Background: The role of glucagon in regulation of hepatic circadian clock is unclear.
Results: Glucagon-CREB/CRTC2 signaling axis plays an important role in regulation of hepatic Bmal1 expression.
Conclusion: We find that the temporal signals of fasting and refeeding hormones regulate the transcription of Bmal1 in the liver.
Significance: We identify a novel transcriptional pathway that links glucagon/insulin metabolic cues and circadian clock.
Keywords: cAMP-response Element-binding Protein (CREB), Circadian Clock, Clock Gene, Glucagon, Glucocorticoid, Insulin, CRTC2
Abstract
Energy metabolism follows a diurnal pattern responding to the cycles of light and food exposures. Although food availability is a potent synchronizer of peripheral circadian clock in mammals, the underlying mechanism remains elusive. Here, we found that the temporal signals of fasting and refeeding hormones regulate the transcription of Bmal1, a key transcription activator of molecular clock, in the liver. During fasting, glucagon, a major fasting hormone, activates CREB/CRTC2 transcriptional complex that is recruited to Bmal1 promoter to induce its expression. Furthermore, we showed that CRTC2 is required for basal transcriptional regulation of Bmal1 by experiments using either adenovirus-mediated CRTC2 RNAi knockdown or primary Crtc2 null hepatocytes. On the other hand, insulin suppresses fasting-induced Bmal1 expression by inhibiting CRTC2 activity after refeeding. Taken together, our results indicate CRTC2 as a key component of the circadian oscillator that integrates the mammalian clock and energy metabolism.
Introduction
On the earth, most living organisms, from the simplest archaebacteria to humans, exhibit behavioral and physiological circadian rhythms (1). Although the master pacemaker that resides in the hypothalamic suprachiasmatic nucleus is entrained directly by light, peripheral circadian oscillators are mainly entrained by diurnal feeding cycles in mammals (2). Both central and peripheral clocks are controlled by self-sustained transcriptional feedback loops (3–5). At the center of this molecular machinery are the heterodimers of two transcription activators, BMAL1 and CLOCK, that stimulate the expression of repressors (Per and Cry) for their own activity (3–5). A stabilizing loop within the clockwork is provided by CLOCK/BMAL1 transactivation of the nuclear receptors RORα2 and REV-ERBα, which feed back to activate or repress BMAL1 transcription respectively by competing for a shared ROR element. Besides, many other cyclic inputs, such as rhythmic access to food, may also act as entraining agents (6–13). Remarkably, mice with defective clock function develop obesity and exhibit impaired glucose homeostasis (14, 15), suggesting that the regulation of circadian clocks is linked to the pathways of energy metabolism and potentially to the pathogenesis of metabolic diseases.
In the liver of fasted animals, glucagon activates cAMP-response element-binding protein (CREB) and its coactivator CREB-regulated transcriptional coactivator 2 (CRTC2) through cAMP signaling. Fasting activates CRTC2 activity through its dephosphorylation, resulting in its nuclear localization and increased association with CREB on the CRE sites of the promoters of gluconeogenic genes (16–18). During feeding, insulin inactivates this coactivator by promoting its phosphorylation and nuclear export (18–20). Thus, CRTC2 is an inducible transcriptional coactivator that regulates adaptive energy metabolism in the liver (18, 19, 21–23). The critical role of this factor in linking the environment to metabolism prompted us to examine whether CRTC2 regulates the circadian clock and serves as a link between the clock and energy metabolism.
Here, we showed that Bmal1 expression is induced in the liver after 4-, 24-, 26-, and 28-h fasting (initiated at zeitgeber time 12 (ZT12)), which is reversed to basal level by refeeding. By using quantitative PCR and in vivo imaging experiments with adenovirus-mediated Bmal1-luciferase reporter, we further confirmed that fasting stimulates Bmal1 expression through both glucagon and glucocorticoid signaling pathways. Moreover, we demonstrated that CRTC2 is essential for both fasting and basal Bmal1 transcription. Our data revealed that fasting and refeeding hormones, such as glucagon and insulin, play important roles in the dynamic interplay between metabolism and circadian clock through CREB/CRTC2 signaling pathway.
MATERIALS AND METHODS
Adenoviruses and Animals
CRTC2 RNAi, unspecific RNAi, GFP, CRTC2, and Rous Sarcoma Virus (RSV)-β-galactosidase (β-gal) adenoviruses have been described (24). Ad-Bmal1-Luc reporter was constructed by insertion of Bmal1-Luc containing −2.2–0-kb Bmal1 promoter into pShuttle vector and by transferring this cassette to AdEasy by non-homologous recombination. The 8–10-week-old male C57BL/6 and Slac:KM mice (Shanghai Laboratory Animal Center, China) were housed in the animal facility at the Shanghai Institutes for Biological Sciences (SIBS). Mice were kept in colony cages bedded with sawdust to ensure that no kind of food was available during fasting periods. Mice were maintained on a 12-h light/12-h dark cycle for at least 2 weeks before the study and had free access to water and regular diet (12% fat/68% carbohydrate/20% protein). For live imaging experiments, 1 × 108 pfu Bmal1-Luc and 5 × 107 pfu RSV-β-gal adenoviruses were delivered to 8–12-week-old male mice by tail vein injection. Mice were imaged on days 3–5 after adenovirus delivery. For other in vivo studies, 1 × 108 pfu of overexpressing or RNAi adenovirus was employed. For glucagon receptor antagonist-II (GRA-II) experiments, mice were intraperitoneally injected with GRA-II (6.25 mg/kg, Merck) 2 h before 4-h fasting. Plasma corticosterone levels were measured by ELISA kits (Enzo Life Science). All animal care and use procedures were in accordance with the guidelines of the SIBS Animal Care and Use Committee.
In Vivo Imaging and Analysis
Live imaging experiments were performed as described previously (19). Briefly, mice were injected intraperitoneally with 100 mg/kg sterile firefly d-luciferin (Biosynth AG) and then imaged on the IVIS imaging system and analyzed with Living Image software (Xenogen, Alameda CA). Liver lysates were prepared to determine β-gal activity. Luciferase activity detected for each mouse was normalized with β-gal expression in the liver.
Cell Culture
HEK293T cells were maintained and transfected as described previously, using 50 ng of Bmal1-Luc promoter and 20 ng of β-gal, together with 100 ng of Crtc2 plasmids per well (24-well cell culture plate). Luciferase activity was measured as described previously (24). Mouse primary hepatocytes were prepared, cultured, and infected with adenoviruses as described previously (19).
