates 242-05, 195-12 and 272-12 each had a unique nt site showing constant, double base calls (3–4 × sequence coverage), and there were two contradicting cDNAs from isolate 12-12 (nt site 1873). Although this seems to suggest the presence of two molecular variants in a single host isolate, we fixed the character state for the Median-Joining and Neighbor-Joining analyses according to the more commonly occurring nucleotide in the isolation source or clone.
Spatial distribution of viruses
During the 7-year sampling period, there were no new HetPV7 infections and six cases of within-clone dispersal for HetPV2 (isolation sources 2, 36, 86, 87, 93 and 130; Figure 1a and b; Supplementary Figure S2). Eleven isolation sources contained H. parviporum strains infected by HetPV2 in both 2005 and 2012, and two strains (sources 5 and 133) had lost the virus. In 2012, no cultures were obtained from two isolation sources previously harboring host strains infected by HetPV2. In the case of HetPV7, strains from seven isolation sources were infected by the virus in 2005 and 2012, but no cultures were obtained from three isolation sources in 2012.
Based on microsatellite analysis, most of the five clones inhabited by HetPV2 were not siblings of a single fruitbody (i.e., at least one of the four microsatellite loci did not contain any shared alleles). However, isolate 117-05 seemed to be the homokaryotic constituent of the neighboring heterokaryon, 97-05. The five clones infected by HetPV7 appeared to be more closely related; and isolate 187-05 seemed to be the homokaryotic constituent of the adjacent heterokaryon, 228-05, which, in turn, was a putative sibling of the neighboring isolate, 227-05 (one shared allele at each locus). The clones, including isolates 195-05 and 270-05, were distinct.
The distribution of HetRV6 strains seemed to be more fragmented and temporally variable compared with the partitiviruses (Figure 1b; Supplementary Figure S2). In 2005, three of the five different HetRV6 strains that were found (HetRV6-pa15, HetRV6-pa18 and HetRV6-pa28) occurred solely within single Heterobasidion clones, while HetRV6-pa13 and HetRV6-pa19 were found in two neighboring clones. In 2012, three novel HetRV6 strains had appeared at the study site in two clones that were previously devoid of HetRV6. One of the novel strains occurred in two adjacent clones (isolation sources 12 and 52). A new HetRV6 strain was also found from two neighboring isolates of the same clone, 95-12 and 117-12. There was only one case of within-clone virus dispersal for HetRV6: the virus in isolate 82-05 was newly found in neighboring isolate 83 in 2012. Moreover, two new infections seemed to result from inter-clone transmission from isolation source 5 to isolates 36-12 and 116-12 of the neighboring clone. Eight isolation sources contained H. parviporum strains infected by HetRV6 in both 2005 and 2012, while strains from two (226, 270) isolation sources had lost the virus in 2012. In 2012, no Heterobasidion cultures were obtained from five isolation sources previously harboring HetRV6-infected host strains. None of the clones that shared particular HetRV6 sequence variants (120 and 124 in 2005; 12 and 52 in 2012) were siblings according to microsatellite variation.
HetRV6 was the only viral species that occurred in isolates obtained from spore trapping (8.3% of the 60 established cultures; Supplementary Table S3). Four of these isolates (D4I/5, D5I/4, D6I/4 and D9I/3) were trapped during the first exposure period (30–31.8.2012), and all were infected by HetRV6-pa30, which was also newly detected in wood isolate 133-12 (i.e., HetRV6 was not detected in 133-05). However, all homokaryotic isolates derived from spores contained microsatellite alleles that were distinct from 133-12 and seemed to have originated from a source outside the study site. Isolate D8III/1, which was infected by HetRV6-pa13, was trapped during the second exposure period (5–6.9.2012). The trapped spore most likely originated from the Heterobasidion clone that resided in isolation source 82, which was located ∼3 m from the collection disk. The spore-derived D8III/1 and local isolates shared a common microsatellite marker at all four loci and were infected by HetRV6-pa13 sequence variants that differed by only a single point mutation. A H. parviporum fruitbody was present in stump 82 in 2012.
