Background: Mechanisms by which the mother does not reject the fetus are not fully understood.
Results: In sphingosine kinase-deficient mice, the innate arm of the maternal immune system attacks the fetus, resulting in miscarriage.
Conclusion: Sphingolipid metabolism has an essential role in maternal immunological adaptation to the fetus.
Significance: Our findings may help to develop treatments for unexplained miscarriages in humans.
Keywords: Chemokine, Innate Immunity, Neutrophil, Pregnancy, Sphingolipid, Decidual Cell, Fetomaternal Tolerance, Sphingosine Kinase
Abstract
For a successful pregnancy, the mother's immune system has to tolerate the semiallogeneic fetus. A deleterious immune attack is avoided by orchestration of cellular, hormonal, and enzymatic factors. However, the precise mechanisms underlying fetomaternal tolerance are not yet completely understood. In this study, we demonstrate that sphingolipid metabolism constitutes a novel signaling pathway that is indispensable for fetomaternal tolerance by regulating innate immune responses at the fetomaternal interface. Perturbation of the sphingolipid pathway by disruption of the sphingosine kinase gene (Sphk) during pregnancy caused unusually high expression of neutrophil chemoattractants, CXCL1 and CXCL2, in the decidua, leading to a massive infiltration of neutrophils into the fetomaternal interface with enhanced oxidative damage, resulting in early fetal death. Sphk-deficient mice also exhibited neutrophilia in the peripheral blood, enhanced generation of granulocytes in the bone marrow, and a decrease in the number of decidual natural killer cells. The blockage of neutrophil influx protected Sphk-deficient mice against pregnancy loss. Notably, a similar result was obtained in human decidual cells, in which Sphk deficiency dramatically increased the secretion of CXCL1 and IL-8. In conclusion, our findings suggest that the sphingolipid metabolic pathway plays a critical role in fetomaternal tolerance by regulating innate immunity at the fetomaternal interface both in mice and humans, and it could provide novel insight into the development of therapeutic strategies to treat idiopathic pregnancy loss in humans.
Introduction
Although the paternal antigens presented by the fetus are considered foreign by the maternal immune system, it survives peacefully in the uterine milieu of normal pregnancy. The development of immune tolerance to the semiallogeneic fetus and suppression of inflammation at the fetomaternal interface facilitates the establishment and maintenance of successful pregnancy (1, 2). Multiple mechanisms have been proposed to operate in this process, including complement system, catabolism of tryptophan by indoleamine 2,3-dioxygenase, regulatory T cells, natural killer (NK)2 cells, macrophages, and dendritic cells (1, 2). Notably, a recent study implicated granulocytes in fetomaternal tolerance (3). Maternal decidual tissues play a critical role in this process, as indicated by the fact that pregnancy losses occur during the process of decidualization when endometrial stromal cells surrounding implanting blastocysts undergo dramatic transformation (4, 5). The decidua provides a vascular network for nutrition and gas exchange for the developing embryo prior to the establishment of a functional placenta. An exaggerated maternal immune response in the decidua to fetal antigens has been proposed to cause early pregnancy complications such as pregnancy loss and preeclampsia (6).
The sphingolipid metabolic pathway produces bioactive signaling metabolites (7). A potent, pleiotropic extracellular phospholipid messenger is sphingosine 1-phosphate (S1P) that activates a family of cell-surface G protein-coupled receptors designated S1PR1–5 (8). Recent studies have demonstrated its importance in many physiological and pathological processes, such as the development of the vascular system (9), cancer (10), and the immune system (11, 12) by signaling through S1P receptors. S1P enhances cell survival and growth (13–15), whereas two precursors of S1P, ceramide and sphingosine, have generally been considered pro-apoptotic and anti-growth through modulation of key intracellular signaling pathways (16–18).
Sphingosine kinase (Sphk) is a key enzyme that catalyzes the ATP-dependent phosphorylation of sphingosine to form S1P and exists as two isoforms, Sphk1 and Sphk2 (19, 20). Genetic studies revealed that simultaneous defects in Sphk1 and Sphk2 (Sphk1−/−Sphk2−/−) in mice led to embryonic lethality around E11.5 due to severe defects in neurogenesis and angiogenesis, although Sphk1-null or Sphk2-null mice appeared morphologically and functionally normal (21–23).
In our previous studies, we found that reproductive failure occurred in Sphk1−/−Sphk2+/− female mice (24). The sphingolipid metabolic pathway was highly activated in the decidua during normal pregnancy. Perturbation in the activated pathway by disruption of sphingosine kinase genes, as observed in Sphk1−/−Sphk2+/− mice, caused defective decidualization with increased death of decidual cells, decreased proliferation of undifferentiated stromal cells, and massive breakage of decidual blood vessels, resulting in uterine hemorrhage and maternally derived early pregnancy loss. Moreover, these results suggest that sphingolipid metabolism regulates proper decidualization and blood vessel stability. The crucial role of sphingolipid metabolism in decidualization led us to investigate the detailed mechanism by which it contributes to early pregnancy loss. Here, we demonstrate that Sphk1−/−Sphk2+/− mice exhibit the collapse of fetomaternal tolerance mediated by neutrophils, which is reversed by the suppression of neutrophil recruitment at the fetomaternal interface. In addition, we explore the significance of sphingolipid metabolism in human pregnancy.
EXPERIMENTAL PROCEDURES
Animals
Disruption of Sphk1 and Sphk2 was performed as described (21, 22). Mice were maintained on a mixed genetic background, C57BL/6 × 129Sv. In quantitative analysis of sphingolipids, mice on a C57BL/6 background were also used. In all experiments, 6–12-week-old females were mated with wild-type males, and the day of the vaginal plug was considered day 0.5 pc. All mouse studies were performed in accordance with protocols approved by Musashino University and Kyoto University.
Sample Preparation
Serum and plasma were harvested from whole blood collected from nonpregnant or day 7.5 pc wild-type, Sphk1−/−Sphk2+/+, or Sphk1−/−Sphk2+/− female mice. Nonpregnant whole uteri and deciduas and interimplantation tissues of day 7.5 pc uteri from which the embryos had been removed were immediately frozen.
Quantitative Real Time PCR
Total RNA from uteri was isolated using TRIzol (Invitrogen). Total RNA (1 μg) was reverse-transcribed using the SuperScript First-Strand Synthesis System for RT-PCR (Invitrogen) following the manufacturer's instructions. Mouse CXCL1 and CXCL2 mRNA was quantified using Assays-on-Demand (Applied Biosystems) with an ABI Prism 7500 sequence detection system (Applied Biosystems). β-Actin was used as an internal standard.
Histological Analysis
Uteri were fixed in 10% formalin and processed for embedding in paraffin. Serial sections (5 μm) were made at 10–30-μm intervals and stained with H&E for general morphology. Paraffin sections were deparaffinized and rehydrated. Antigens were retrieved by target retrieval solution (DAKO) at 95 °C for 20 min. Endogenous peroxidase activity was quenched by incubation with 1% hydrogen peroxide in water for 30 min. Specimens were incubated overnight at 4 °C with the following: goat anti-CXCL1 (R&D Systems, catalog no. AF-453-NA, 1:200); goat anti-CXCL2 (R&D Systems, catalog no. AF-452-NA, 1:100); rat anti-CXCR2 (R&D Systems, catalog no. MAB2164, 1:500); rat anti-neutrophil (Abcam, catalog no. ab53457, 1:200); rat anti-F4/80 (Abcam, catalog no. ab6640, 1:100); rabbit anti-CD3 (Abcam, catalog no. ab16669, 1:100); rabbit anti-tissue factor (TF) (Abcam, catalog no. ab104513, 1:100); rabbit anti-myeloperoxidase (MPO) (Abcam, catalog no. ab45977, 1:100), or peroxidase-conjugated anti-Dolichos biflorus agglutinin (DBA) (Vector Laboratories, catalog no. B-1035, 1:500). The anti-DBA, anti-F4/80, and anti-CD3 antibodies were used to label decidual NK (dNK) cells, macrophages, and T cells, respectively. Subsequent incubation with peroxidase-conjugated rabbit anti-goat IgG, rabbit anti-rat IgG, or goat anti-rabbit IgG was performed for 30 min at room temperature. An avidin/biotin horseradish peroxidase system (Vector Laboratories) was used with diaminobenzidine to visualize the positive staining. Images were captured with an Eclipse E600 microscope (Nikon) equipped with a DP21 camera (Olympus). Magnification for each captured image is specified for each experiment in the figure legends.
Mouse Chemokine and Cytokine Arrays
The uteri were homogenized in PBS containing complete protease inhibitor mixture (Roche Applied Science). The assays were conducted using Q-Plex mouse chemokine or cytokine array (Quansys Biosciences) according to the manufacturer's instructions.
ELISA for Mouse Specimens
The uteri were homogenized in PBS containing complete protease inhibitor mixture (Roche Applied Science) for chemokines, lactoferrin, and TF, in a solution of 200 mm NaCl, 5 mm EDTA, 10 mm Tris, 10% glycerin, 1 mm phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, and 28 μg/ml aprotinin (pH 7.4) for MPO. The concentrations were determined using ELISA for CXCL1, CXCL2 (R&D Systems), MPO (Hycult Biotech), lactoferrin, and TF (USCN Life Science Inc.) according to the manufacturer's instructions.
Flow Cytometric Dihydrorhodamine Assay
Heparinized whole blood from mice was loaded with 10 μm dihydrorhodamine (Sigma) for 15 min at 37 °C. After that, the whole blood was stimulated with 1 μg/ml phorbol myristate acetate (Sigma) for 15 min at 37 °C. Red blood cells were lysed with ACK lysing buffer (Invitrogen), and reactive oxygen species (ROS) production was determined by flow cytometry using a FACSCaliburTM (BD Biosciences).
Flow Cytometry Analysis of Bone Marrow Cells
Single-cell suspensions were prepared from mouse bone marrow collected from bilateral femurs. Cells (2 × 106) were incubated at 4 °C for 30 min in PBS with anti-Gr-1 or anti-Ly6G antibody conjugated with phycoerythrin (BD Biosciences). Following antibody labeling, cells were washed with FACS buffer, containing 2% FBS, 0.05% sodium azide, 2 mm EDTA (pH 8.0), and resuspended in 500 μl of FACS buffer. Flow cytometry analysis was performed using a FACSCalibur (BD Biosciences).
NK Cytotoxicity Assay
Spleens were removed from mice, pressed through a 40-μm cell strainer, and resuspended in RPMI 1640 medium with 10% FBS to make a single cell suspension. Mononuclear cells from spleens were prepared using Ficoll-Hypaque gradient centrifugation. YAC-1 cells (NK-sensitive target cells) were labeled by incubation at 37 °C for 1 h with Na251CrO4 (200 μCi). Effector cells (splenocytes) and target cells were added to v-shaped 96-well plates to obtain an effector/target cell ratio of 50:1. After 4 h of incubation, the supernatant was harvested and analyzed on a γ-counter (ARC-370M; Aloka). The % specific cytotoxicity was calculated as follows: % specific cytotoxicity = (cpmexp − cpmspontaneous)/(cpmmaximum − cpmspontaneous) × 100.
