Background: The ability of TMP to serve as a precursor of mitochondrial synthesis of TTP is not well understood.
Results: TMP cannot be converted to TTP except by breakdown to thymidine in isolated mitochondria.
Conclusion: Thymidine is the sole source for TTP synthesis in the mitochondrial matrix.
Significance: Thymidine salvage in mitochondria is crucial to understand mitochondrial DNA depletion diseases caused by mitochondrial thymidine kinase (TK2) deficiency.
Keywords: Mitochondria, Mitochondrial Metabolism, Nucleoside/Nucleotide Biosynthesis, Nucleoside/Nucleotide Metabolism, Thymidine Kinase 2
Abstract
The primary pathway of TTP synthesis in the heart requires thymidine salvage by mitochondrial thymidine kinase 2 (TK2). However, the compartmentalization of this pathway and the transport of thymidine nucleotides are not well understood. We investigated the metabolism of [3H]thymidine or [3H]TMP as precursors of [3H]TTP in isolated intact or broken mitochondria from the rat heart. The results demonstrated that [3H]thymidine was readily metabolized by the mitochondrial salvage enzymes to TTP in intact mitochondria. The equivalent addition of [3H]TMP produced far less [3H]TTP than the amount observed with [3H]thymidine as the precursor. Using zidovudine to inhibit TK2, the synthesis of [3H]TTP from [3H]TMP was effectively blocked, demonstrating that synthesis of [3H]TTP from [3H]TMP arose solely from the dephosphorysynthase pathway that includes deoxyuridine triphosphatelation of [3H]TMP to [3H]thymidine. To determine the role of the membrane in TMP metabolism, mitochondrial membranes were disrupted by freezing and thawing. In broken mitochondria, [3H]thymidine was readily converted to [3H]TMP, but further phosphorylation was prevented even though the energy charge was well maintained by addition of oligomycin A, phosphocreatine, and creatine phosphokinase. The failure to synthesize TTP in broken mitochondria was not related to a loss of membrane potential or inhibition of the electron transport chain, as confirmed by addition of carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone and potassium cyanide, respectively, in intact mitochondria. In summary, these data, taken together, suggest that the thymidine salvage pathway is compartmentalized so that TMP kinase prefers TMP synthesized by TK2 over medium TMP and that this is disrupted in broken mitochondria.
Introduction
Eukaryotic cells must maintain and replicate nuclear and mitochondrial DNA from two distinct pools of dNTPs. In dividing cells, nuclear DNA replication requires a high concentration of dNTP molecules (1). Outside of S phase, and in non-dividing cells, dNTPs are required only for DNA repair and for mitochondrial DNA replication, which occurs continuously in all cells. However, the amount of dNTPs required outside of S phase is decreased substantially (2).
Depending on the cell type and physiological conditions, dNTPs may be provided from ribonucleoside salvage or the de novo synthesis of ribonucleotide diphosphates followed by reduction to dNDPs by ribonucleotide reductase (RNR)2 with subsequent phosphorylation by cellular nucleoside diphosphokinases (NDPKs) to dNTPs (for a review, see Ref. 3). TTP is distinct from the other dNTPs because it cannot be generated directly by reduction of ribonucleotide diphosphates by the RNR enzyme. TTP synthesis requires an additional thymidylate synthase pathway that includes deoxyuridine triphosphate pyrophosphorylase, thymidylate synthase, and dihydrofolate reductase (Fig. 1) (3). RNR activity and the thymidylate synthase pathway have generally been considered to be cytosolic enzymes present in dividing cells. However, there are recent reports of both RNR activity (4) and the thymidylate synthase pathway (5) in mammalian liver mitochondria. In contrast, in previous studies from our laboratory in which adult rat hearts were perfused for 2 h with multiple appropriate radioactive precursors, we were unable to detect evidence of RNR or thymidylate synthase activity in either the cytosol or mitochondria (6, 7), suggesting that the heart may be considerably different from the liver.
FIGURE 1.
Model of thymidine metabolism in the cytosol and mitochondrial matrix. Blue pathways represent potential contributions to TMP synthesis from non-thymidine precursors. Black pathways represent potential contributions to TTP synthesis from thymidine. The green pathway represents thymidine breakdown. The thymidylate synthase pathway is generally known to be cytosolic. However, recent evidence has suggested its presence in liver mitochondria. Previous work from our laboratory has been unable to detect either a cytosolic or mitochondrial thymidylate synthase pathway in the perfused heart or in isolated heart mitochondria. TP, thymidine phosphorylase; dUTPase, deoxyuridine pyrophosphorylase; CDA, cytidine deaminase; cdN, cytosolic deoxynucleotidase; mdN, mitochondrial deoxynucleotidase; DHFR, dihydrofolate reductase; TYMS, thymidylate synthase; dCK, deoxycytidine kinase; TMPK, cytosolic thymidine monophosphate kinase; ENT1/2, equilibrative nucleoside transporter 1/2; methylene-THF, methylene-tetrahydrofolate; DHF, dihydrofolate.