Total RNA Isolation and Analysis of mRNA Expression by Quantitative PCR
Total RNAs from whole livers or primary cultured hepatocytes were extracted using TRIzol (Invitrogen) and reverse-transcribed into cDNAs by the PrimeScript RT regent kit with gDNA eraser (Takara). mRNA levels were determined by the SYBR Green PCR kit with an ABI PRISM 7900HT sequence detector (Perkin Elmer). Ribosomal L32 mRNA levels were used as internal control. The primer sequences are as shown: Bmal1, 5′-GCCCGCTGAACATCACAAGT-3′, 5′-CCTGAGCCTGCCCTGGTAA-3′; Clock, 5′-GAGGTCGTCCTTCAGCAGTC-3′, 5′-TGTGACATGCCTTGTGGAAT-3′; Nr1d1, 5′-TCCCCAAGAGAGAGAAGCAA-3′, 5′-CTGAGAGAAGCCCACCAAAG-3′; Cry1, 5′-CATCGTGCGCATTTCACATAC-3′, 5′-TCCAGTGGCTCCATCTTGCT-3′; Per1, 5′-AGCAGGACAACCCATCTACCA-3′, 5′-CGAAGTTTGAGCTCCCGAAGT-3′; Crtc2, 5′-CACCAGAACTTGACCCACTGT-3′, 5′-CACAGGGGTCACTCAGCATAG-3′; Pck1, 5′-GTGCTGGAGTGGATGTTCGG-3′, 5′-CTGGCTGATTCTCTGTTTCAGG-3′; G6Pase, 5′-TCTGTCCCGGATCTACCTTG-3′, 5′-GTAGAATCCAAGCGCGAAAC-3′; and L32, 5′-TCTGGTGAAGCCCAAGATCG-3′, 5′-CTCTGGGTTTCCGCCAGTT-3′.
Immunoblot
Immunoblot was performed as described previously (24). AKT, phospho-AKT (Ser-473), CREB, and phospho-CREB (Ser-133) antibodies were from Cell Signaling, HSP90 and phosphoenolpyruvate carboxykinase (PEPCK) antibodies were from Santa Cruz Biotechnology, BMAL1 antibody was from Abcam, CLOCK antibody was from Millipore, α-tubulin and β-actin antibodies were from Sigma, CRTC2 antibody was from Calbiochem, and HA antibody was from Covance.
Chromatin Immunoprecipitation
Primary hepatocytes were treated with glucagon for 30 min and then cross-linked with 1% formaldehyde. CRTC2 antibody was used for immunoprecipitations along with rabbit IgG for negative controls. After removing the cross-link, DNA was extracted using phenol-chloroform and ethanol-precipitated. Target promoters were analyzed by agarose gel electrophoresis or quantified with real-time PCR using SYBR Green and normalized to input chromatin signals. The primer sequences are shown as follows: G6Pase, 5′-GGCAGCCTCTAGCACTGTC-3′, 5′-AACCACTTTTGTCTAAAAT-3′; Bmal1, 5′-TCCGGTTAGTCGTCGGAAGG-3′, 5′-CTGTGGATTTCTTGCCCCAGC-3′; and Bmal1-NS, 5′-AGCAGGCTTCCCAAGATGGG-3′, 5′-TGAAAGAAAAACTAGGAACT-3′.
Statistical Analyses
Results are reported as mean ± S.E. The comparison of different groups was carried out using two-way analysis of variance or two-tailed unpaired Student's t test by GraphPad Prism 6 (GraphPad Software, San Diego, CA). Differences were considered statistically significant at p < 0.05. All experiments were performed on at least two independent occasions.
RESULTS
Fasting and Refeeding Signals Modulate Hepatic Bmal1 Expression
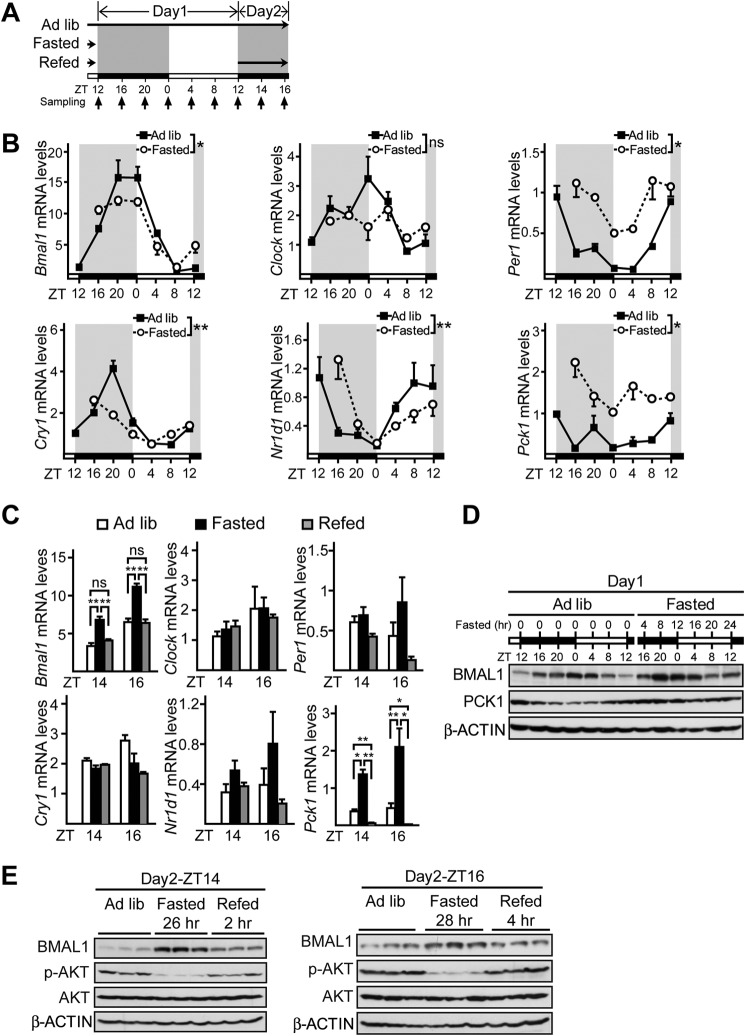
Although several studies have investigated the effects of fasting or refeeding on hepatic clock gene expression (7, 25–28), none of them initiated fasting periods around ZT12 when food was usually removed in daily restricted feeding experiments. Considering the temporal variations in the diurnal cycles of clock gene expression and hormonal signaling, we reexamined the transcriptional responses of circadian clock genes, including Bmal1, Clock, Per1, Cry1, Nr1d1, and gluconeogenic gene Pck1, as a positive control to fasting and refeeding in livers from mice fasted from ZT12. Mice were sacrificed at 4-h intervals around the clock during 28-h fasting from Day1-ZT12 to Day2-ZT16 and 2-h intervals after refeeding from Day2-ZT12 to Day2-ZT16 (Fig. 1A). During the first 24-h fasting, the temporal patterns of almost all clock genes were changed as compared with those in ad libitum fed mice, except Clock (Fig. 1B). In contrast, among the five core clock genes, only Bmal1 expression was significantly affected by prolonged fasting and refeeding, whereas the other four were relatively insensitive to the modification of feeding status (from Day2-ZT14 to Day2-ZT16, Fig. 1C). We further confirmed that the oscillation of BMAL1 protein (Fig. 1, D and E) was also altered by following its mRNA changes (Fig. 1, B and C). Consistent with previous studies (29, 30), it seemed that fasting advanced the phase of Bmal1 expression in day 2, which was readjusted back by refeeding.
