Background: Aip1 cooperates with cofilin to disassemble actin filaments.
Results: Aip1 increases the rate of filament severing by cofilin by binding the sides of actin filaments, and Δaip1 mutants have cytokinesis defects.
Conclusion: Aip1 promotes actin filament severing by the high concentrations of cofilin in cells.
Significance: We provide the first evidence that Aip1 promotes filament severing and illustrate its importance to cytokinesis.
Keywords: Actin, Cofilin, Cytokinesis, Myosin, Yeast, Aip1, Severing
Abstract
Aip1 (actin interacting protein 1) is ubiquitous in eukaryotic organisms, where it cooperates with cofilin to disassemble actin filaments, but neither its mechanism of action nor its biological functions have been clear. We purified both fission yeast and human Aip1 and investigated their biochemical activities with or without cofilin. Both types of Aip1 bind actin filaments with micromolar affinities and weakly nucleate actin polymerization. Aip1 increases up to 12-fold the rate that high concentrations of yeast or human cofilin sever actin filaments, most likely by competing with cofilin for binding to the side of actin filaments, reducing the occupancy of the filaments by cofilin to a range favorable for severing. Aip1 does not cap the barbed ends of filaments severed by cofilin. Fission yeast lacking Aip1 are viable and assemble cytokinetic contractile rings normally, but rings in these Δaip1 cells accumulate 30% less myosin II. Further, these mutant cells initiate the ingression of cleavage furrows earlier than normal, shortening the stage of cytokinetic ring maturation by 50%. The Δaip1 mutation has negative genetic interactions with deletion mutations of both capping protein subunits and cofilin mutations with severing defects, but no genetic interaction with deletion of coronin.
Introduction
Actin filament polymerization and turnover drive a variety of essential cellular processes, including motility, endocytosis, cytokinesis, and the establishment of cell polarity. Regulation of filament disassembly maintains pools of monomeric actin available for polymerization. The small actin-binding protein cofilin, a member of the ADF (actin depolymerizing protein) family, promotes filament turnover by binding cooperatively to the sides of ADP-actin filaments and severing at interfaces between decorated and undecorated segments (1–4).
Cofilin is ubiquitous in eukaryotes and essential for viability of yeast, fruit flies, nematodes, and vertebrates where it contributes to endocytosis, cytokinesis, cell motility, neurite growth, and tissue formation (5). Mammals possess ADF and two cofilin isoforms: nonmuscle cofilin-1 and muscle cofilin-2. Fission yeast has one cofilin gene (6), making it favorable for studying the biological functions of cofilin. Fission yeast depend on actin filament severing by cofilin to remove filaments from sites of endocytosis (actin patches) and to produce actin filament fragments that promote assembly of new actin patches (7). Fission yeast cofilin also participates in both the assembly and disassembly of the cytokinetic contractile ring (6, 8).
Paradoxically, cofilin concentrations in cells, >10 μm (7), are high enough to stabilize rather than sever actin filaments (2). Cells might promote severing by phosphorylating a large fraction of the cofilin pool (9), which greatly reduces the affinity for actin filaments (10). Although some yeast cofilin is phosphorylated (11), the phosphorylation is not essential for the regulation of its cellular activities (12), so phosphorylation cannot be a universal mechanism. Alternatively, high cofilin concentrations in cells may sever newly polymerized filaments only transiently as the first few cofilins bind ADP-actin after phosphate dissociates, or other actin binding proteins might promote severing by enhancing the activity of cofilin or by competing with cofilin to reduce the density of cofilin on filaments (13).
Aip1 (Actin interacting protein 1), a candidate for regulation of the activity of cofilin, was discovered in a two-hybrid screen for budding yeast actin binding proteins (14, 15) and later identified in a broad spectrum of eukaryotes. The 65-kDa Aip1 protein consists of two seven-bladed β-propeller domains (16, 17), so it is also called WDR1 (WD domain repeat protein 1) in some species.
Aip1 participates in cell division, endocytosis and cellular locomotion. Cytokinesis is slow and sometimes fails in Dictyostelium lacking Aip1 (18) and mammalian cells depleted of Aip1 (19). Both budding yeast and Dictyostelium aip1 null mutants have endocytosis defects (18, 20, 21), whereas aip1 mutations reduce cellular motility in both Dictyostelium and human tissue cells (18, 22). Mutation or depletion of aip1 causes a wide range of developmental defects in multicellular organisms including muscles of nematodes and fruit flies (23, 24), epidermal cells of fruit flies (25), the immune system of mice (26), and root hairs of plants (27). In several cases aip1 mutant cells were noted to have abundant thick actin cables.
Mutations of aip1 interact genetically with cofilin mutations (14, 28) and genes for many other actin cytoskeletal proteins. A budding yeast aip1 deletion mutation had negative genetic interactions with mutations of srv2/CAP (29), capping protein and fimbrin (14, 30), and positive interaction with a mutation of tropomyosin (31). Dictyostelium aip1 deletion mutations also had negative genetic interactions with a coronin deletion mutation (32), and the null mutation of one of the two aip1 isoforms in nematode had positive genetic interactions with both tropomyosin and muscle myosin II mutations (33).
Aip1 is proposed to enhance actin filament severing by cofilin and then cap the newly created barbed ends, but these biochemical activities are far from clear. When added to mixtures of actin filaments and cofilin, Aip1 from both budding yeast and nematodes increased the amount of actin in high speed supernatants (14, 34), originally interpreted as depolymerization. Direct observation by fluorescence microscopy showed that Aip1 reduced the average length of filaments in the presence of cofilin (35, 36). Other microscopic observations suggested that Aip1 promotes depolymerization (37). Some have proposed that a trimeric complex of cofilin, Aip1, and actin enhances the activity of cofilin (14), but no evidence exists for such a complex or direct physical interaction of Aip1 and cofilin except for yeast two-hybrid assays. Purified Xenopus Aip1 enhanced actin filament disassembly by cofilin, and the newly created barbed ends did not elongate in a bulk polymerization assay (35). This apparent capping activity of Aip1 depended on cofilin (35). Aip1 and capping protein complemented each other's activities in an in vitro bead motility assay with budding yeast cellular extracts (38).
Here we compare the biochemical activities of fission yeast Aip1 (SpAip1) and human Aip1 (HsAip1). Both Aip1 proteins bind the sides of actin filaments and have modest effects on nucleation, filament elongation, and depolymerization. Aip1 enhances actin filament severing by cofilin but does not cap the severed fragments. Although fission yeast cells lacking Aip1 are viable, Δaip1 deletion mutants recruited less myosin II to their cytokinetic contractile rings, which initiated constriction prematurely.
MATERIALS AND METHODS
Cloning
SpAip1 cDNA was cloned from a fission yeast cDNA library. Human HsAip1 cDNA was cloned from a human full-length cDNA library (SC320182; Origene). Both cDNAs were subcloned into the maltose-binding protein (MBP)2 expression vector (NEB). A TEV protease cleavage site (ENLYFQG) was engineered between MBP and the recombinant proteins by PCR.
Protein Purification
Recombinant SpAip1 and HsAip1 proteins were expressed in BL21(DE3)-RIL cells (Agilent), induced with 0.5 mm isopropyl β-d-thiogalactopyranoside at 16 °C overnight. Cells were lysed by sonication in MBP buffer (400 mm NaCl, 20 mm Tris-Cl, pH 7.4, 1 mm EDTA, 1 mm DTT) plus 0.5 mm PMSF and protease inhibitor mixture (Complete; Roche). Bacterial lysates were clarified and incubated with amylose resin (NEB) for 1 h at 4 °C on a rocking platform. The lysate and resin were poured into an empty column and washed with 10 column volumes of MBP buffer followed by five column volumes of TEV buffer (50 mm NaCl, 50 mm Tris-Cl, pH 7.4, 0.5 mm EDTA). The MBP-tagged recombinant protein was digested on the column with MBP-tagged TEV protease overnight at 4 °C. The untagged recombinant protein was eluted from the column with three column volumes of MBP buffer, dialyzed against DEAE buffer 1 (50 mm NaCl, 20 mm Tris-Cl, pH 8.0, 1 mm DTT) and loaded on a DEAE-Sepharose column (GE Healthcare). The protein was eluted from the column with a gradient of 50–500 mm NaCl in DEAE buffer and concentrated to 20 μm with a centrifuge filter device (Amicon, Ultra-15, 30-kDa molecular mass cutoff; Millipore). Aliquots of concentrated protein were flash-frozen in liquid nitrogen for storage at −80 °C.
Actin was purified from an acetone powder of chicken breast muscle by one cycle of polymerization and depolymerization followed by gel filtration in buffer G (2 mm Tris-Cl, pH 8.0, 0.5 mm ATP, 0.5 mm DTT, 0.1 mm CaCl2, and 1 mm NaN3) on a 100 × 2.5-cm column of Sephacryl S-300 (GE Healthcare) (39). For each experiment Ca-ATP-actin was converted to Mg-ATP-actin by incubating for at least 2 min at 25 °C after adjusting buffer G to 50 μm MgCl2 and 0.2 mm EGTA. For bulk fluorescence experiments, purified actin filaments were labeled on cysteine 374 with pyrene iodoacetamide (Invitrogen), depolymerized, clarified, and gel-filtrated on Sephacryl S-300 (40). For microscopy experiments, actin filaments in 50 mm PIPES, pH 6.8, 50 mm KCl, 0.2 mm CaCl2, 0.2 mm ATP were labeled on lysines by incubating overnight at 4 °C with a 1:13 molar ratio of actin to Alexa Fluor 488 carboxylic acid succinimidyl ester (A-20000; Invitrogen). After depolymerization, clarification, and gel filtration on Sephacryl S-300, purified Alexa 488-actin monomers were typically ∼30–50% labeled.