Irregular distribution of viruses within Heterobasidion clones
Large clones inhabiting multiple (5–18) isolation sources were all infected by at least one viral species, while clones residing in only one isolation source were mostly virus-free (70.6% in 2005, 71.4% in 2012) and usually homokaryotic. The only homokaryons infected by viruses were 117-05, 187-05 and 187-12, each of which was positioned next to a heterokaryotic clone sharing a common microsatellite haplotype and a common virus strain (HetPV2 or HetPV7, respectively).
Large Heterobasidion clones spread over multiple isolation sources were also heterogeneous in their viral content. Isolates of two large clones with 8 and 18 isolation sources showed infection rates of 37.5% and 44.4%, respectively, for HetPV2 in 2005. Similarly, isolates of clones with five to eight isolation sources harboring strains of HetRV6 showed infection rates of 12.5–80% in 2005. Notably, because of novel HetRV6 infections in 2012, each of the two large clones harbored two to three distinct strains of HetRV6, but each virus strain occurred only in one to two isolates, suggesting that the new HetRV6 infections were sporadic (Figure 1b). The possibility of a spontaneous appearance of intact viral RNA from host genomic DNA does not seem likely, as we did not detect viral amplification products using host genomic DNA as a PCR template.
Co-infections by distantly related and congeneric viruses
In 2005, we found natural double infections of HetPV2/HetRV6 (isolate 5) and HetPV7/HetRV6 (isolates 195, 226, 241, 270 and 274) and triple infections of HetRV6/HetPV7/HetPV9 (isolates 227 and 242). In 2012, five new co-infections were observed, in most cases resulting from new HetRV6 infections of Heterobasidion strains that already harbored HetPV2 (isolates 12, 95, 116 and 117). Because of viral gains and losses, only two host strains (95 and 274) were infected by the same two viral strains in 2005 and 2012. All viral losses occurred in strains that harbored a second viral species, and in three of four cases, one of the co-infecting viruses was present in both 2005 and 2012.
The congeneric strains HetPV2 and HetPV7 did not naturally form co-infections. To test whether this was due to viral interference, we conducted transmission experiments that produced co-infections of HetPV2 and HetPV7 in isolate 228-05 (all four replicates).
Mutual exclusion of conspecific viral strains
Only one HetRV6 strain was detected from each Heterobasidion isolate using HetRV6-specific primers, which suggests the mutual exclusion of closely related HetRV6 strains in the same host. This hypothesis is also supported by the lack of recombination among HetRV6 strains. To reveal putative co-infections, we designed strain-specific primer pairs for HetRV6-pa15 and HetRV6-pa19 (Supplementary Table S5). These viral strains occurred in two adjacent clones, both of which were infected by HetPV7-pa1, suggesting that lateral transfer might have occurred between them. Strain-specific primers and sequence analysis revealed co-infections of HetRV6-pa15 and HetRV6-pa19 in Heterobasidion isolates 241-05 and 226-05 (Supplementary Table S5). However, the more rare strain (HetRV6-pa15) occurred in only a few of the tested replicate cDNA samples (12.5–18.2%). All other isolates from the two neighboring clones harbored single strains of HetRV6.
Discussion
In this investigation, we show that Heterobasidion isolates at a forest site with a long history of root and butt rot had a viral infection rate of 48–67%, which was considerably higher than the typical 15–17% that was observed in earlier studies (Ihrmark, 2001; Vainio et al., 2012) and suggests that viruses accumulate over time in Heterobasidion clones. Our 7-year follow-up work also revealed the appearance of novel viral infections in aging clones. The observation is interesting, as the lifespan of Heterobasidion clones is estimated to be considerably shorter (<200 years; Stenlid and Redfern, 1998) than that of some other wood decay fungi that form large clones in the forest (e.g., Phellinus weirii and Armillaria spp., with a lifespan of more than 1000 years; Dickman and Cook, 1989; Ferguson et al., 2003), and suggests a hypothesis that viruses effectively shorten the lifespan of Heterobasidion clones because of their increasing frequency and small but negative overall effect (Hyder et al., 2013).
