Background: Low concentrations of HDAC inhibitors suppress inflammation, but HDAC inhibitors are toxic at higher concentrations.
Results: ITF3056 specifically inhibits HDAC8 activity and reduces proinflammatory cytokine production from human blood monocytes and in vivo but lacks cell toxicity.
Conclusion: Specific inhibition of HDAC8 reduces inflammation with low cell toxicity.
Significance: Inhibition of specific HDACs is preferred for treating inflammatory diseases, with fewer side effects.
Keywords: Cell Death, Cytokine, Histone Deacetylase Inhibitor (HDAC Inhibitor) (HDI), Inflammation, Lipopolysaccharide (LPS), ITF2357, ITF3056, Anti-inflammatory, Human Peripheral Blood Mononuclear Cells
Abstract
ITF2357 (generic givinostat) is an orally active, hydroxamic-containing histone deacetylase (HDAC) inhibitor with broad anti-inflammatory properties, which has been used to treat children with systemic juvenile idiopathic arthritis. ITF2357 inhibits both Class I and II HDACs and reduces caspase-1 activity in human peripheral blood mononuclear cells and the secretion of IL-1β and other cytokines at 25–100 nm; at concentrations >200 nm, ITF2357 is toxic in vitro. ITF3056, an analog of ITF2357, inhibits only HDAC8 (IC50 of 285 nm). Here we compared the production of IL-1β, IL-1α, TNFα, and IL-6 by ITF2357 with that of ITF3056 in peripheral blood mononuclear cells stimulated with lipopolysaccharide (LPS), heat-killed Candida albicans, or anti-CD3/anti-CD28 antibodies. ITF3056 reduced LPS-induced cytokines from 100 to 1000 nm; at 1000 nm, the secretion of IL-1β was reduced by 76%, secretion of TNFα was reduced by 88%, and secretion of IL-6 was reduced by 61%. The intracellular levels of IL-1α were 30% lower. There was no evidence of cell toxicity at ITF3056 concentrations of 100–1000 nm. Gene expression of TNFα was markedly reduced (80%), whereas IL-6 gene expression was 40% lower. Although anti-CD3/28 and Candida stimulation of IL-1β and TNFα was modestly reduced, IFNγ production was 75% lower. Mechanistically, ITF3056 reduced the secretion of processed IL-1β independent of inhibition of caspase-1 activity; however, synthesis of the IL-1β precursor was reduced by 40% without significant decrease in IL-1β mRNA levels. In mice, ITF3056 reduced LPS-induced serum TNFα by 85% and reduced IL-1β by 88%. These data suggest that specific inhibition of HDAC8 results in reduced inflammation without cell toxicity.
Introduction
DNA is tightly wrapped around nuclear histones and maintained in a state of deacetylation by histone deacetylases (HDACs),2 silencing gene expression. In humans, there are 18 HDACs divided into classes based on their dependence on zinc for enzyme activity (1). Class I (HDAC1, -2, -3, and -8) and Class II (HDAC4, -5, -6, -7, -9, -10, and -11) are zinc-dependent enzymes, whereas class III (sirtuins 1–7) are NAD+-dependent (2, 3). Although HDACs deacetylate the highly conserved N-terminal lysines on nuclear histones, HDACs also target cytosolic proteins, such as transcription factors and proteins, that regulate cell proliferation, migration, and death (4). HDAC inhibitors hyperacetylate signal transducers and activators of transcription (STAT) (5) and NFκB, both of which are associated with decreased inflammation (6).
Synthetic inhibitors of HDACs are orally active and well tolerated and have been used widely in medicine (reviewed in Ref. 7). Although synthetic HDAC inhibitors were developed to increase pro-apoptotic genes in cancer (8), we have studied HDAC inhibitors as anti-inflammatory agents using concentrations significantly lower than those used for anti-tumor effects. In both in vitro cellular assays as well as in animal models of inflammatory and autoimmune diseases, low concentrations of HDAC inhibitors reduce cytokine production and severity of disease. ITF2357 (generic givinostat) is a hydroxamic acid-containing, orally active inhibitor that is anti-inflammatory in the low nanomolar range, either in vitro or in vivo (9–16). For example, in mice with inducible (17) or spontaneous (6) diabetes, a low dose of oral ITF2357 of 1.5 mg/kg/day protected the insulin-producing islets as well as the incidence of clinical diabetes. In a Phase I trial in healthy males, oral givinostat reduced the production of proinflammatory cytokines from whole blood cultures (18). Children with systemic juvenile idiopathic arthritis have been successfully treated with givinostat (19, 20). In addition, patients with hematopoietic bone marrow transplantation have been treated with a related HDAC inhibitor vorinostat, and a significant reduction in graft versus host disease was observed (21). Based on in vitro data (22), HDAC inhibitors are also being tested in HIV-1 infections to decrease the population of latently infected T-cells but also to reduce in indolent inflammation of the disease (23–26).
Givinostat and vorinostat are pan-HDAC inhibitors in that they inhibit Class I and Class II HDACs. In developing more specific HDAC inhibitors, analogues of givinostat (ITF2357) were generated. In the present study, we compared the effect on cytokine production by an HDAC8-specific inhibitor ITF3056 with that of the pan-HDAC inhibitor ITF2357. Freshly obtained human peripheral blood mononuclear cells (PBMCs) were stimulated with LPS (for TLR4 responses), heat-killed Candida albicans (for broad stimulation by TLRs and C-type lectin receptors as well as NOD-like receptors (27)) or anti-CD3 in combination with anti-CD28 antibodies (for T-cell responses); the effect of the two analogues on cytokine production was determined. In addition, circulating TNFα and IL-1β were measured in mice treated with ITF2357 or ITF3056 prior to LPS.
EXPERIMENTAL PROCEDURES
Reagents
ITF2357 and ITF3056 were synthesized at Italfarmaco (Cinisello Balsamo, Italy), and purity was confirmed by high performance liquid chromatography. ITF2357 was dissolved in water and kept at room temperature (11). ITF3056 was dissolved in DMSO to a concentration of 20 mm, divided into aliquots, and stored at −80 °C. Frozen aliquots of ITF3056 (20 mm in 100% DMSO) were freshly thawed and further diluted in warm RPMI to different concentrations for experiments. The concentrations used in these studies were 1000, 500, 200, 100, 50, and 25 nm. For 1000 nm ITF3056, the final concentration of DMSO in cell culture is 0.005%. In parallel, 0.005% DMSO alone in RPMI was used as vehicle control, and no effect was observed compared with RPMI alone. Lipopolysaccharide (LPS) Escherichia coli (055:B5) was purchased from Sigma. Lactate dehydrogenase (LDH) cytotoxicity assay kit was purchased from Biovision (Mountain View, CA). All related antibodies for electrochemiluminescence (ECL) or ELISA were from R&D Systems (Minneapolis, MN). Heat-killed C. albicans UC820 was kindly provided by Professor Mihai Netea (Radboud University Medical Centre, Nijmegen, The Netherlands). Anti-human CD3 and anti-human CD28 antibodies were purchased from eBioscience (San Diego, CA).
PBMC Cultures
The study was approved by the Colorado Medical Institutional Review Board. Venous blood from healthy consenting donors was drawn into lithium heparin-containing tubes, and PBMCs were isolated using centrifugation over Ficoll-Hypaque cushions as described previously (11). Cells were washed three times with 0.9% saline and resuspended in RPMI at 5 × 106/ml. For LPS stimulation, 0.5 × 106 cells were seeded per well in 96-flat bottom well plates and cultured in a total of 200 μl of RPMI for 24 h, with or without 10 ng/ml LPS or increasing concentrations of the analogues. For cultures with Candida or anti-CD3/CD28, 0.5 × 106 cells were seeded per well in 96-round bottom well plates and cultured in a total of 200 μl of RPMI with 10% pooled human serum for 5 days, with or without Candida (106 colonies/ml) or 5 μg/ml anti-CD3 plus 1 μg/ml anti-CD28 as stimuli (28, 29), in the presence of increasing concentrations of the analogues. The HDAC inhibitors were added 30 min before the stimuli. After incubation times were completed, supernatants were collected by centrifugation at 1000 rpm for 5 min and stored at −80 °C. Cells remaining in the wells were lysed in 100 μl of 0.5% Triton X-100 in water and stored at −80 °C for later analysis.
