Abstract
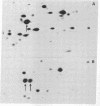
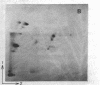
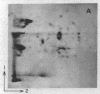
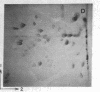
A previously isolated mutant of E. coli K12 HAK 88 [Kuwano, M., Endo, h & yamamoto, M. (1972) J. Bacteriol, 112, 1150-1156], contains a new protein that in two-dimensional gel electropherorgrams has the same molecular weight as normal elongation factor Tu, but whose isoelectric point is altered approximately 0.1 pH unit in the acidic direction. Peptide mapping, purification properties and the ratio of leucyl plus isoleucyl to methionyl plus cysteinyl residues of the normal elongation factor Tu protein and the new protein show a close similarity between the two. The mutation causing the altered electrophoretic mobility is located between argH and rif (79 min on the E. coli genetic map). These biochemical and genetic data indicate that strain HAK 88 has a mutationally altered tufB gene.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai K. I., Kawakita M., Kaziro Y. Studies on polypeptide elongation factors from Escherichia coli. II. Purification of factors Tu-guanosine diphosphate, Ts, and Tu-Ts, and crystallization of Tu-guanosine diphosphate and Tu-Ts. J Biol Chem. 1972 Nov 10;247(21):7029–7037. [PubMed] [Google Scholar]
- Hori K., Harada K., Kuwano M. Function of bacteriophage Qbeta replicase containing an altered subunit IV. J Mol Biol. 1974 Jul 15;86(4):699–708. doi: 10.1016/0022-2836(74)90347-7. [DOI] [PubMed] [Google Scholar]
- Jaskunas S. R., Lindahl L., Nomura M. Identification of two copies of the gene for the elongation factor EF-Tu in E. coli. Nature. 1975 Oct 9;257(5526):458–462. doi: 10.1038/257458a0. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Endo H., Kamiya T., Hori K. A mutant of Escherichia coli blocked in peptide elongation: altered elongation factor Ts. J Mol Biol. 1974 Jul 15;86(4):689–698. doi: 10.1016/0022-2836(74)90346-5. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Endo H., Yamamoto M. Temperature-sensitive mutation in regulation of ribonucleic acid synthesis in Escherichia coli. J Bacteriol. 1972 Dec;112(3):1150–1156. doi: 10.1128/jb.112.3.1150-1156.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Lenard J. Protein biosynthesis. Annu Rev Biochem. 1971;40:409–448. doi: 10.1146/annurev.bi.40.070171.002205. [DOI] [PubMed] [Google Scholar]
- Miller D. L., Weissbach H. Studies on the purification and properties of factor Tu from E. coli. Arch Biochem Biophys. 1970 Nov;141(1):26–37. doi: 10.1016/0003-9861(70)90102-5. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]