The distribution of viruses among large H. parviporum clones was irregular and suggested that viruses were both acquired and lost during vegetative growth of the host and/or that the mycelial network comprising a single clone might be partly disconnected. This distribution resembles the uneven distribution of viruses within clones of the pathogenic root rot fungi Helicobasidium mompa (Ikeda et al., 2005) and Rosellinia necatrix (Yaegashi et al., 2013) and the ascomycetous pathogen C. parasitica (Shain and Miller, 1992; Hoegger et al., 2003). We have shown that the partitivirus HetPV4 (formerly HetRV4) was transmitted via hyphal contact from an introduced strain of H. parviporum into native strains inhabiting Norway spruce stumps (Vainio et al., 2013a). In the present study, partitiviruses seemed to disperse slowly by hyphal contacts between adjacent Heterobasidion mycelia, while strains of HetRV6 were able to rapidly infect new clones via airborne spores. Notably, the appearance of novel virus strains in aging clones suggests that anastomoses between Heterobasidion strains are more frequent than what was inferred from genotype data.
We observed an unexpectedly high frequency of homokaryotic isolates in 2005, although many of them were not detected again in 2012. The occurrence of H. parviporum homokaryons in living trees is regarded as a rare phenomenon in Europe, but homokaryons of the closely related H. occidentale are known to colonize two or more nearby trees in North America (Garbelotto et al., 1997). Here, we observed several homokaryons, most of which were virus-free, which supports the notion that viruses are relatively rare in basidiospores. However, some of the airborne spores hosted strains of HetRV6, while no partitivirus infections were detected among the spore-trapped isolates. The ability to be transmitted by basidiospores may strongly affect the dispersal of viruses and enables rapid spread into new sites. Ihrmark et al. (2004) showed that, depending on the fungal strain, dsRNA viruses are present in 10–84% of basidiospores produced by a virus-infected Heterobasidion fruitbody; however, the viral species were not identified. Considering the immense spore deposit from H. parviporum fruitbodies (Möykkynen et al., 1997), a continuous spore load could represent a major route of virus transmission, and this is supported by our observation that stumps have a higher viral incidence compared with standing trees, which is expected, as stumps have more exposed and unprotected surfaces that are suitable for spore-derived infections than uninjured trees.
We observed double or triple viral infections in several Heterobasidion isolates. Co-infections by distantly related or congeneric mycoviruses have been described (Peever et al., 1997; Lakshman et al., 1998; Preisig et al., 1998; Hong et al., 1999; Ghabrial et al., 2002; Osaki et al., 2004; Park et al., 2005; Tuomivirta and Hantula, 2005; Vainio et al., 2012, 2013a), and the overall similarity of the co-infecting viruses has been relatively low (30–53% at the aa level or <55% at the nt level). Sun et al. (2006) have described that the transmission of Mycoreovirus 1 is enhanced by co-infection with Cryphonectria hypovirus 1 in C. parasitica. Our results suggested that co-infections by distantly related viral species were more stable than those between conspecific strains, and strains of HetRV6 were able to coexist only transitionally within a single fungal mycelium. If this is a general phenomenon, pre-existing viral infections may affect the dispersal of similar strains and could affect the prevalence of each virus strain within a host population. Partitivirus infections are relatively rare in Heterobasidion and occur only in ∼5% of isolates in culture collections (Vainio et al., 2011b). Therefore, a single partitivirus strain might potentially spread into several neighboring clones without being restricted through encounters with conspecific strains. In contrast, HetRV6 infections occur in ∼12.5% of Heterobasidion strains (Vainio et al., 2012), and dispersing viruses are more likely to meet conspecific strains.
The level of intraspecific sequence variation was low for the three partitivirus species, while the HetRV6 population consisted of eight viral strains, three of which showed spatial or temporal microvariations among isolates of single Heterobasidion clones. Based on sequence comparisons to previously described viral strains, we deduced that the minor sequence polymorphisms of 1–4 nt were in situ mutational events, rather than parallel infections by highly similar strains. Among plants, a single individual may be infected by a swarm of viruses with sequence polymorphisms or so-called ‘quasispecies' (Domingo and Holland, 1997), but this phenomenon has been poorly shown in mycoviruses.