Lastly, dNTPs can be synthesized by taking up deoxynucleosides from the blood stream and salvaging them via deoxynucleoside kinases. In the salvage pathway, the first phosphorylation reaction is typically irreversible and, usually, the rate-limiting reaction (8). Two of the enzymes responsible for this activity are cytosolic: thymidine kinase 1 (TK1), which phosphorylates thymidine, and deoxycytidine kinase, which phosphorylates deoxycytidine, deoxyguanosine, and deoxyadenosine. The other two kinases are mitochondrial: thymidine kinase 2 (TK2), which phosphorylates thymidine and deoxycytidine, and deoxyguanosine kinase, which phosphorylates deoxyguanosine and deoxyadenosine (for a review, see Ref. 9). Therefore, it is possible to generate all four of the naturally occurring deoxynucleoside monophosphates in either the cytoplasm or the mitochondria.
As noted above and illustrated in Fig. 1 and Ref. 3, thymidine salvage may take place in either the cytosolic or the mitochondrial compartment. However, the cytosolic salvage of thymidine by TK1 takes place only during S phase of dividing cells because the enzyme is inactivated and not expressed outside of S phase (1). Therefore, thymidine salvage in differentiated non-dividing cells must occur solely within the mitochondrial compartment via TK2. Alternatively, in some tissues, TMP can be synthesized from the thymidylate synthase pathway from salvage of deoxyuridine to dUMP or deoxycytidine to dCMP, followed by deamination to dUMP (Fig. 1). However, as noted above, the thymidylate synthase pathway does not appear to be active in either the cytosol or mitochondria in adult rat hearts.
When TMP is formed, it can take part in two different reactions. TMP can be cleaved back to the nucleoside by 5′ nucleotidases that are located in both the cytoplasm (10) and the mitochondria (11), or TMP can be phosphorylated again to its diphosphate form through a cytosolic TMP kinase (12) or mitochondrial TMP kinase (TMPK2) (Fig. 1) (13). The addition of the third phosphate is a function of nucleoside diphosphate kinases, known to be present in both the intermembrane space and matrix, and rapidly equilibrates the nucleoside and deoxynucleoside triphosphate pools with ATP (14, 15). The capability of isolated intact mitochondria from the heart (16), liver (17), and brain (18) to carry out these reactions has been demonstrated by showing that thymidine can be readily converted to TTP and that the conversion appears to occur in the matrix.
There is evidence in the literature from human cell culture studies (19–22) to suggest that cytosolic and mitochondrial dNTP pools communicate via specific inner mitochondrial membrane transporters. TMP synthesized within the cytosolic compartment can either be phosphorylated within the cytosolic compartment, or, as suggested for isolated mouse liver mitochondria by Ferraro et al. (23), it can be transported across the inner mitochondrial membrane to be phosphorylated in the matrix. However, work by McKee et al. (16) has shown that a large bolus of unlabeled TMP added to the medium of isolated heart mitochondria did not inhibit the conversion of [3H]TMP formed from [3H]thymidine to [3H]TTP, indicating that medium TMP did not mix well with TMP synthesized from thymidine in the matrix, suggesting that TMP may not be transported by heart mitochondria. This investigation was undertaken to elucidate and understand the fate of TMP in isolated heart mitochondria in comparison with thymidine. The importance of this investigation is illustrated by human mutations in TK2, which have been well described and lead to a lethal muscle mitochondrial DNA depletion disease in young children (24). Because TMP would bypass the mutant TK2 enzyme, it has been proposed as a treatment (25). This research was accomplished by measuring the conversion of [3H]thymidine and [3H]TMP to [3H]TTP in intact and broken mitochondria isolated from the rat heart. Our data demonstrated that TMP synthesized from thymidine does not mix with exogenously added TMP and that TMP cannot be used for synthesis of TTP except by dephosphorylation to thymidine first as an intermediate.
EXPERIMENTAL PROCEDURES
Chemicals and Biochemicals
All the unlabeled chemicals used in this study were purchased from Sigma-Aldrich, except zidovudine (AZT), which was purchased from Synthonix (Wake Forest, NC). [3H]Thymidine and [3H]TMP were purchased from Moravek Biochemicals (Brea, CA).
Isolation of Mitochondria from Rat Heart
In-house outbred Harlan-Sprague-Dawley rats were raised in the institution vivarium according to the Guide for the Care and Use of Animals, and the experiments were conducted as described in an Institutional Animal Care and Use Committee-approved animal protocol. Coupled mitochondria from the heart were isolated from female rats as described previously (26), except that differential centrifugation at 4 °C was adjusted to 1000 × g and 8500 × g for low- and high-speed centrifugations, which improved the recovery of mitochondria. The intactness of the isolated mitochondria was determined by measuring the respiratory control ratio using high-resolution respirometry (Oxygraph-2K, OROBOROS) with glutamate and malate as substrates, as described previously (18). Mitochondria with a respiratory control ratio value of 6 and above were used for these experiments. The protein concentration of the isolated mitochondria was obtained by the Lowry method using BSA as a standard.
Preparation of Broken Mitochondria
Mitochondria were isolated as described above, and all additions to the incubation were made prior to freezing and thawing. The broken mitochondria were obtained by freezing the incubation sample in liquid nitrogen for 30 s, followed by thawing for 1 min in a 30 °C water bath. The incubation sample was mixed, and this process was repeated three more times. The broken mitochondria were incubated, and aliquots were removed exactly as described for the intact mitochondria.