Measurement of S1P, Dihydro-S1P, Sphingosine, Dihydrosphingosine, and Ceramide Levels
These sphingolipids were measured by high performance liquid chromatography-tandem mass spectrometry LC-MS by Lipidomics Core at the Medical University of South Carolina on a ThermoFinnigan TSQ Quantum (Thermo Fisher Scientific) triple quadrupole mass spectrometer, operating in a multiple reaction monitoring-positive ionization mode using tissue homogenates corresponding to 1.3–5.8 mg of protein as described (25).
Animal Treatment
Female mice were treated with intraperitoneal injections of N-(2-hydroxy-4-nitrophenyl)-N9-(2-bromophenyl) urea (SB225002; 25 mg/kg) (Cayman Chemicals) twice per day (9:00 and 17:00) on days 5 and 6. Uteri and embryos were inspected on day 7.5 pc. SB225002 was dissolved in 0.9% NaCl solution containing 1% Tween 80 immediately before use, and control mice were treated with this vehicle. In neutrophil depletion experiments, mice were injected on day 4.5 intraperitoneally with 0.1 mg of rat anti-mouse granulocyte RB6–8C5 monoclonal antibody (Pharmingen) that reacts with Gr-1. The mice were then injected intraperitoneally with SB225002 (25 mg/kg) twice a day (9:00 and 17:00) from day 5 to 7 or day 8. Mice were sacrificed on days 8.5, 9.5, 10.5, or 11.5 of pregnancy; uteri were dissected, and fetal resorption rates were calculated. Control mice were intraperitoneally injected with 0.9% NaCl solution. The dose and frequency of SB225002 and RB6–8C5 were determined so as to maximize therapeutic effects and minimize unwanted side effects.
Human Tissues
Decidual specimens from elective terminations or spontaneous abortions of first-trimester pregnancy (7–10 weeks) from healthy patients, aged 25–41 years, were used. The specimens were obtained by vaginal curettage. Written informed consent was obtained from both patients and their spouses, and the study was approved by the Institutional Review Board at Kyoto University and Adachi Hospital (E1275).
Decidual Stromal Cell (DSC) Cultures
Decidual tissues were thoroughly washed in PBS solution and carefully freed from the villous cytotrophoblasts. Decidual fragments were finely minced between two scalpels in a small volume of RPMI 1640 medium with 100 units/ml penicillin and 100 μg/ml streptomycin and digested in 0.2% collagenase type I (WOR) and 0.01% DNase (Roche Applied Science) in a 37 °C shaking water bath for 60 min. The digestate was then subjected to consecutive filtration through 70- and 40-μm Millipore filters. Cells were resuspended in RPMI 1640 medium with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin and incubated in culture dishes for 1 h to allow macrophages and granulocytes to adhere to the dish. The supernatant was resuspended in RPMI 1640 medium and then seeded on culture dishes. Purity was confirmed using immunofluorescent staining to detect the expression of vimentin and the lack of cytokeratin 7 and CD45 by 95–100% of DSCs. After 1–2 passages, the cells were seeded onto 24-well culture plates at an initial density of 2 × 105/ml, grown to near confluence, and treated with 0.01% vehicle (ethanol) or the indicated concentrations (0–10 μm) of d-erythro-N,N-dimethylsphingosine (DMS) (Cayman Chemical) for 48 h. Supernatants were subsequently collected and stored at −30 °C. In additional experiments, confluent decidual cells seeded on 24-well plates were stimulated with 10 nm estrogen (E2) (Sigma) and 1 μm progesterone (P4) (Sigma) for 6 days with one change of medium. Decidual cells maintained under the same culture conditions but without the addition of E2/P4 served as nonstimulated controls. After 6 days, the cells were treated with 0.01% vehicle (ethanol) or indicated concentrations (0–50 μm) of DMS for 48 h, and supernatants were collected.
Human Chemokine and Cytokine Levels
Supernatants from human DSC cultures treated with various amounts of DMS were harvested, and particulates were removed by centrifugation. The chemokine/cytokine levels in cell culture supernatants were determined using ELISA for CXCL1, IL-8, IL-6, TNF-α, IL-1β, or IFN-γ (R&D Systems) according to the manufacturer's instructions.
Statistics
Data are expressed as means ± S.E. Results having p values of <0.05 by the unpaired Student's t test were considered significant.
RESULTS
Increased Expression of CXCL1 and CXCL2 in the Decidua from Sphk1−/−Sphk2+/− Mice
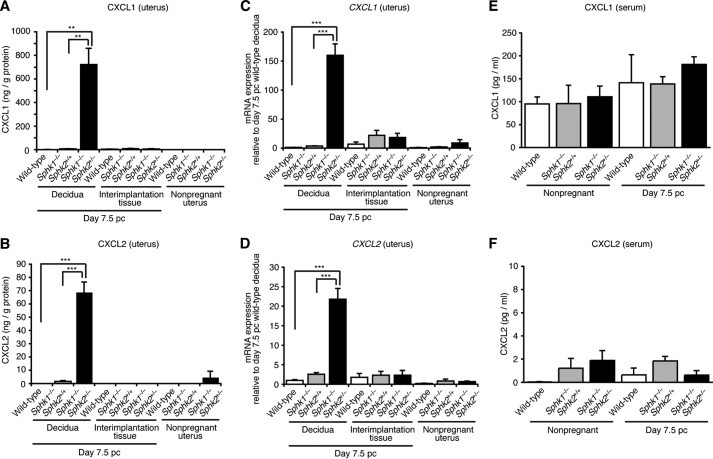
To decipher the mechanism of pregnancy loss in Sphk1−/−Sphk2+/− mice, we performed microarray analysis of day 7.5 pc decidual mRNA from wild-type and Sphk1−/−Sphk2+/− mice using Affymetrix mouse genome GeneChips. The genes for chemokine ligands and their receptors were found to be highly elevated in the decidua of Sphk1−/−Sphk2+/− mice compared with those in the decidua of wild-type mice. We performed chemokine and cytokine arrays using homogenized pregnant day 7.5 pc deciduas and day 7.5 pc interimplantation tissues (uterine tissues lacking implantation sites) from wild-type, Sphk1−/−Sphk2+/+, and Sphk1−/−Sphk2+/− female mice, from which the embryos had been removed. Chemokine arrays, including nine major chemokines, revealed that expression of CXCL1, also known as keratinocyte-derived chemokine, was markedly increased in Sphk1−/−Sphk2+/− deciduas, compared with wild-type or Sphk1−/−Sphk2+/+ deciduas (Fig. 1, A and C). CXCL1 expression was barely detectable in wild-type, Sphk1−/−Sphk2+/+, or Sphk1−/−Sphk2+/− interimplantation tissues (Fig. 1A). CXC chemokines, CXCL1 and CXCL2, also known as macrophage inflammatory protein-2 (MIP-2), are potent chemotactic mediators for neutrophils and are speculated to be functional homologs of human IL-8. Notably, Sphk1−/−Sphk2+/− mice did not display any altered serum or plasma chemokine levels compared with wild-type or Sphk1−/−Sphk2+/+ mice, irrespective of pregnancy, indicative of a local chemokine response (Fig. 1A). Cytokine arrays, including 16 major cytokines, revealed that none of the major cytokines examined were substantially up-regulated in Sphk1−/−Sphk2+/− uteri compared with wild-type or Sphk1−/−Sphk2+/+ uteri, irrespective of whether they included deciduas or interimplantation tissue (Fig. 1, B and D). To verify the results obtained by the semiquantitative chemokine/cytokine arrays, we next performed quantitative ELISAs. Strikingly, CXCL1 expression in pregnant day 7.5 pc Sphk1−/−Sphk2+/− deciduas was increased ∼1000- or 113-fold compared with that in pregnant day 7.5 pc wild-type or Sphk1−/−Sphk2+/+ deciduas, respectively (Fig. 2A). Expression of CXCL2 was also highly elevated in day 7.5 pc Sphk1−/−Sphk2+/− deciduas, in contrast to the negligible levels of expression measured in day 7.5 pc wild-type and Sphk1−/−Sphk2+/+ deciduas (Fig. 2B). Similar results were obtained by quantitative real time PCR analysis, in which the CXCL1 and CXCL2 mRNA levels were much higher in pregnant day 7.5 pc Sphk1−/−Sphk2+/− deciduas than in day 7.5 pc wild-type or Sphk1−/−Sphk2+/+ deciduas (Fig. 2, C and D). Conversely, CXCL1 levels in the circulating serum of pregnant day 7.5 pc Sphk1−/−Sphk2+/− females were not significantly different from those in wild-type or Sphk1−/−Sphk2+/+ females on day 7.5 pc, although an upward trend was observed as pregnancy was established (Fig. 2E). CXCL2 levels in serum were virtually undetectable in any of the mice, irrespective of their genotype (Fig. 2F).
FIGURE 1.
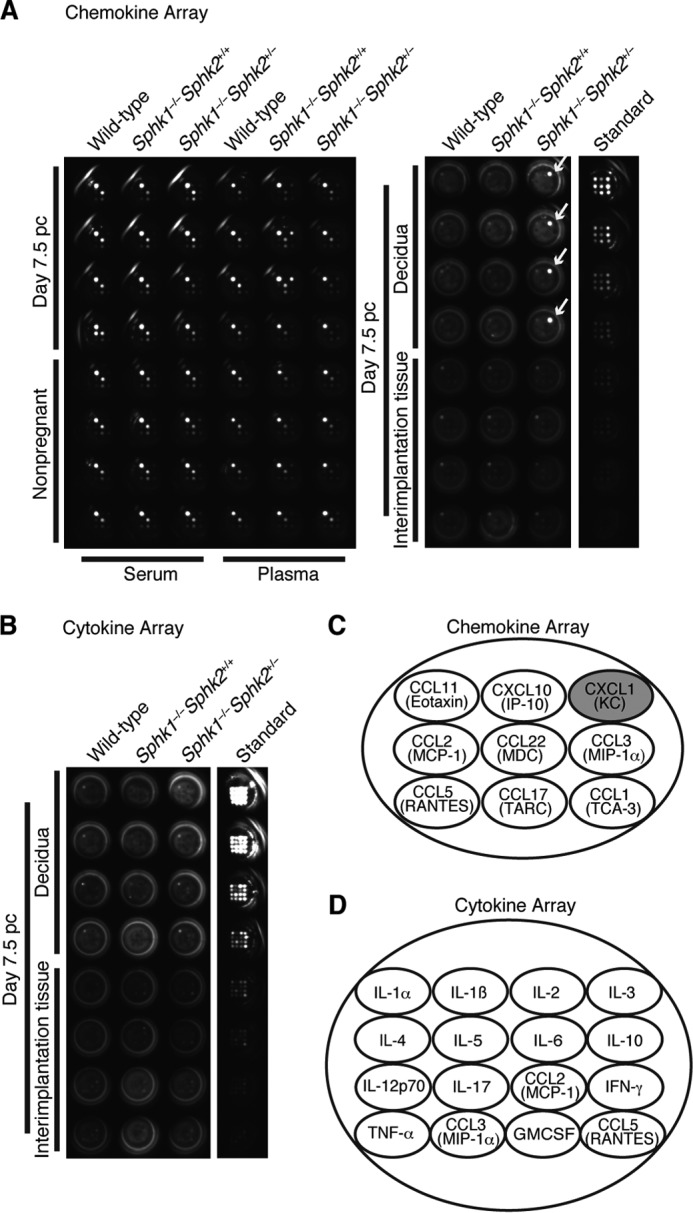
Chemokine and cytokine arrays. A, chemokine array. The samples analyzed were day 7.5 pc deciduas and day 7.5 pc interimplantation tissues from wild-type, Sphk1−/−Sphk2+/+, and Sphk1−/−Sphk2+/− females (n = 4) and serum and plasma samples from wild-type, Sphk1−/−Sphk2+/+, and Sphk1−/−Sphk2+/− females during nonpregnancy and on day 7.5 pc (n = 4). Note the extraordinary increase in the CXCL1 expression in Sphk1−/−Sphk2+/− deciduas on day 7.5 pc (white arrows). B, cytokine array. The samples analyzed were day 7.5 pc deciduas and day 7.5 pc interimplantation tissues from wild-type, Sphk1−/−Sphk2+/+, and Sphk1−/−Sphk2+/− females (n = 4). C, diagram depicts the chemokine location in each well in A. Each well has nine spots. D, diagram depicts the cytokine location in each well in B. Each well has 16 spots.