Cytokine Measurement
Supernatant human mature IL-1β, TNFα, IL-6, IFNγ, and intracellular IL-1α were measured with ECL assays (Bioveris, Gaithersburg, MD) as described previously (11, 30, 31). Supernatant and intracellular human IL-1β precursor and mouse serum TNFα were measured by specific ELISAs (R&D Systems). Total IL-1β was calculated by adding extracellular and intracellular compartments of supernatant and lysates for mature as well as precursor IL-1β. The percentage of each compartment for either mature or precursor IL-1β was then determined for each concentration of HDAC inhibitor.
Enzymatic Assay for HDAC Activity
Recombinant human HDAC enzymes (HDAC1–10) were purchased from BPS (San Diego, CA). HDAC11 was purchased from Enzo Life Sciences (Farmingdale, NY). Activity of HDAC1, -2, -3, -6, -10, and -11 was assayed using the Fluor de Lys deacetylase substrate (Enzo Life Sciences). HDAC8 activity was assayed using Fluor de Lys Green deacetylase substrate (Enzo Life Science). Nϵ-Trifluoroacetyl-l-lysine was used to assay activity of HDAC 4, 5, 7, and 9. Recombinant enzymes were preincubated with ITF2357 or ITF3056 at 30 °C in a volume of 25 μl in wells of a microtiter plate. After a brief incubation, 25 μl of substrate was added, and the fluorescent signal was generated by the addition of 50 μl of developer (Fluor de Lys Developer (Enzo Life Science) containing 2 μm Trichostatin A (Sigma-Aldrich). For each assay, the amount of enzyme, incubation times, assay buffer, and concentration of the substrates were optimized. Positive control for enzyme activity consisted of enzyme plus substrate without ITF2357 or ITF3056. The fluorescence signal was detected using a Victor multilabel plate reader (PerkinElmer Life Sciences).
Caspase-1 Activity Assay
Human PBMCs from four donors were stimulated with LPS (10 ng/ml) for 4 and 24 h. Based on the reduction by ITF2357 and ITF3056 of LPS-induced IL-1β production and secretion, 25 nm ITF2357 and 1000 nm ITF3056 were selected as the optimal concentrations to assess effects on caspase-1 activity. ITF2357 (25 nm) or ITF3056 (1000 nm) were added 30 min prior to LPS. After the supernatant was removed, the cells were lysed using radioimmune precipitation assay buffer (Sigma-Aldrich) containing a mixture of protease inhibitors and centrifuged at 12,000 rpm for 20 min at 4 °C. The supernatants were assayed for protein content using the Bio-Rad method. Protein was processed for caspase-1 activity using the fluorogenic substrate A2452 (Sigma-Aldrich). The fluorescence was reported as arbitrary fluorescence units generated by 1 μg of sample/min (fluorescence/μg/min). The data were expressed as the percentage change in caspase-1 activity present in the lysates of LPS-stimulated PBMCs incubated with analogues, with the lysates from LPS only set as 100%.
Assays for Cytotoxicity
Three assays were used to compare the cytotoxic effects of ITF2357 with those of ITF3056. LDH release was measured in supernatants from freshly obtained PBMCs cultured in 96-flat bottom well plates for 24 h in the presence of LPS (10 ng/ml) without fetal calf serum as described previously using the LDH cytotoxicity assay kit from Biovision (Mountain View, CA) (22). The percentage of LDH release was calculated according to the manufacturer's instructions. In addition, cell viability was assessed in PBMCs cultured in RPMI supplemented with 1% FCS in the presence of LPS (10 ng/ml) at 400,000 cells/ml in a 96-flat bottom well plate for 24 h. At the end of incubation, cell viability was determined by the CellTiter 96® Aqueous One solution cell proliferation assay (Promega, Madison, WI) according to the manufacturer's instructions. We also assessed cell viability in PBMC isolated from buffy coat cells of human citrated blood. PBMCs isolated from buffy coats were seeded at 500,000 cells/well (96-flat bottom well plate) in RPMI with 10% FCS and incubated for 72 h in the presence of increasing concentrations of ITF2357 or ITF3056 using the CellTiter assay as described above.
Annexin V Staining for Monocyte Apoptosis
Monocytes were isolated from fresh PBMCs by magnetic separation (Monocyte Isolation Kit II, Miltenyi Biotec, Germany) and resuspended in RPMI containing 10% FCS. Purified monocytes were seeded at 250,000 cells/well and incubated in the presence of increasing concentrations of ITF2357 or ITF3056 with LPS (10 ng/ml). After 24 h, the cells were labeled with annexin V-FLUOS (Roche Applied Science) and propidium iodide (PI) following the manufacturer's instructions. The percentages of annexin V-positive and annexin V/PI-double-positive cells were determined by flow cytometric analysis.
Caspase-3/7 Determinations for Monocyte Apoptosis
Monocytes, isolated as described above, were incubated at 50,000 cells/well in 96-flat bottom well plates in the presence of increasing concentrations of ITF2357 or ITF3056 with LPS (10 ng/ml) for 24 h of incubation. The activity of caspase-3/7 was then determined by the Apo-ONE homogeneous caspase-3/7 assay (Promega), and the amount of fluorescence was detected by a fluorimetric plate reader (Victor-2, PerkinElmer Life Sciences).
Steady State mRNA Levels and Real-time PCR
To obtain sufficient RNA for analysis of steady state mRNA levels of different cytokines, 3 × 106 cells were seeded per well in 24-well plates and cultured in a total of 1200 μl of RPMI for 20 h, with or without 10 ng/ml LPS and increasing concentrations of the analogues. The HDAC inhibitors were added 30 min before the addition of LPS. After 20 h of incubation at 37 °C, the supernatants were collected, and cytokine levels were measured. The cells were washed with PBS and lysed in RLT Plus lysis buffer. Total RNA was collected with the RNeasy mini plus kit from Qiagen (Hilden, Germany). Approximately 300–500 ng of total RNA was reverse-transcribed using the Superscript first-strand synthesis system to obtain the cDNA (Invitrogen). Real-time PCR was then performed on a 7300 Real-time PCR system with a cycle of 40 using Power SYBR Green PCR Master Mix (Applied Biosystems). PCR using GAPDH-specific primers as an internal control was performed for each RNA sample. The forward primer for IL-1β real-time PCR was GCA CGA TGC ACC TGT ACG AT, and the reverse primer was AGA CAT CAC CAA GCT TTT TTG CT. The forward primer used for TNFα real-time PCR was CCC AGG CAG TCA GAT CAT CTT C, and the reverse primer was AGC TGC CCC TCA GCT TGA. The forward primer for IL-6 was GGT ACA TCC TCG ACG GCA TCT, and the reverse primer was GTG CCT CTT TGC TGC TTT CAC. For GAPDH, the forward primer was TGC ACC ACC AAC TGC TTA GC, and the reverse primer was GGC ATG GAC TGT GGT CAT GAG.
The relative ratio of the mRNA from each cytokine gene to internal control (GAPDH) was calculated as: 1/2ΔCt (cytokine gene minus the internal control gene). The -fold change of mRNA from treated cells over the negative control (untreated cells) was further calculated based on the relative ratio. Levels of LPS-induced mRNA were set at 100%, and the percentage change for each concentration of ITF2357 or ITF3056 was calculated. The data are depicted as the mean percentage change compared with LPS alone.