Recombinant fission yeast cofilin was purified (2) from bacteria induced with 1 mm isopropyl β-d-thiogalactopyranoside at 37 °C for 5 h, lysed by sonication, and clarified. Protein was precipitated with 70% ammonium sulfate, resuspended in buffer D (10 mm Tris-Cl, 1 mm NaN3, 1 mm EDTA, 250 mm NaCl, 2 mm DTT, pH 8.0), and gel-filtered on a 400-ml column of Sephacryl S-200. Peak cofilin fractions were dialyzed against DEAE buffer 2 (25 mm Tris-Cl, pH 8.4, 2 mm DTT), were loaded on a DEAE-Sepharose column, and eluted with a 500-ml gradient of 0–500 mm NaCl in DEAE buffer 2. Human cofilin 1 was expressed in pLysS cells induced with 1 mm isopropyl β-d-thiogalactopyranoside for 4 h at 37 °C. Cell pellets were resuspended in 10 mm Tris-Cl (pH 7.5), 1 mm EGTA, 2 mm DTT; lysed by sonication; and clarified. The lysate supernatant was then applied to DEAE-Sepharose. The flow-through was collected; dialyzed against 150 mm NaCl, 10 mm Tris-Cl (pH 7.5), 2 mm DTT; and gel-filtered on Sephacryl S-200 in the same buffer. Both fission yeast and human cofilin were dialyzed into KMEI buffer (50 mm KCl, 1 mm MgCl2, 1 mm EGTA, 10 mm imidazole, pH 7.0), flash-frozen in aliquots, and stored at −80 °C.
Equilibrium Binding Experiments
We measured the affinity of Aip1 for pyrenyl-actin filaments by fluorescence quenching. The reactants were mixed manually in KMEI buffer in a 96-well plate and incubated for 30 min at room temperature, and fluorescence was measured with a Spectra Max Gemini XPS (Molecular Devices, Downingtown, PA) plate reader with excitation at 365 nm and emission at 407 nm.
Bulk Assembly and Disassembly of Actin Filaments
We monitored the assembly of pyrenyl-actin filaments using a PTI Alphascan fluorimeter (Photo Technology International) with excitation at 365 nm and emission at 407 nm. At the start of each reaction, 2 μm pyrenyl-ATP-Ca2+-actin (10% labeled) in buffer G was brought to 50 μm MgCl2, 0.2 mm EGTA for 2 min at 25 °C to exchange Mg2+ for Ca2+ and then was polymerized in KMEI buffer. The concentration of barbed ends was calculated using the equation [ends] = assembly rate/(k+ [actin monomer]), where k+ is the elongation rate constant measured under the same conditions by TIRF microscopy (see below), and the actin monomer concentration is the total actin concentration minus the polymerized actin concentration.
Total Internal Reflection Fluorescence Microscopy
Glass flow chambers were prepared as described previously (41). Before introducing actin, each chamber was incubated for 1 min with two washes of 8 μl of 0.5% Tween 80 in high salt TBS (50 mm Tris-Cl, pH 7.5, 600 mm KCl), followed by two washes with 10 μl of high salt TBS, two 30-s incubations with 8 μl of 250 nm N-ethylmaleimide-inactivated chicken skeletal muscle myosin, two washes with 10 μl of high salt TBS, and two 30-s incubations with 8 μl of 10% BSA in high salt TBS. The standard microscopy buffer consisted of 10 mm imidazole, pH 7.0, 50 mm KCl, 1.0 mm EGTA, 1 mm MgCl2, 1 mm EGTA, 0.3 mm ATP, 15 mm glucose, 50 mm DTT, 0.02 μm CaCl2, 20 μg/ml catalase, 100 μg/ml glucose oxidase, and 0.5% methylcellulose (4,000 centipoise at 2% (w/v)).
Polymerization was initiated by introducing actin into the chamber with or without Aip1 in microscopy buffer. For depolymerization and severing experiments, actin was polymerized in the chamber for ∼5 min, and then the solution was replaced with a fresh sample of proteins (Aip1 and/or cofilin) in microscopy buffer, and imaging was continued. We generated time lapse movies of growing or shortening actin filaments using prism-style TIRF microscopy on an Olympus IX70 inverted microscope and a Hamamatsu C4742-95 CCD camera controlled by MetaMorph software (Molecular Devices) (41). Specimens were illuminated for 1 s at intervals of 10 s for polymerization and depolymerization experiments and 5 s for severing experiments. Images were processed with NIH ImageJ software. For severing experiments, we monitored the elongation of each filament by time lapse imaging prior to introduction of cofilin and Aip1 into the chamber, allowing us to distinguish faster growing barbed ends from slowly growing pointed ends. For each sample, we measured the rates of barbed end elongation, shortening or severing of 10–15 filaments, typically over a span of at least 300 s.
Genetics and Molecular Genetics
The Δaip1 and Δcrn1 mutants were generated by replacing the ORFs of the fission yeast genes with the ura4+ cassette in pFA6a-URA4 vector using a PCR-based homologous recombination method (42). Overexpression of Aip1 or GFP-Aip1 was driven by the strong 3nmt1 promoter, which replaced the endogenous promoter in the genome, but only overexpression of Aip1 rescued the growth defect of cofilin mutants. Genetic crosses were carried out at 25 °C for 3 days before tetrad dissections. For dilution assays, we used the cell cultures grown at 25 °C with A600 between 0.3 and 0.6. Table 1 lists the yeast strains used in this study.
TABLE 1.
Fission yeast strains used in this study
| Name | Genotype | Source |
|---|---|---|
| FY527 | h- leu1-32 ura4-D18 his3-D1 ade6-M216 | Lab stock |
| QC101 | h- aip1::ura4+ leu1-32 ura4-D18 his3-D1 ade6-M216 | This study |
| QC514 | aip1::ura4+ sad1-mEGFP-KanMX6 rlc1-tdTomato-NatMX6 | This study |
| QC274 | rlc1-tdTomato-NatMX6 sad1-mEGFP-KanMX6 | Lab stock |
| QC109 | aip1::ura4+ rlc1-3GFP-kanMX6 | This study |
| QC289 | h− wsp1Δ::kanMX6 leu1-32 ura4-D18 his3-D1 ade6-M216 | Lab stock |
| QC313 | aip1::ura4+ wsp1::KanMX6 | This study |
| QC40 | h− cdc12-112 ade6-M210 leu1-32 ura4-D18 | Lab stock |
| QC155 | aip1::ura4+ cdc12-112 | This study |
| KV7 | h− acp2Δ::kanMX6 ade6-M216 his7-366 leu1-32 ura4-D18 | Lab stock |
| KV112 | h− acp1Δ::kanMX6 ade6-M216 his7-366 leu1-32 ura4-D18 | Lab stock |
| QC152 | aip1::ura4+ acp1Δ::kanMX6 | This study |
| QC546 | h+ crn1::ura4+ leu1-32 ura4-D18 his3-D1 ade6-M? | This study |
| QC550 | crn1::ura4+ aip1::ura4+ | This study |
| QC102 | h− adf1M2-KanMX6 leu1-32 ura4-D18 his3-D1 ade6-M216 | Ref. 8 |
| QC93 | h− aip1::KanMX6-3nmt1-mEGFP-aip1 leu1-32 ura4-D18 his3-D1 ade6-M216 | This study |
| QC517 | h− aip1::KanMX6-P3nmt1-aip1 leu1-32 ura4-D18 his3-D1 ade6-M216 | This study |
| QC518 | aip1::KanMX6-P3nmt1-aip1 adf1-M2-kanMX6 | This study |
| JW1558 | h− kanMX6-P3nmt1-mEGFP-lifeact ade6-M210 leu1-32 ura4-D18 | Jianqiu Wu |
| QC463 | kanMX6-P3nmt1-mEGFP-lifeact aip1::ura4+ | This study |
| KGY978 | h− arp3-c1 ura4-D leu1-32 ade6-M210 | Lab stock |
| QC312 | aip1::ura4+ arp3-c1 | This study |
| MB9 | cdc8-27 his7-366 leu1-32 ura4-D18 ade6-M216 | Lab stock |
| QC154 | aip1::ura4+ cdc8-27 | This study |
| QC168 | gmf1::ura4+ leu1-32 ura4-D18 his3-D1 ade6-M216 | This study |
| QC311 | gmf1::ura4+ aip1::ura4+ | This study |
| QC341 | h+ end4::end4Δ663-1102-NatMX6 | Ref. 7 |
| QC453 | aip1::ura4+ end4::end4Δ663-1102-NatMX6 | This study |
| QC441 | ent1:ent1Δ647-706-mEGFP-KanMX6 | Ref. 7 |
| QC452 | aip1::ura4+ ent1:ent1Δ647-706-mEGFP-KanMX6 | This study |
| QC437 | h+ pan1::pan1Δ1743-1794-NatMX6 | Ref. 7 |
| QC454 | aip1::ura4+ pan1::pan1Δ1743-1794-NatMX6 | This study |
| TP190 | h− leu1-32 ura4-D18 his3-D1 ade6-M216 myo1::KanMX6 | Lab stock |
| QC314 | aip1::ura4+ myo1::KanMX6 | This study |
Fluorescence Microscopy
Yeast cells were cultured by standard methods at 25 °C. To visualize actin filaments, cells were fixed with 4% formaldehyde in TEMK buffer (50 mm Tris-Cl, pH 7.4, 1 mm EGTA, 2 mm MgCl2, 50 mm KCl) and permeabilized with 1% Triton X-100 in TEMK buffer before staining with 2 μm Bodipy-phallacidin (Invitrogen) in TEMK buffer. For imaging live cells, actively growing cultures in YE5s medium with A600 between 0.4 and 0.5 were harvested by centrifugation at 3000 rpm for 1 min and washed briefly three times with EMM5s before resuspending, applying to a 25% gelatin pad made with EMM5s and sealing under a coverslip. Cells were imaged with a 100× Plan Apochromat objective lens (NA 1.40) on an Olympus IX-71 microscope equipped with a CSU-X1 confocal spinning disk unit (Yokogawa) with either an Orca-ER camera (Hamamatsu) for fixed cells or electron multiplying charge-coupled device camera (Andor, iXon) for live cells. We measured the cytoplasmic concentration of Aip1 to be 1.0 ± 0.3 μm (n = 81) in aip1::Paip1-mGFP-aip1 cells (43) by confocal fluorescence microscopy (44).