Incubation of Isolated Mitochondria
Mitochondria were incubated at a final concentration of 4 mg mitochondrial protein/ml in medium described previously (26) with [3H]thymidine or [3H]TMP at a final concentration of 100 nm (∼2200 dpm/pmol). Other additions to the incubation are described under “Results” and in the figure legends where appropriate. The incubation was terminated at specific times by removing an incubation aliquot with addition of an equal volume of 10% TCA to lyse the mitochondria and precipitate the macromolecules. TCA-treated samples were centrifuged to remove the precipitates, and the resultant supernatant was neutralized to at least pH 6.5 with ion exchange resin (AG-11A8, Bio-Rad) and filtered using a 0.45-μm Whatman syringe filter. The amount of radioactivity in each sample was determined by counting an aliquot in scintillation fluid (Insta-Gel Plus, PerkinElmer Life Sciences) on a liquid scintillation analyzer (Tri-Carb 2800TR, PerkinElmer Life Sciences).
UPLC Deoxynucleotide Analysis and Radioactivity Quantification
[3H]Thymidine, [3H]TMP, and their intermediates and phosphorylated products were analyzed by UPLC (1290 Infinity, Agilent) equipped with a C18 reverse-phase column (ZORBAX Eclipse Plus, 3.0 × 150 mm, 1.8 μm, Agilent) coupled to an inline diode array and liquid scintillation counter (β-RAM5, LabLogic). The mobile phases were composed of 5 mm tetrabutylammonium acetate, 60 mm ammonium acetate (pH 5.0), and 5 mm tetrabutylammonium acetate in methanol using a gradient program. The flow rate and column temperature were set at 0.5 ml/min and 30 °C, respectively. A diode array was used to quantitate levels of ADP and ATP, and the 3H signals were detected by β-RAM and quantified using Laura software (LabLogic).
Data Analysis
The rate of conversion of each precursor to products was dependent on the amount of mitochondrial protein added. Therefore, the amounts of products were expressed as disintegrations per minute per milligram of mitochondrial protein. These values were subsequently converted to picomole per milligram of mitochondrial protein by dividing by the specific radioactivity of the 3H precursor. Data presented in the figures represent the mean and S.E. of at least three independent determinations from three individual rat heart mitochondrial isolates. Significant differences in results were computed via Student's t test.
RESULTS
Conversion of [3H]Thymidine and [3H]TMP to [3H]TTP in Isolated Intact Rat Heart Mitochondria
The extent of phosphorylation of thymidine and TMP was determined by incubating isolated intact heart mitochondria (4 mg/ml) for 0, 30, 60, 90, and 120 min with 100 nm of either [3H]thymidine or [3H]TMP, both added at a specific radioactivity of ∼2200 dpm/pmol (Fig. 2). The conversion of these precursors to breakdown products or phosphorylated intermediates was quantitated from UPLC chromatograms and expressed as picomole per milligram of mitochondrial protein as described under “Experimental Procedures.” All three phosphorylated products of thymidine were observed. However, the overall detection of [3H]TDP was small and negligible in all cases. Therefore, the small amount of [3H]TDP measured was added to the much larger amount measured as [3H]TTP and reported as [3H]TTP. About 7.2% of the exogenously added [3H]TMP was found as [3H]thymidine after 2 h of incubation, but there was no breakdown of [3H]thymidine. Although both substrates were converted to [3H]TTP linearly with time, it was clear (Fig. 2) that addition of 100 nm (25 pmol/mg of protein) [3H]thymidine made four times more [3H]TTP than observed with a 100 nm addition of [3H]TMP even though the level of [3H]TMP, when added directly to the medium, was four times higher than the level of [3H]TMP produced from [3H]thymidine after 2 h of incubation. Because TMP is a more immediate precursor to TDP and TTP than thymidine, these data strongly suggest that the [3H]TMP synthesized from [3H]thymidine is much more readily available to mitochondrial TMPK and NDPK than [3H]TMP added exogenously. Furthermore, because 7.2% of the exogenously added [3H]TMP was found dephosphorylated to [3H]thymidine, it is possible that some of the [3H]TTP synthesis observed from the exogenous addition of [3H]TMP may have occurred via a thymidine intermediate, further increasing the difference between phosphorylation rates.
FIGURE 2.
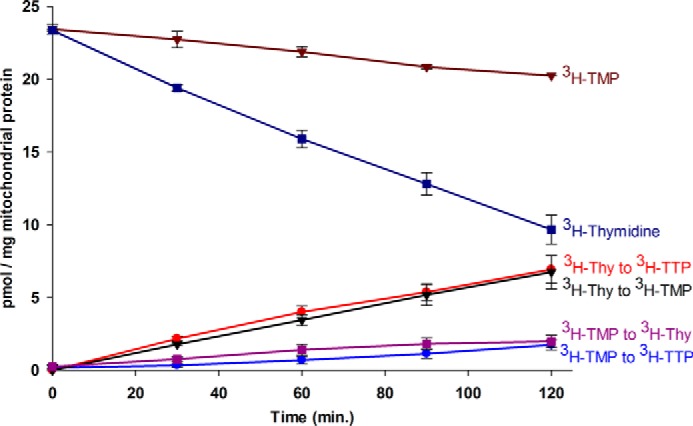
The metabolism of [3H]thymidine and [3H]TMP in isolated intact rat heart mitochondria. Freshly isolated mitochondria (4 mg of protein/ml) were incubated at various time points in incubation medium at 30 °C containing 100 nm of either [3H]thymidine or [3H]TMP (∼2200 dpm/pmol) and processed as described under “Experimental Procedures.” The 3H products formed were detected and quantified using UPLC and an inline liquid scintillation counter. The amount of 3H products formed were expressed as picomole of product per milligram of mitochondrial protein and plotted against time. All data points represent the mean and S.E. of three independent determinations from three individual rat tissue mitochondrial isolates.