FIGURE 1.
Temporal analysis of hepatic BMAL1 expression in response to fasting and refeeding signals. A, experimental schedule of fasting and refeeding experiments. Mice were subjected to fasting (from Day1-ZT12 to Day2-ZT16) and refeeding (from Day2-ZT12 to Day2-ZT16), or kept under ad libitum (Ad lib), and sacrificed at the time points indicated by the small arrows. White bars indicate the light phase. Black bars indicate the dark phase. Long black arrow indicates food availability. B, quantitative PCR analysis of mRNA levels of Bmal1, Clock, Per1, Cry1, Nr1d1, and Pck1 (n = 3) in mice fed ad libitum, fasted for 4, 8, 12, 16, 20, or 24 h (from ZT12). *, p < 0.05, two-way analysis of variance, **, p < 0.01, two-way analysis of variance, ns, no significance. C, quantitative PCR analysis of mRNA levels of Bmal1, Clock, Per1, Cry1, Nr1d1, and Pck1 (n = 3) in mice fed ad libitum, fasted for 26 and 28 h, or refed for 2 and 4 h (from Day2-ZT12).D, immunoblot analysis of BMAL1 protein amounts (n = 3) in mice fed ad libitum and fasted. Tissues from three mice per time point were homogenized and pooled together for immunoblot analysis. E, immunoblot analysis of BMAL1 protein amounts in liver protein extracts at Day2-ZT14 and Day2-ZT16. p-AKT, phospho-AKT. Data are represented as mean ± S.E., *, p < 0.05, **, p < 0.01, ns, no significance. White and dark bars indicate light and dark phases, respectively.
Fasting Induces BMAL1 through Glucagon and Glucocorticoid Signaling Pathways
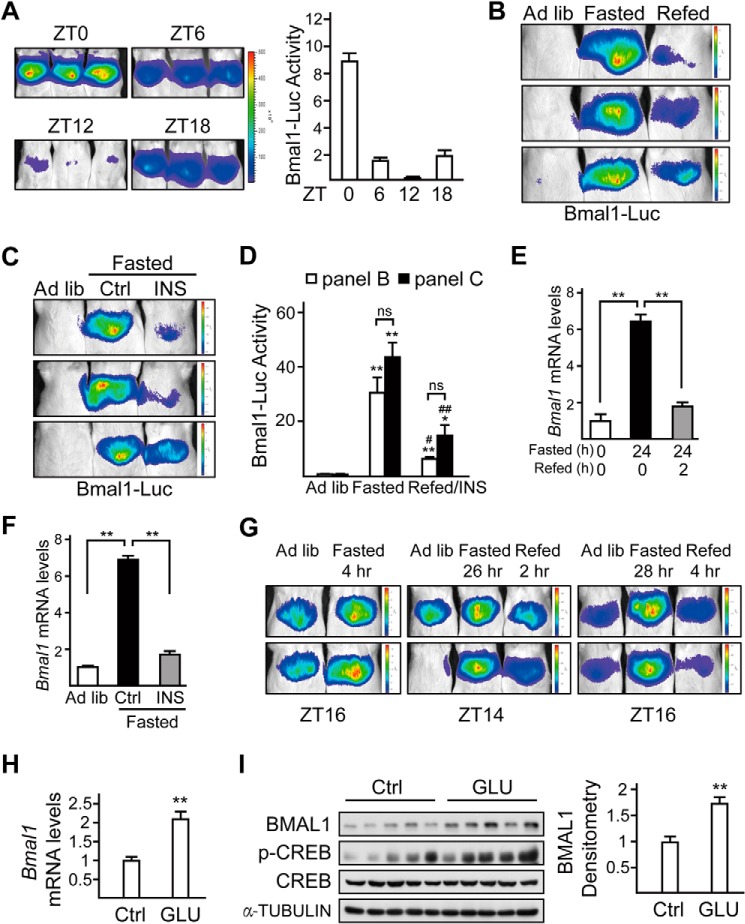
To further explore the underlying mechanism in vivo, we monitored Bmal1 expression in the liver by performing live imaging experiments with an adenovirus containing a luciferase reporter driven by Bmal1 promoter (Ad-Bmal1-Luc). We first verified the feasibility of this system by determining daily oscillation of hepatic Bmal1-Luc activity in mice fed ad libitum (Fig. 2A), which was consistent with the circadian pattern of Bmal1 mRNA levels as reported previously (31). By utilizing this method, we confirmed that fasting stimulated hepatic Bmal1-Luc activity, which was significantly suppressed in refed animals (Fig. 2B). Correspondingly, the hepatic mRNA levels of Bmal1 were elevated in fasted mice, whereas they were decreased in refed mice (Fig. 2E).
FIGURE 2.