RESULTS
We purified recombinant fission yeast Aip1 (SpAip1) and human Aip1 (HsAip1) (Fig. 1, A and B) and studied their interactions with actin filaments. These homologs share 33% sequence identity, and homology models based on the crystal structure of Saccharomyces cerevisiae Aip1 predict that both consist of two seven-bladed β-propeller domains.
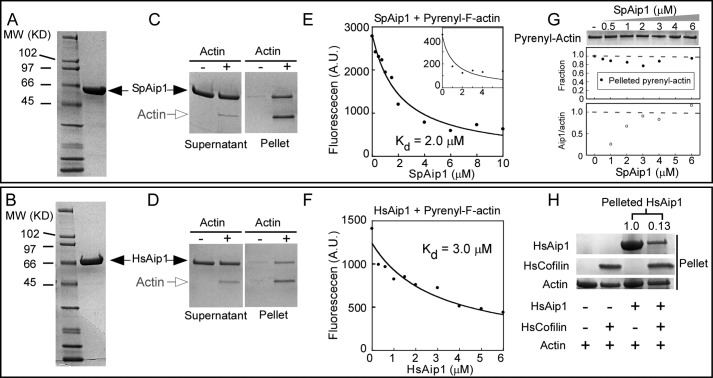
FIGURE 1.
Fission yeast SpAip1 and human HsAip1 bind actin filaments. A and B, SDS-PAGE of standards and purified SpAip1 (A) and HsAip1 (B) stained with Coomassie Blue. C and D, cosedimentation of Aip1 with actin filaments. Samples of 1.5 μm SpAip1 (C) or 1.5 μm HAip1 (D) were incubated with 1 μm actin filaments for 1 h and then centrifuged at 100,000 × g for 25 min. Supernatant and pellet samples were analyzed by SDS-PAGE and stained with Coomassie Blue. The SpAip1 samples were twice the volumes of the HsAip1 samples. E and F, fluorescence quenching to measure equilibrium binding of a range of concentrations of SpAip1 (E) or HsAip1 (F) to 1 μm 100% labeled pyrenyl-actin filaments in KMEI buffer at 25 °C. The smooth curves are fits of the binding equation to the data yielding Kd values of 2.0 μm for SpAip1 and 3.0 μm for HsAip1. The inset in E shows fluorescence quenching of 1 μm 20% pyrenyl-actin filaments SpAip1 with the best fit (smooth curve) yielding a Kd of 1.2 μm. G, pelleting assay: 1 μm 94% labeled pyrenyl-actin filaments were incubated with a range of concentrations of SpAip1 and centrifuged at 100,000 for 25 min. Actin in the pellet samples were analyzed by SDS-PAGE and stained with Coomassie Blue (top panel) and quantified using NIH ImageJ (middle panel). The ratio of SpAip1 to actin in the pellet samples was quantified to measure the stoichiometry between SpAip1 and actin (bottom panel). H, pelleting assay: 2 μm actin filaments were incubated with either 15 μm HsCofilin or 10 μm HsAip1 or both and centrifuged at 100,000 × g for 1 h. Pellet samples were analyzed by SDS-PAGE and stained with Coomassie Blue. The intensities of the HsAip1 bands were quantified with NIH ImageJ.
Aip1 Binds to the Sides of Actin Filaments
Like Aip1 of other organisms (16, 35, 45, 46), both SpAip1 and HsAip1 pellet with chicken muscle actin filaments (Fig. 1, C and D). At saturation, the stoichiometry was 1 SpAip1 per polymerized actin subunit (Fig. 1G). We measured the affinities of these interactions with fluorescence spectroscopy by taking advantage of the fact that Aip1 binding quenches the fluorescence of pyrenyl-actin filaments (Fig. 1, E and F). Both fission yeast and human Aip1 reduced the fluorescence of 1 μm pyrenyl-actin filaments in a concentration-dependent fashion (Fig. 1, E and F) but did not depolymerize the filaments (Fig. 1G). Quenching was similar for 20 and 100% pyrenyl-labeled actin filaments (Fig. 1E, inset). Fitting binding curves to the dependence of the fluorescence quenching on Aip1 concentration gave Kd values of 2.0 μm for SpAip1 and 3.0 μm for HsAip1. Binding of HsAip1 to actin filaments was significantly lower in the presence of HsCofilin (Fig. 1H), showing competition between the two proteins for binding actin filaments.
Effects of Aip1 on Actin Polymerization and Depolymerization
Direct observations by total internal reflection fluorescence (TIRF) microscopy showed that actin filament barbed ends elongated at all concentrations of SpAip1 and HsAip1 that we tested (Fig. 2, A and A′). Submicromolar concentrations of SpAip1 increased the rate of barbed end elongation by ∼50%, a small but reproducible effect, whereas higher concentrations slowed polymerization (Fig. 2A). In contrast, HsAip1 modestly slowed but did not completely halt barbed end elongation at all concentrations tested (Fig. 2A′). Thus, neither human nor fission yeast (recombinant) Aip1 caps barbed ends on their own at the concentrations tested.
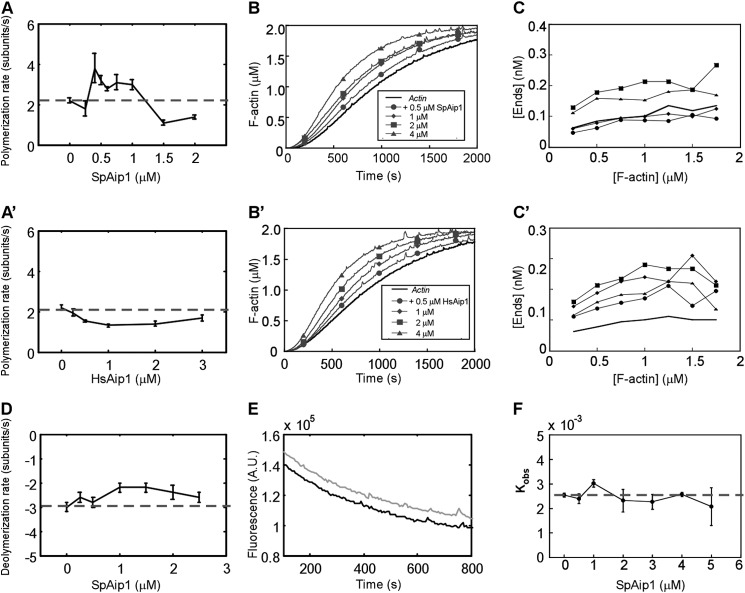
FIGURE 2.
Effects of Aip1 on actin polymerization. A and A′, dependence of the rate of barbed end elongation of 0.5 μm Mg-ATP-actin on the concentration of SpAip1 (A) and HsAip1 (A′) measured by TIRF microscopy. The error bars are ±1 standard deviation of the mean elongation rates of at least 10 filaments. The dashed line is the polymerization rate of actin alone. The dashed line is the polymerization rate of actin alone. B and B′, time course of spontaneous polymerization measured by fluorescence of 2 μm Mg-ATP-actin (10% pyrene-labeled) with a range of concentrations of SpAip1 (B) or HsAip1 (B′). C and C′, concentrations of actin filament barbed ends as actin polymerized in B and B′, taking into account the elongation rates measured in A and A′. D, dependence of barbed end depolymerization rates on the concentration of SpAip1, measured by TIRF microscopy. Filaments were grown in the observation chamber from 0.5 μm Mg-ATP-actin monomers in the presence of SpAip1, followed by replacement with SpAip1 in polymerization buffer without actin monomers. The error bars are ±1 standard deviation of the mean elongation rate of at least 10 filaments. E, time course of depolymerization of pyrenyl-actin filaments diluted to 0.1 μm in KMEI buffer at 25 °C with no SpAip1 (black line) or with 400 nm SpAip1 (gray line). The dashed line is the depolymerization rate of actin alone. F, the depolymerization rate constants of 0.1 μm pyrenyl-actin filaments over a range of concentrations of SpAip1. The dashed line is the depolymerization rate of actin alone. The error bars are ±1 standard deviation of the mean depolymerization rates.