Animal Studies
Animal protocols were approved by the University of Colorado Health Sciences Center Animal Care and Use Committee. C57BL/6 mice were purchased from Charles River (Reading, MA) and housed in the animal facility for at least 5 days before use. For the comparison study, ITF2357 at 10 mg/kg was administered orally as reported previously (11), and ITF3056 was injected intraperitoneally. One hour after administration of the compounds, the animals were treated intraperitoneally with LPS from Salmonella typhimurium (Sigma-Aldrich) at a dose of 2.5 mg/kg. 90 min after the LPS treatment, mice were sacrificed, and sera were collected and stored at −80 °C until further analysis of cytokine productions. A dose-response study of ITF3056 at 4, 8, and 16 mg/kg was performed in mice injected intraperitoneallly with LPS from E. coli (055:B5) (Sigma-Aldrich) at 10 mg/kg. After 4 h, the serum was collected for cytokine levels. Another dose study of ITF3056 at 1 and 5 mg/kg used a lower dose of LPS (2.5 mg/kg) given intraperitoneally after ITF3056 injection. 4 h later, the mice were sacrificed, and blood was collected. Blood was collected in EDTA, separated into plasma for cytokine levels, or diluted in RPMI for whole blood culture as described previously (33).
Statistical Analysis
Results are expressed as mean ± S.E. (n is noted in the figure legends). Statistical significance was evaluated with Student's two-tailed t test.
RESULTS
A Comparison of the Effect of ITF2357 with That of Its Analog ITF3056 on LPS-induced IL-1β Production and Secretion
To find more selective HDAC inhibitors, analogues of ITF2357 were generated and assessed for inhibition of enzymatic activity (34). Analog ITF3056 was highly specific for inhibition of HDAC8, with IC50 at 285 nm with no significant inhibition of other HDACs (Table 1). By comparison, ITF2357 primarily inhibits Class I and II HDACs, and, as shown, ITF2357 inhibits HDAC1 and -3, with an IC50 of 198 and 157 nm, respectively.
TABLE 1.
Inhibition (IC50) of HDACs by ITF2357 or the analogue ITF3056
| ITF | hHDAC1 | hHDAC2 | hHDAC3 | hHDAC8 | hHDAC4 | hHDAC5 | hHDAC7 | hHDAC9 | hHDAC6 | hHDAC10 | hHDAC11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 2357 | 198 | 325 | 157 | 854 | 1059 | 532 | 524 | 541 | 315 | 340 | 292 |
| 3056 | 2033 | >3000 | >3000 | 285 | >10000 | >10000 | >10000 | >10000 | >3000 | >3000 | >3000 |
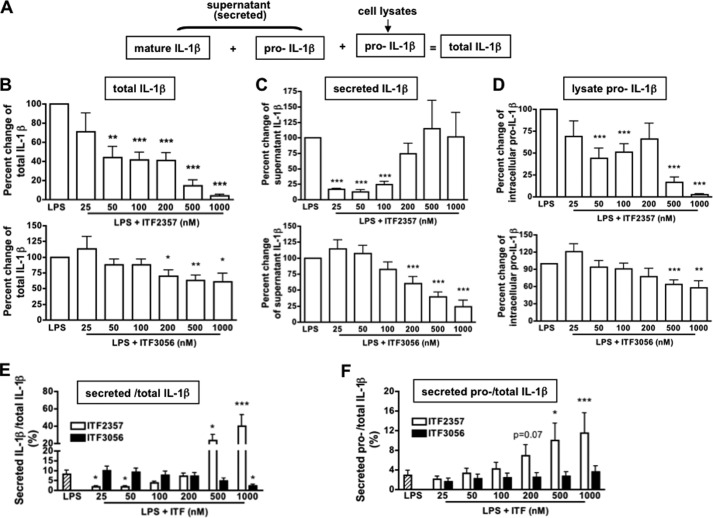
Fig. 1A illustrates the different compartments of mature and precursor IL-1β produced in LPS-stimulated PBMC. We used specific, non-cross-reacting ELISAs to measure the mature and precursor forms of IL-1β and calculated the percentage in each compartment of the total. As shown in Fig. 1B, ITF2357 suppressed total LPS-induced IL-1β production robustly compared with the reduction by ITF3056. At 25, 50, and 100 nm, ITF2357 reduced IL-1β secretion more than 70% (Fig. 1C), consistent with previous studies (11). However, at concentrations of 200, 500, or 1000 nm, we observed greater variability in donor responses (Fig. 1C, top). In contrast, ITF3056 exhibited a linear reduction in IL-1β secretion from 200 to 1000 nm (Fig. 1C, bottom).
FIGURE 1.
Comparison of the effect of ITF2357 with that of the analog ITF3056 on LPS-induced IL-1β production in PBMC cultures. A, diagram depicts LPS (10 ng/ml) stimulation of PBMC cultures from nine healthy subjects. Shown is IL-1β as measured in the different compartments using specific assays for mature IL-1β and the IL-1β precursor. B, mean ± S.E. dose-response of total IL-1β as a percentage change of ITF2357 (top) or ITF3056 (bottom). C, mean ± S.E. percentage change of secreted mature IL-1β as measured in the supernatants. Data are derived from cultures shown in B. D, mean ± S.E. percentage change of lysate containing the intracellular IL-1β precursor in cultures shown in B. E, mean ± S.E. of the percentage of the secreted mature IL-1β specific over the total IL-1β of data derived from B–D. F, mean ± S.E. of the percentage of the IL-1β precursor as measured in the supernatants over total IL-1β. ***, p < 0.001; **, p < 0.01; *, p < 0.05, compared with LPS alone.
We next compared the effect of ITF2357 and 3056 on the level of the intracellular IL-1β precursor. From previous studies (35, 36), in freshly obtained human PBMCs, ∼10–15% of the total intracellular content of the IL-1β precursor is processed by caspase-1 and secreted during a 24-h culture of monocytes, whereas most of the precursor remains unprocessed in the cells. This was also observed in the present study. Although at 50 and 100 nm, ITF2357 reduced the level of the IL-1β precursor by 50% (Fig. 1D, top), at 500 and 1000 nm, the reduction is probably due to cell death (11). In contrast, there was a linear reduction of the precursor by the analog ITF3056, which reached statistical significance at 500 and 1000 nm (Fig. 1D, bottom). These data indicate that ITF3056 at 500–1000 nm concentrations reduces the synthesis of IL-1β and are consistent with the reduction in LPS-induced mRNA (see Fig. 4B).
FIGURE 4.
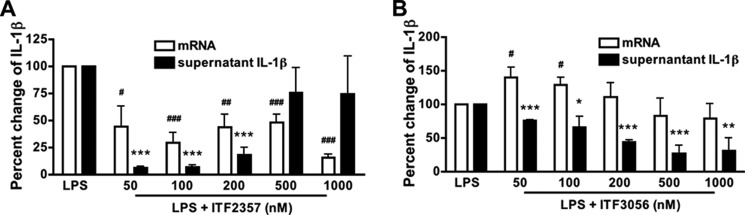
Comparison of the effect of ITF2357 with that of ITF3056 on LPS-induced IL-1β steady state mRNA levels. Shown is dose response of ITF2357 (A) and ITF3056 (B) in PBMC cultured in a 24-well plate with 10 ng/ml LPS for 20 h. Shown is mean ± S.E. percentage change of LPS-induced IL-1β mRNA levels compared with the corresponding supernatant levels of mature IL-1β with increasing concentrations. n = 4 donors; ### or ***, p < 0.001; ## or **, p < 0.01; # or *, p < 0.05, compared with LPS alone.
We further compared the effects of ITF2357 and ITF3056 on the efficiency of IL-1β secretion by calculating the percentage of secreted IL-1β over total IL-1β production (Fig. 1E). Although ITF2357 inhibited the percentage of secreted IL-1β at 25 and 50 nm, with increasing concentrations, there was more IL-1β compared with LPS without ITF2357. In contrast, ITF3056 reduced the percentage of secreted IL-1β in a linear fashion, which reached significance at 1000 nm (p < 0.05).