Clearly thymidine is the preferred substrate for the salvage pathway in isolated heart mitochondria. This raises the question of the extent to which exogenously added TMP mixes with the endogenously synthesized pool of TMP. Support for compartmentalization of TMP pools was observed by incubating [3H]thymidine (100 nm) with a 100-fold excess of unlabeled TMP (10 μm) in intact mitochondria and comparing the level of phosphorylation observed with that obtained when [3H]thymidine was added alone (Fig. 3). The bolus addition of unlabeled TMP substantially reduced the conversion of [3H]thymidine to both [3H]TMP and [3H]TTP. Because unlabeled TMP was expected to break down to unlabeled thymidine and dilute the specific radioactivity of the [3H]thymidine pool, the reduction in conversion of [3H]thymidine to [3H]TMP was anticipated. However, if the exogenously added unlabeled TMP mixed readily with the [3H]TMP made from [3H]thymidine, then it would be expected to reduce the specific radioactivity of this pool more than 400-fold, and conversion of [3H]TMP to [3H]TDP and [3H]TTP would be effectively blocked. As shown in Fig. 3, this clearly was not the case. Although the amount of [3H]TTP synthesized from [3H]thymidine was significantly lower than what was observed in the absence of the unlabeled TMP addition, the amount observed was consistent with the amount of [3H]TMP produced from [3H]thymidine. Taken together, these data demonstrate that exogenously added TMP and TMP synthesized from thymidine do not mix readily and must be compartmentalized.
FIGURE 3.

The effect of unlabeled TMP on [3H]thymidine metabolism in isolated intact rat heart mitochondria. Mitochondria were incubated and processed and data were plotted as described under “Experimental Procedures” and in the legend for Fig. 2, except that 10 μm of unlabeled TMP was added to the incubations. Dashed lines represent results from Fig. 2 obtained in the absence of 10 μm unlabeled TMP for comparison. All data points represent the mean and S.E. of three independent determinations from three individual rat tissue mitochondrial isolates.
Conversion of [3H]Thymidine and [3H]TMP to [3H]TTP in Broken Rat Heart Mitochondria
Transport of TMP across the inner mitochondrial membrane represents an obvious mechanism of compartmentation of TMP pools. Clearly [3H]thymidine was much more readily accessible to the mitochondrial matrix and metabolized by the mitochondrial salvage enzymes to [3H]TTP than the equivalent addition of [3H]TMP. Therefore, one interpretation of these data is that TMP is not transported across the inner mitochondrial membrane until it is broken down to thymidine.
To determine whether the mitochondrial inner membrane was a limiting factor in [3H]thymidine or [3H]TMP metabolism, incubations were performed using the same experimental conditions, precursor concentrations, and specific radioactivities as outlined under “Experimental Procedures” for intact mitochondria except that the mitochondrial membranes were disrupted by repeated freezing and thawing. This allowed [3H]thymidine and [3H]TMP free access to the enzyme systems existing within the mitochondrial matrix. As observed by UPLC analysis, the broken mitochondrial incubations were associated with a drastic drop in ATP from a large, distinct peak of predominantly ATP observed in intact mitochondrial incubation samples to near depletion observed in the broken mitochondrial incubation samples (Fig. 4, A versus B). Because dNTP synthesis depends on ATP as the donor for phosphorylation, the ATP levels were normalized by addition of oligomycin A (50 μm, ATPase inhibitor), 12 mm phosphocreatine, and 50 units/ml of creatine phosphokinase enzyme (energy generating system) to the broken mitochondria incubation. These additions had no effect on intact mitochondria (Fig. 4C) but dramatically normalized the ATP levels in the broken mitochondria to that observed in the intact system (Fig. 4D).
FIGURE 4.

Energy charge in the mitochondrial incubation systems. The distribution (percent) of AMP/ADP/ATP in the intact and broken mitochondrial systems after 2 h of incubation is shown. A, intact mitochondria (no additions). B, broken mitochondria (no additions). C, intact mitochondria + oligomycin A (50 nm), phosphocreatine (PC, 12 mm), and creatine phosphokinase (CPK, 50 units/ml). D, broken mitochondria + oligomycin A (50 nm), phosphocreatine (12 mm), and creatine phosphokinase (50 units/ml). E, intact mitochondria as in C + FCCP (3 μm). F, intact mitochondria as in C + KCN (6 mm). All data points represent the mean and S.E. of three independent determinations from three individual rat tissue mitochondrial isolates.