FIGURE 2.
Profiles of chemokine production. A and B, levels of chemokines, CXCL1 (A) and CXCL2 (B), in nonpregnant whole uteri, day 7.5 pc deciduas, and day 7.5 pc interimplantation tissues from wild-type, Sphk1−/−Sphk2+/+, and Sphk1−/−Sphk2+/− females, as determined by ELISA. The data represent mean values ± S.E. (n = 4, **, p < 0.01, ***, p < 0.001, unpaired t test). C and D, relative mRNA expression of CXCL1 (C) and CXCL2 (D) in nonpregnant whole uteri, pregnant day 7.5 pc deciduas and day 7.5 pc interimplantation tissues from wild-type, Sphk1−/−Sphk2+/+, and Sphk1−/−Sphk2+/− females, as determined by real time PCR. The expression levels are shown relative to those in day 7.5 pc wild-type decidua. The data represent mean values ± S.E. (n = 4, ***, p < 0.001, unpaired t test). E and F, serum levels of chemokines, CXCL1 (E) and CXCL2 (F), in wild-type, Sphk1−/−Sphk2+/+, and Sphk1−/−Sphk2+/− females during nonpregnancy and on day 7.5 pc. The data represent mean values ± S.E. (n = 4). A–F are representative of two independent experiments.
Decidual Cells in the Secondary Decidual Zone Are the Major Source of CXCL1 and CXCL2
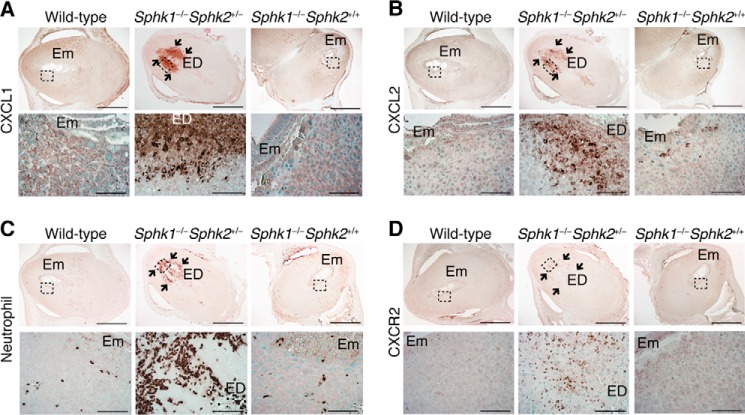
To determine the location of CXCL1 and CXCL2 expression, we performed immunohistochemical analysis on pregnant day 7.5 pc deciduas from wild-type, Sphk1−/−Sphk2+/+, and Sphk1−/−Sphk2+/− females. Both CXCL1 and CXCL2 were highly expressed in the secondary decidual zone immediately surrounding embryos in Sphk1−/−Sphk2+/− females, whereas these chemokines were weakly expressed in the cytoplasm of decidual cells in wild-type and Sphk1−/−Sphk2+/+ females (Fig. 3, A and B). Decidual cells were identified by immunostaining with an anti-desmin antibody (data not shown). The expression patterns of the chemokines observed in Sphk1−/−Sphk2+/− females were same as those of Sphk, previously reported by us (24), suggesting that the strong expression of CXCL1 and CXCL2 in the decidua was induced by Sphk deficiency. It is notable that excessive chemokine expression was undetectable on day 6.5 pc in Sphk1−/−Sphk2+/− deciduas (data not shown).
FIGURE 3.
Increased expression of neutrophil chemoattractants and neutrophil recruitment into the fetomaternal interface in the Sphk1−/−Sphk2+/− decidua. A–D, immunostaining with anti-CXCL1 (A), anti-CXCL2 (B), anti-neutrophil (C), and anti-CXCR2 (D) antibodies on wild-type, Sphk1−/−Sphk2+/−, and Sphk1−/−Sphk2+/+ uteri on day 7.5 pc. In each part of the figure (A–D), the lower panels represent high power views of the boxed areas in corresponding upper panels. Arrows indicate the increased number of cells immunopositive for CXCL1 (A), CXCL2 (B), neutrophil (C), and CXCR2 (D) in the Sphk1−/−Sphk2+/− decidua. Em, embryo; ED, embryonic demise. Scale bars, 1000 μm (upper panels in A–D) and 100 μm (lower panels in A–D). Data are representative of three independent experiments with similar results.
Neutrophil Recruitment into the Fetomaternal Interface in the Decidua from Sphk1−/−Sphk2+/− Mice
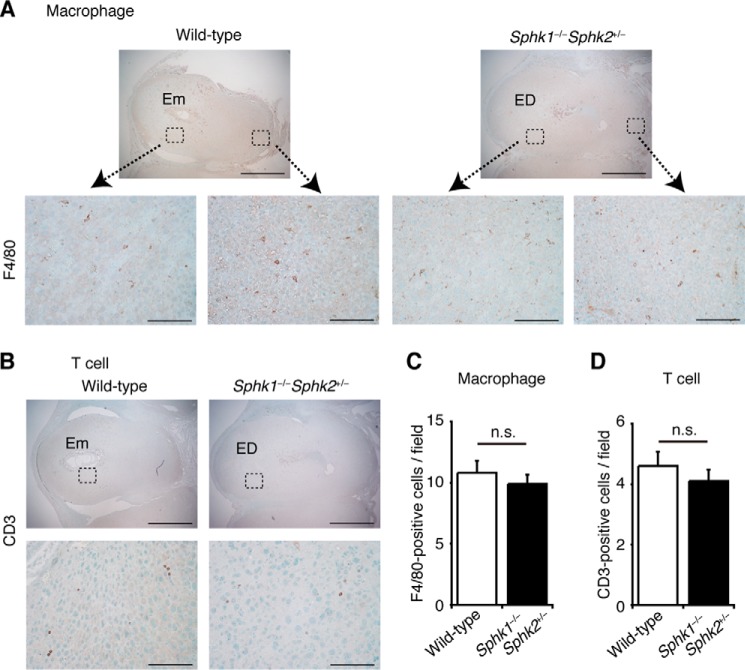
In line with the abundant expression of neutrophil chemoattractants, Sphk1−/−Sphk2+/− uteri exhibited a massive infiltration of neutrophils into the fetomaternal interface, as denoted by immunostaining with an anti-neutrophil antibody (Fig. 3C). The infiltrating neutrophils expressed CXCR2, a G protein-coupled CXC chemokine receptor that binds CXCL1 and CXCL2 in mice (Fig. 3D). In contrast, neutrophils from wild-type and Sphk1−/−Sphk2+/+ females remained inside decidual blood vessels (Fig. 3C). It is also worth noting that the populations of macrophages (Fig. 4, A and C) and T cells (Fig. 4, B and D) recruited to the fetomaternal interface on day 7.5 pc Sphk1−/−Sphk2+/− uteri were not significantly different from those on day 7.5 pc wild-type uteri.
FIGURE 4.
Expression of macrophages and T cells on deciduas. A and B, immunostaining with anti-F4/80 (A) and anti-CD3 (B) antibodies to label macrophages and T cells, respectively, on wild-type and Sphk1−/−Sphk2+/− uteri on day 7.5 pc. In each part of the figure (A and B), lower panels represent high power views of the boxed areas in corresponding upper panels. Em, embryo; ED, embryonic demise. Scale bars, 1000 μm (upper panels in A and B) and 100 μm (lower panels in A and B). C and D, number of F4/80- (C) or CD3-positive cells (D) per field (n = 10, n.s., not significant, unpaired t test).
Enhanced Oxidative Damage in the Decidua from Sphk1−/−Sphk2+/− Mice
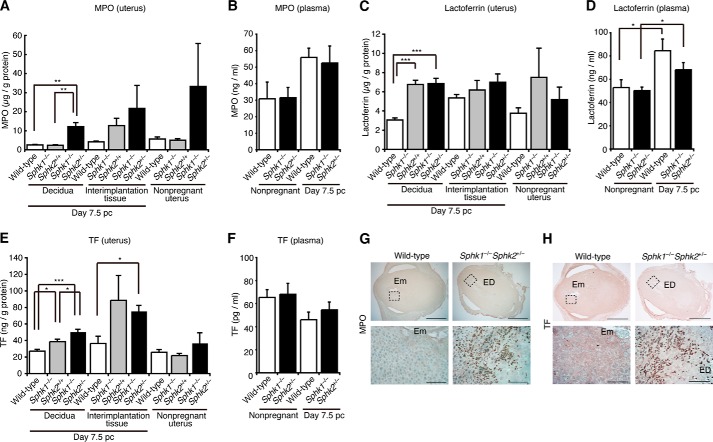
To determine whether neutrophils are activated in the decidua from Sphk1−/−Sphk2+/− females, we measured MPO levels. MPO is a key enzyme in the ROS production, catalyzing the production of hypochlorous acid from hydrogen peroxide and chloride ion during the neutrophil's respiratory burst. ELISA revealed that MPO levels were ∼5-fold higher in pregnant day 7.5 pc Sphk1−/−Sphk2+/− deciduas than in day 7.5 pc wild-type or Sphk1−/−Sphk2+/+ deciduas, suggesting that neutrophils were activated in the Sphk1−/−Sphk2+/− deciduas (Fig. 5A). An upward trend of MPO levels was also observed in pregnant day 7.5 pc interimplantation tissues or nonpregnant uteri from Sphk1−/−Sphk2+/− mice compared with those from wild-type mice (Fig. 5A). MPO was detected in the neutrophils of Sphk1−/−Sphk2+/− deciduas by immunostaining (Fig. 5G). Lactoferrin, another marker for neutrophil activation, is released from secondary granules of neutrophils upon inflammation. Lactoferrin levels were increased 2.2-fold in pregnant day 7.5 pc Sphk1−/−Sphk2+/− deciduas compared with those in pregnant day 7.5 pc wild-type deciduas, although a similar increase was observed in pregnant day 7.5 pc Sphk1−/−Sphk2+/+ deciduas (Fig. 5C). TF is the primary cellular initiator of the blood coagulation cascade, but it also plays an important role in inflammation (26). Thus, we examined the TF expression in the decidua. The TF levels in day 7.5 pc Sphk1−/−Sphk2+/− deciduas were significantly higher than those in day 7.5 pc wild-type or Sphk1−/−Sphk2+/+ deciduas, as denoted by ELISA (Fig. 5E). Immunostaining using an anti-TF antibody revealed that activated neutrophils expressed TF within the Sphk1−/−Sphk2+/− deciduas (Fig. 5H). There were no significant differences in circulating plasma MPO, lactoferrin, or TF levels between wild-type and Sphk1−/−Sphk2+/− mice, whether they were pregnant or not (Fig. 5, B, D, and F). Taken together, these results suggest that Sphk deficiency predisposes mice to pregnancy loss by rendering the fetomaternal interface vulnerable to oxidative damage.