In the presence of HsAip1, more filaments appeared in TIRF microscopy experiments, so we analyzed the time course of spontaneous polymerization of 2 μm actin monomers with a range of concentrations of SpAip1 or HsAip1 (Fig. 2, B and B′) to measure the rates of nucleation (Fig. 2, C and C′). We normalized these curves to correct for quenching of the pyrene fluorescence. Calculating the number of filament ends from the polymerization rate depended on knowing the rate of barbed end elongation at each Aip1 concentration (Fig. 2, A and A′). Both SpAip1 and HsAip1 modestly increased the number of ends in polymerization reactions (Fig. 2, C and C′), suggesting that they stabilize nuclei.
A range of SpAip1 concentrations did not change the rate that subunits dissociated from barbed ends (Fig. 2D) or the total depolymerization rate of actin filaments in bulk assays (Fig. 2, E and F). This is additional evidence that (recombinant) Aip1 does not cap the barbed ends of actin filaments on its own, unlike capping protein, which caps barbed ends with low nanomolar affinity (47).
Aip1 Enhances Severing of Actin Filaments by Cofilin
Aip1 was reported to enhance cofilin-mediated filament severing (14, 48), but this process was not observed directly, so we examined the effects of Aip1 and cofilin on actin filament severing by TIRF microscopy (Fig. 3, A and B). To keep severing slow, we used 100 nm fission yeast cofilin, a concentration well above the 10 nm optimum. SpAip1 dramatically stimulated severing by 100 nm cofilin, with a maximum rate at 1.5 μm SpAip1 and lower rates at higher concentrations (Fig. 3C). At all SpAip1 concentrations tested ∼80% of new barbed ends created by severing events depolymerized (Fig. 3D) at rates that decreased insignificantly with SpAip1 concentration (Fig. 3E). These depolymerization rates were higher than published values (2), likely because severing near barbed ends was difficult to distinguish from filament depolymerization.
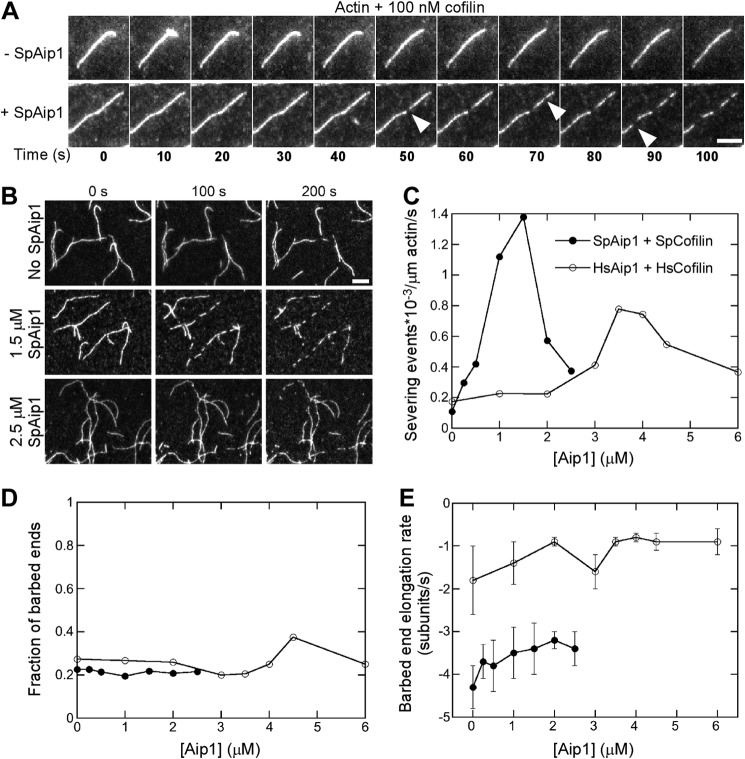
FIGURE 3.
Aip1 enhances actin filament severing by cofilin observed by TIRF microscopy in standard microscopy buffer. A and B, time series of images showing actin filament severing by 100 nm fission yeast cofilin with or without SpAip1. Bars, 5 μm. A, time lapse images at 10-s intervals of single actin filaments with 0 or 1.5 μm Aip1. B, images at 100-s intervals of a field of actin filaments being severed with 0, 1.5, or 2.5 μm SpAip1. Filament seeds were grown from 0.5 μm Mg-ATP-actin monomers in the presence of SpAip1, followed by a wash and replacement with 100 nm cofilin with SpAip1 in polymerization buffer without actin monomers. C, dependence of the severing activity of 100 nm fission yeast cofilin or 7 μm human cofilin 1 on the concentration of SpAip1 (filled circles) or HsAip1 (open circles). D, dependence of the fraction of new barbed ends that do not shorten following severing on the concentration of SpAip1 (filled circles) and HsAip1 (open circles). E, dependence of the depolymerization rates of new barbed ends created by fission yeast cofilin severing events on the concentration of SpAip1 (filled circles) and HsAip1 (open circles). The data were collected as for A. The error bars are ±1 standard deviation of the mean elongation rates of at least 6–10 filaments for experiments done with SpAip1 or HsAip1. The depolymerization rates do not vary significantly across this range of concentrations of either SpAip1 or HsAip1 (p > 0.1, single factor analysis of variance test).
HsAip1 stimulated severing by human cofilin 1 with an optimum concentration of 3.5 μm (Fig. 3C). We used 7 μm human cofilin 1 in these experiments, because of its lower affinity for actin filaments and its higher optimal concentration for severing. In the absence of HsAip1, basal levels of severing were similar to those of 100 nm fission yeast cofilin. HsAip1 did not increase the fraction of barbed ends of severed filaments that did not shorten, consistent with a lack of capping activity in the presence of cofilin (Fig. 3D). HsAip1 did not significantly slow the rate of depolymerization of barbed ends created by severing (Fig. 3E). These results and the evidence that HsAip1 competes with HsCofilin for binding actin filaments (Fig. 1H) suggest that Aip1 increases severing by reducing the binding density of cofilin on the filaments into a range that favors severing.
Genetic Interactions of Δaip1
Cells lacking Aip1 grew normally (Fig. 5A), but genetic crosses revealed negative interactions between Δaip1 and mutations of a subset of genes for proteins involved with endocytosis (Fig. 4; Table 2). The strongest negative interactions were with two cofilin mutations that reduce the severing activity of cofilin (7). Reciprocally, overexpression of Aip1 partially rescued the slow growth of these cofilin mutant strains (Fig. 4C). The Δaip1 mutation also had strong negative genetic interactions with deletion of either subunit of capping protein, Acp1 and Acp2 (Fig. 4B). Although both interacted with Δaip1, the capping protein deletion mutations did not interact with the cofilin mutant adf1-M2, and overexpression of Acp2 did not rescue the growth defect of adf1-M2. Aip1 had no genetic interaction with genes for several other proteins that participate in endocytosis (Arp2/3 complex, Ent1p, End4p, and Pan1p), including the coronin deletion mutant Δcrn1, even though Δcrn1 is synthetically lethal with the cofilin mutants (Fig. 4E). The Δaip1 deletion had positive genetic interactions with temperature-sensitive formin mutation cdc12-112 and deletion mutation of the Arp2/3 complex nucleation promoting factor Δwsp1 (Fig. 4A).
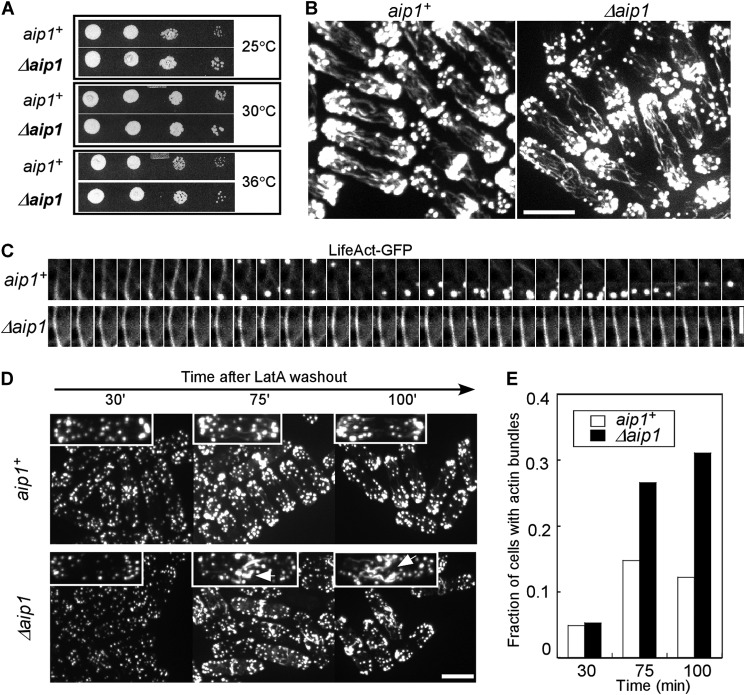
FIGURE 5.