When broken mitochondria were incubated under these conditions with [3H]thymidine (Fig. 5), twice as much [3H]TMP was made at the end of 2 h than was observed in the intact mitochondria (broken, 13.8 versus intact, 6.7 pmol/mg of protein). However, very little [3H]TTP was observed (broken, 1.3 versus intact, 6.9 pmol/mg of protein), indicating that total phosphorylation in the broken versus intact mitochondria (broken = 15.1 versus intact = 13.6 pmol/mg of protein) was similar. These data demonstrate that TK2 activity was unaffected in broken mitochondria but that subsequent phosphorylation was mostly lost. When broken mitochondria were incubated with [3H]TMP, about the same amount of [3H]TTP was observed after 2 h as in intact mitochondria (Fig. 5). However, the time courses of phosphorylation of each were different. Phosphorylation of [3H]TMP in intact mitochondria started at zero and increased linearly to 1.75 pmol/mg protein over 2 h (Fig. 2), whereas, in the broken mitochondrial preparation, a substantial amount of [3H]TTP (1.07 pmol/mg of protein) was formed during the freeze-thaw process recorded at the initial time point. This increased somewhat within the next 30 min and plateaued at 1.79 pmol/mg of protein (Fig. 5).
FIGURE 5.

Metabolism of [3H]thymidine and [3H]TMP in broken rat heart mitochondria. Broken mitochondria were prepared, incubated, processed, and plotted as described under “Experimental Procedures” and in the legend for Fig. 2. The energy charge of the experiments was maintained as described in Fig. 4D. All data points represent the mean and S.E. of three independent determinations from three individual rat tissue mitochondrial isolates.
The data shown in Fig. 5 were obtained in the presence of oligomycin A, phosphocreatine, and creatine phosphokinase, which preserved ATP levels (Fig. 4, B versus C). However, the rate of phosphorylation of [3H]thymidine and [3H]TMP within broken mitochondria was not changed when these components were not added (data not shown), and the ATP levels fell precipitously.
Conversion of [3H]TMP to [3H]TTP in the Presence of AZT
From the data shown in Figs. 2 and 5, it was observed that a small amount of [3H]TTP was formed when [3H]TMP was used as a precursor in both the intact (1.75 pmol/mg of protein) and broken (1.79 pmol/mg of protein) mitochondrial incubations after 2 h of incubation. Because some of the [3H]TMP was known to break down to [3H]thymidine, it was not clear whether the [3H]TTP formed came from the direct phosphorylation of [3H]TMP or from the breakdown to [3H]thymidine and subsequent rephosphorylation. To test this possibility, AZT, a known potent inhibitor of TK2, was added to the incubations with [3H]TMP to determine the total extent of [3H]TMP breakdown by preventing the rephosphorylation of [3H]thymidine back to [3H]TMP. As shown in Fig. 6A, the presence of AZT significantly increased the amount of [3H]thymidine while significantly decreasing the amount of [3H]TMP observed compared with control incubations in the absence of AZT. The difference in the amount of [3H]thymidine was calculated and plotted in Fig. 6B. This calculated line represented the amount of [3H]thymidine that would have been rephosphorylated to [3H]TMP, [3H]TDP, and [3H]TTP, if AZT were not present. The difference in the amount of [3H]TMP was also calculated, and the line plotted in Fig. 6B represents the amount of [3H]TMP that would have arisen directly from rephosphorylation of [3H]thymidine back to [3H]TMP in the absence of AZT that would have remained as [3H]TMP. From the difference in these two calculated plots, the amounts of [3H]TDP and [3H]TTP that arose from [3H]TMP breakdown followed by rephosphorylation can be predicted, and the predicted results are plotted in Fig. 6B. In intact mitochondria, (Fig. 6B), the actual observed plot of [3H]TDP and [3H]TTP closely matches the amounts of [3H]TDP and [3H]TTP predicted to have been made via [3H]thymidine rephosphorylation, indicating that all of the [3H]TDP and [3H]TTP produced from the addition of [3H]TMP was produced via a [3H]thymidine intermediate and none produced directly from exogenously added [3H]TMP. In the broken system, the amount of [3H]TDP and [3H]TTP made from either exogenous [3H]thymidine or [3H]TMP is quite low, and about 65% of the [3H]TDP and [3H]TTP made from exogenous [3H]TMP appears to be made via the rephosphorylation of [3H]thymidine route (data not shown). Finally, as these data demonstrate, only a negligible amount of [3H]TDP and [3H]TTP was produced from exogenously added [3H]TMP in the presence of AZT. These data confirm that [3H]TMP added to the medium does not mix with [3H]TMP synthesized from [3H]thymidine.
FIGURE 6.

The effect of AZT on [3H]TMP metabolism in intact rat heart mitochondria. A, mitochondria were prepared, incubated, processed, and plotted as described under “Experimental Procedures” and in the legend for Fig. 2 except for the presence and absence of 200 μm AZT. Because AZT inhibits the conversion of [3H]thymidine to [3H]TMP, [3H]thymidine arising from the dephosphorylation of [3H]TMP is trapped as [3H]thymidine. B, the data in A was used to calculate the difference in [3H]thymidine and [3H]TMP levels observed in the presence and absence of 200 μm AZT (see text for details). All data points represent the mean and S.E. of three independent determinations from three individual rat tissue mitochondrial isolates.