Our detailed analysis of a mycovirus community over a 7-year interval has shown that the distribution of H. parviporum viruses is spatially influenced by (i) host clonal structure and (ii) lateral transmission of viruses between neighboring clones. We have also shown that (iii) viruses accumulate in aging Heterobasidion clones, (iv) the presence of pre-existing viral infections may restrict the dispersal of similar strains, and (v) viral strains acquire point mutations during persistent infections. In conclusion, this study gives unique insights into the dispersal patterns of mycoviruses, which are essential for understanding their biology, their effects on forest mycology and the development of their use as biocontrol agents.
Acknowledgments
This study was financially supported by the Academy of Finland (decision numbers 251193 and 258520) and the Finnish Forest Research Institute. We thank Marja-Leena Santanen, Juha Puranen, Nieves Lorenzo Gotor, Heidi Ylinenpää, Sonja Sarsila and Suvi Saloranta for technical assistance and Jaana Jurvansuu and Taina Pennanen for commenting on the manuscript.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on The ISME Journal website (http://www.nature.com/ismej)
Supplementary Material
References
- Ahn IP, Lee YH. A viral double-stranded RNA up regulates the fungal virulence of Nectria radicicola. Mol Plant-Microbe Interact. 2001;14:496–507. doi: 10.1094/MPMI.2001.14.4.496. [DOI] [PubMed] [Google Scholar]
- Anagnostakis S, Day P. Hypovirulence conversion in Endothia parasitica. Phytopathology. 1979;69:1226–1229. [Google Scholar]
- Chiba S, Kondo H, Tani A, Saisho D, Sakamoto W, Kanematsu S, et al. Widespread endogenization of genome sequences of non-retroviral RNA viruses into plant genomes. PLoS Pathog. 2011;7:e1002146. doi: 10.1371/journal.ppat.1002146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y-C, Vainio EJ, Hantula J, Niemelä T, Korhonen K. Investigations on Heterobasidion annosum s.lat. in central and eastern Asia with the aid of mating tests and DNA fingerprinting. Forest Pathol. 2003;33:269–286. [Google Scholar]
- Dickman A, Cook S. Fire and fungus in a Mountain Hemlock forest. Can J Bot. 1989;67:2005–2016. [Google Scholar]
- Domingo EJJH, Holland JJ. RNA virus mutations and fitness for survival. Annu Rev Microbiol. 1997;51:151–178. doi: 10.1146/annurev.micro.51.1.151. [DOI] [PubMed] [Google Scholar]
- Ferguson BA, Dreisbach TA, Parks CG, Filip GM, Schmitt CL. Coarse-scale population structure of pathogenic Armillaria species in a mixed-conifer forest in the Blue Mountains of northeast Oregon. Can J For Res. 2003;33:612–623. [Google Scholar]
- Garbelotto MM, Lee HK, Slaughter G, Popenuck T, Cobb FW, Bruns TD. Heterokaryosis is not required for virulence of Heterobasidion annosum. Mycologia. 1997;89:92–102. [Google Scholar]
- Ghabrial SA, Soldevila AI, Havens WM.2002Molecular genetics of the viruses infecting the plant pathogenic fungus Helminthosporium victoriaeIn: Tavantzis S, (ed)Molecular biology of double-stranded RNA: concepts and applications in agriculture, forestry and medicine CRC Press: Boca Raton; 213–236. [Google Scholar]