We next measured the level of caspase-1 activity in the lysates of PBMC after LPS stimulation in the presence of ITF2357 compared with ITF3056. As shown in Fig. 2, ITF2357 reduced caspase-1 activity by ∼50% 24 h after LPS (p = 0.002), whereas ITF3056 showed no inhibition of caspase-1 activity (p = 0.19). At 4 h following LPS, both ITF2357 and ITF3056 showed no significant effect on caspase-1 activity when compared with LPS alone.
FIGURE 2.
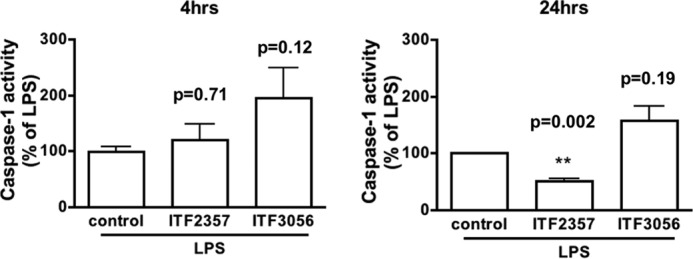
Differential effects of ITF2357 and ITF3056 on caspase-1 activity. Mean ± S.E. of caspase-1 activity in PBMC lysates 4 h (left) and 24 h (right) after LPS (10 ng/ml). Caspase-1 activity is expressed as the percentage change of the mean value of each assay with LPS set at 100% (see “Experimental Procedures” for details). ITF2357 (25 nm) and ITF3056 (1000 nm) were added 30 min before LPS; the concentrations were based on the data shown in Fig. 1. n = 4 donors; significant (p < 0.05) or non-significant (p > 0.05) p values compared with control condition are provided above the corresponding graph bars. ***, p < 0.001; **, p < 0.01; *, p < 0.05, compared with control.
From our previous study, ITF2357 at concentrations of 250 nm or higher can be injurious to PBMCs (11); thus, the percentage of secreted IL-1β over total IL-1β production at 500 and 1000 nm could be related to increased release of the IL-1β precursor from the disrupted cell membrane and cell death. The amount of secreted precursor IL-1β was calculated as the percentage of total IL-1β production (Fig. 1F). ITF2357 at 500 and 1000 nm specifically enhanced precursor IL-1β release from the cell but not at lower concentrations (<200 nm).
We also examined the levels of intracellular IL-1α in cell lysates. Immunoassays for IL-1α detect both the precursor and processed forms of the cytokine. ITF2357 reduced IL-1α levels significantly from 25 nm (35% reduction) to 200 nm (31% reduction). However, the reductions of 80 and 90% at 500 and 1000 nm are probably due to cell death. The analog ITF3056 exhibited a linear reduction, reaching statistical significance at 500 nm (24% reduction) and 1000 nm (32% reduction).
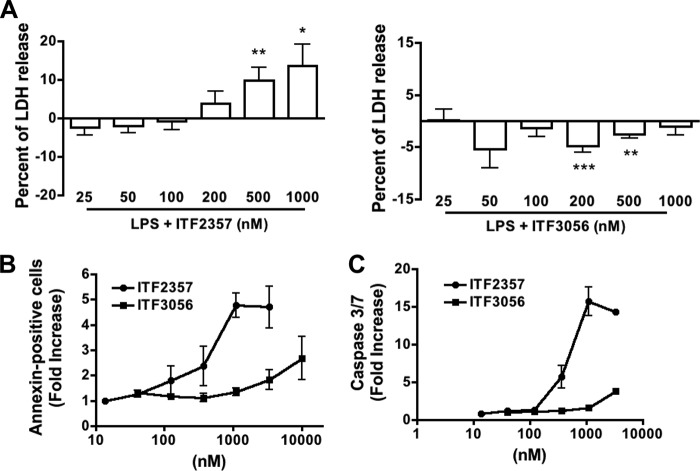
Lack of Cytotoxicity by HDAC-8 Inhibition
We used the release of LDH to first assess the cytotoxic effects of these analogues. As shown in Fig. 3A, ITF2357 resulted in the release of LDH at 200, 500, and 1000 nm, whereas ITF3056 at these same concentrations had no effect on LDH release. Similar findings were observed in PBMCs isolated from either fresh blood or buffy coat cells using a cell proliferation assay. For LPS-stimulated PBMC obtained from fresh blood, 87% of cells were viable with 123 nm ITF2357 but only 63% viable at 370 nm and only 52.5% viable at 1000 nm. In contrast, more than 93% cells were viable even at 1000 nm ITF3056. For PBMC obtained from buffy coats, at 123, 370, and 1111 nm ITF2357, there were correspondingly 63, 40, and 32% viable cells, whereas 87% of cells remained viable at 1111 nm ITF3056. Because monocytes are the major producers of the cytokines induced by LPS, we determined annexin V staining in isolated monocytes (Fig. 3B) as well as activation of caspase-3/7 (Fig. 3C). Consistently, ITF3056 lacked cell toxicity even at 1000 nm, whereas ITF2357 was toxic beginning at 200 nm.
FIGURE 3.
Cytotoxicity differences between ITF2357 and ITF3056. A, mean ± S.E. percentage change of LPS-induced LDH release with increasing concentrations of ITF2357 (left) or ITF3056 (right) in nine donors derived from data shown in Fig. 1. ***, p < 0.001; **, p < 0.01; *, p < 0.05, compared with LPS alone. B, apoptosis and necrosis in monocytes by annexin V/PI staining. Purified monocytes (see “Experimental Procedures”) in RPMI were incubated with increasing concentrations of ITF2357 or ITF3056 in the presence of LPS. After 24 h, the percentage of annexin V and PI-positive cells was determined. C, 24 h mean ± S.E. caspase-3/7 activation in purified monocytes incubated with increasing concentrations of ITF2357 or 3056 in the presence of LPS (n = 3 donors). The data of B and C are expressed as mean -fold increase compared with monocytes without ITF2357 or ITF3056.
A Comparison of the Effect of ITF2357 with That of ITF3056 on LPS-induced IL-1β Steady State mRNA Levels
As shown in Fig. 4A, at 50 nm, IL-1β mRNA was reduced by nearly 70% by ITF2357. However, as shown above, at 500 nm, the reduction in mRNA is due to cell injury. Although ITF3056 reduced IL-1β mRNA levels dose-dependently, the reduction did not reach significance. Fig. 4B also shows the reduction of secreted IL-1β in the same cultures. Unlike ITF2357, the inhibitory effect of ITF3056 on mRNA levels as well as secretion of IL-1β is linear and without significant cell death.
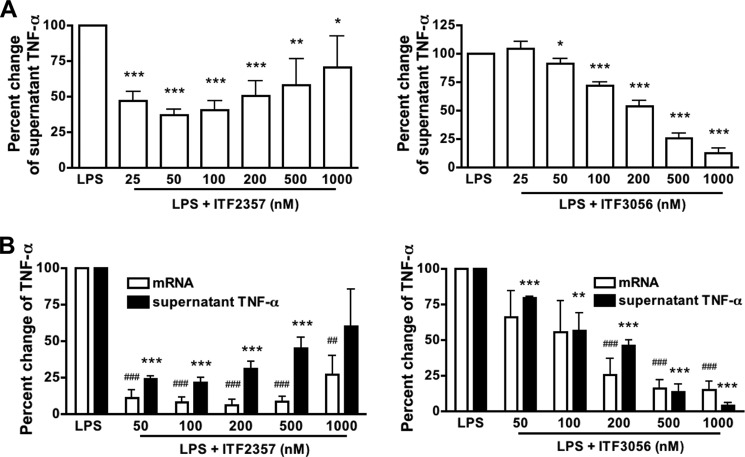
Effect of ITF3056 on LPS-induced TNFα Secretion and Gene Expression Compared with ITF2357
Fig. 5A (left) depicts the dose-response of ITF2357 inhibition of secreted TNFα following 24-h LPS stimulation. As shown, the greater reductions were at 50 and 100 nm, but at 200, 500, and 1000 nm, increasing levels of TNFα are present, suggesting cell injury. Starting at 50 nm, there is a progressive reduction of TNFα in the presence of ITF3056 with inhibition of 75% at 500 nm and 85% at 1000 nm (Fig. 5A, right). In a separate PBMCs culture, 20-h LPS-induced TNFα mRNA levels were also determined. There was a reduction in TNFα mRNA of 85% at 50 nm ITF2357 (Fig. 5B, left). ITF3056 also suppressed TNFα gene expression starting at 100 nm and dose-dependently reduced TNFα mRNA levels by 80% at 1000 nm (Fig. 5B, right).