Effects of aip1 deletion on fission yeast. A, serial of dilutions of wild type (aip1+) and deletion mutants (Δaip1) grown at three different temperatures on YE5S plates. B, wild type and Δaip1 cells fixed and stained with Bodipy-phallacidin are indistinguishable in spinning disk confocal fluorescence micrographs. Bar, 5 μm. C, time lapse at 2 s interval of confocal fluorescence micrographs of a single plane of actin cables in a wild type cell and a Δaip1 cell expressing LifeAct-mGFP as a marker for actin filaments. Bar, 2 μm. D, fluorescence micrographs of the time course of the recovery of wild type and Δaip1 cells from treatment with 10 μm LatA for 15 min. Cells were stained with Bodipy-phallacidin 30, 75, and 100 min after washing out LatA. One representative cell is enlarged for each time point. Arrows, thick actin bundles. Bar, 5 μm. E, the histogram shows the fraction of wild type and Δaip1 cells (n > 100) with thick actin bundles at times after LatA washout.
FIGURE 4.
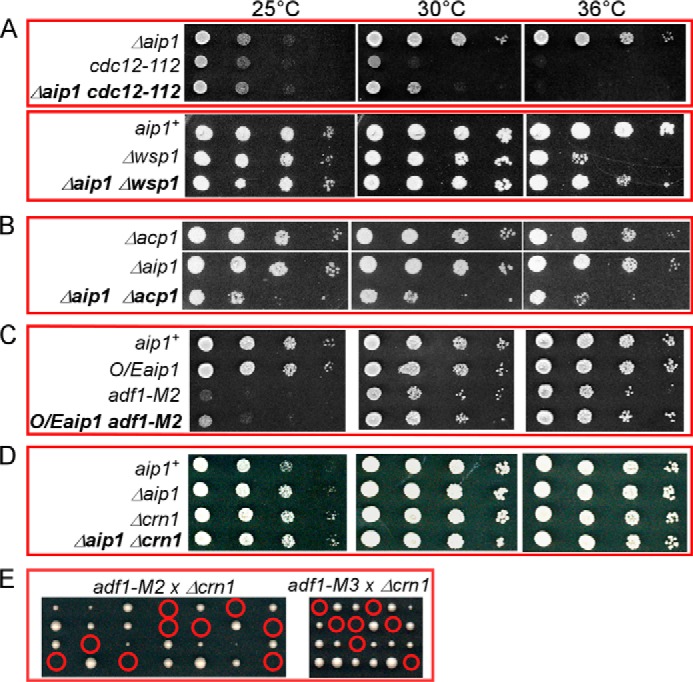
Analysis of genetic interactions of Δaip1. A–D, ten fold serial dilutions of cells were grown for 2 days on YE5s plates at three temperatures, related to Table 2. A, the Aip1 null mutation Δaip1 partially rescued the growth defects of the cdc12-112 and Δwsp1 mutants. B, the Aip1 null mutation had negative genetic interactions with deletion of the genes for each subunit of capping protein; Δaip1Δacp1 grew slowly, whereas Δaip1Δacp2 was not viable. C, overexpression (O/E) of Aip1 partially rescued the slow growth of adf1-M2 mutant. D, the Aip1 null mutation had no genetic interaction with the deletion mutant of coronin (crn1). E, tetrad dissection plates of Δcrn1 crossed with either adf1-M2 or adf1-M3 show that the Crn1 null mutation is synthetically lethal with cofilin mutants. Red circles highlight inviable double mutants.
TABLE 2.
Genetic interactions of the Aip1 deletion mutation Δaip1
| Gene | Protein | Mutations | Genetic interaction |
|---|---|---|---|
| adf1 | Cofilin | adf1-M2, adf1-M3 | Synthetically lethal (8) |
| arp3 | Arp2/3 complex subunit | arp3-c1 | None |
| acp1 | Capping protein subunit | Δacp1 | Slow growth at all temperatures |
| acp2 | Capping protein subunit | Δacp2 | Synthetically lethal |
| cdc8 | Tropomyosin | cdc8-27 | None |
| cdc12 | Formin | cdc12-112 | Partial rescue of growth defect at 30 °C |
| crn1 | Coronin | Δcrn1 | None |
| end4 | Endocytosis adapter | end4Δ663-1102 | None |
| ent1 | Endocytosis adapter | ent1Δ647-706 | None |
| gmf1 | Glial maturation factor | Δgmf1 | None |
| pan1 | Endocytosis adapter | pan1Δ1743-1794 | None |
| myo1 | Myosin-I | Δmyo1 | None |
| wsp1 | WASp | Δwsp1 | Partial rescue of growth defect at 36 °C |
Aip1 Increases the Turnover of Actin Cables in Cells
The fission yeast lacking Aip1 have normal appearing actin patches, cables, and contractile rings (Fig. 5B). Because the rapid turnover of actin cables in budding yeast relies on the activity of cofilin (31), we tested whether Aip1 influences the dynamics of cables in fission yeast. Actin cables labeled with LifeAct-GFP turned over in <20 s in wild type cells but persisted for more than 100 s in Δaip1 mutants (Fig. 5C). Treating cells with 10 μm latrunculin A (LatA) depolymerized actin cables, contractile rings, and most actin patches in 15 min. After washing out LatA, actin patches, followed by contractile rings and actin cables, recovered in most wild type cells within 100 min (Fig. 5, D and E). After LatA treatment, most Δaip1 cells reassembled actin patches normally, but 30% assembled large bundles of actin filaments either perpendicular or parallel to the long axis of cells (Fig. 5, D and E).
Contributions of Aip1 to Cytokinesis
The severing of actin filaments by cofilin plays a critical role in both endocytosis and cytokinesis in fission yeast (7, 8). Because Aip1 enhances the severing of actin filaments by cofilin in vitro, we expected that it participates in these two cellular processes as well. Another study presents an analysis of Aip1 in endocytosis (43). Our study is focused on the contribution of Aip1 to cytokinesis. Mutations in aip1 genes cause cytokinesis defects in many organisms (18, 22, 25), although a mechanistic understanding is lacking.
We used time lapse fluorescence microscopy to compare the assembly, maturation, constriction, and disassembly of actomyosin contractile rings in fission yeast cells with and without Aip1 (Fig. 6A and Table 3). We used cells expressing both a contractile ring marker, the myosin II regulatory light chain Rlc1p-tdTomato, and a spindle pole body (SPB) marker, Sad1p-mGFP, to track these events in time. Separation of the SPBs was defined as time 0.
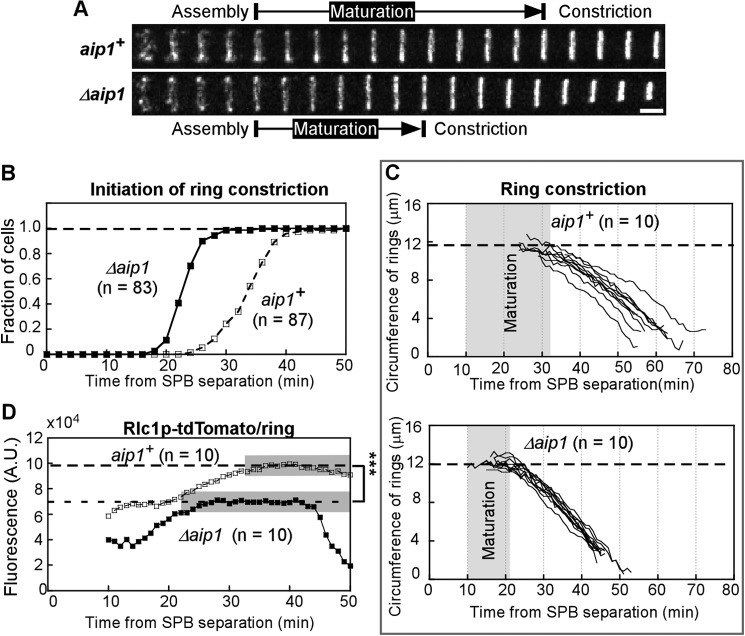
FIGURE 6.