FIGURE 5.
Neutrophil activation in the decidua from Sphk1−/−Sphk2+/− mice. A, C, and E, levels of MPO (A), lactoferrin (C), and TF (E) in nonpregnant whole uteri, day 7.5 pc deciduas, and day 7.5 pc interimplantation tissues from wild-type, Sphk1−/−Sphk2+/+, and Sphk1−/−Sphk2+/− females, as determined by ELISA. The data represent mean values ± S.E. (n = 4, *, p < 0.05, **, p < 0.01, ***, p < 0.001, unpaired t test). B, D, and F, plasma MPO (B), lactoferrin (D), and TF (F) levels in wild-type and Sphk1−/−Sphk2+/− females during nonpregnancy and on day 7.5 pc. The data represent mean values ± S.E. (n = 4, *, p < 0.05, unpaired t test). G and H, immunostaining with anti-MPO (G) and anti-TF (H) antibodies on wild-type and Sphk1−/−Sphk2+/− uteri on day 7.5 pc. Lower panels represent high power views of the boxed areas in corresponding upper panels. Em, embryo; ED, embryonic demise. Scale bars, 1000 μm (upper panels) and 100 μm (lower panels). A–F are representative of two and G and H of three independent experiments.
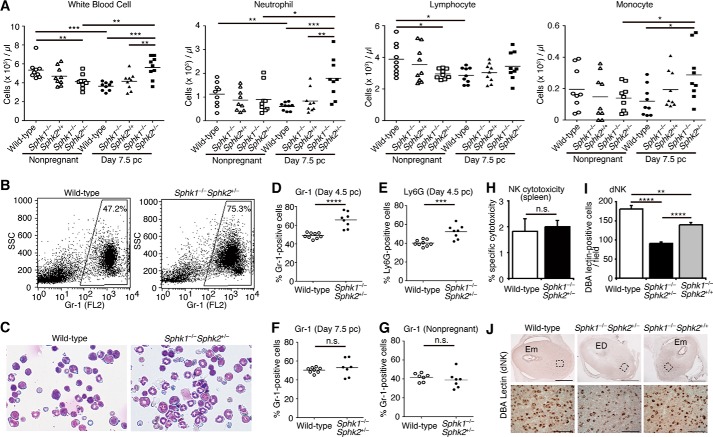
Neutrophilia and Enhanced Generation of Granulocytes in Pregnant Sphk1−/−Sphk2+/− Mice
We determined the number of blood cells in the peripheral blood. Compared with day 7.5 pc wild-type or Sphk1−/−Sphk2+/+ females, white blood cell and neutrophil levels were elevated in day 7.5 pc Sphk1−/−Sphk2+/− females (Fig. 6A). Notably, these levels were significantly higher in day 7.5 pc Sphk1−/−Sphk2+/− females than those detected in nonpregnant Sphk1−/−Sphk2+/− females, while those were lower in day 7.5 pc wild-type females than those in nonpregnant wild-type females, suggesting that neutrophil numbers may be strictly controlled during normal pregnancy. There was no significant difference in the number of lymphocytes between the three types of mice examined on day 7.5 pc (Fig. 6A). The number of monocytes was slightly increased in day 7.5 pc Sphk1−/−Sphk2+/− females compared with day 7.5 pc wild-type females (Fig. 6A). We next examined ROS production of neutrophils in the peripheral blood by using a fluorescent dye, dihydrorhodamine 123, in a flow cytometric assay. ROS production in neutrophils from day 7.5 pc Sphk1−/−Sphk2+/− females was not significantly different from that in day 7.5 pc wild-type neutrophils, regardless of whether they were stimulated in vitro with phorbol myristate acetate or not (data not shown). We next examined whether granulocyte differentiation and maturation in bone marrow were affected in pregnant Sphk1−/−Sphk2+/− mice using flow cytometry with anti-Gr-1 and anti-Ly6G antibodies. The proportion of Gr-1- (Fig. 6, B and D) and Ly6G-positive cells (Fig. 6E) in the bone marrow from day 4.5 pc Sphk1−/−Sphk2+/− mice was significantly increased compared with that in day 4.5 pc wild-type mice, although no difference in Gr-1 expression was detected between wild-type and Sphk1−/−Sphk2+/− females on day 7.5 pc (Fig. 6F) and during nonpregnancy (Fig. 6G). To verify these findings, we also performed cytospin analysis of bone marrow cells. Consistent with the flow cytometry data, a trend of increased band and segmented neutrophils in the bone marrow of day 4.5 pc Sphk1−/−Sphk2+/− mice was observed (Fig. 6C). On day 4.5 pc, Sphk1−/−Sphk2+/− mice had not yet displayed neutrophilia in the peripheral blood (data not shown). These results suggest the enhanced generation of granulocytes in day 4.5 pc Sphk1−/−Sphk2+/− bone marrow.
FIGURE 6.
Analysis of leukocytes. A, number of white blood cells, neutrophils, lymphocytes, and monocytes in the peripheral blood of wild-type, Sphk1−/−Sphk2+/+, and Sphk1−/−Sphk2+/− females during nonpregnancy and on day 7.5 pc (n = 9, *, p < 0.05; **, p < 0.01; ***, p < 0.001, unpaired t test). B, representative flow cytometry data showing Gr-1 expression in the bone marrow from day 4.5 pc wild-type and Sphk1−/−Sphk2+/− females. C, representative micrographs of bone marrow cytospins from day 4.5 pc wild-type and Sphk1−/−Sphk2+/− females. Data are representative of three independent experiments. D and E, percentage of Gr-1-positive cells (D) or Ly6G-positive cells (E) in the bone marrow from day 4.5 pc wild-type (n = 9) and Sphk1−/−Sphk2+/− females (n = 8) (***, p < 0.001; ****, p < 0.0001, unpaired t test). F, percentage of Gr-1-positive cells in the bone marrow from day 7.5 pc wild-type (n = 8) and Sphk1−/−Sphk2+/− females (n = 7) (n.s., not significant, unpaired t test). G, percentage of Gr-1-positive cells in the bone marrow from nonpregnant wild-type and Sphk1−/−Sphk2+/− females (n = 7, n.s., not significant, unpaired t test). H, 51Cr release assay for splenic NK cytotoxicity against YAC-1 tumor cells in day 7.5 pc wild-type and Sphk1−/−Sphk2+/− females. The % cytotoxicity is shown on the y axis. The data represent mean values ± S.E. (n = 4, n.s., not significant, unpaired t test). I, number of DBA lectin-positive cells per field (n = 10, **, p < 0.01; ****, p < 0.0001, unpaired t test). J, immunostaining with anti-DBA lectin antibody to label dNK cells on wild-type, Sphk1−/−Sphk2+/−, and Sphk1−/−Sphk2+/+ uteri on day 7.5 pc. Lower panels represent high power views of the boxed areas in corresponding upper panels. Em, embryo; ED, embryonic demise. Scale bars, 1000 μm (upper panels) and 100 μm (lower panels).
Decreased Number of dNK Cells in Pregnant Sphk1−/−Sphk2+/−Mice
Previous studies reported that elevated levels of NK cell cytotoxicity were associated with increased rates of spontaneous abortion in humans (27, 28). Thus, we examined the cytotoxicity of splenic NK cells using a 51Cr release assay. We observed no significant differences in NK cytotoxicity between day 7.5 pc Sphk1−/−Sphk2+/− and wild-type females (Fig. 6H). We next examined the number of dNK cells by anti-DBA immunostaining, because the dNK cells differ from their peripheral counterpart in phenotype and function. The DBA stains specifically dNK cells in mice but not peripheral NK cells (29). The number of dNK cells, which abundantly accumulate on the mesometrial side of the decidua, was reduced in day 7.5 pc Sphk1−/−Sphk2+/− decidua compared with that in day 7.5 pc wild-type or Sphk1−/−Sphk2+/+ decidua (Fig. 6, I and J).
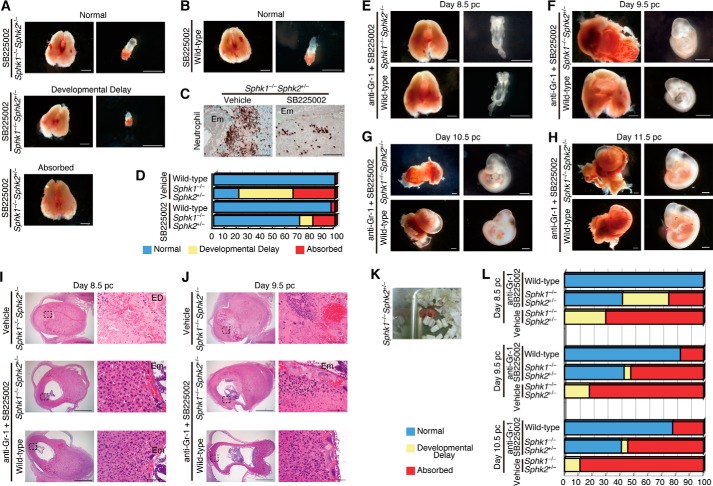
CXCR2 Antagonist Ameliorates Detrimental Inflammatory Responses in the Decidua from Pregnant Sphk1−/−Sphk2+/− Females
In pregnant Sphk1−/−Sphk2+/− mice, migration of neutrophils toward the highest concentration of chemoattractants is a critical cellular effector of fetal damage. To examine the importance of neutrophilic chemoattractants in sphingolipid-mediated pregnancy loss, we treated mice with a selective and competitive CXCR2 antagonist, SB225002, twice a day (9:00 and 17:00) on days 5 and 6 pc and evaluated pregnant uteri and embryos on day 7.5 pc. Developmental analysis revealed that 71% of embryos examined were normal in SB225002-treated Sphk1−/−Sphk2+/− uteri, whereas 18 and 11% of embryos in SB225002-treated Sphk1−/−Sphk2+/− uteri were absorbed and considerably smaller than those found in wild-type uteri, respectively, at this stage (Fig. 7, A and D). In sharp contrast, only 21% of embryos examined were normal in vehicle-treated Sphk1−/−Sphk2+/− uteri (Fig. 7D). Embryos in SB225002-treated wild-type uteri did not significantly differ from those in vehicle-treated wild-type uteri, suggesting that the SB225002 treatment had no adverse effects on embryonic development (Fig. 7, B and D). Moreover, these macroscopic observations were consistent with immunohistochemical findings where neutrophil influx into the fetomaternal interface was remarkably reduced in SB225002-treated Sphk1−/−Sphk2+/− uteri compared with vehicle-treated Sphk1−/−Sphk2+/− uteri (Fig. 7C). Interestingly, the expression of CXCL1 and CXCL2 was reduced in the area surrounding embryos from SB225002-treated Sphk1−/−Sphk2+/− uteri compared with vehicle-treated Sphk1−/−Sphk2+/− uteri and is probably attributable to the diminution of neutrophil infiltration (data not shown). These results were substantiated by ELISA for CXCL1 and CXCL2 (data not shown). Nevertheless, very few embryos in SB225002-treated Sphk1−/−Sphk2+/− uteri survived beyond day 8.5 pc, suggesting that SB225002 treatment alone is not sufficient to prevent pregnancy loss.