Fasting and refeeding signals modulate hepatic Bmal1 expression via glucagon and insulin, respectively. A, imaging analysis of the hepatic activity of adenoviral luciferase reporter driven by Bmal1 promoter (Bmal1-Luc) in mice fed ad libitum at ZT0, ZT6, ZT12, and ZT18. Luciferase activity was normalized to co-infected Ad-RSV-β-gal reporter activity in the liver (right, n = 3 per group). B, in vivo imaging analysis of Ad-Bmal1-Luc reporter activity in mice fed ad libitum (Ad lib), 24-h fasted (from Day1-ZT10), or 2-h refed (from Day2-ZT8). C, imaging analysis of the hepatic activity of Bmal1-Luc in mice fed ad libitum AND fasted for 22 h (from ZT12) followed by 2-h injection of saline (Ctrl) or insulin (INS, 0.5 units/kg). D, bar graph showing relative Ad-Bmal1-Luc activity in B and C. Luciferase activity was normalized to co-infected Ad-RSV-β-gal reporter activity in the liver (n = 3 per condition, * as compared with Ad lib group, # as compared with fasted group, ns, no significance). E, the effects of fasting (from Day1-ZT10) and refeeding (from Day2-ZT8) on hepatic mRNA levels of Bmal1 (n = 3). F, quantitative PCR analysis of Bmal1 mRNA levels in mice fed ad libitum, fasted for 22 h (from ZT12) followed by 2-h injection of saline (Ctrl) or insulin (0.5 units/kg). G, in vivo imaging analysis of Ad-Bmal1-Luc reporter activity in mice fed ad libitum, fasted for 4, 26, and 28 h (from Day1-ZT12), or refed for 2 and 4 h (from Day2-ZT12) relative to Fig. 1. H and I, quantitative PCR analysis of Bmal1 mRNA level (H) and immunoblot analysis of BMAL1 protein amounts (left, n = 5) and densitometry analysis of BMAL1 protein (right, I) in mice injected with glucagon (GLU, 100 μg/kg) for 4 h (from ZT4) or saline (Ctrl). p-CREB, phospho-CREB. Data are represented as mean ± S.E., *or # p < 0.05, **or ## p < 0.01.
Because of the dominant inhibitory effect of refeeding on fasting hormones, we thus tested whether insulin is also involved in the suppression of Bmal1 expression after refeeding. Indeed, intraperitoneal administration of insulin (0.5 units/kg) not only suppressed the stimulating effect of fasting on Bmal1-Luc activity, but also decreased BMAL1 mRNA and protein levels in the liver as well (Fig. 2, C, D, and F). To confirm the effect of fasting and refeeding on Bmal1 expression, we performed live imaging experiments at Day1-ZT16, Day2-ZT14, and Day2-ZT16. Consistent with the above results, Bmal1-Luc activity was induced in mice fasted for 4, 26, and 28 h (Day1-ZT16, Day2-ZT14, and Day2-ZT16, correspondingly), which was suppressed in mice refed for 2 and 4 h (Fig. 2G).
On the basis of its role in regulating fasting-induced hepatic gene expression, glucagon would be expected to mediate the effects of fasting on BMAL1 in the liver. We then analyzed mRNA and protein levels of BMAL1 after intraperitoneal injection of glucagon in mice. As expected, exposure to glucagon increased both mRNA and protein levels of BMAL1 (Fig. 2, H and I).
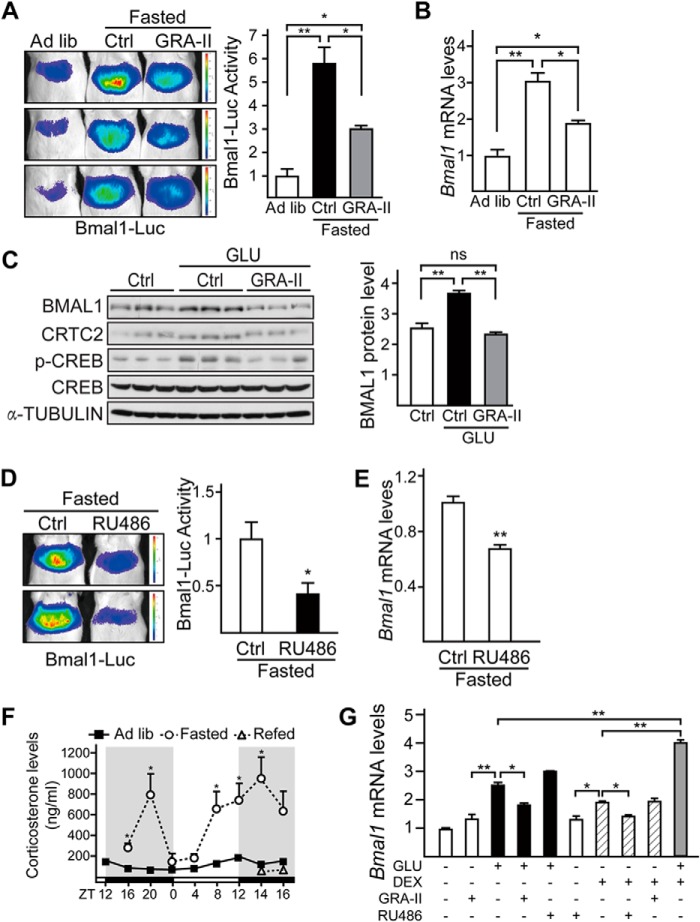
We further confirmed the effect of glucagon-receptor antagonist on expression of Bmal1 in this regard. Similarly, intraperitoneal injection of GRA-II significantly reduced fasting induction of Bmal1-Luc activity and Bmal1 mRNA levels in the liver (Fig. 3, A and B). Consistently, exposure of primary hepatocytes to glucagon increased the protein levels of BMAL1, which was blocked by pretreatment of GRA-II (Fig. 3C). Next, we investigated the potential role of another kind of fasting hormone, glucocorticoids, in this process. Consistent with the previous report that glucocorticoid signaling regulates Bmal1 expression (32), glucocorticoid receptor antagonist (RU486) significantly decreased fasting-induced Bmal1-Luc activity and Bmal1 mRNA levels in the liver (Fig. 3, D and E). Meanwhile, we found that the temporal change of plasma corticosterone levels did not completely fit the BMAL1 pattern affected by fasting and refeeding (Fig. 3F). Interestingly, the additive induction effects of glucagon and dexamethasone on Bmal1 mRNA levels suggest that these two fasting hormonal signals induce Bmal1 expression via two separate signaling pathways (Fig. 3G).
FIGURE 3.
Fasting induces Bmal1 expression through glucagon and glucocorticoid signaling pathways. A and B, in vivo imaging analysis of Ad-Bmal1-Luc reporter activity (A) and quantitative PCR analysis of Bmal1 mRNA levels (B) in mice fed ad libitum (Ad lib), 4-h fasted (from ZT12) with 2-h pre-injection of GRA-II (10 mg/kg) or saline (Ctrl). Luciferase activity was normalized to co-infected Ad-RSV-β-gal reporter activity in the liver (n = 3 per condition). C, immunoblotting analysis of BMAL1 protein (left) in primary mouse hepatocytes pretreated with GRA-II (50 μg/ml) for 2 h and followed by 4-h glucagon (GLU, 100 nm) stimulation and densitometry analysis of BMAL1 protein levels (right). (n = 3 per condition, ns, no significance). p-CREB, phospho-CREB. D and E, imaging analysis of hepatic Bmal1-Luc activity (D) and quantitative PCR analysis of Bmal1 mRNA levels (E) in mice fasted for 4 h (from ZT12) with 2-h pre-injection of glucocorticoid-receptor antagonist (RU486, 10 mg/kg) or saline (Ctrl). Luciferase activity was normalized to co-infected Ad-RSV-β-gal reporter activity in the liver. (n = 3). F, the effect of fasting and refeeding on plasma corticosterone levels relative to Fig. 1. G, quantitative PCR analysis of Bmal1 mRNA levels (n = 3) in primary mouse hepatocytes exposed to glucagon (GLU, 100 nm), dexamethasone (DEX, 100 nm), GRA-II (20 nm), or RU486 (20 nm) as indicated. Data are represented as mean ± S.E., * p < 0.05, ** p < 0.01.