FIGURE 5.
Comparison of the effect of ITF2357 with that of ITF3056 on LPS-induced TNFα secretion and steady state mRNA levels. A, mean ± S.E. percentage change from LPS-induced TNFα secreted (472 ± 149 pg/ml, n = 15 donors) with increasing concentrations of ITF2357 (left) or ITF3056 (right). The cells were seeded on 96-well plates with 0.5 × 106 PBMCs/well and stimulated with or without 10 ng/ml LPS for 24 h before the supernatant was collected for cytokine measurement. Data are derived from nine donors shown in Fig. 1 with an additional six donors (15 total donors). B, mean ± S.E. percentage change from LPS-induced TNFα mRNA synthesis and the corresponding supernatant TNFα with increasing concentrations of ITF2357 (left) or ITF3056 (right) (n = 4 donors). For this study, 3 × 106 PBMCs were seeded on a 24-well plate with or without 10 ng/ml LPS for 20 h. For all panels, ### or ***, p < 0.001; ## or **, p < 0.01; # or *, p < 0.05, compared with LPS alone.
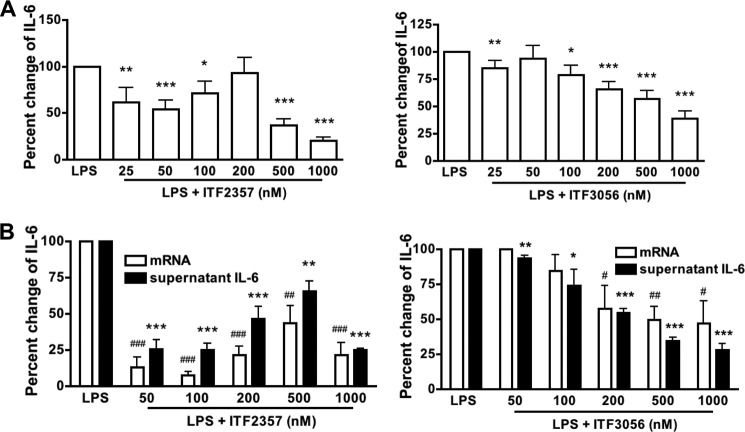
Effect of ITF3056 on LPS-induced IL-6 Secretion and Gene Expression Compared with ITF2357
ITF2357 suppresses the production of IL-6 in PBMCs stimulated with TLR agonists as well as the combination of IL-12 plus IL-18 (11). As shown in Fig. 6A (left), IL-6 secretion decreased to 50% at 50 nm ITF2357, but at 100 and 200 nm, there was no reduction. The reductions at 500 and 1000 nm (70 and 80%) are probably due to cell death. The effect of the HDAC8 inhibitor ITF3056 is shown in Fig. 6A (right) and reveals a linear dose response with maximal reduction of 60% at 1000 nm. In Fig. 6B (left), the levels of LPS-induced IL-6 mRNA in a 20-h PBMC culture (as in Fig. 5B) treated with ITF2357 parallel those of the protein. There is a reduced level at 50 and 100 nm, but the reductions at higher concentrations are related to cell death. In contrast, in PBMCs exposed to ITF3056, the steady state levels of IL-6 mRNA exhibit the same linear dose-response curve with a 50% reduction at 1000 nm, and the mRNA levels parallel those of the protein (Fig. 6B, right).
FIGURE 6.
Comparison of the effect of ITF2357 with that of ITF3056 on LPS-induced IL-6 secretion and steady state mRNA synthesis. A, mean ± S.E. percentage change from LPS-induced supernatant IL-6 (22,569 ± 8149 pg/ml, n = 15 donors) with increasing concentrations of ITF2357 (left) or ITF3056 (right). Data are derived from cultures shown in Fig. 5A. B, mean ± S.E. percentage change from LPS-induced IL-6 mRNA synthesis and the corresponding supernatant IL-6 with increasing concentrations of ITF2357 (left) or ITF3056 (right) (n = 4 donors). Data are derived from cultures shown in Fig. 5B. For all panels, ### or ***, p < 0.001; ## or **, p < 0.01; # or *, p < 0.05, compared with LPS alone.
The Effects of ITF Analogues 2357 and 3056 on Candida-induced Cytokine Secretion
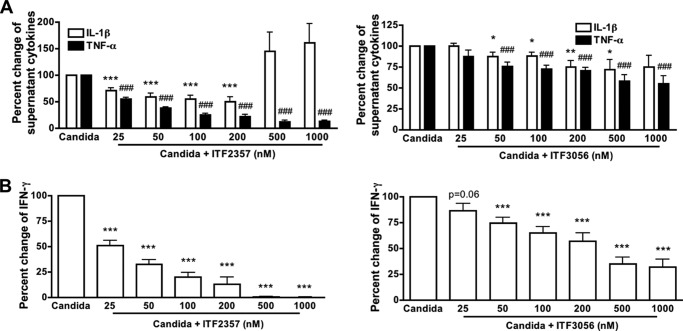
We next assessed the T-cell population employing antigen recall using heat-killed C. albicans. There are two components of the C. albicans cell wall: an outer layer that is composed of glycoproteins and an inner layer that contains polysaccharides. Immune responses can be induced by components from the outer layer of the cell wall but also by components from the inner layer. Several classes of pattern recognition receptors have been implicated in the recognition of the Candida pathogen-associated molecular patterns and the induction of innate host responses. These include the Toll-like receptors (TLR2/4/9); the C-type lectin receptors, such as dectin-1/2 and the macrophage mannose receptor; and the NOD-like receptors (27, 37). In this model, there is a requirement for activation of the monocyte and IL-1 dependence (28, 38, 39). Both ITF2357 and ITF3056 reduced Candida-induced IL-1β and TNFα secretion (Fig. 7A). IFNγ in the same cultures was reduced for both ITF2357 and ITF3056 (Fig. 7B).
FIGURE 7.
Comparison of ITF2357 with the analog ITF3056 on Candida-induced cytokines. Supernatants from PBMC were cultured in a 96-round bottom well plate and stimulated with heat-killed C. albicans for 5 days. A, mean ± S.E. percentage change from Candida-induced IL-1β or TNFα with increasing concentrations of ITF2357 (left) or ITF3056 (right). B, mean ± S.E. percentage change from Candida-induced IFNγ with increasing concentrations of ITF2357 (left) or ITF3056 (right). n = 6 donors; ### or ***, p < 0.001; ## or **, p < 0.01; # or *, p < 0.05, compared with Candida alone.
Suppression of Anti-CD3/28-induced IL-1β and TNFα by HDAC Inhibition
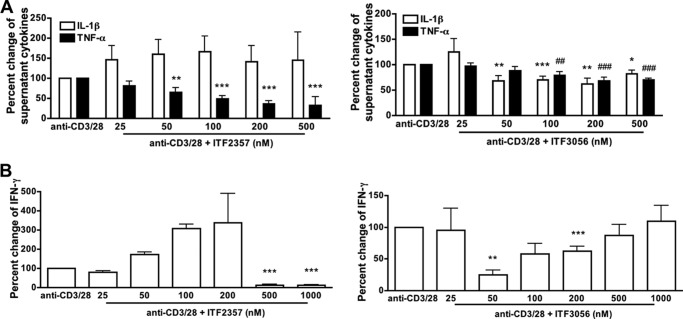
Anti-CD3 together with anti-CD28 is widely used to trigger T cells via the T-cell receptor and CD28 co-stimulatory signal (29, 37, 40), thereby mimicking T cell activation during antigen presentation. During a 5-day co-culture with PBMCs, T cells are activated by anti-CD3/28 and induce production of macrophage proinflammatory cytokines, such as IL-1β and TNFα (29). As shown in Fig. 8A, ITF2357 significantly inhibited anti-CD3/28-induced TNFα secretion in PBMCs by 75% but not IL-1β (Fig. 8A, left). The reduction by ITF3056 of IL-1β and TNFα secretion was observed at 100 nm and higher (Fig. 8A, right). We also measured anti-CD3/28-induced IFNγ in the same cultures and observed partial inhibition for ITF3056 but not ITF2357 (Fig. 8B). At 500 nm ITF2357, there is significant cell death (see Fig. 3).