- Ghabrial SA. Advances in Virus Research 86: Mycoviruses. Academic Press, Elsevier; 2013. [DOI] [PubMed] [Google Scholar]
- Hammond TM, Andrewski MD, Roossinck MJ, Keller NP. Aspergillus mycoviruses are targets and suppressors of RNA silencing. Eukaryot Cell. 2008;7:350–357. doi: 10.1128/EC.00356-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen EM, Stenlid J, Johansson M. Genetic control of somatic incompatibility in the root-rotting basidiomycete Heterobasidion annosum. Mycol Res. 1993;97:1229–1233. [Google Scholar]
- Hoegger PJ, Heiniger U, Holdenrieder O, Rigling D. Differential transfer and dissemination of hypovirus and nuclear and mitochondrial genomes of a hypovirus-infected Cryphonectria parasitica strain after introduction into a natural population. Appl Environ Microb. 2003;69:3767–3771. doi: 10.1128/AEM.69.7.3767-3771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Y, Dover SL, Cole TE, Brasier CM, Buck KW. Multiple mitochondrial viruses in an isolate of the Dutch Elm disease fungus Ophiostoma novo-ulmi. Virology. 1999;258:118–127. doi: 10.1006/viro.1999.9691. [DOI] [PubMed] [Google Scholar]
- Huang S, Ghabrial SA. Organization and expression of the double-stranded RNA genome of Helminthosporium victoriae 190S virus, a totivirus infecting a plant pathogenic filamentous fungus. Proc Natl Acad Sci USA. 1996;93:12541–12546. doi: 10.1073/pnas.93.22.12541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyder R, Pennanen T, Hamberg L, Vainio EJ, Piri T, Hantula J. Two viruses of Heterobasidion confer beneficial, cryptic or detrimental effects to their hosts in different situations. Fungal Ecol. 2013;6:387–396. [Google Scholar]
- Ihrmark K. Double-stranded RNA elements in the root rot fungus Heterobasidion annosum. PhD Dissertation. Swedish University of Agricultural Sciences: Uppsala, Sweden; 2001. [Google Scholar]
- Ihrmark K, Johannesson H, Stenström E, Stenlid J. Transmission of double-stranded RNA in Heterobasidion annosum. Fungal Genet Biol. 2002;36:147–154. doi: 10.1016/S1087-1845(02)00011-7. [DOI] [PubMed] [Google Scholar]
- Ihrmark K, Stenström E, Stenlid J. Double-stranded RNA transmission through basidiospores of Heterobasidion annosum. Mycol Res. 2004;108:149–153. doi: 10.1017/s0953756203008839. [DOI] [PubMed] [Google Scholar]
- Ikeda K-I, Nakamura H, Arakawa M, Koiwa T, Matsumoto N. Dynamics of double-stranded RNA segments in a Helicobasidium mompa clone from a tulip tree plantation. FEMS Microbiol Ecol. 2005;51:293–301. doi: 10.1016/j.femsec.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Johannesson H, Stenlid J. Nuclear reassortment between vegetative mycelia in natural populations of the basidiomycete Heterobasidion annosum. Fungal Genet Biol. 2004;41:563–570. doi: 10.1016/j.fgb.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Kaarna-Vuorinen L.2000. Kuusen (Picea abies (L.) Karst.) lahoisuus, sen taloudelliset vaikutukset ja syyt Kaakkois-Suomen päätehakkuissa. Rot frequency and ensuing economic losses, and the cause of butt rot in final fellings in Norway spruce (Picea abies (L.) Karst.) stands in south-eastern Finland. Helsingin yliopiston metsäekonomian laitoksen julkaisuja 8. 82 p. [In Finnish with English summary].