Conversion of [3H]Thymidine to [3H]TTP in the Presence of Mitochondrial Electron Transport and Membrane Potential Inhibitors
To determine whether the disruption in the [3H]TTP synthesis in the broken mitochondria was related to a disruption of mitochondrial electron transport and/or mitochondrial membrane potential, [3H]TTP synthesis from [3H]thymidine was measured in intact mitochondria in the presence of KCN (which blocks electron transport) or FCCP (which collapses the proton gradient) (Fig. 7). The energy charges (ATP levels) of these experiments were maintained by adding oligomycin A (50 μm), phosphocreatine (12 mm), and creatine phosphokinase (50 unites/ml) to the incubation medium (Fig. 4, D versus E). The addition of KCN had no effect on [3H]TTP synthesis, whereas FCCP treatment significantly increased [3H]TTP. Although the effect of FCCP on increasing [3H]TTP synthesis is unknown, neither treatment blocked the conversion of [3H]thymidine to [3H]TTP, as observed in broken mitochondria.
FIGURE 7.

The effect of KCN and FCCP on [3H]thymidine metabolism in intact rat heart mitochondria. Mitochondria were prepared, incubated, processed, and plotted as described in the legend for Fig. 4D except that KCN and FCCP were added as indicated. Shown is the [3H]thymidine metabolism in the presence of KCN (6 mm) and FCCP (3 μm), respectively after 2 h of incubation. The energy charge of these incubations are shown in Fig. 4, C, E, and F. *, p < 0.005; **, p < 0.001. All data points represent the mean and S.E. of three independent determinations from three individual rat tissue mitochondrial isolates.
DISCUSSION
Although others have shown that enzymes of the de novo pathway, including RNR (4) and thymidylate synthase (5), may be present in some mitochondria, studies from our laboratory in the perfused rat heart suggested that TTP can be synthesized solely through the salvage of thymidine via TK2 (6, 7). The goal of this study was to understand the fate of TMP with respect to transport, compartmentalization, and conversion to TTP. The importance of this pathway is illustrated by human mutations in TK2, which have been well described and lead to a lethal muscle mitochondrial DNA depletion disease in young children (24). Because TMP would bypass the mutant TK2 enzyme, it has been proposed as a treatment (25).
This investigation set out to determine whether TMP would serve as a potential entry point of the salvage pathway that results in TTP synthesis in isolated mitochondria. If TMP serves as an entry point for TTP synthesis, then it would be anticipated that TMP would lead to faster, more effective conversion to TTP than thymidine as it bypasses the first phosphorylation step, which is usually considered as the rate-limiting step. However, the data from this investigation clearly demonstrated that [3H]thymidine was converted to [3H]TTP much more readily than [3H]TMP. Furthermore, the [3H]TMP breakdown to [3H]thymidine was evident from the results obtained, suggesting that the small amount of TTP observed may be synthesized via thymidine as an intermediate. The data shown in Fig. 3 demonstrated that a large bolus of exogenous unlabeled TMP in the medium did not mix with the [3H]TMP synthesized from [3H]thymidine in the matrix because [3H]TTP synthesis, although reduced, was still observed.
To obtain a clear picture, AZT was used to inhibit TK2 activity. The data shown in Fig. 6 indicate that all of the [3H]TTP observed in the incubation with [3H]TMP arose via [3H]thymidine as an intermediate. One possible mechanism accounting for TMP compartmentalization is that TMP is not transported across the mitochondrial inner membrane. Therefore, TMP in the mitochondrial matrix can only be provided by TK2 phosphorylation of thymidine.
To investigate the TMP transport hypothesis, mitochondrial membrane intactness was disrupted by freezing and thawing. Although the synthesis of [3H]TMP from [3H]thymidine was normal in the broken mitochondria, [3H]TTP could not be formed from either [3H]thymidine or [3H]TMP (Fig. 5). The loss of TTP synthesis did not appear to be related to the energy charge of the system because the energy charge was as well maintained in the broken mitochondria as in the intact mitochondria by the addition of oligomycin A, phosphocreatine, and creatine phosphokinase (Fig. 4C). The results shown in Fig. 7 demonstrate that the failure to synthesize TTP from thymidine in broken mitochondria is not related to the mitochondrial membrane potential (collapsed with FCCP) or to the activity of the electron transport chain (inhibited by KCN).
Because it is impossible to demonstrate TTP synthesis from either thymidine or TMP in broken mitochondria, it was not possible to conclude that transport of TMP across the mitochondrial inner membrane accounted for the failure of TMP to be phosphorylated to TDP and TTP. The transport of TMP across the inner membrane has been demonstrated in mouse liver mitochondria (23). An alternative mechanism that supports the transport of TMP across the inner membrane is suggested in the model (Fig. 8) in which we hypothesize that the mitochondrial TMPK utilizes TMP presented by TK2 preferentially over TMP transported from the medium to the matrix. The suggested mechanism of mitochondrial TTP synthesis may occur by direct interaction of the two enzymes, i.e. TMPK and TK2. Further, disruption of the mitochondria by freezing and thawing may interfere with this enzyme-enzyme interaction. Mitochondrial TMPK has been identified by Chen et al. (13), and the recombinant TMPK2 protein was synthesized. Interestingly, they were unable to show TMPK2 activity using the purified recombinant TMPK2 in vitro. Our proposed model would suggest that TMPK2 may not be functional in the absence of TMP presented by TK2. This would explain the use of thymidine as the only source for TTP synthesis in the mitochondrial salvage pathway.