FIGURE 8.
Comparison of the effect of ITF2357 with that of ITF3056 on anti-CD3/28-induced cytokine production. Cells were cultured with the combination of 5 μg/ml anti-CD3 plus 1 μg/ml anti-CD28 for 5 days. A, mean ± S.E. percentage change from anti-CD3/28-induced secretion of IL-1β or TNFα as measured in the supernatants with increasing concentrations of ITF2357 (left) or ITF3056 (right). B, mean ± S.E. percentage change from anti-CD3/28-induced IFNγ with increasing concentrations of ITF2357 (left) or ITF3056 (right). n = 5 donors; ### or ***, p < 0.001; ## or **, p < 0.01; # or *, p < 0.05, compared with anti-CD3/28 alone.
Effect of ITF3056 on Cytokine Production during Endotoxemia
The ability of ITF3056 to affect circulating TNFα and IL-1β following LPS challenge was assessed. ITF3056 at 0.1, 1, and 10 mg/kg was injected intraperitoneally into mice 1 h before an intraperitoneal injection of LPS (2.5 mg/kg). After 90 min, the mice were sacrificed, and blood was collected. ITF2357 at 10 mg/kg was used as a positive control and, as expected, reduced serum TNFα by 60%. Strikingly, pretreatment of ITF3056 starting at 0.1 mg/kg significantly reduced the circulating TNFα by nearly 90% (Fig. 9A). To achieve a significant increase in serum IL-1β production, a higher dose of LPS was injected (10 mg/kg), and blood was collected after 4 h. ITF3056 suppressed circulating IL-1β linearly, by 45% at 4 mg/kg, 70% at 8 mg/kg, and more than 80% at 16 mg/kg (Fig. 9B). Similarly, when pretreated with lower doses of ITF3056 (1 or 5 mg/kg), there was a 22% reduction for 1 mg/kg and 40% for 5 mg/kg.
FIGURE 9.
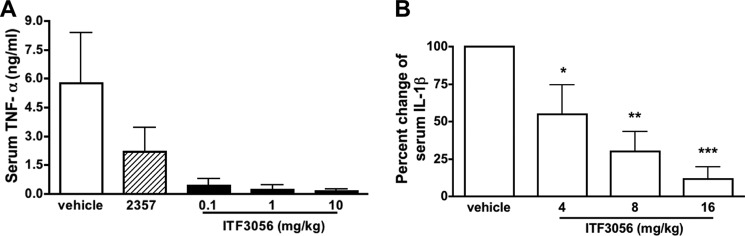
Effect of ITF3056 on in vivo endotoxemia-induced cytokine production. A, mean serum TNFα levels of ITF2357 (10 mg/kg)- or ITF3056-treated mice (5 mice/group) 90 min after intraperitoneal injection of 2.5 mg/kg LPS. B, mean ± S.E. percentage change of plasma IL-1β from ITF3056-treated mice 4 h after intraperitoneal injection of 10 mg/kg LPS compared with vehicle-treated mice (5 mice/group).
In parallel, we used an assay of LPS-induced IL-1β secretion from whole blood cultured from ITF3056-pretreated mice and observed a 24 and 23% reduction for 1 and 5 mg/kg. These data are consistent with LPS-stimulated PBMC culture (Fig. 1).
DISCUSSION
Although HDAC inhibitors have been and are still being developed to treat cancer, their ability to reduce the production of cytokines in vitro and disease severity in animal models of inflammatory and autoimmune conditions (as reviewed in Ref. 9) has prompted renewed interest in compounds that target specific HDACs. It is expected that monospecific inhibition of HDACs will offer optimal safety and efficacy, depending on the dominant cell type or mechanism in a particular disease (9). In this present study, we have compared ITF2357 with the analog ITF3056 on cytokine production as well as gene expression in freshly obtained primary human PBMC. ITF2357 inhibits several HDACs (both class I and class II HDACs), but ITF3056 is unique in that it is highly specific for inhibition of HDAC8. Suppression of cytokine production by ITF3056 compared with that of the pan-HDAC inhibitor ITF2357 was evaluated in PBMCs from the same donor in the same cultures.
One important difference between ITF2357 and ITF3056 is the lack of cell injury by ITF3056. We have reported that concentrations of ITF2357 of 250 nm or higher are injurious to PBMCs, as determined by LDH release as well as annexin staining (11), and presently confirm this observation. Measurements with annexin V staining or caspase-3/7 activation also indicated that concentrations of ITF2357 greater than 250 nm lead to monocyte apoptosis (Fig. 3). In marked contrast, ITF3056 was devoid of cell injury using either assay. In fact, at 200 nm, PBMCs exposed to ITF3056 released less LDH compare with the LPS alone, suggesting that ITF3056 may enhance cell survival under these conditions. Therefore, the reductions in cytokine production as well as gene expression by ITF3056 at 1000 nm can be assumed to be due to HDAC inhibition and independent of cell injury.
The other notable difference between ITF2357 and ITF3056 is the dose-response relationship. For each cytokine or cytokine gene expression, ITF3056 consistently exhibited a linear decrease to 1000 nm. On the other hand, ITF2357, which consistently inhibited cytokines at 25, 50, and 100 nm, failed to inhibit at 200 nm. This loss in inhibition can be observed in Figs. 1 and 5, for IL-1β and TNFα, respectively. This effect of ITF2357 is probably due to cell injury in that higher concentrations increase the release of LDH (Fig. 3A) and therefore leakage of the IL-1β precursor from the cell. Because the intracellular levels of the IL-1β precursor are markedly low at these higher concentrations (Fig. 1D), the increase in secreted IL-1β is not due to greater caspase-1 processing but rather to the release of the precursor itself. Indeed, we did detect increased release of the IL-1β precursor as a percentage of the total IL-1β produced by the cells, at 500 and 1000 nm ITF2357 (Fig. 1F). Thus, the release of the IL-1β precursor also serves as an indicator of loss of membrane integrity and early cell death.
The increase in the release of the IL-1β precursor observed at high concentrations of ITF2357 is probably not relevant to the effective dose of ITF2357 in the treatment of systemic onset juvenile idiopathic arthritis. The children received a daily oral dose of 1.5 mg/kg, which results in a peak blood level less than 100 nm (18, 20). Animal studies with ITF2357 also demonstrated optimal efficacy of oral ITF2357 in protecting mouse islets from streptozotocin toxicity at 1.25 and 2.5 mg/kg. In vitro, ITF2357 at 62.5 and 125 nm reduced cytokine-induced toxicity in rat and mouse beta cells (17, 41). In the non-obese diabetic mouse model of Type 1 diabetes, ITF2357 added to the drinking water prevented the spontaneous development of diabetes (6). The daily dose of ITF2357 in that study was calculated at 1 mg/kg. Therefore, the efficacy of ITF2357 is observed at concentrations considerably less than 200 nm both in vitro and in vivo. In contrast, ITF3056 in vitro at 1000 nm is not toxic.