- Korhonen K. Intersterility groups of Heterobasidion annosum. Commun Inst Forest Fenn. 1978;94:1–25. [Google Scholar]
- Lakshman DK, Jian J, Tavantzis M. A double-stranded RNA element from a hypovirulent strain of Rhizoctonia solani occurs in DNA form and is genetically related to the pentafunctional AROM protein of the shikimate pathway. Proc Natl Acad Sci USA. 1998;95:6425–6429. doi: 10.1073/pnas.95.11.6425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambden PR, Cooke SJ, Caul EO, Clarke IN. Cloning of noncultivatable human rotavirus by single primer amplification. J Virol. 1992;66:1817–1822. doi: 10.1128/jvi.66.3.1817-1822.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fu Y, Jiang D, Li G, Xie J, Peng Y, et al. A novel mycovirus that is related to the human pathogen hepatitis E virus and rubi-like viruses. J Virol. 2009;83:1981–1991. doi: 10.1128/JVI.01897-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Fu Y, Jiang D, Li G, Xie J, Cheng J, et al. Widespread horizontal gene transfer from double-stranded RNA viruses to eukaryotic nuclear genomes. J Virol. 2010;84:11876–11887. doi: 10.1128/JVI.00955-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Márquez LM, Redman RS, Rodriguez RJ, Roossinck MJ. A virus in a fungus in a plant: three-way symbiosis required for thermal tolerance. Science. 2007;315:513–515. doi: 10.1126/science.1136237. [DOI] [PubMed] [Google Scholar]
- MacDonald WL, Fulbright DW. Biological control of chestnut blight: use and limitations of transmissible hypovirulence. Plant Dis. 1991;75:656–661. [Google Scholar]
- Martin DP, Lemey P, Lott M, Moulton V, Posada D, Lefeuvre P. RDP3: a flexible and fast computer program for analyzing recombination. Bioinformatics. 2010;26:2462–2463. doi: 10.1093/bioinformatics/btq467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris TJ, Dodds JA. Isolation and analysis of double-stranded RNA from virus-infected plant and fungal tissue. Phytopathology. 1979;69:854–858. [Google Scholar]
- Möykkynen T, Weissenberg KV, Pappinen A. Estimation of dispersal gradients of S-and P-type basidiospores of Heterobasidion annosum. Eur J Forest Pathol. 1997;27:291–300. [Google Scholar]
- Nibert ML, Ghabrial SA, Maiss E, Lesker T, Vainio EJ, Jiang D, et al. 2014Taxonomic reorganization of family Partitiviridae and other recent progress in partitivirus research Virus Rese-pub ahead of print 21 April 2014doi: 10.1016/j.virusres.2014.04.007 [DOI] [PubMed]
- Niemelä T, Korhonen K.1998Taxonomy of the genus HeterobasidionIn: Woodward S, Stenlid J, Karjalainen R, Hüttermann A, (eds)Heterobasidion annosum: Biology, Ecology, Impact and Control CAB International: UK; 27–41. [Google Scholar]
- Osaki H, Nomura K, Matsumoto N, Ohtsu Y. Characterization of double-stranded RNA elements in the violet root rot fungus Helicobasidium mompa. Mycol Res. 2004;108:635–640. doi: 10.1017/s095375620400005x. [DOI] [PubMed] [Google Scholar]
- Park Y, James D, Punja ZK. Co-infection by two distinct totivirus-like double-stranded RNA elements in Chalara elegans (Thielaviopsis basicola) Virus Res. 2005;109:71–85. doi: 10.1016/j.virusres.2004.10.011. [DOI] [PubMed] [Google Scholar]
- Pearson MN, Beever RE, Boine B, Arthur K. Mycoviruses of filamentous fungi and their relevance to plant pathology. Mol Plant Pathol. 2009;10:115–128. doi: 10.1111/j.1364-3703.2008.00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peever TL, Liu Y-C, Milgroom MG. Diversity of hypoviruses and other double-stranded RNAs in Cryphonectria parasitica in North America. Phytopathology. 1997;87:1026–1033. doi: 10.1094/PHYTO.1997.87.10.1026. [DOI] [PubMed] [Google Scholar]
- Piri T, Korhonen K. The effect of winter thinning on the spread of Heterobasidion parviporum in Norway spruce stands. Can J For Res. 2008;38:2589–2595. [Google Scholar]