CREB/CRTC2 Mediates Fasting Stimulation of Hepatic BMAL1
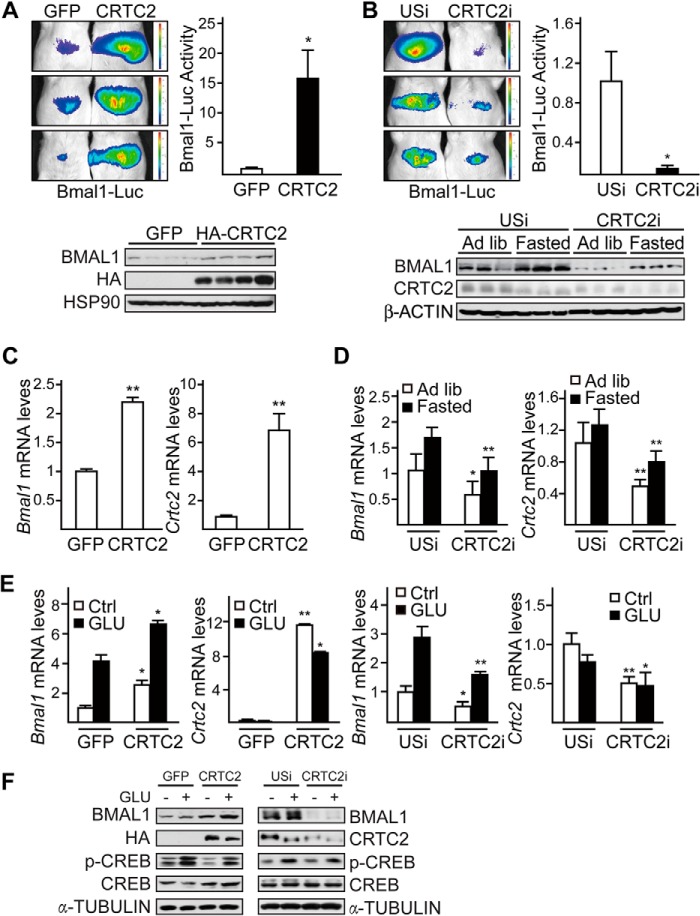
Having seen the involvement of glucagon in fasting-induced Bmal1 expression, we explored the underlying molecular mechanism. CRTC2 has been found to promote glucagon-regulated gene expression after its association with CREB (18, 33). Thus, we evaluated the role of CRTC2 in mediating the effects of glucagon on hepatic Bmal1 during fasting.
Overexpression of CRTC2 dramatically elevated Bmal1-Luc activity, the mRNA levels, and protein amounts of BMAL1 in the liver (Fig. 4A and 4C). In contrast, adenovirus-mediated RNAi of hepatic CRTC2 markedly reduced Bmal1-Luc activity in fasting mice. The mRNA levels and protein amounts of hepatic BMAL1 were also decreased in CRTC2 knockdown mice under both ad libitum and fasting conditions (Fig. 4, B and D), suggesting that CREB/CRTC2 regulates the basal transcription of Bmal1. Moreover, we obtained similar results of Bmal1 mRNA and protein levels in in vitro experiments by using primary hepatocytes (Fig. 4, E and F). These results point to an essential role of CRTC2 in regulating core clock gene Bmal1.
FIGURE 4.
CRTC2 mediates fasting-induced BMAL1 expression in the liver. A and C, imaging analysis of hepatic Bmal1-Luc activity (A) and quantitative PCR analysis of Bmal1 and Crtc2 mRNA levels (C) in Ad-GFP or Ad-HA-CRTC2 co-infected mice with 8-h fasting (from ZT0, top, n ≥ 3) and BMAL1 protein levels in liver homogenates collected around ZT9 from ad libitum fed mice (A, bottom). B and D, imaging analysis of hepatic Bmal1-Luc activity (B) and quantitative PCR analysis of Bmal1 and Crtc2 mRNA levels (D) in Ad-unspecific (USi) or Ad-CRTC2 RNAi (CRTC2i) co-infected mice with 20-h fasting (from ZT7, top, n = 3) as well as BMAL1 protein levels in liver homogenates collected around ZT10 from ad libitum (Ad lib) fed or 22-h fasted mice (B, bottom). The effects of Ad-CRTC2i relative to Ad-USi control and Ad-CRTC2 relative to Ad-GFP control are indicated. E and F, quantitative PCR analysis of Bmal1 and Crtc2 mRNA levels (E) and immunoblot analysis (F) in primary hepatocytes exposed to glucagon (GLU, 100 nm) for 6 h, amounts of CRTC2, HA-CRTC2, phospho-CREB (p-CREB), CREB, and α-tubulin are shown. Data are represented as mean ± S.E., n = 3, * p < 0.05, ** p < 0.01.
CRTC2 Is Recruited to Bmal1 Promoter
As Bmal1 promoter contains no canonic cAMP-response element (CREs), we performed promoter deletion analysis and found that the sequence from −0.5 to −0.1 kb of Bmal1 promoter was required for CREB/CRTC2 to up-regulate Bmal1-Luc expression (Fig. 5A). Supporting this notion, the results of ChIP assays indicated that CRTC2 was present on this portion of Bmal1 promoter in primary hepatocytes exposed to glucagon (Fig. 5B). In line with the previously reported reduction of CRTC2 activity after long term fasting (24), the enhanced recruitment of CRTC2 to CRE-containing region on G6pc promoter was attenuated in livers from 24-h fasted mice, as compared with that from 4-h fasted mice. However, both 4-h and 24-h fasting induced relatively weaker but similar levels of CRTC2 presence on Bmal1 promoter (Fig. 5C), further suggesting the basal function of CREB/CRTC2 in Bmal1 transcriptional regulation.
FIGURE 5.