FIGURE 7.
Depletion of neutrophils protects against pregnancy loss in Sphk1−/−Sphk2+/− females. A and B, photographs of whole uteri (including embryos) (left panels) and embryos (right panels) from day 7.5 pc Sphk1−/−Sphk2+/− (A) or wild-type (B) female mice. Mice were treated with a CXCR2 antagonist, SB225002. Scale bars, 1 mm. C, immunostaining with anti-neutrophil antibody in day 7.5 pc Sphk1−/−Sphk2+/− uteri treated with SB225002 or vehicle. Scale bars, 100 μm. Em, embryo. Data are representative of three independent experiments. D, summary of the embryonic phenotype from day 7.5 pc (wild-type with SB225002 (n = 32), wild-type with vehicle (n = 6), Sphk1−/−Sphk2+/− with SB225002 (n = 55), Sphk1−/−Sphk2+/− with vehicle (n = 29)) uteri. Embryos were counted according to the following classification: normal, developmental delay (the length of long axis, <1.0 mm), or absorbed, and the percentages were calculated for inclusion in the figure. E--H, photographs of whole uteri (including embryos) (left panels) and embryos (right panels) on day 8.5 pc (E), day 9.5 pc (F), day 10.5 pc (G), and day 11.5 pc (H). Mice were treated with anti-Gr-1 neutralizing antibody and SB225002. Scale bars, 1 mm. I and J, H&E staining of longitudinal sections from wild-type and Sphk1−/−Sphk2+/− uteri on day 8.5 pc (I) and day 9.5 pc (J). Wild-type mice were treated with anti-Gr-1 antibody and SB225002. Sphk1−/−Sphk2+/− mice were treated with both anti-Gr-1 antibody and SB225002 or vehicle. Right panels represent high power views of the boxed areas in corresponding left panels. Scale bars represent 1 mm (left panels) and 100 μm (right panels). Em, embryo; ED, embryonic demise. Data are representative of three independent experiments. K, photograph of a neonate from Sphk1−/−Sphk2+/− female mice. L, summary of the embryonic phenotype from day 8.5 pc uteri (wild-type with anti-Gr-1 and SB225002 (n = 5), Sphk1−/−Sphk2+/− with anti-Gr-1 and SB225002 (n = 24), and Sphk1−/−Sphk2+/− with vehicle (n = 27)), day 9.5 pc (wild-type with anti-Gr-1 and SB225002 (n = 6), Sphk1−/−Sphk2+/− with anti-Gr-1 and SB225002 (n = 21), and Sphk1−/−Sphk2+/− with vehicle (n = 28)), and day 10.5 pc (wild-type with anti-Gr-1 and SB225002 (n = 9), Sphk1−/−Sphk2+/− with anti-Gr-1 and SB225002 (n = 22), and Sphk1−/−Sphk2+/− with vehicle (n = 18)). Embryos were counted according to the following classification: normal, developmental delay (the length of long axis: <2.0 mm (day 8.5 pc), <2.5 mm (day 9.5 pc), and <3.5 mm (day 10.5 pc)), or absorbed, and the percentages calculated for inclusion in the figure.
Depletion of Neutrophils by Co-administration of Anti-neutrophil Neutralizing Antibody and CXCR2 Antagonist Protects Sphk1−/−Sphk2+/− Female Mice against Pregnancy Loss
In this study, we found that Sphk1−/−Sphk2+/− mice showed enhanced generation of granulocytes in the bone marrow on day 4.5 pc and subsequent neutrophilia on day 7.5 pc. Thus, we administered both anti-neutrophil neutralizing antibody and CXCR2 antagonist to pregnant mice to alleviate the neutrophil infiltration. We treated mice with anti-Gr-1 RB6–8C5 antibody on day 4.5, followed by SB225002 administration twice per day (9:00 and 17:00) on day 5–7 pc or day 8 pc, and we evaluated pregnant uteri and embryos after day 8.5 pc. Strikingly, ∼40% of embryos in Sphk1−/−Sphk2+/− uteri treated with both inhibitory reagents were found alive with normal morphology on days 8.5, 9.5, 10.5, or day 11.5 pc (Fig. 7, E–H and L), despite the fact that decidual tissues were moderately impaired and exhibited increased decidual cell death by histological examination (Fig. 7, I and J). In contrast, most embryos in vehicle-treated Sphk1−/−Sphk2+/− uteri were found absorbed at all stages examined (Fig. 7, I, J, and L). Of note, several Sphk1−/−Sphk2+/− females treated with both inhibitory reagents successfully delivered pups on day 19, although they appeared to be relatively premature (Fig. 7K). Embryos in wild-type uteri were not adversely affected by treatment with both inhibitory reagents (Fig. 7, E–H and L).
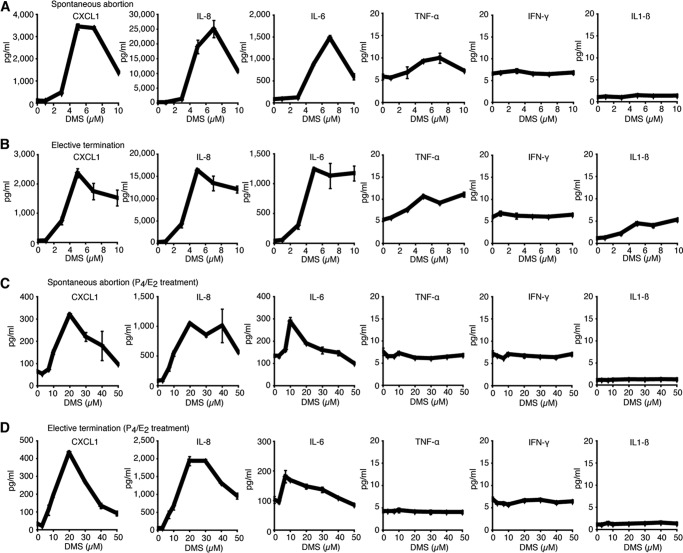
Increased Secretion of Neutrophil Chemoattractants from Human DSCs Deficient in Sphk
To examine the effects of Sphk on the expression of inflammatory cytokines/chemokines during human pregnancy, primary cultures of human first-trimester DSCs were treated with increasing concentrations (0–10 μm) of a competitive inhibitor for Sphk, DMS, for 48 h. DMS is a potent competitive inhibitor of Sphk that suppresses activity of both Sphk1 and Sphk2 (30, 31). Following DMS treatment, cell culture supernatants were collected, and cytokine/chemokine levels were determined by ELISA. Purity of DSCs was verified by immunofluorescence using antibodies against vimentin, cytokeratin 7, and CD45, detecting stromal cells, epithelial cells, and leukocytes, respectively (data not shown). We analyzed 12 cases with either elective terminations (three cases) or spontaneous abortions (nine cases) of first-trimester pregnancy. All cases examined exhibited a similar outcome with DMS treatment dramatically increasing the secretion of neutrophil chemoattractants, CXCL1 and IL-8, in a dose-dependent manner (Fig. 8, A and B). A maximal response was achieved with 5–10 μm DMS after 48 h of treatment, although peak values varied depending on the individual cases. Representative results are shown in Fig. 8. Intriguingly, the levels of major proinflammatory cytokines, TNF-α, IFN-γ, and IL-1β, were very low and increased slightly with DMS treatment (Fig. 8, A and B). Conversely, DMS treatment caused a remarkable elevation in IL-6 secretion (Fig. 8, A and B).
FIGURE 8.
Profiles of chemokine and cytokine production by Sphk-deficient human DSCs. A–D, human DSCs isolated from patients with spontaneous abortions (A and C) or elective terminations (B and D) were treated with increasing concentrations (0–10 μm in (A and B) and 0–50 μm in (C and D)) of a Sphk inhibitor, DMS, for 48 h. Culture supernatants were subjected to ELISAs for CXCL1, IL-8, IL-6, TNF-α, IFN-γ, and IL-1β in duplicate. Dose-response curves in representative cases are shown. C and D, human DSCs were primed with 10 nm estrogen (E2) and 1 μm progesterone (P4) for 6 days before DMS treatment. A is representative of nine, B of three, and C and D of three individual cases with similar results.
We primed human DSCs with 10 nm E2 and 1 μm P4 for 6 days to mimic the hormonal milieu of pregnancy, followed by increasing concentrations (0–50 μm) of DMS for 48 h. In contrast to naive DSCs, the addition of less than 10 μm DMS induced only slight chemokine/cytokine secretion from P4/E2-treated DSCs (Fig. 8, C and D). However, application of >10 μm DMS induced larger amounts of CXCL1, IL-8, and IL-6 secretion, although peak values were much lower than those in naive DSCs (Fig. 8, C and D). In sharp contrast, the secretion of proinflammatory cytokines, TNF-α, IFN-γ, and IL-1β, was not increased above the baseline level in P4/E2-treated DSCs, even with high concentrations of DMS (Fig. 8, C and D). These results re-highlight the ability of Sphk-deficient DSCs to secrete increased amounts of CXCL1 and IL-8. In addition, steroid hormones may protect Sphk-deficient DSCs by inhibiting chemokine/cytokine release.
Sphk Deficiency Does Not Interfere with the Reproductive Success of Syngeneic Matings
Thus far, all mutant and control mice used in this study are on a 129Sv/C57BL/6 mixed background. It is known that, in this setting, each animal will have a different mixture of the two original backgrounds (32), suggesting that our experimental design is similar to an allogeneic mouse model of pregnancy (33, 34). Notably, C57BL/6 Sphk1−/−Sphk2+/− females mated to C57BL/6 males (syngeneic) did not show any reproductive failure (Fig. 9B). In sharp contrast, complete fetal loss was observed in Sphk1−/−Sphk2+/− females on a 129Sv/C57BL/6 mixed background mated to males on 129Sv/C57BL/6 mixed background (Fig. 9A). This indicates that the defect of Sphk does not preclude the reproductive success of syngeneic matings.
FIGURE 9.