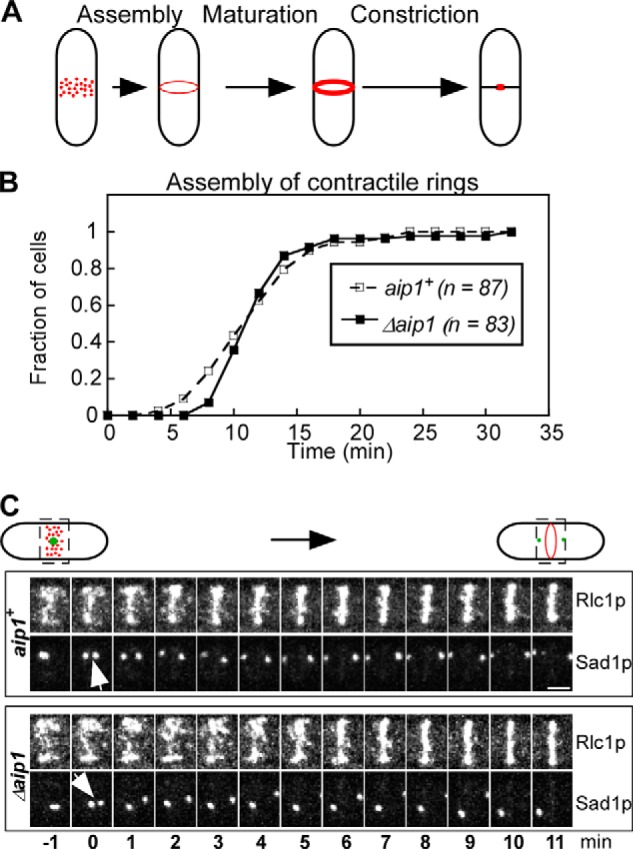
Analysis of contractile ring assembly in wild type and Δaip1 cells expressing Rlc1p-tdTomato and Sad1p-mGFP, related to Table 3. A, cartoon representation of three stages of ring assembly in fission yeast: assembly of the contractile ring from nodes (red dots), maturation of the ring, and constriction. B, outcomes plot of the accumulation of cells with a complete contractile ring over time after the separation of SPBs (time 0). Open squares, wild type cells; filled squares, Δaip1 cells. The difference between the two plots is not statistically significant (p > 0.1), according to a log rank test. C, time series of fluorescence micrographs (maximum intensity projections of Z-series of whole cells) of the division plane (rectangular areas outlined by dashed lines) in a wild type (aip1+) and Δaip1 cell during the assembly of contractile rings. Bar, 2 μm. Numbers are times in min. Arrows point to the separation of SPBs at time 0.
TABLE 3.
Comparison of the time for cytokinesis stages in aip1+ and Δaip1 cells
| Genotype | Ring assembly | Ring maturation | Ring constriction |
|---|---|---|---|
| min | min | min | |
| aip1+ | 11.1 ± 0.5 (n = 89) | 22.6 ± 6.1 (n = 53) | 34.2 ± 4.8 (n = 66) |
| Δaip1 | 11.9 ± 0.4 (n = 83) | 11.4 ± 3.1 (n = 51) | 29.3 ± 2.4 (n = 59)a |
a p < 0.0001 (two-tailed Student's test).
The time course of contractile ring assembly did not differ significantly (log rank test) in wild type and Δaip1 cells (Figs. 6B and C and Table 3). However, newly formed contractile rings in Δaip1 cells had fewer Rlc1p molecules than the wild type cells (Fig. 7D).
FIGURE 7.
Analysis of contractile ring constriction in wild type and Δaip1 mutant cells expressing Rlc1p-tdTomato and Sad1p-mGFP, related to Table 3. A, time series at 2 min interval of fluorescence micrographs (maximum intensity projections of Z-series of whole cells) of the division plane of a wild type (aip1+) and Δaip1 cell during cytokinesis. Bar, 2 μm. B, outcomes plot of the accumulation of cells that have initiated the constriction of contractile rings after the separation of SPBs (time 0). Open squares, wild type cells; filled squares, Δaip1 cells. C, time courses of circumferences of constricting rings in wild type cells (top panel) and Δaip1 cells (bottom panel). The dashed line is the average circumference of fully formed contractile rings. The period of ring maturation is shaded gray. D, average fluorescence intensities of Rlc1p-tdTomato in contractile rings over time after spindle pole body separation (n = 10). The period of ring constriction is shaded gray. Open squares, wild type cells; filled squares, Δaip1 cells. The peak fluorescence intensities (dashed lines) of Rlc1p-tdTomato in wild type contractile rings (98,000 ± 27,000, n = 50) are significantly higher than those in Δaip1 mutant rings (69,000 ± 13,000, n = 50) (p < 0.0001, two-tailed Student's test).
Surprisingly, contractile rings began to constrict earlier in Δaip1 cells than wild type cells (Fig. 7, A and B, and Table 3), foreshortening the maturation period before constriction (49) when several proteins are recruited to rings. Maturing contractile rings accumulated Rlc1p at similar rates in Δaip1 and wild type cells, but the rings in Δaip1 cells ended up with ∼30% fewer Rlc1p molecules, because they started with fewer Rlc1p molecules and because constriction started early (Fig. 7D).
As in wild type cells (49), the total fluorescence of Rlc1p-tdTomato in rings of Δaip1 cells was constant through most of ring constriction (Fig. 7D, gray shaded area). Contractile rings in Δaip1 cells constricted at 0.42 ± 0.01 μm/min (n = 10), slightly faster than rings in wild type cells (0.31 ± 0.01 μm/min, n = 10, p < 0.001) (Fig. 7C). As a result, ring constriction in Δaip1 cells takes ∼ 5 min less than in wild type cells (Table 3). Overall, cytokinesis was ∼16 min (24%) faster in Δaip1 cells than wild type cells, because the maturation period was shorter and the rings constricted faster.
When the circumference of rings decreased to ∼3 μm (Fig. 8A, shaded area), myosin II marked by Rlc1p-tdTomato rapidly dispersed into the cytoplasm as constriction finished. This loss of Rlc1p-tdTomato fluorescence followed single exponential time courses with similar rate constants in wild type and Δaip1 cells (Fig. 8B). Because rings finished constricting ∼5 min faster in Δaip1 than wild type cells, dissociation of Rlc1p-tdTomato was incomplete in most Δaip1 cells (Fig. 8C). The contractile ring remnants marked with Rlc1p-tdTomato moved away from the division plane through the cytoplasm toward the poles before disappearing over 8 min (Fig. 8C). In contrast, such movements of ring remnants were only seen in 40% of wild type cells and lasted only ∼4 min on average. Fast imaging of the ring remnants labeled by Rlc1p-3GFP confirmed their rapid movement in the cytoplasm of Δaip1 cells (Fig. 8D).
FIGURE 8.
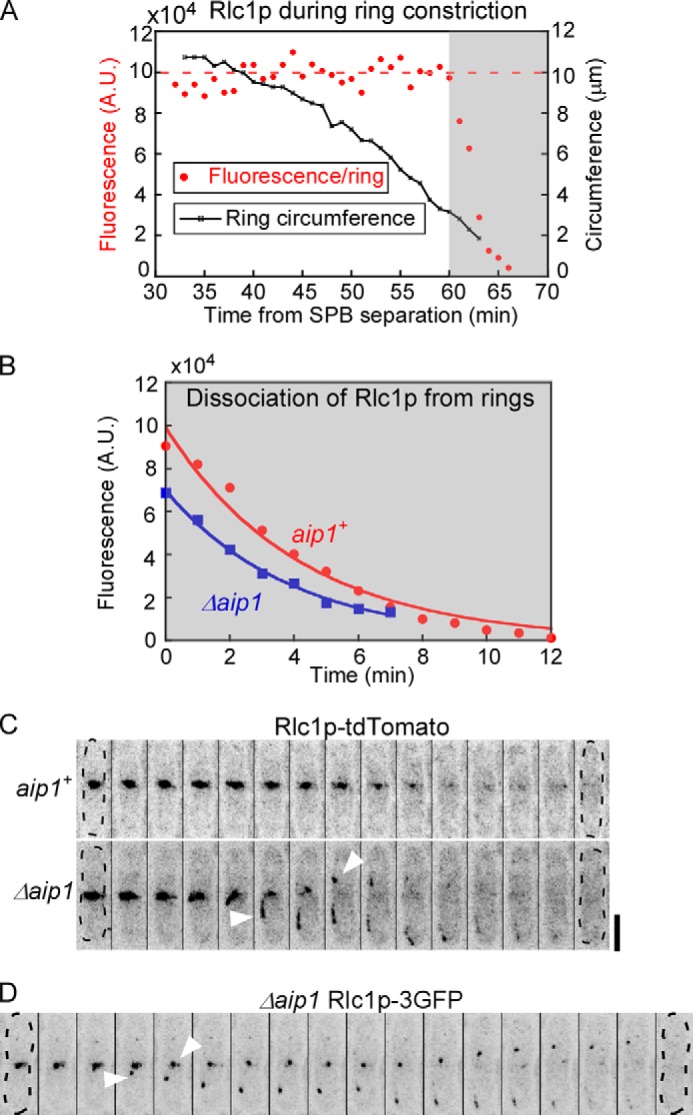
Defective dissociation of myosin II from contractile rings in Δaip1 cells. A, time courses of the circumference (open squares) and the fluorescence intensity (red circles) of contractile rings in a wild type cell expressing Rlc1p-tdTomato. Time is relative to the separation of SPBs at time 0. The shaded area highlights the rapid dissociation of Rlc1p from the contractile rings as their circumference decreased to <4 μm. B, time courses of the average fluorescence intensities of Rlc1p-tdTomato that is dissociating from the rings of (red circles) aip1+ cells and (blue squares) Δaip1 cells; both data sets fit with single exponential curves (solid lines). C and D, time series of fluorescence micrographs (reverse contrast, maximum intensity projections) of a wild type (aip1+) and a Δaip1 cell expressing Rlc1p-tdTomato (C) and a Δaip1 cell expressing Rlc1p-3GFP (D). Arrowheads mark ring remnants dissociating from the ring and moving toward the cell poles in Δaip1 cells. Cells are outlined in dashed lines. The time intervals are 60 s in (C) and 30 s in (D). Bar, 5 μm.