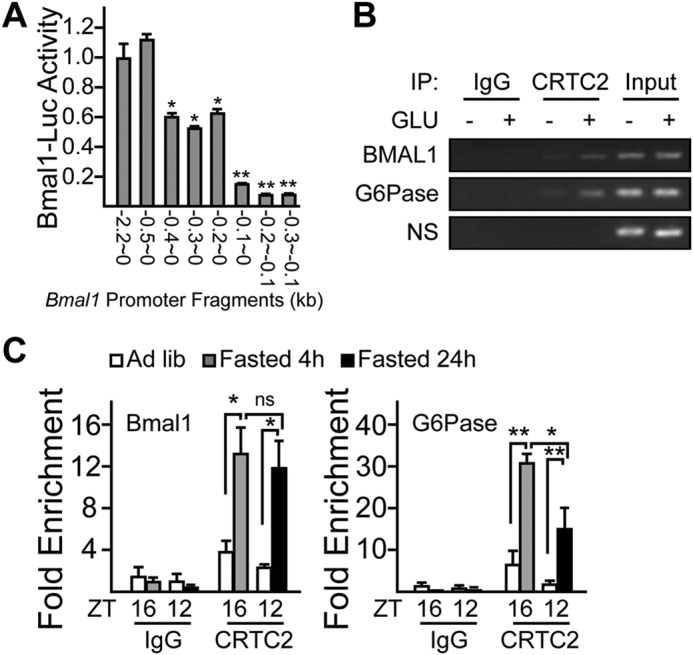
CRTC2 binds Bmal1 promoter. A, Bmal1 promoter deletion analysis. HEK293T cells were co-transfected with CRTC2, β-gal, and luciferase reporter plasmids driven by different Bmal1 promoter fragments as indicated. Luciferase activity was measured after 4-h forskolin (10 nm) treatment and normalized to β-gal activity. B, ChIP analysis showing the effect of 30-min glucagon treatment on recruitment of CRTC2 to the promoters of Bmal1 and G6Pase in primary hepatocytes (NS: nonspecific binding). IP, immunoprecipitation. C, ChIP analysis showing effect of 4- and 24-h fasting on the recruitment of CRTC2 to the promoters of Bmal1 and G6Pase in mice liver (n = 3 per condition, * p < 0.05, ** p < 0.01, ns, no significance). Ad lib, ad libitum.
CRTC2 Ablation Blocks Glucagon-induced Hepatic Bmal1 Expression
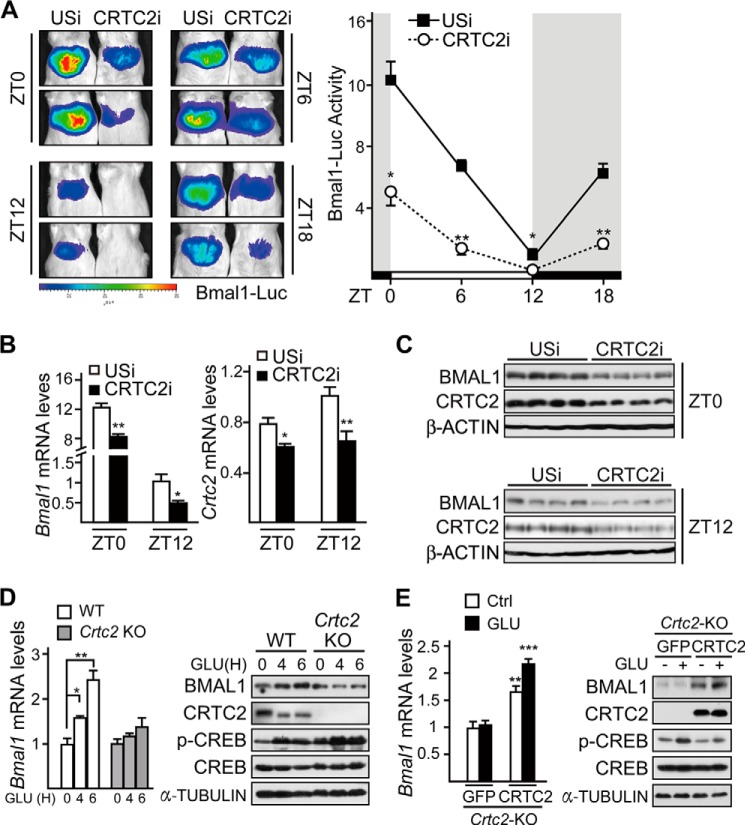
The above results suggest that CRTC2 is involved in the regulation of Bmal1 basal expression, which prompted us to explore whether CRTC2 regulates the circadian rhythm of Bmal1 in vivo. As expected, Ad-CRTC2i significantly reduced hepatic Bmal1-Luc activity at ZT0, ZT6, ZT12, and ZT18 under ad libitum conditions (Fig. 6A), and the mRNA levels and protein amounts of BMAL1 at ZT0 and ZT12 were decreased correspondingly (Fig. 6, B and C).
FIGURE 6.
CRTC2 is required for glucagon-induced hepatic Bmal1 expression. A, imaging analysis of hepatic Bmal1-Luc activity around ZT0, ZT6, ZT12, and ZT18 in Ad-USi or Ad-CRTC2i co-infected mice fed ad libitum (n = 3). B and C, quantitative PCR analysis of Bmal1 mRNA levels (B) and immunoblot analysis of BMAL1 protein (C) in mice liver samples collected around ZT0 and ZT12 from Ad-USi or Ad-CRTC2i infected mice fed ad libitum (n ≥ 3). D, quantitative PCR analysis of Bmal1 mRNA levels (left, n = 3) and immunoblot analysis of BMAL1 protein amounts (right) in primary WT or Crtc2 null (Crtc2 KO) hepatocytes with glucagon (100 nm) treatment. GLU(H) indicates the periods (hours) of glucagon treatment. p-CREB, phospho-CREB. E, quantitative PCR analysis of Bmal1 mRNA levels (left, n = 3) and immunoblot analysis of BMAL1 protein amounts (right) in primary Crtc2 null hepatocytes infected with Ad-GFP or Ad-CRTC2 with glucagon (GLU, 100 nm, 6 h) treatment. Data are represented as mean ± S.E., * p < 0.05, ** p < 0.01.
To further confirm the effects of the CRTC2 deficiency on hepatic BMAL1, we checked Bmal1 expression in the primary hepatocytes from Crtc2 null mice and their WT littermates. As compared with WT hepatocytes, glucagon exposure had little effect on both mRNA and protein levels of BMAL1 in Crtc2-depleted hepatocytes (Fig. 6D). Similar results of Bmal1-Luc activity were confirmed in experiments by using these primary hepatocytes (data not shown).