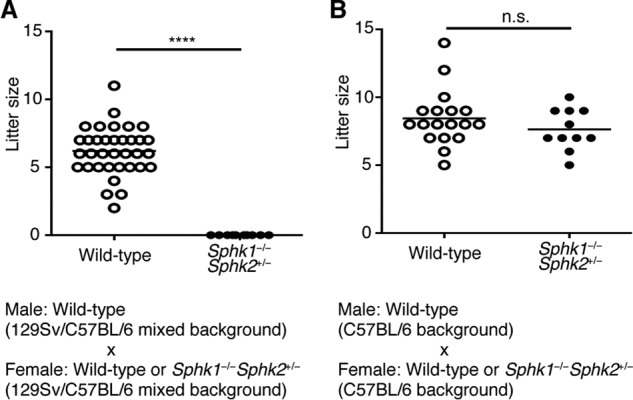
Effect of Sphk deficiency on litter size. Number of pups at birth is shown as a scatter dot plot. A, wild-type (n = 35) or Sphk1−/−Sphk2+/− (n = 10) females on 129Sv/C57BL/6 mixed background were mated to wild-type males on 129Sv/C57BL/6 mixed background (****, p < 0.0001, unpaired t test). B, wild-type (n = 18) or Sphk1−/−Sphk2+/− (n = 11) females on C57BL/6 background were mated to wild-type males on C57BL/6 background (n.s., not significant, unpaired t test).
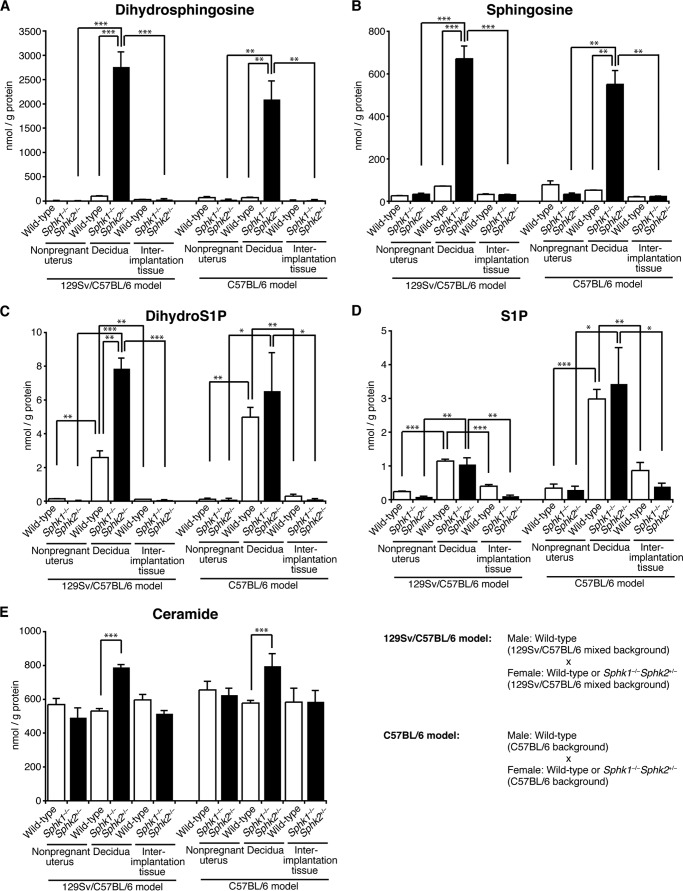
Sphk1−/−Sphk2+/− Deciduas in the C57BL/6 Mating Model Exhibit Similar Sphingolipid Profiles to Those of Sphk1−/−Sphk2+/− Deciduas in the 129Sv/C57BL/6 Mating Model
—Sphingolipid levels were measured by mass spectrometry in homogenates of nonpregnant whole uteri, pregnant day 7.5 pc deciduas, and day 7.5 pc interimplantation tissues from wild-type and Sphk1−/−Sphk2+/− females, either in the 129Sv/C57BL/6 mating model (females on 129Sv/C57BL/6 mixed background mated to wild-type males on 129Sv/C57BL/6 mixed background) or C57BL/6 mating model (C57BL/6 females mated to wild-type C57BL/6 males). As reported previously by us (24), both dihydrosphingosine (sphinganine) and sphingosine levels were remarkably increased in day 7.5 pc Sphk1−/−Sphk2+/− deciduas as compared with those in day 7.5 pc wild-type deciduas in the 129Sv/C57BL/6 mating model (Fig. 10, A and B). Intriguingly, such remarkable increases were similarly observed in day 7.5 pc Sphk1−/−Sphk2+/− deciduas without reproductive failure in the C57BL/6 mating model (Fig. 10, A and B). The aberrant accumulation of dihydrosphingosine and sphingosine was detected only in Sphk1−/−Sphk2+/− deciduas, and not in nonpregnant Sphk1−/−Sphk2+/− uteri or Sphk1−/−Sphk2+/− interimplantation tissues (Fig. 10, A and B). In day 7.5 pc Sphk1−/−Sphk2+/− deciduas, dihydro-S1P levels were slightly higher than in day 7.5 pc wild-type deciduas in the 129Sv/C57BL/6 mating model but not in the C57BL/6 mating model (Fig. 10C). S1P levels in Sphk1−/−Sphk2+/− deciduas were not significantly different from those in wild-type diciduas, either in the 129Sv/C57BL/6 mating model or C57BL/6 mating model (Fig. 10D). Both dihydro-S1P and S1P levels in Sphk1−/−Sphk2+/− deciduas were higher than those in nonpregnant Sphk1−/−Sphk2+/− uteri or Sphk1−/−Sphk2+/− interimplantation tissues (Fig. 10, C and D). Such increases were also observed in wild-type deciduas compared with nonpregnant wild-type uteri or wild-type interimplantation tissues (Fig. 10, C and D). Ceramide levels in Sphk1−/−Sphk2+/− deciduas were slightly higher than those in wild-type deciduas, either in the 129Sv/C57BL/6 mating model or C57BL/6 mating model (Fig. 10E). As a summary, Sphk1−/−Sphk2+/− deciduas in the C57BL/6 mating model exhibited similar sphingolipid profiles to those of Sphk1−/−Sphk2+/− deciduas in the 129Sv/C57BL/6 mating model. It is worthy of note that dihydrosphingosine and sphingosine were abnormally accumulated in Sphk1−/−Sphk2+/− deciduas in the C57BL/6 mating model, regardless of normal fertility. Taken together, these results suggest that the aberrant accumulation of sphingoid bases may not account for the early fetal loss observed in Sphk1−/−Sphk2+/− female mice in the 129Sv/C57BL/6 mating model.
FIGURE 10.
Measurement of sphingolipid levels. Dihydrosphingosine (A), sphingosine (B), dihydro-S1P (C), S1P (D), and ceramide (E) levels were determined in nonpregnant whole uteri (wild-type and Sphk1−/−Sphk2+/−), day 7.5 pc deciduas (wild-type and Sphk1−/−Sphk2+/−), and day 7.5 pc interimplantation tissues (wild-type and Sphk1−/−Sphk2+/−) either in the 129Sv/C57BL/6 mating model (females on 129Sv/C57BL/6 mixed background mated to wild-type males on 129Sv/C57BL/6 mixed background) or C57BL/6 mating model (C57BL/6 females mated to wild-type C57BL/6 males) by mass spectrometry. The data represent mean values ± S.E. (n = 3, *, p < 0.05; **, p < 0.01; ***, p < 0.001, unpaired t test).
DISCUSSION
In this study, we discovered that the disturbance in the sphingolipid pathway by disruption of sphingosine kinase genes during pregnancy (Fig. 11A), as depicted in Sphk1−/−Sphk2+/− female mice, caused the unusually high expression of neutrophil chemoattractants, CXCL1 and CXCL2, in the decidual region immediately surrounding the embryos. Overexpression of CXCL1 and CXCL2 induced a massive infiltration of neutrophils into the fetomaternal interface, with an enhanced respiratory burst in the decidua, resulting in fetal death by rendering the fetomaternal interface vulnerable to oxidative damage (Fig. 11, B and C). The Sphk deficiency also caused the decrease in the number of decidual NK cells (Fig. 11, B and C). The blockage of neutrophil influx by the in vivo use of CXCR2 antagonist and an anti-neutrophil neutralizing antibody restored normal embryonic development in pregnant Sphk1−/−Sphk2+/− female mice. A similar result was obtained in primary human decidual cell cultures in which the Sphk deficiency dramatically increased the secretion of neutrophil chemoattractants, such as CXCL1 and IL-8, from decidual cells. These results suggest that neutrophil-mediated tissue damage plays a key role in the pathogenesis of pregnancy loss in Sphk1−/−Sphk2+/− female mice.
FIGURE 11.
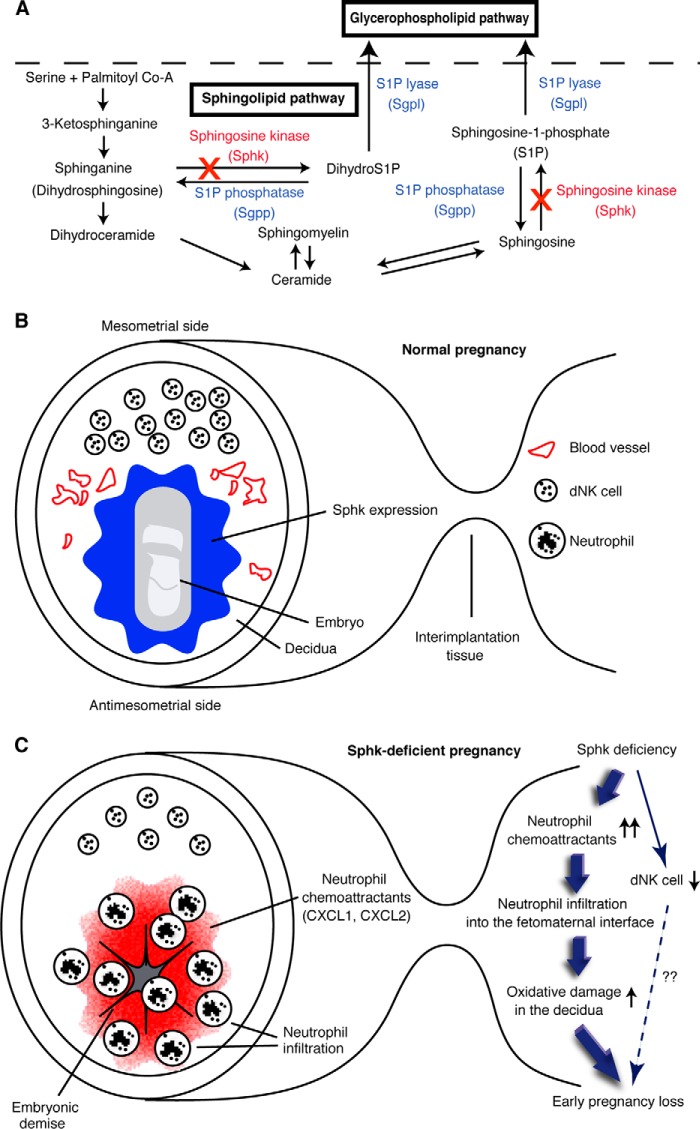
Model of the role of sphingolipid metabolism in fetomaternal tolerance. A, sphingolipid biosynthetic pathway. × indicates genetic disruption of sphingosine kinase genes. B and C, schematic diagrams depicting cross-sections of implantation sites on day 7 of normal pregnancy (B) or Sphk-deficient pregnancy (C) in mice. B, during normal pregnancy, sphingolipid signaling pathway is highly activated, and Sphk is abundantly expressed at the fetomaternal interface to protect the semiallogeneic fetus from assault by the maternal immune system. C, with the loss of Sphk activity during pregnancy, the innate arm of maternal immune system recognizes the fetus as a potential target for immunological attack, resulting in pregnancy loss.