DISCUSSION
Previous work provided evidence that Aip1 helps cofilin disassemble actin filaments and might cap the newly formed barbed ends. Our evidence suggests that Aip1 enhances severing by limiting binding of cofilin to ADP-actin filaments. Aip1 did not cap or slow depolymerization of the new barbed ends under the conditions of our assays. Our experiments on fission yeast show that Aip1 participates in the turnover of actin cables and constriction of actomyosin contractile rings.
Aip1 Enhances the Severing Activity of Physiological Concentrations of Cofilin
Our evidence supports the hypothesis that Aip1 enhances the severing of actin filaments by competing with cofilin for binding sites on the actin filament, as originally shown for phalloidin and myosin (13). Competition by Aip1 lowers the binding density of high concentrations of cofilin into a range where severing is favored. The ∼20 μm concentration of cofilin in the cytoplasm of fission yeast (7) is more than 3 orders of magnitude higher than the optimal concentration for steady state severing (2). High concentrations of cofilin can sever newly polymerized filaments transiently before they saturate the filament (50), so competition from Aip1 should prolong this period of severing.
However, other mechanisms must participate in cells, because the 1 μm cytoplasmic concentration of Aip1 is lower than the 20 μm concentration of cofilin, and the ratio of GFP-Aip1 molecules to cofilin in endocytic actin patches is <1:20 (7, 43), much lower than what is required to enhance the severing activity of cofilin in our in vitro experiments. Another potential competitor, fimbrin, is present in greater numbers than Aip1 (44). The β-propeller protein coronin also facilitates severing filaments by cofilin (51), and deletion of coronin was synthetically lethal with our cofilin severing mutants (Fig. 4E), but not with deletion of Aip1.
In addition to direct competition, binding of the two β-propeller domains of Aip1 may change the flexibility of actin filaments, making them more favorable for severing by cofilin. In addition, a complex of Aip1 and cofilin may sever actin filaments more efficiently than cofilin alone (30, 52).
Aip1 purified from Xenopus eggs capped the barbed ends of actin filaments severed by cofilin (35), but we detected no capping activity in our preparations of recombinant human or fission yeast Aip1. Similarly, purified recombinant worm Aip1 did not cap the ends of actin filaments (16). On the other hand, quantitative analysis of actin patch dynamics in cells with aip1+ and capping protein deletions supports the hypothesis that both proteins cap actin filaments (43). The simplest explanation is that Aip1 cooperates with other factors in cells to cap actin filaments and that Aip1 prepared from Xenopus eggs contained those factors that helped to cap the filament ends.
The Role of Aip1 in the Turnover of Actin Cables and Actomyosin Contractile Rings
Our evidence that Aip1 contributes to the turnover of actin cables and contractile rings in fission yeast adds to previous work showing that Aip1 mutations lead to a wide range of defects in many model organisms. Actin cables in fission yeast are bundles of actin filaments nucleated by formin. Cables turn over slower in Δaip1 cells than in wild type cells. The Δaip1 cells are also hypersensitive to LatA, similar to budding yeast with other deletion mutations, including Δcap2 (capping protein), Δtpm1 (tropomyosin), Δsac6 (fimbrin), and Δsla2 (Hip1R) (31, 53), which can be explained by slower turnover of actin filaments in these mutants.
We discovered that Aip1 is also important for the initiation of contractile ring constriction. The contractile rings of Δaip1 mutants constrict prematurely after a shortened maturation stage of ring assembly. Nothing about the known activities of Aip1 or the assembly of contractile rings predicted this striking phenotype, so the Δaip1 mutation has revealed a new feature regulating cytokinesis. Further studies of this function of Aip1 may provide clues in understanding its role in regulating the contractility of both body wall muscle and the somatic gonad of C. elegans (33, 54). Additionally, Δaip1 mutants display a striking phenotype with ring remnants moving away from the division site at the end of cytokinesis as a result of slightly accelerated ring constriction.
Acknowledgments
We thank all the members of Pollard lab for helpful discussions in preparing the manuscript. We thank James Bamburg and Jian-Qiu Wu for sharing materials.
This work was supported, in whole or in part, by National Institutes of Health Grant R01GM026338.
- MBP
- maltose-binding protein
- SPB
- spindle pole body
- TIRF
- total internal reflection fluorescence
- LatA
- latrunculin A.
REFERENCES
- 1. Suarez C., Roland J., Boujemaa-Paterski R., Kang H., McCullough B. R., Reymann A. C., Guérin C., Martiel J. L., De la Cruz E. M., Blanchoin L. (2011) Cofilin tunes the nucleotide state of actin filaments and severs at bare and decorated segment boundaries. Curr. Biol. 21, 862–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andrianantoandro E., Pollard T. D. (2006) Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol. Cell 24, 13–23 [DOI] [PubMed] [Google Scholar]
- 3. De La Cruz E. M. (2005) Cofilin binding to muscle and non-muscle actin filaments: isoform-dependent cooperative interactions. J. Mol. Biol. 346, 557–564 [DOI] [PubMed] [Google Scholar]
- 4. Pavlov D., Muhlrad A., Cooper J., Wear M., Reisler E. (2007) Actin filament severing by cofilin. J. Mol. Biol. 365, 1350–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bernstein B. W., Bamburg J. R. (2010) ADF/cofilin: a functional node in cell biology. Trends Cell Biol. 20, 187–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nakano K., Mabuchi I. (2006) Actin-depolymerizing protein Adf1 is required for formation and maintenance of the contractile ring during cytokinesis in fission yeast. Mol. Biol. Cell 17, 1933–1945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Q., Pollard T. D. (2013) Actin filament severing by cofilin dismantles actin patches and produces mother filaments for new patches. Curr. Biol. 23, 1154–1162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Q., Pollard T. D. (2011) Actin filament severing by cofilin is more important for assembly than constriction of the cytokinetic contractile ring. J. Cell Biol. 195, 485–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohta Y., Nishida E., Sakai H., Miyamoto E. (1989) Dephosphorylation of cofilin accompanies heat shock-induced nuclear accumulation of cofilin. J. Biol. Chem. 264, 16143–16148 [PubMed] [Google Scholar]
- 10. Blanchoin L., Robinson R. C., Choe S., Pollard T. D. (2000) Phosphorylation of Acanthamoeba actophorin (ADF/cofilin) blocks interaction with actin without a change in atomic structure. J. Mol. Biol. 295, 203–211 [DOI] [PubMed] [Google Scholar]
- 11. Chi A., Huttenhower C., Geer L. Y., Coon J. J., Syka J. E., Bai D. L., Shabanowitz J., Burke D. J., Troyanskaya O. G., Hunt D. F. (2007) Analysis of phosphorylation sites on proteins from Saccharomyces cerevisiae by electron transfer dissociation (ETD) mass spectrometry. Proc. Natl. Acad. Sci. U.S.A. 104, 2193–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lappalainen P., Fedorov E. V., Fedorov A. A., Almo S. C., Drubin D. G. (1997) Essential functions and actin-binding surfaces of yeast cofilin revealed by systematic mutagenesis. EMBO J. 16, 5520–5530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Elam W. A., Kang H., De La Cruz E. M. (2013) Competitive displacement of cofilin can promote actin filament severing. Biochem. Biophys. Res. Commun. 438, 728–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rodal A. A., Tetreault J. W., Lappalainen P., Drubin D. G., Amberg D. C. (1999) Aip1p interacts with cofilin to disassemble actin filaments. J. Cell Biol. 145, 1251–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Amberg D. C., Basart E., Botstein D. (1995) Defining protein interactions with yeast actin in vivo. Nat. Struct. Biol. 2, 28–35 [DOI] [PubMed] [Google Scholar]
- 16. Mohri K., Vorobiev S., Fedorov A. A., Almo S. C., Ono S. (2004) Identification of functional residues on Caenorhabditis elegans actin-interacting protein 1 (UNC-78) for disassembly of actin depolymerizing factor/cofilin-bound actin filaments. J. Biol. Chem. 279, 31697–31707 [DOI] [PubMed] [Google Scholar]
- 17. Voegtli W. C., Madrona A. Y., Wilson D. K. (2003) The structure of Aip1p, a WD repeat protein that regulates Cofilin-mediated actin depolymerization. J. Biol. Chem. 278, 34373–34379 [DOI] [PubMed] [Google Scholar]