We next investigated whether the phenotype in Crtc2 null hepatocytes could be rescued by the overexpression of CRTC2. As expected, both mRNA and protein levels of BMAL1 were remarkably increased in Crtc2 null hepatocytes overexpressing CRTC2 (Fig. 6E). Consistent results were also obtained by detecting Ad-Bmal1-Luc activity (data not shown). Taken together, our results suggest that glucagon-CREB/CRTC2 signaling axis stimulates Bmal1 mRNA and protein accumulations during fasting stage.
DISCUSSION
During fasting, one of the major effects of both glucagon and glucocorticoids is to stimulate hepatic glucose production. Glucocorticoids have been shown to reset circadian rhythms in peripheral tissues (25). In contrast, the involvement of glucagon in regulating circadian oscillations is still unclear. Here, we demonstrated that glucagon stimulates the expression of Bmal1 through activating the CREB coactivator CRTC2, which is essential for maintaining normal circadian rhythms. We further identified CRTC2 as a key component of the circadian clock that integrates the mammalian clock and energy metabolism.
Several groups, including ours, found that BMAL1 expression is affected by fasting, whereas the altered patterns were not consistent with each other (26–30). This is mainly due to the different initiating ZT points and various durations of fasting periods that were applied in these studies. Bmal1 expression is inhibited by NR1D1, a nuclear receptor that targets ROR-response elements in the promoter of the Bmal1 gene (34), which may contribute to the reduction of Bmal1 expression in mice during fasting from Day1-ZT20 to Day1-ZT0 (Fig. 1B). The results of ChIP assays indicate that CRTC2 is present on the −0.5- to ∼−0.1-kb region of Bmal1 promoter (Fig. 5A), where the ROR-binding site is not contained (35), suggesting that CREB/CRTC2 regulation of Bmal1 may be at least partially independent of PGC-1α/RORα.
Although the expression of Bmal1 is enhanced in ad libitum fed mice at ZT20 to ZT0, when glucagon and glucocorticoid signaling is low, the decreased expression of repressor proteins of the negative limb, such as NR1D1, probably led to a net increase in the transcription of Bmal1 (Fig. 1B). Meanwhile, the increased expression of activator proteins, such as PGC-1α and RORα, may also contribute to the rising Bmal1 levels throughout the night (data not shown).
Fasting and refeeding have been considered to function as a crucial environmental synchronizer of the peripheral circadian clock for decades. However, the molecular mechanism is still elusive. Our study demonstrates that the temporal fluctuations of key fasting and refeeding hormones (glucagon/glucocorticoids and insulin) serve as a feeding-responsive oscillator, which coordinates with the autonomous circadian machinery to control the rhythmic Bmal1 expression in the liver. Thus, we have uncovered CRTC2 as a potential molecular target that could simultaneously modulate circadian clocks and energy metabolism.
This work was supported by grants from the National Basic Research 973 (NBR973) Program (2014CB910500), National Natural Science Foundation of China (81390351 and 31222028), NBR973 (2012CB524900), the Chinese Academy of Sciences (KSCX2-EW-R-09 and 2010OHTP08), and Shanghai Pujiang Program (10PJ1411200).
- ROR
- retinoid-related orphan receptor
- CREB
- cAMP-response element-binding protein
- CRE
- cAMP-response element
- CRTC2
- CREB-regulated transcriptional coactivator 2
- ZT
- zeitgeber time
- GRA-II
- glucagon receptor antagonist-II
- Luc
- luciferase
- Ad
- adenovirus
- Ctrl
- control
- USi
- unspecific.
REFERENCES
- 1. Dunlap J. C. (1999) Molecular bases for circadian clocks. Cell 96, 271–290 [DOI] [PubMed] [Google Scholar]
- 2. Schibler U., Sassone-Corsi P. (2002) A web of circadian pacemakers. Cell 111, 919–922 [DOI] [PubMed] [Google Scholar]
- 3. Gekakis N., Staknis D., Nguyen H. B., Davis F. C., Wilsbacher L. D., King D. P., Takahashi J. S., Weitz C. J. (1998) Role of the CLOCK protein in the mammalian circadian mechanism. Science 280, 1564–1569 [DOI] [PubMed] [Google Scholar]
- 4. Hogenesch J. B., Gu Y. Z., Jain S., Bradfield C. A. (1998) The basic-helix-loop-helix-PAS orphan MOP3 forms transcriptionally active complexes with circadian and hypoxia factors. Proc. Natl. Acad. Sci. U.S.A. 95, 5474–5479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yagita K., Tamanini F., van Der Horst G. T., Okamura H. (2001) Molecular mechanisms of the biological clock in cultured fibroblasts. Science 292, 278–281 [DOI] [PubMed] [Google Scholar]
- 6. Damiola F., Le Minh N., Preitner N., Kornmann B., Fleury-Olela F., Schibler U. (2000) Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 14, 2950–2961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Stokkan K. A., Yamazaki S., Tei H., Sakaki Y., Menaker M. (2001) Entrainment of the circadian clock in the liver by feeding. Science 291, 490–493 [DOI] [PubMed] [Google Scholar]
- 8. Vollmers C., Gill S., DiTacchio L., Pulivarthy S. R., Le H. D., Panda S. (2009) Time of feeding and the intrinsic circadian clock drive rhythms in hepatic gene expression. Proc. Natl. Acad. Sci. U.S.A. 106, 21453–21458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Panda S., Antoch M. P., Miller B. H., Su A. I., Schook A. B., Straume M., Schultz P. G., Kay S. A., Takahashi J. S., Hogenesch J. B. (2002) Coordinated transcription of key pathways in the mouse by the circadian clock. Cell 109, 307–320 [DOI] [PubMed] [Google Scholar]
- 10. Storch K. F., Lipan O., Leykin I., Viswanathan N., Davis F. C., Wong W. H., Weitz C. J. (2002) Extensive and divergent circadian gene expression in liver and heart. Nature 417, 78–83 [DOI] [PubMed] [Google Scholar]
- 11. Ueda H. R., Chen W., Adachi A., Wakamatsu H., Hayashi S., Takasugi T., Nagano M., Nakahama K., Suzuki Y., Sugano S., Iino M., Shigeyoshi Y., Hashimoto S. (2002) A transcription factor response element for gene expression during circadian night. Nature 418, 534–539 [DOI] [PubMed] [Google Scholar]