Following LPS stimulation of freshly obtained human PBMCs, activated caspase-1 cleaves the IL-1β precursor, and the processed IL-1β, together with the uncleaved precursor as well as other contents of the secretory lysosomes, is released by exocytosis in the absence of cell death (42). Although ITF2357 inhibits LPS-induced IL-1β release by preventing exocytosis of IL-1β-containing secretory lysosomes (16), ITF2357 does not directly inhibit the enzymatic activity of caspase-1 (11, 16). In the present study, we measured caspase-1 activity in the cell lysates from ITF2357- and ITF3056-treated PBMC and found that after 24 h, but not after 4 h, ITF2357 at 25 nm significantly reduced LPS-induced caspase-1 activity by 50% (Fig. 2), most likely due to a reduction in the amount of the enzyme. Although ITF3056 at 1000 nm had no effect on caspase-1 activity in cell lysates, the analog reduced the level of intracellular IL-1β precursor (Fig. 1D), which may partially explain the reduction in mature IL-1β secreted into the extracellular space.
We also observed a significant inhibition of IL-1β steady state mRNA synthesis by ITF2357 in LPS-stimulated PBMCs after 24 h (Fig. 4A). The percentage reduction in IL-1β mRNA at 100 nm is 70%, which differs from a former study in which ITF2357 did not affect LPS-induced IL-1β mRNA levels as assessed by Northern blot analysis (11). In contrast, ITF3056 exhibited non-significant reductions in IL-1β mRNA levels with only 20% at 1000 nm. Nevertheless, there was a 40% reduction in IL-1β secretion at 200 nm and 80% at 1000 nm (Figs. 1C and 4B).
Similar to the previous study (11), we observed a reduction in mRNA levels of TNFα by ITF2357 (Fig. 5B). ITF3056 reduced LPS-induced TNFα and IL-6 mRNA synthesis in parallel to cytokine secretion (Figs. 5B and 6B). A marked (80%) reduction in TNFα mRNA synthesis and nearly 95% reduction in its secretion were observed. For IL-6, mRNA levels were reduced by 50%, and secretion of the cytokine was reduced by 70%. ITF2357 also inhibited IL-6 mRNA synthesis (Fig. 6B, left), but similar to its reduction in IL-6 secretion, the inhibition is observed at the lower concentrations of 50 and 100 nm.
In addition to the LPS-induced cytokines, we also observed significant inhibition of Candida-induced release of IL-1β, TNFα, and IFNγ by both ITF2357 and ITF3056 (Fig. 7). Because Candida activates glucan and mannose receptors as well as Toll-like receptors (37), it was not surprising that we observed similar trends in the reduction of IL-1β and TNFα induced by Candida and LPS. ITF2357 had been shown to inhibit LPS- as well as IL-12/IL-18-induced IFNγ but not anti-CD3-induced IFNγ (11). Here, we demonstrate that both ITF2357 and ITF3056 reduced Candida-induced IFNγ but not by anti-CD3/28 antibodies, which directly stimulate T cells (Figs. 7B and 8B). These consistent data indicate that the Candida-induced IFNγ is macrophage-dependent and that the effects of ITF2357 and ITF3056 are mainly on macrophages in the PBMC culture. Although we detected a low level of inhibition by ITF3056 on anti-CD3/28-induced IL-1β and TNFα (Fig. 8A), the effect is probably through an interaction between T cells and macrophages. In the 5-day culture of PBMCs, which is a mixed population of monocytes and lymphocytes, there is prolonged interaction between macrophages and T cells. For example, IL-18 and IL-12 secreted by activated macrophages could further activate the T cells in the co-culture to induce IFNγ production (32). In fact, ITF2357 inhibits IL-18- and IL-12-induced IFNγ in PBMCs, with a 50% reduction at 25 nm (11).
We tested the in vivo effect of ITF2357 and ITF3056 on circulating IL-1β and TNFα after LPS challenge. Previous studies showed that ITF2357 reduces LPS-induced circulating TNFα (11), which was confirmed in the present study. However, ITF3056 was highly effective in reducing LPS-induced plasma TNFα levels at low doses of 0.1 mg/kg (Fig. 9A). The exact differences between the in vitro and in vivo effects of ITF3056 are not clear. However, consistent with the in vitro study, a linear dose-response relationship is again observed in IL-1β production in vivo (Fig. 9).
Acknowledgments
We thank Kristina Barber, Benjamin Swartzwelter, and Isak Tengesdal for technical assistance.
Note Added in Proof
Maria Luisa Moras' contributions to this article fulfill the JBC authorship criteria, but her authorship was inadvertently omitted from the version of the article that was published on December 1, 2014 as a Paper in Press.
This work was supported, in whole or in part, by National Institutes of Health Grants AI-15614 and AR-45584 (to C. A. D.). This work was also supported by American Heart Association Postdoctoral Fellowship Grant 12POST12030134 (to S. L.).
- HDAC
- histone deacetylase
- PBMC
- peripheral blood mononuclear cell
- LDH
- lactate dehydrogenase
- PI
- propidium iodide.
REFERENCES
- 1. Khan N., Jeffers M., Kumar S., Hackett C., Boldog F., Khramtsov N., Qian X., Mills E., Berghs S. C., Carey N., Finn P. W., Collins L. S., Tumber A., Ritchie J. W., Jensen P. B., Lichenstein H. S., Sehested M. (2008) Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem. J. 409, 581–589 [DOI] [PubMed] [Google Scholar]
- 2. Bertos N. R., Wang A. H., Yang X. J. (2001) Class II histone deacetylases: structure, function, and regulation. Biochem. Cell Biol. 79, 243–252 [PubMed] [Google Scholar]
- 3. Gregoretti I. V., Lee Y. M., Goodson H. V. (2004) Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J. Mol. Biol. 338, 17–31 [DOI] [PubMed] [Google Scholar]
- 4. Marks P. A., Breslow R. (2007) Dimethyl sulfoxide to vorinostat: development of this histone deacetylase inhibitor as an anticancer drug. Nat. Biotechnol. 25, 84–90 [DOI] [PubMed] [Google Scholar]
- 5. Icardi L., De Bosscher K., Tavernier J. (2012) The HAT/HDAC interplay: multilevel control of STAT signaling. Cytokine Growth Factor Rev. 23, 283–291 [DOI] [PubMed] [Google Scholar]
- 6. Christensen D. P., Gysemans C., Lundh M., Dahllöf M. S., Noesgaard D., Schmidt S. F., Mandrup S., Birkbak N., Workman C. T., Piemonti L., Blaabjerg L., Monzani V., Fossati G., Mascagni P., Paraskevas S., Aikin R. A., Billestrup N., Grunnet L. G., Dinarello C. A., Mathieu C., Mandrup-Poulsen T. (2014) Lysine deacetylase inhibition prevents diabetes by chromatin-independent immunoregulation and beta-cell protection. Proc. Natl. Acad. Sci. U.S.A. 111, 1055–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dinarello C. A. (2010) Anti-inflammatory agents: present and future. Cell 140, 935–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Marks P., Rifkind R. A., Richon V. M., Breslow R., Miller T., Kelly W. K. (2001) Histone deacetylases and cancer: causes and therapies. Nat. Rev Cancer 1, 194–202 [DOI] [PubMed] [Google Scholar]
- 9. Dinarello C. A., Fossati G., Mascagni P. (2011) Histone deacetylase inhibitors for treating a spectrum of diseases not related to cancer. Mol. Med. 17, 333–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Leoni F., Zaliani A., Bertolini G., Porro G., Pagani P., Pozzi P., Donà G., Fossati G., Sozzani S., Azam T., Bufler P., Fantuzzi G., Goncharov I., Kim S. H., Pomerantz B. J., Reznikov L. L., Siegmund B., Dinarello C. A., Mascagni P. (2002) The antitumor histone deacetylase inhibitor suberoylanilide hydroxamic acid exhibits antiinflammatory properties via suppression of cytokines. Proc. Natl. Acad. Sci. U.S.A. 99, 2995–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leoni F., Fossati G., Lewis E. C., Lee J. K., Porro G., Pagani P., Modena D., Moras M. L., Pozzi P., Reznikov L. L., Siegmund B., Fantuzzi G., Dinarello C. A., Mascagni P. (2005) The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo. Mol. Med. 11, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Skov S., Rieneck K., Bovin L. F., Skak K., Tomra S., Michelsen B. K., Ødum N. (2003) Histone deacetylase inhibitors: a new class of immunosuppressors targeting a novel signal pathway essential for CD154 expression. Blood 101, 1430–1438 [DOI] [PubMed] [Google Scholar]