In our previous report, we described Sphk1−/−Sphk2+/− female mice as exhibiting excessive accumulation of sphingoid base in the decidua, followed by severe defects in decidual cells and decidual blood vessels, leading to maternally derived early pregnancy loss. Defective vascular endothelial cells were probably the cause of hemorrhage and subsequent passive extravasation of neutrophils into the decidua. Under these circumstances, we presumed that sphingoid base accumulation in decidual cells and blood vessels would be a primary determinant of pregnancy loss because the sphingoid base is proapoptotic and cytotoxic. However, it was unclear why Sphk1−/−Sphk2+/+ female mice exhibited apparently normal fertility, despite the substantial sphingoid base accumulation in their uteri at a nearly comparable level to that in Sphk1−/−Sphk2+/− female mice. In this study, we found that Sphk1−/−Sphk2+/+ deciduas as well as wild-type deciduas revealed neither abnormal expression of neutrophil chemoattractants nor neutrophil infiltration into the fetomaternal interface. This was in sharp contrast to the severe decidual tissue injury caused by neutrophil infiltration and activation in Sphk1−/−Sphk2+/− deciduas. Given the findings in this study, we speculate that pregnancy loss observed in Sphk1−/−Sphk2+/− female mice is primarily due to abrogated fetomaternal tolerance, leading to tissue injury by activated neutrophils rather than sphingoid base accumulation. This idea may be further substantiated by our finding that Sphk1−/−Sphk2+/− female mice in the C57BL/6 mating model exhibit an abnormal accumulation of sphingoid bases, irrespective of the absence of reproductive failure. Taken together, sphingolipid signaling pathway may contribute to immunological tolerance of a genetically incompatible fetus by controlling innate immune responses at the fetomaternal interface.
Activated neutrophils have been implicated in pregnancy loss in patients with anti-phospholipid syndrome (APS), which is clinically characterized by systemic thrombosis and recurrent pregnancy loss due to the presence of antiphospholipid antibodies. In a murine model, passive transfer of human IgG isolated from antiphospholipid antibody-positive sera from women with APS revealed that complement component C5a induced TF expression in neutrophils, contributing to respiratory burst, trophoblast injury, and pregnancy loss (35, 36). Moreover, statins, simvastatin and pravastatin, prevented APS-induced pregnancy loss in mice by down-regulating TF expression (37). At present, inflammatory processes, particularly through neutrophil activation, are considered more important in the pathogenesis of APS than thrombophilia. The identification of novel inflammatory mediators involved in APS would provide a new target for therapy to prevent pregnancy loss in patients with APS, as an alternative to the current application of anticoagulants throughout pregnancy.
In this study, we also found that the Sphk deficiency caused the decrease in the number of dNK cells in mice. It is now known that dNK cells secrete cytokines and growth factors that contribute to successful pregnancy outcome and a reduction in dNK cell number is associated with preeclampsia and intrauterine growth restriction in humans (38, 39). In contrast, mice lacking dNK cells, Il15−/− mice, are fertile, suggesting that dNK cells are not required for successful pregnancy in mice (40). Notably, a recent study reveals the importance of the S1P pathway in the regulation of dNK cell physiology during normal pregnancy in humans (41). The authors report that S1P receptor 5 (S1PR5), rather than S1PR1, expressed by dNK cells plays a critical role in the regulation of trophoblast migration and endothelial angiogenesis in humans (41). The significance of the decreased number of dNK cells in the early pregnancy loss observed in pregnant Sphk1−/−Sphk2+/− mice awaits further investigations.
Spontaneous pregnancy loss in humans is a common complication in ∼15% of clinically recognized pregnancies. Recurrent pregnancy loss (RPL), defined as three or more consecutive abortions prior to 20 weeks gestation, affects 1–5% of women of childbearing age. Although the etiology consists of fetal chromosomal anomalies, maternal endocrine defects, uterine abnormalities, infections, and autoimmune disorders such as APS described above, the pathogenesis is unknown despite an extensive evaluation of >50% RPL cases (42–44). An exaggerated maternal immune response, involving the effect of cytokines on fetal antigens, has been proposed to be one of the mechanisms underlying idiopathic RPL. The orchestration of the cytokine cascade is an important determinant of successful pregnancy, and dysregulation of the cytokine network in the decidua is a possible cause of pregnancy failure (45). Thus, the decidua is a locus of extensive regulatory control critical to normal pregnancy. Cytokines are produced by decidual stromal cells as well as trophoblastic cells and lymphomyeloid cells in the decidua, which include T lymphocytes, macrophages, and natural killer cells. A favorable pregnancy outcome relies on a discrete balance between proinflammatory Th1-type cytokines (e.g. TNF-α, IFN-γ, IL-1, and IL-2) and anti-inflammatory Th2-type cytokines (e.g. IL-4, IL-5, IL-10, and IL-12). Therefore, overstimulation of Th1 or Th2 immunity may be harmful for successful pregnancy (45). Indeed, TNF-α and IFN-γ have been reported to inhibit fetal development (46, 47). In addition, circulating levels of TNF-α and IFN-γ were higher in patients with a subsequent abortion compared with those with normal pregnancy (48, 49). In this study, we found that proinflammatory chemokines, CXCL1 and CXCL2, were profoundly increased in the decidua surrounding developing embryos in pregnant Sphk1−/−Sphk2+/− female mice. These chemokines, most likely homologs of human IL-8, have neutrophil activation and chemoattraction properties. Similar results were observed in human first-trimester DSCs in which the Sphk deficiency dramatically increased the secretion of CXCL1, IL-8, and IL-6. Recent microarray analysis demonstrated for the first time that genes in the IL-8 pathway were up-regulated in deciduas from patients with idiopathic RPL compared with those from control miscarriage patients with aneuploid embryonic demises who had experienced at least one term delivery and no previous miscarriages (50). Taken together, these results indicate that Sphk-deficient mice may be a useful animal model for gaining a comprehensive understanding of the mechanisms underlying pregnancy loss and assist in the development of treatment for human idiopathic RPL.
At present, an increasing range of immunomodulatory therapies are applied to women with idiopathic RPL, including glucocorticoid therapy, intravenous immunoglobulin therapy, anti-TNF-α therapy, and intralipid therapy, although the exact mechanisms of these therapies are unclear (51). Immunoglobulin therapy has been shown to be clinically beneficial in some selected groups of women with RPL by reducing NK cell cytotoxicity or favorably decreasing Th1/Th2 ratios (52–54). Our preliminary data revealed that the immunoglobulin therapy was effective for decreasing pregnancy loss in Sphk1−/−Sphk2+/− female mice, further supporting the similarity between our mouse model and human idiopathic RPL. Finally, taking all data into consideration, the CXCL1/IL-8 pathway may be a novel target for the development of more specific and effective therapies for human RPL. Moreover, detailed analysis of sphingolipid metabolism in normal pregnancy and idiopathic RPL in human is a promising area for future investigation.
Acknowledgment
We thank Dr. Richard L Proia for helpful discussions.
This work was supported by Grants-in-aid for Scientific Research 21591414 and 24591600 from the Japan Society for the Promotion of Science, the Naito Foundation, the Japan Foundation for Pediatric Research, and the Cell Science Research Foundation (to K. M.).
- NK
- natural killer
- APS
- anti-phospholipid syndrome
- DBA
- Dolichos biflorus agglutinin
- DMS
- d-erythro-N,N-dimethylsphingosine
- dNK
- decidual natural killer
- DSC
- decidual stromal cell
- E2
- estrogen
- MPO
- myeloperoxidase
- P4
- progesterone
- ROS
- reactive oxygen species
- RPL
- recurrent pregnancy loss
- S1P
- sphingosine 1-phosphate
- Sphk
- sphingosine kinase
- TF
- tissue factor
- pc
- post-coitum.
REFERENCES
- 1. Trowsdale J., Betz A. G. (2006) Mother's little helpers: mechanisms of maternal-fetal tolerance. Nat. Immunol. 7, 241–246 [DOI] [PubMed] [Google Scholar]
- 2. Arck P. C., Hecher K. (2013) Fetomaternal immune cross-talk and its consequences for maternal and offspring's health. Nat. Med. 19, 548–556 [DOI] [PubMed] [Google Scholar]
- 3. Chabtini L., Mfarrej B., Mounayar M., Zhu B., Batal I., Dakle P. J., Smith B. D., Boenisch O., Najafian N., Akiba H., Yagita H., Guleria I. (2013) TIM-3 regulates innate immune cells to induce fetomaternal tolerance. J. Immunol. 190, 88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cha J., Sun X., Dey S. K. (2012) Mechanisms of implantation: strategies for successful pregnancy. Nat. Med. 18, 1754–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lim H. J., Wang H. (2010) Uterine disorders and pregnancy complications: insights from mouse models. J. Clin. Invest. 120, 1004–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Warning J. C., McCracken S. A., Morris J. M. (2011) A balancing act: mechanisms by which the fetus avoids rejection by the maternal immune system. Reproduction 141, 715–724 [DOI] [PubMed] [Google Scholar]
- 7. Hannun Y. A., Obeid L. M. (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 [DOI] [PubMed] [Google Scholar]
- 8. Kluk M. J., Hla T. (2002) Signaling of sphingosine-1-phosphate via the S1P/EDG-family of G-protein-coupled receptors. Biochim. Biophys. Acta 1582, 72–80 [DOI] [PubMed] [Google Scholar]
- 9. Argraves K. M., Wilkerson B. A., Argraves W. S. (2010) Sphingosine-1-phosphate signaling in vasculogenesis and angiogenesis. World J. Biol. Chem. 1, 291–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Pyne N. J., Pyne S. (2010) Sphingosine 1-phosphate and cancer. Nat. Rev. Cancer 10, 489–503 [DOI] [PubMed] [Google Scholar]
- 11. Cyster J. G., Schwab S. R. (2012) Sphingosine-1-phosphate and lymphocyte egress from lymphoid organs. Annu. Rev. Immunol. 30, 69–94 [DOI] [PubMed] [Google Scholar]