FIGURE 8.
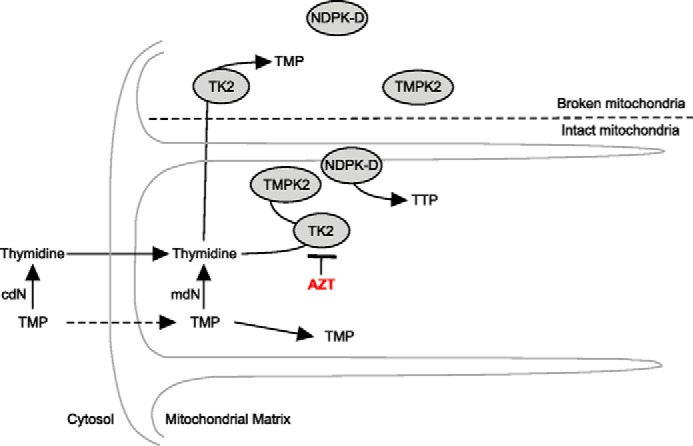
Proposed model of thymidine and TMP salvage in rat heart mitochondria. Shown is the proposed model to account for TMP compartmentalization. cdN, cytosolic deoxynucleotidase; mdN, mitochondrial deoxynucleotidase.
A TK2 knockin mouse line carrying the human TK2 mutation has been shown to recapitulate the human mitochondrial DNA depletion syndrome (27). A recent publication from Garone et al. (25) has shown that TMP supplementation in TK2 knockin mice significantly prolonged the lifespan of the knockin mice, although they still died from the disease. From these studies, the authors proposed that the exogenous TMP bypasses the mutant TK2 step in mitochondrial TTP synthesis. However, it should be noted that TMP in these studies was also shown to be degraded to thymidine, potentially increasing thymidine levels. We have shown previously (16) that TTP synthesized by mitochondria is robustly related to the exogenous thymidine concentrations. In summary, our data suggest an alternative possibility for the TMP-mediated increase in longevity in which increased thymidine levels may increase residual TK2 enzyme activity and produce more TTP.
This work was supported, in whole or in part, by National Institute of Health Grant R01/HL096480. This work was also supported by the Central Michigan University Office of Research Sponsored Programs summer fellowship (to Z. J. L.).
- RNR
- ribonucleotide reductase
- NDPK
- nucleoside diphosphate kinase
- AZT
- zidovudine
- TMPK
- cytosolic thymidine monophosphate kinase
- KCN
- potassium cyanide
- FCCP
- carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone
- UPLC
- ultra performance liquid chromatography.
REFERENCES
- 1. Coppock D. L., Pardee A. B. (1987) Control of thymidine kinase mRNA during the cell cycle. Mol. Cell. Biol. 7, 2925–2932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gandhi V. V., Samuels D. C. (2011) A review comparing deoxyribonucleoside triphosphate (dNTP) concentrations in the mitochondrial and cytoplasmic compartments of normal and transformed cells. Nucleosides Nucleotides Nucleic Acids 30, 317–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mathews C. K. (2014) Deoxyribonucleotides as genetic and metabolic regulators. FASEB J. 10.1096/fj.14-251249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chimploy K., Song S., Wheeler L. J., Mathews C. K. (2013) Ribonucleotide reductase association with mammalian liver mitochondria. J. Biol. Chem. 288, 13145–13155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anderson D. D., Quintero C. M., Stover P. J. (2011) Identification of a de novo thymidylate biosynthesis pathway in mammalian mitochondria. Proc. Natl. Acad. Sci. U.S.A. 108, 15163–15168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morris G. W., Iams T. A., Slepchenko K. G., McKee E. E. (2009) Origin of pyrimidine deoxyribonucleotide pools in perfused rat heart: implications for 3′-azido-3′-deoxythymidine-dependent cardiotoxicity. Biochem. J. 422, 513–520 [DOI] [PubMed] [Google Scholar]
- 7. Morris G. W., Laclair D. D., McKee E. E. (2010) Pyrimidine deoxynucleoside and nucleoside reverse transcriptase inhibitor metabolism in the perfused heart and isolated mitochondria. Antivir Ther. 15, 587–597 [DOI] [PubMed] [Google Scholar]
- 8. Johansson M., van Rompay A. R., Degrève B., Balzarini J., Karlsson A. (1999) Cloning and characterization of the multisubstrate deoxyribonucleoside kinase of Drosophila melanogaster. J. Biol. Chem. 274, 23814–23819 [DOI] [PubMed] [Google Scholar]
- 9. Arnér E. S., Eriksson S. (1995) Mammalian deoxyribonucleoside kinases. Pharmacol Ther. 67, 155–186 [DOI] [PubMed] [Google Scholar]
- 10. Hunsucker S. A., Mitchell B. S., Spychala J. (2005) The 5′-nucleotidases as regulators of nucleotide and drug metabolism. Pharmacol Ther. 107, 1–30 [DOI] [PubMed] [Google Scholar]
- 11. Rampazzo C., Gallinaro L., Milanesi E., Frigimelica E., Reichard P., Bianchi V. (2000) A deoxyribonucleotidase in mitochondria: involvement in regulation of dNTP pools and possible link to genetic disease. Proc. Natl. Acad. Sci. U.S.A. 97, 8239–8244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Van Rompay A. R., Johansson M., Karlsson A. (2000) Phosphorylation of nucleosides and nucleoside analogs by mammalian nucleoside monophosphate kinases. Pharmacol Ther. 87, 189–198 [DOI] [PubMed] [Google Scholar]