- Preisig O, Wingfield BD, Wingfield MJ. Coinfection of a fungal pathogen by two distinct double-stranded RNA viruses. Virology. 1998;252:399–406. doi: 10.1006/viro.1998.9480. [DOI] [PubMed] [Google Scholar]
- Preisig O, Moleleki N, Smit WA, Wingfield BD, Wingfield MJ. A novel RNA mycovirus in a hypovirulent isolate of the plant pathogen Diaporthe ambigua. J Gen Virol. 2000;81:3107–3114. doi: 10.1099/0022-1317-81-12-3107. [DOI] [PubMed] [Google Scholar]
- Shain L, Miller JB. Movement of cytoplasmic hypovirulence agents in chestnut blight cankers. Can J Bot. 1992;70:557–561. [Google Scholar]
- Stenlid J, Redfern DB.1998Spread within the tree and standIn: Woodward S, Stenlid J, Karjalainen R, Hüttermann A, (eds)Heterobasidion annosum: Biology, Ecology, Impact and Control CAB International: UK; 125–141. [Google Scholar]
- Stenlid J. Population structure of Heterobasidion annosum as determined by somatic incompatibility, sexual incompatibility, and isoenzyme patterns. Can J Bot. 1985;63:2268–2273. [Google Scholar]
- Sun L, Nuss DL, Suzuki N. Synergism between a mycoreovirus and a hypovirus mediated by the papain-like protease p29 of the prototypic hypovirus CHV1-EP713. J Gen Virol. 2006;87:3703–3714. doi: 10.1099/vir.0.82213-0. [DOI] [PubMed] [Google Scholar]
- Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomivirta TT, Hantula J. Three unrelated viruses occur in a single isolate of Gremmeniella abietina var. abietina type A. Virus Res. 2005;110:31–39. doi: 10.1016/j.virusres.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Vainio EJ, Korhonen K, Hantula J. Genetic variation in Phlebiopsis gigantea as detected with random amplified microsatellite (RAMS) markers. Mycol Res. 1998;102:187–192. [Google Scholar]
- Vainio EJ, Korhonen K, Tuomivirta TT, Hantula J. A novel putative partitivirus of the saprotrophic fungus Heterobasidion ecrustosum infects pathogenic species of the Heterobasidion annosum complex. Fungal Biol. 2010;114:955–965. doi: 10.1016/j.funbio.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Vainio EJ, Keriö S, Hantula J. Description of a new putative virus infecting the conifer pathogenic fungus Heterobasidion parviporum with resemblance to Heterobasidion annsoum P-type partitivirus. Arch Virol. 2011;156:79–86. doi: 10.1007/s00705-010-0823-9. [DOI] [PubMed] [Google Scholar]
- Vainio EJ, Hakanpää J, Dai Y-C, Hansen E, Hantula J. Species of Heterobasidion host a diverse pool of partitiviruses with global distribution and interspecies transmission. Fungal Biol. 2011;115:1234–1243. doi: 10.1016/j.funbio.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Vainio EJ, Hyder R, Aday G, Hansen E, Piri T, Dogmus-Lehtijärvi T, et al. Population structure of a novel putative mycovirus infecting the conifer root-rot fungus Heterobasidion annosum sensu lato. Virology. 2012;422:366–376. doi: 10.1016/j.virol.2011.10.032. [DOI] [PubMed] [Google Scholar]
- Vainio EJ, Piri T, Hantula J. Virus community dynamics in the conifer pathogenic fungus Heterobasidion parviporum following an artificial introduction of a partitivirus. Microb Ecol. 2013;65:28–38. doi: 10.1007/s00248-012-0118-7. [DOI] [PubMed] [Google Scholar]
- Vainio EJ, Capretti P, Motta E, Hantula J. Molecular characterization of HetRV8-ir1, a partitivirus of the invasive conifer pathogenic fungus H. irregulare. Arch Virol. 2013;158:1613–1615. doi: 10.1007/s00705-013-1643-5. [DOI] [PubMed] [Google Scholar]
- Yaegashi H, Nakamura H, Sawahata T, Sasaki A, Iwanami Y, Ito T, et al. Appearance of mycovirus-like double-stranded RNAs in the white root rot fungus, Rosellinia necatrix, in an apple orchard. FEMS Microbiol Ecol. 2013;83:49–62. doi: 10.1111/j.1574-6941.2012.01454.x. [DOI] [PubMed] [Google Scholar]
- Yu X, Li B, Fu Y, Jiang D, Ghabrial SA, Li G, et al. A geminivirus-related DNA mycovirus that confers hypovirulence to a plant pathogenic fungus. Proc Natl Acad Sci USA. 2010;107:8387–8839. doi: 10.1073/pnas.0913535107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.