- 12. Spiegel S., Milstien S. (2011) The outs and the ins of sphingosine-1-phosphate in immunity. Nat. Rev. Immunol. 11, 403–415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cuvillier O., Pirianov G., Kleuser B., Vanek P. G., Coso O. A., Gutkind S., Spiegel S. (1996) Suppression of ceramide-mediated programmed cell death by sphingosine-1-phosphate. Nature 381, 800–803 [DOI] [PubMed] [Google Scholar]
- 14. Olivera A., Spiegel S. (1993) Sphingosine-1-phosphate as second messenger in cell proliferation induced by PDGF and FCS mitogens. Nature 365, 557–560 [DOI] [PubMed] [Google Scholar]
- 15. Olivera A., Kohama T., Edsall L., Nava V., Cuvillier O., Poulton S., Spiegel S. (1999) Sphingosine kinase expression increases intracellular sphingosine-1-phosphate and promotes cell growth and survival. J. Cell Biol. 147, 545–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hannun Y. A., Obeid L. M. (2002) The ceramide-centric universe of lipid-mediated cell regulation: stress encounters of the lipid kind. J. Biol. Chem. 277, 25847–25850 [DOI] [PubMed] [Google Scholar]
- 17. Merrill A. H., Jr. (2002) De novo sphingolipid biosynthesis: a necessary, but dangerous, pathway. J. Biol. Chem. 277, 25843–25846 [DOI] [PubMed] [Google Scholar]
- 18. Nava V. E., Cuvillier O., Edsall L. C., Kimura K., Milstien S., Gelmann E. P., Spiegel S. (2000) Sphingosine enhances apoptosis of radiation-resistant prostate cancer cells. Cancer Res. 60, 4468–4474 [PubMed] [Google Scholar]
- 19. Kohama T., Olivera A., Edsall L., Nagiec M. M., Dickson R., Spiegel S. (1998) Molecular cloning and functional characterization of murine sphingosine kinase. J. Biol. Chem. 273, 23722–23728 [DOI] [PubMed] [Google Scholar]
- 20. Liu H., Sugiura M., Nava V. E., Edsall L. C., Kono K., Poulton S., Milstien S., Kohama T., Spiegel S. (2000) Molecular cloning and functional characterization of a novel mammalian sphingosine kinase type 2 isoform. J. Biol. Chem. 275, 19513–19520 [DOI] [PubMed] [Google Scholar]
- 21. Allende M. L., Sasaki T., Kawai H., Olivera A., Mi Y., van Echten-Deckert G., Hajdu R., Rosenbach M., Keohane C. A., Mandala S., Spiegel S., Proia R. L. (2004) Mice deficient in sphingosine kinase 1 are rendered lymphopenic by FTY720. J. Biol. Chem. 279, 52487–52492 [DOI] [PubMed] [Google Scholar]
- 22. Mizugishi K., Yamashita T., Olivera A., Miller G. F., Spiegel S., Proia R. L. (2005) Essential role for sphingosine kinase in neural and vascular development. Mol. Cell. Biol. 25, 11113–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zemann B., Kinzel B., Müller M., Reuschel R., Mechtcheriakova D., Urtz N., Bornancin F., Baumruker T., Billich A. (2006) Sphingosine kinase type 2 is essential for lymphodepletion induced by the immunomodulatory drug FTY720. Blood 107, 1454–1458 [DOI] [PubMed] [Google Scholar]
- 24. Mizugishi K., Li C., Olivera A., Bielawski J., Bielawska A., Deng C. X., Proia R. L. (2007) Maternal disturbance in activated sphingolipid metabolism causes pregnancy loss in mice. J. Clin. Invest. 117, 2993–3006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bielawski J., Pierce J. S., Snider J., Rembiesa B., Szulc Z. M., Bielawska A. (2009) Comprehensive quantitative analysis of bioactive sphingolipids by high-performance liquid chromatography-tandem mass spectrometry. Methods Mol. Biol. 579, 443–467 [DOI] [PubMed] [Google Scholar]
- 26. Girardi G. (2011) Role of tissue factor in pregnancy complications: crosstalk between coagulation and inflammation. Thromb. Res. 127, S43–S46 [DOI] [PubMed] [Google Scholar]
- 27. Aoki K., Kajiura S., Matsumoto Y., Ogasawara M., Okada S., Yagami Y., Gleicher N. (1995) Preconceptional natural-killer-cell activity as a predictor of miscarriage. Lancet 345, 1340–1342 [DOI] [PubMed] [Google Scholar]
- 28. Yamada H., Morikawa M., Kato E. H., Shimada S., Kobashi G., Minakami H. (2003) Pre-conceptional natural killer cell activity and percentage as predictors of biochemical pregnancy and spontaneous abortion with normal chromosome karyotype. Am. J. Reprod. Immunol. 50, 351–354 [DOI] [PubMed] [Google Scholar]
- 29. Paffaro V. A., Jr., Bizinotto M. C., Joazeiro P. P., Yamada A. T. (2003) Subset classification of mouse uterine natural killer cells by DBA lectin reactivity. Placenta 24, 479–488 [DOI] [PubMed] [Google Scholar]
- 30. Edsall L. C., Van Brocklyn J. R., Cuvillier O., Kleuser B., Spiegel S. (1998) N,N-Dimethylsphingosine is a potent competitive inhibitor of sphingosine kinase but not of protein kinase C: modulation of cellular levels of sphingosine 1-phosphate and ceramide. Biochemistry 37, 12892–12898 [DOI] [PubMed] [Google Scholar]
- 31. Kim J. W., Kim Y. W., Inagaki Y., Hwang Y. A., Mitsutake S., Ryu Y. W., Lee W. K., Ha H. J., Park C. S., Igarashi Y. (2005) Synthesis and evaluation of sphingoid analogs as inhibitors of sphingosine kinases. Bioorg. Med. Chem. 13, 3475–3485 [DOI] [PubMed] [Google Scholar]
- 32. Doetschman T. (2009) Influence of genetic background on genetically engineered mouse phenotypes. Methods Mol. Biol. 530, 423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Munn D. H., Zhou M., Attwood J. T., Bondarev I., Conway S. J., Marshall B., Brown C., Mellor A. L. (1998) Prevention of allogeneic fetal rejection by tryptophan catabolism. Science 281, 1191–1193 [DOI] [PubMed] [Google Scholar]
- 34. Mellor A. L., Sivakumar J., Chandler P., Smith K., Molina H., Mao D., Munn D. H. (2001) Prevention of T cell-driven complement activation and inflammation by tryptophan catabolism during pregnancy. Nat. Immunol. 2, 64–68 [DOI] [PubMed] [Google Scholar]
- 35. Girardi G., Berman J., Redecha P., Spruce L., Thurman J. M., Kraus D., Hollmann T. J., Casali P., Caroll M. C., Wetsel R. A., Lambris J. D., Holers V. M., Salmon J. E. (2003) Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J. Clin. Invest. 112, 1644–1654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Redecha P., Tilley R., Tencati M., Salmon J. E., Kirchhofer D., Mackman N., Girardi G. (2007) Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood 110, 2423–2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Redecha P., Franzke C. W., Ruf W., Mackman N., Girardi G. (2008) Neutrophil activation by the tissue factor/Factor VIIa/PAR2 axis mediates fetal death in a mouse model of antiphospholipid syndrome. J. Clin. Invest. 118, 3453–3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hanna J., Goldman-Wohl D., Hamani Y., Avraham I., Greenfield C., Natanson-Yaron S., Prus D., Cohen-Daniel L., Arnon T. I., Manaster I., Gazit R., Yutkin V., Benharroch D., Porgador A., Keshet E., Yagel S., Mandelboim O. (2006) Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat. Med. 12, 1065–1074 [DOI] [PubMed] [Google Scholar]
- 39. Williams P. J., Bulmer J. N., Searle R. F., Innes B. A., Robson S. C. (2009) Altered decidual leucocyte populations in the placental bed in pre-eclampsia and foetal growth restriction: a comparison with late normal pregnancy. Reproduction 138, 177–184 [DOI] [PubMed] [Google Scholar]
- 40. Barber E. M., Pollard J. W. (2003) The uterine NK cell population requires IL-15 but these cells are not required for pregnancy nor the resolution of a Listeria monocytogenes infection. J. Immunol. 171, 37–46 [DOI] [PubMed] [Google Scholar]
- 41. Zhang J., Dunk C. E., Lye S. J. (2013) Sphingosine signalling regulates decidual NK cell angiogenic phenotype and trophoblast migration. Hum. Reprod. 28, 3026–3037 [DOI] [PubMed] [Google Scholar]
- 42. Branch D. W., Gibson M., Silver R. M. (2010) Clinical practice. Recurrent miscarriage. N. Engl. J. Med. 363, 1740–1747 [DOI] [PubMed] [Google Scholar]
- 43. Ford H. B., Schust D. J. (2009) Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev. Obstet. Gynecol. 2, 76–83 [PMC free article] [PubMed] [Google Scholar]
- 44. Jaslow C. R., Carney J. L., Kutteh W. H. (2010) Diagnostic factors identified in 1020 women with two versus three or more recurrent pregnancy losses. Fertil. Steril. 93, 1234–1243 [DOI] [PubMed] [Google Scholar]
- 45. Saini V., Arora S., Yadav A., Bhattacharjee J. (2011) Cytokines in recurrent pregnancy loss. Clin. Chim. Acta 412, 702–708 [DOI] [PubMed] [Google Scholar]
- 46. Haimovici F., Hill J. A., Anderson D. J. (1991) The effects of soluble products of activated lymphocytes and macrophages on blastocyst implantation events in vitro. Biol. Reprod. 44, 69–75 [DOI] [PubMed] [Google Scholar]
- 47. Suffys P., Beyaert R., Van Roy F., Fiers W. (1989) TNF in combination with interferon-γ is cytotoxic to normal, untransformed mouse and rat embryo fibroblast-like cells. Anticancer Res. 9, 167–171 [PubMed] [Google Scholar]
- 48. Mueller-Eckhardt G., Mallmann P., Neppert J., Lattermann A., Melk A., Heine O., Pfeiffer R., Zingsem J., Domke N., Mohr-Pennert A. (1994) Immunogenetic and serological investigations in nonpregnant and in pregnant women with a history of recurrent spontaneous abortions. German RSA/IVIG Study Group. J. Reprod. Immunol. 27, 95–109 [DOI] [PubMed] [Google Scholar]
- 49. Jenkins C., Roberts J., Wilson R., MacLean M. A., Shilito J., Walker J. J. (2000) Evidence of a T(H) 1 type response associated with recurrent miscarriage. Fertil. Steril. 73, 1206–1208 [DOI] [PubMed] [Google Scholar]
- 50. Krieg S. A., Fan X., Hong Y., Sang Q. X., Giaccia A., Westphal L. M., Lathi R. B., Krieg A. J., Nayak N. R. (2012) Global alteration in gene expression profiles of deciduas from women with idiopathic recurrent pregnancy loss. Mol. Hum. Reprod. 18, 442–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bansal A. S., Bajardeen B., Thum M. Y. (2012) The basis and value of currently used immunomodulatory therapies in recurrent miscarriage. J. Reprod. Immunol. 93, 41–51 [DOI] [PubMed] [Google Scholar]
- 52. Kotlan B., Padanyi A., Batorfi J., Fulop V., Szigetvari I., Rajczy K., Penzes M., Gyodi E., Reti M., Petranyi G. (2006) Alloimmune and autoimmune background in recurrent pregnancy loss–successful immunotherapy by intravenous immunoglobulin. Am. J. Reprod. Immunol. 55, 331–340 [DOI] [PubMed] [Google Scholar]
- 53. van den Heuvel M. J., Peralta C. G., Hatta K., Han V. K., Clark D. A. (2007) Decline in number of elevated blood CD3(+) CD56(+) NKT cells in response to intravenous immunoglobulin treatment correlates with successful pregnancy. Am. J. Reprod. Immunol. 58, 447–459 [DOI] [PubMed] [Google Scholar]
- 54. Winger E. E., Reed J. L., Ashoush S., El-Toukhy T., Ahuja S., Taranissi M. (2011) Elevated preconception CD56+ 16+ and/or Th1:Th2 levels predict benefit from IVIG therapy in subfertile women undergoing IVF. Am. J. Reprod. Immunol. 66, 394–403 [DOI] [PubMed] [Google Scholar]