- 13. Chen Y. L., Lin D. W., Chang Z. F. (2008) Identification of a putative human mitochondrial thymidine monophosphate kinase associated with monocytic/macrophage terminal differentiation. Genes Cells 13, 679–689 [DOI] [PubMed] [Google Scholar]
- 14. Steeg P. S., Zollo M., Wieland T. (2011) A critical evaluation of biochemical activities reported for the nucleoside diphosphate kinase/Nm23/Awd family proteins: opportunities and missteps in understanding their biological functions. Naunyn Schmiedebergs Arch. Pharmacol. 384, 331–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Schlattner U., Tokarska-Schlattner M., Epand R. M., Boissan M., Lacombe M. L., Klein-Seetharaman J., Kagan V. E. (2014) Mitochondrial NM23-H4/NDPK-D: a bifunctional nanoswitch for bioenergetics and lipid signaling. Naunyn Schmiedebergs Arch. Pharmacol. 10.1007/s00210-014-1047-4 [DOI] [PubMed] [Google Scholar]
- 16. McKee E. E., Bentley A. T., Hatch M., Gingerich J., Susan-Resiga D. (2004) Phosphorylation of thymidine and AZT in heart mitochondria: elucidation of a novel mechanism of AZT cardiotoxicity. Cardiovasc. Toxicol. 4, 155–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lynx M. D., Bentley A. T., McKee E. E. (2006) 3′-Azido-3′-deoxythymidine (AZT) inhibits thymidine phosphorylation in isolated rat liver mitochondria: a possible mechanism of AZT hepatotoxicity. Biochem. Pharmacol. 71, 1342–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McCann K. A., Williams D. W., McKee E. E. (2012) Metabolism of deoxypyrimidines and deoxypyrimidine antiviral analogs in isolated brain mitochondria. J. Neurochem. 122, 126–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rampazzo C., Fabris S., Franzolin E., Crovatto K., Frangini M., Bianchi V. (2007) Mitochondrial thymidine kinase and the enzymatic network regulating thymidine triphosphate pools in cultured human cells. J. Biol. Chem. 282, 34758–34769 [DOI] [PubMed] [Google Scholar]
- 20. Leanza L., Ferraro P., Reichard P., Bianchi V. (2008) Metabolic interrelations within guanine deoxynucleotide pools for mitochondrial and nuclear DNA maintenance. J. Biol. Chem. 283, 16437–16445 [DOI] [PubMed] [Google Scholar]
- 21. Bridges E. G., Jiang Z., Cheng Y. C. (1999) Characterization of a dCTP transport activity reconstituted from human mitochondria. J. Biol. Chem. 274, 4620–4625 [DOI] [PubMed] [Google Scholar]
- 22. Franzolin E., Miazzi C., Frangini M., Palumbo E., Rampazzo C., Bianchi V. (2012) The pyrimidine nucleotide carrier PNC1 and mitochondrial trafficking of thymidine phosphates in cultured human cells. Exp. Cell Res. 318, 2226–2236 [DOI] [PubMed] [Google Scholar]
- 23. Ferraro P., Nicolosi L., Bernardi P., Reichard P., Bianchi V. (2006) Mitochondrial deoxynucleotide pool sizes in mouse liver and evidence for a transport mechanism for thymidine monophosphate. Proc. Natl. Acad. Sci. U.S.A. 103, 18586–18591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Saada A., Shaag A., Elpeleg O. (2003) mtDNA depletion myopathy: elucidation of the tissue specificity in the mitochondrial thymidine kinase (TK2) deficiency. Mol. Genet. Metab. 79, 1–5 [DOI] [PubMed] [Google Scholar]
- 25. Garone C., Garcia-Diaz B., Emmanuele V., Lopez L. C., Tadesse S., Akman H. O., Tanji K., Quinzii C. M., Hirano M. (2014) Deoxypyrimidine monophosphate bypass therapy for thymidine kinase 2 deficiency. EMBO Mol. Med. 6, 1016–1027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McKee E. E., Grier B. L., Thompson G. S., McCourt J. D. (1990) Isolation and incubation conditions to study heart mitochondrial protein synthesis. Am. J. Physiol. 258, E492–E502 [DOI] [PubMed] [Google Scholar]
- 27. Akman H. O., Dorado B., López L. C., García-Cazorla A., Vilà M. R., Tanabe L. M., Dauer W. T., Bonilla E., Tanji K., Hirano M. (2008) Thymidine kinase 2 (H126N) knockin mice show the essential role of balanced deoxynucleotide pools for mitochondrial DNA maintenance. Hum. Mol. Genet. 17, 2433–2440 [DOI] [PMC free article] [PubMed] [Google Scholar]