- 12. Lowrey P. L., Takahashi J. S. (2004) Mammalian circadian biology: elucidating genome-wide levels of temporal organization. Annu. Rev. Genomics Hum. Genet. 5, 407–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wijnen H., Young M. W. (2006) Interplay of circadian clocks and metabolic rhythms. Annu. Rev. Genet. 40, 409–448 [DOI] [PubMed] [Google Scholar]
- 14. Rudic R. D., McNamara P., Curtis A. M., Boston R. C., Panda S., Hogenesch J. B., Fitzgerald G. A. (2004) BMAL1 and CLOCK, two essential components of the circadian clock, are involved in glucose homeostasis. PLoS Biol. 2, e377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turek F. W., Joshu C., Kohsaka A., Lin E., Ivanova G., McDearmon E., Laposky A., Losee-Olson S., Easton A., Jensen D. R., Eckel R. H., Takahashi J. S., Bass J. (2005) Obesity and metabolic syndrome in circadian Clock mutant mice. Science 308, 1043–1045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang Y., Li G., Goode J., Paz J. C., Ouyang K., Screaton R., Fischer W. H., Chen J., Tabas I., Montminy M. (2012) Inositol-1,4,5-trisphosphate receptor regulates hepatic gluconeogenesis in fasting and diabetes. Nature 485, 128–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yoon Y. S., Lee M. W., Ryu D., Kim J. H., Ma H., Seo W. Y., Kim Y. N., Kim S. S., Lee C. H., Hunter T., Choi C. S., Montminy M. R., Koo S. H. (2010) Suppressor of MEK null (SMEK)/protein phosphatase 4 catalytic subunit (PP4C) is a key regulator of hepatic gluconeogenesis. Proc. Natl. Acad. Sci. U.S.A. 107, 17704–17709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Koo S. H., Flechner L., Qi L., Zhang X., Screaton R. A., Jeffries S., Hedrick S., Xu W., Boussouar F., Brindle P., Takemori H., Montminy M. (2005) The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature 437, 1109–1111 [DOI] [PubMed] [Google Scholar]
- 19. Dentin R., Liu Y., Koo S. H., Hedrick S., Vargas T., Heredia J., Yates J., 3rd, Montminy M. (2007) Insulin modulates gluconeogenesis by inhibition of the coactivator TORC2. Nature 449, 366–369 [DOI] [PubMed] [Google Scholar]
- 20. Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., Depinho R. A., Montminy M., Cantley L. C. (2005) The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Screaton R. A., Conkright M. D., Katoh Y., Best J. L., Canettieri G., Jeffries S., Guzman E., Niessen S., Yates J. R., 3rd, Takemori H., Okamoto M., Montminy M. (2004) The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell 119, 61–74 [DOI] [PubMed] [Google Scholar]
- 22. Le Lay J., Tuteja G., White P., Dhir R., Ahima R., Kaestner K. H. (2009) CRTC2 (TORC2) contributes to the transcriptional response to fasting in the liver but is not required for the maintenance of glucose homeostasis. Cell Metab. 10, 55–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ryu D., Oh K. J., Jo H. Y., Hedrick S., Kim Y. N., Hwang Y. J., Park T. S., Han J. S., Choi C. S., Montminy M., Koo S. H. (2009) TORC2 regulates hepatic insulin signaling via a mammalian phosphatidic acid phosphatase, LIPIN1. Cell Metab. 9, 240–251 [DOI] [PubMed] [Google Scholar]
- 24. Liu Y., Dentin R., Chen D., Hedrick S., Ravnskjaer K., Schenk S., Milne J., Meyers D. J., Cole P., Yates J., 3rd, Olefsky J., Guarente L., Montminy M. (2008) A fasting inducible switch modulates gluconeogenesis via activator/coactivator exchange. Nature 456, 269–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balsalobre A., Brown S. A., Marcacci L., Tronche F., Kellendonk C., Reichardt H. M., Schütz G., Schibler U. (2000) Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 289, 2344–2347 [DOI] [PubMed] [Google Scholar]
- 26. Kawamoto T., Noshiro M., Furukawa M., Honda K. K., Nakashima A., Ueshima T., Usui E., Katsura Y., Fujimoto K., Honma S., Honma K., Hamada T., Kato Y. (2006) Effects of fasting and re-feeding on the expression of Dec1, Per1, and other clock-related genes. J. Biochem. 140, 401–408 [DOI] [PubMed] [Google Scholar]
- 27. Froy O., Chapnik N., Miskin R. (2009) Effect of intermittent fasting on circadian rhythms in mice depends on feeding time. Mech. Ageing Dev. 130, 154–160 [DOI] [PubMed] [Google Scholar]
- 28. Oike H., Nagai K., Fukushima T., Ishida N., Kobori M. (2011) Feeding cues and injected nutrients induce acute expression of multiple clock genes in the mouse liver. PLoS One 6, e23709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Barnea M., Madar Z., Froy O. (2009) High-fat diet delays and fasting advances the circadian expression of adiponectin signaling components in mouse liver. Endocrinology 150, 161–168 [DOI] [PubMed] [Google Scholar]
- 30. Tahara Y., Otsuka M., Fuse Y., Hirao A., Shibata S. (2011) Refeeding after fasting elicits insulin-dependent regulation of Per2 and Rev-erbα with shifts in the liver clock. J. Biol. Rhythms 26, 230–240 [DOI] [PubMed] [Google Scholar]
- 31. Preitner N., Damiola F., Lopez-Molina L., Zakany J., Duboule D., Albrecht U., Schibler U. (2002) The orphan nuclear receptor REV-ERBα controls circadian transcription within the positive limb of the mammalian circadian oscillator. Cell 110, 251–260 [DOI] [PubMed] [Google Scholar]
- 32. Reddy A. B., Maywood E. S., Karp N. A., King V. M., Inoue Y., Gonzalez F. J., Lilley K. S., Kyriacou C. P., Hastings M. H. (2007) Glucocorticoid signaling synchronizes the liver circadian transcriptome. Hepatology 45, 1478–1488 [DOI] [PubMed] [Google Scholar]
- 33. Vander Kooi B. T., Onuma H., Oeser J. K., Svitek C. A., Allen S. R., Vander Kooi C. W., Chazin W. J., O'Brien R. M. (2005) The glucose-6-phosphatase catalytic subunit gene promoter contains both positive and negative glucocorticoid response elements. Mol. Endocrinol. 19, 3001–3022 [DOI] [PubMed] [Google Scholar]
- 34. Crumbley C., Wang Y., Kojetin D. J., Burris T. P. (2010) Characterization of the core mammalian clock component, NPAS2, as a REV-ERBα/RORα target gene. J. Biol. Chem. 285, 35386–35392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Liu C., Li S., Liu T., Borjigin J., Lin J. D. (2007) Transcriptional coactivator PGC-1α integrates the mammalian clock and energy metabolism. Nature 447, 477–481 [DOI] [PubMed] [Google Scholar]