- 18. Konzok A., Weber I., Simmeth E., Hacker U., Maniak M., Müller-Taubenberger A. (1999) DAip1, a Dictyostelium homologue of the yeast actin-interacting protein 1, is involved in endocytosis, cytokinesis, and motility. J. Cell Biol. 146, 453–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fujibuchi T., Abe Y., Takeuchi T., Imai Y., Kamei Y., Murase R., Ueda N., Shigemoto K., Yamamoto H., Kito K. (2005) AIP1/WDR1 supports mitotic cell rounding. Biochem. Biophys. Res. Commun. 327, 268–275 [DOI] [PubMed] [Google Scholar]
- 20. Lin M. C., Galletta B. J., Sept D., Cooper J. A. (2010) Overlapping and distinct functions for cofilin, coronin and Aip1 in actin dynamics in vivo. J. Cell Sci. 123, 1329–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Okreglak V., Drubin D. G. (2010) Loss of Aip1 reveals a role in maintaining the actin monomer pool and an in vivo oligomer assembly pathway. J. Cell Biol. 188, 769–777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kato A., Kurita S., Hayashi A., Kaji N., Ohashi K., Mizuno K. (2008) Critical roles of actin-interacting protein 1 in cytokinesis and chemotactic migration of mammalian cells. Biochem. J. 414, 261–270 [DOI] [PubMed] [Google Scholar]
- 23. Ono S. (2001) The Caenorhabditis elegans unc-78 gene encodes a homologue of actin-interacting protein 1 required for organized assembly of muscle actin filaments. J. Cell Biol. 152, 1313–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schnorrer F., Schönbauer C., Langer C. C., Dietzl G., Novatchkova M., Schernhuber K., Fellner M., Azaryan A., Radolf M., Stark A., Keleman K., Dickson B. J. (2010) Systematic genetic analysis of muscle morphogenesis and function in Drosophila. Nature 464, 287–291 [DOI] [PubMed] [Google Scholar]
- 25. Ren N., Charlton J., Adler P. N. (2007) The flare gene, which encodes the AIP1 protein of Drosophila, functions to regulate F-actin disassembly in pupal epidermal cells. Genetics 176, 2223–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kile B. T., Panopoulos A. D., Stirzaker R. A., Hacking D. F., Tahtamouni L. H., Willson T. A., Mielke L. A., Henley K. J., Zhang J. G., Wicks I. P., Stevenson W. S., Nurden P., Watowich S. S., Justice M. J. (2007) Mutations in the cofilin partner Aip1/Wdr1 cause autoinflammatory disease and macrothrombocytopenia. Blood 110, 2371–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ketelaar T., Allwood E. G., Anthony R., Voigt B., Menzel D., Hussey P. J. (2004) The actin-interacting protein AIP1 is essential for actin organization and plant development. Curr. Biol. 14, 145–149 [DOI] [PubMed] [Google Scholar]
- 28. Iida K., Yahara I. (1999) Cooperation of two actin-binding proteins, cofilin and Aip1, in Saccharomyces cerevisiae. Genes Cells 4, 21–32 [DOI] [PubMed] [Google Scholar]
- 29. Balcer H. I., Goodman A. L., Rodal A. A., Smith E., Kugler J., Heuser J. E., Goode B. L. (2003) Coordinated regulation of actin filament turnover by a high-molecular-weight Srv2/CAP complex, cofilin, profilin, and Aip1. Curr. Biol. 13, 2159–2169 [DOI] [PubMed] [Google Scholar]
- 30. Clark M. G., Teply J., Haarer B. K., Viggiano S. C., Sept D., Amberg D. C. (2006) A genetic dissection of Aip1p's interactions leads to a model for Aip1p-cofilin cooperative activities. Mol. Biol. Cell 17, 1971–1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Okada K., Ravi H., Smith E. M., Goode B. L. (2006) Aip1 and cofilin promote rapid turnover of yeast actin patches and cables: a coordinated mechanism for severing and capping filaments. Mol. Biol. Cell 17, 2855–2868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ishikawa-Ankerhold H. C., Gerisch G., Müller-Taubenberger A. (2010) Genetic evidence for concerted control of actin dynamics in cytokinesis, endocytic traffic, and cell motility by coronin and Aip1. Cytoskeleton 67, 442–455 [DOI] [PubMed] [Google Scholar]
- 33. Yu R., Ono S. (2006) Dual roles of tropomyosin as an F-actin stabilizer and a regulator of muscle contraction in Caenorhabditis elegans body wall muscle. Cell Motil. Cytoskeleton 63, 659–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mohri K., Ono S. (2003) Actin filament disassembling activity of Caenorhabditis elegans actin-interacting protein 1 (UNC-78) is dependent on filament binding by a specific ADF/cofilin isoform. J. Cell Sci. 116, 4107–4118 [DOI] [PubMed] [Google Scholar]
- 35. Okada K., Blanchoin L., Abe H., Chen H., Pollard T. D., Bamburg J. R. (2002) Xenopus actin-interacting protein 1 (XAip1) enhances cofilin fragmentation of filaments by capping filament ends. J. Biol. Chem. 277, 43011–43016 [DOI] [PubMed] [Google Scholar]
- 36. Ono S., Mohri K., Ono K. (2004) Microscopic evidence that actin-interacting protein 1 actively disassembles actin-depolymerizing factor/cofilin-bound actin filaments. J. Biol. Chem. 279, 14207–14212 [DOI] [PubMed] [Google Scholar]
- 37. Brieher W. M., Kueh H. Y., Ballif B. A., Mitchison T. J. (2006) Rapid actin monomer-insensitive depolymerization of Listeria actin comet tails by cofilin, coronin, and Aip1. J. Cell Biol. 175, 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Michelot A., Grassart A., Okreglak V., Costanzo M., Boone C., Drubin D. G. (2013) Actin filament elongation in Arp2/3-derived networks is controlled by three distinct mechanisms. Dev. Cell 24, 182–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. MacLean-Fletcher S. D., Pollard T. D. (1980) Viscometric analysis of the gelation of Acanthamoeba extracts and purification of two gelation factors. J. Cell Biol. 85, 414–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cooper J. A., Walker S. B., Pollard T. D. (1983) Pyrene actin: documentation of the validity of a sensitive assay for actin polymerization. J. Muscle Res. Cell Motil. 4, 253–262 [DOI] [PubMed] [Google Scholar]
- 41. Kuhn J. R., Pollard T. D. (2005) Real-time measurements of actin filament polymerization by total internal reflection fluorescence microscopy. Biophys. J. 88, 1387–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bähler J., Wu J. Q., Longtine M. S., Shah N. G., McKenzie A., 3rd, Steever A. B., Wach A., Philippsen P., Pringle J. R. (1998) Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast 14, 943–951 [DOI] [PubMed] [Google Scholar]
- 43. Berro J., Pollard T. D. (2014) Synergies between Aip1p and capping protein subunits (Acp1p and Acp2p) in clathrin-mediated endocytosis and cell polarization in fission yeast. Mol. Biol. Cell 25, 3515–3527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wu J. Q., Pollard T. D. (2005) Counting cytokinesis proteins globally and locally in fission yeast. Science 310, 310–314 [DOI] [PubMed] [Google Scholar]
- 45. Aizawa H., Katadae M., Maruya M., Sameshima M., Murakami-Murofushi K., Yahara I. (1999) Hyperosmotic stress-induced reorganization of actin bundles in Dictyostelium cells over-expressing cofilin. Genes Cells 4, 311–324 [DOI] [PubMed] [Google Scholar]
- 46. Shi M., Xie Y., Zheng Y., Wang J., Su Y., Yang Q., Huang S. (2013) Oryza sativa actin-interacting protein 1 is required for rice growth by promoting actin turnover. Plant J. 73, 747–760 [DOI] [PubMed] [Google Scholar]
- 47. Cooper J. A., Blum J. D., Pollard T. D. (1984) Acanthamoeba castellanii capping protein: properties, mechanism of action, immunologic cross-reactivity, and localization. J. Cell Biol. 99, 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Okada K., Obinata T., Abe H. (1999) XAIP1: a Xenopus homologue of yeast actin interacting protein 1 (AIP1), which induces disassembly of actin filaments cooperatively with ADF/cofilin family proteins. J. Cell Sci. 112, 1553–1565 [DOI] [PubMed] [Google Scholar]
- 49. Wu J. Q., Kuhn J. R., Kovar D. R., Pollard T. D. (2003) Spatial and temporal pathway for assembly and constriction of the contractile ring in fission yeast cytokinesis. Dev. Cell 5, 723–734 [DOI] [PubMed] [Google Scholar]
- 50. Chan C., Beltzner C. C., Pollard T. D. (2009) Cofilin dissociates Arp2/3 complex and branches from actin filaments. Curr. Biol. 19, 537–545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gandhi M., Achard V., Blanchoin L., Goode B. L. (2009) Coronin switches roles in actin disassembly depending on the nucleotide state of actin. Mol. Cell 34, 364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Aggeli D., Kish-Trier E., Lin M. C., Haarer B., Cingolani G., Cooper J. A., Wilkens S., Amberg D. C. (2014) Coordination of the filament stabilizing versus destabilizing activities of cofilin through its secondary binding site on actin. Cytoskeleton 71, 361–379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ayscough K. R., Stryker J., Pokala N., Sanders M., Crews P., Drubin D. G. (1997) High rates of actin filament turnover in budding yeast and roles for actin in establishment and maintenance of cell polarity revealed using the actin inhibitor latrunculin-A. J. Cell Biol. 137, 399–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ono K., Ono S. (2014) Two actin-interacting protein 1 isoforms function redundantly in the somatic gonad and are essential for reproduction in Caenorhabditis elegans. Cytoskeleton 71, 36–45 [DOI] [PMC free article] [PubMed] [Google Scholar]