- 13. Mishra N., Reilly C. M., Brown D. R., Ruiz P., Gilkeson G. S. (2003) Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J. Clin. Invest. 111, 539–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Reddy P., Maeda Y., Hotary K., Liu C., Reznikov L. L., Dinarello C. A., Ferrara J. L. (2004) Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versus-leukemia effect. Proc. Natl. Acad. Sci. U.S.A. 101, 3921–3926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Reilly C. M., Mishra N., Miller J. M., Joshi D., Ruiz P., Richon V. M., Marks P. A., Gilkeson G. S. (2004) Modulation of renal disease in MRL/lpr mice by suberoylanilide hydroxamic acid. J. Immunol. 173, 4171–4178 [DOI] [PubMed] [Google Scholar]
- 16. Carta S., Tassi S., Semino C., Fossati G., Mascagni P., Dinarello C. A., Rubartelli A. (2006) Histone deacetylase inhibitors prevent exocytosis of interleukin-1β-containing secretory lysosomes: role of microtubules. Blood 108, 1618–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lewis E. C., Blaabjerg L., Størling J., Ronn S. G., Mascagni P., Dinarello C. A., Mandrup-Poulsen T. (2011) The oral histone deacetylase inhibitor ITF2357 reduces cytokines and protects islet beta cells in vivo and in vitro. Mol. Med. 17, 369–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Furlan A., Monzani V., Reznikov L. L., Leoni F., Fossati G., Modena D., Mascagni P., Dinarello C. A. (2011) Pharmacokinetics, safety and inducible cytokine responses during a phase 1 trial of the oral histone deacetylase inhibitor ITF2357 (givinostat). Mol. Med. 17, 353–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vojinovic J., Damjanov N. (2011) HDAC inhibition in rheumatoid arthritis and juvenile idiopathic arthritis. Mol. Med. 17, 397–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Vojinovic J., Damjanov N., D'Urzo C., Furlan A., Susic G., Pasic S., Iagaru N., Stefan M., Dinarello C. A. (2011) Safety and efficacy of an oral histone deacetylase inhibitor in systemic onset juvenile idiopathic arthritis. Arthritis Rheum. 63, 1452–1458 [DOI] [PubMed] [Google Scholar]
- 21. Choi S. W., Braun T., Chang L., Ferrara J. L., Pawarode A., Magenau J. M., Hou G., Beumer J. H., Levine J. E., Goldstein S., Couriel D. R., Stockerl-Goldstein K., Krijanovski O. I., Kitko C., Yanik G. A., Lehmann M. H., Tawara I., Sun Y., Paczesny S., Mapara M. Y., Dinarello C. A., DiPersio J. F., Reddy P. (2014) Vorinostat plus tacrolimus and mycophenolate to prevent graft-versus-host disease after related-donor reduced-intensity conditioning allogeneic haemopoietic stem-cell transplantation: a phase 1/2 trial. Lancet Oncol. 15, 87–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Matalon S., Palmer B. E., Nold M. F., Furlan A., Kassu A., Fossati G., Mascagni P., Dinarello C. A. (2010) The histone deacetylase inhibitor ITF2357 decreases surface CXCR4 and CCR5 expression on CD4(+) T-cells and monocytes and is superior to valproic acid for latent HIV-1 expression in vitro. J. Acquir. Immune Defic. Syndr. 54, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rasmussen T. A., Schmeltz Søgaard O., Brinkmann C., Wightman F., Lewin S. R., Melchjorsen J., Dinarello C., Østergaard L., Tolstrup M. (2013) Comparison of HDAC inhibitors in clinical development: effect on HIV production in latently infected cells and T-cell activation. Hum. Vaccin. Immunother. 9, 993–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Barton K. M., Archin N. M., Keedy K. S., Espeseth A. S., Zhang Y. L., Gale J., Wagner F. F., Holson E. B., Margolis D. M. (2014) Selective HDAC inhibition for the disruption of latent HIV-1 infection. PLoS One 9, e102684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manson McManamy M. E., Hakre S., Verdin E. M., Margolis D. M. (2014) Therapy for latent HIV-1 infection: the role of histone deacetylase inhibitors. Antivir. Chem. Chemother. 23, 145–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Archin N. M., Bateson R., Tripathy M. K., Crooks A. M., Yang K. H., Dahl N. P., Kearney M. F., Anderson E. M., Coffin J. M., Strain M. C., Richman D. D., Robertson K. R., Kashuba A. D., Bosch R. J., Hazuda D. J., Kuruc J. D., Eron J. J., Margolis D. M. (2014) HIV-1 expression within resting CD4+ T cells after multiple doses of vorinostat. J. Infect. Dis. 210, 728–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gow N. A., van de Veerdonk F. L., Brown A. J., Netea M. G. (2012) Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat. Rev. Microbiol. 10, 112–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. van de Veerdonk F. L., Joosten L. A., Devesa I., Mora-Montes H. M., Kanneganti T. D., Dinarello C. A., van der Meer J. W., Gow N. A., Kullberg B. J., Netea M. G. (2009) Bypassing pathogen-induced inflammasome activation for the regulation of interleukin-1β production by the fungal pathogen Candida albicans. J. Infect. Dis. 199, 1087–1096 [DOI] [PubMed] [Google Scholar]
- 29. van de Veerdonk F. L., Marijnissen R. J., Kullberg B. J., Koenen H. J., Cheng S. C., Joosten I., van den Berg W. B., Williams D. L., van der Meer J. W., Joosten L. A., Netea M. G. (2009) The macrophage mannose receptor induces IL-17 in response to Candida albicans. Cell Host Microbe 5, 329–340 [DOI] [PubMed] [Google Scholar]
- 30. Rivera V. R., Gamez F. J., Keener W. K., White J. A., Poli M. A. (2006) Rapid detection of Clostridium botulinum toxins A, B, E, and F in clinical samples, selected food matrices, and buffer using paramagnetic bead-based electrochemiluminescence detection. Anal. Biochem. 353, 248–256 [DOI] [PubMed] [Google Scholar]
- 31. Qiu Z. J., Ying Y., Fox M., Peng K., Lewin-Koh S. C., Coleman D., Good J., Lowe J., Rahman A., Yang J., Jiang J., Quarmby V., Song A. (2010) A novel homogeneous Biotin-digoxigenin based assay for the detection of human anti-therapeutic antibodies in autoimmune serum. J. Immunol. Methods 362, 101–111 [DOI] [PubMed] [Google Scholar]
- 32. Netea M. G., Stuyt R. J., Kim S. H., Van der Meer J. W., Kullberg B. J., Dinarello C. A. (2002) The role of endogenous interleukin (IL)-18, IL-12, IL-1β, and tumor necrosis factor-α in the production of interferon-γ induced by Candida albicans in human whole-blood cultures. J. Infect. Dis. 185, 963–970 [DOI] [PubMed] [Google Scholar]
- 33. Nold M. F., Nold-Petry C. A., Zepp J. A., Palmer B. E., Bufler P., Dinarello C. A. (2010) IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 11, 1014–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kölle D., Brosch G., Lechner T., Lusser A., Loidl P. (1998) Biochemical methods for analysis of histone deacetylases. Methods 15, 323–331 [DOI] [PubMed] [Google Scholar]
- 35. Netea M. G., Nold-Petry C. A., Nold M. F., Joosten L. A., Opitz B., van der Meer J. H., van de Veerdonk F. L., Ferwerda G., Heinhuis B., Devesa I., Funk C. J., Mason R. J., Kullberg B. J., Rubartelli A., van der Meer J. W., Dinarello C. A. (2009) Differential requirement for the activation of the inflammasome for processing and release of IL-1β in monocytes and macrophages. Blood 113, 2324–2335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Levandowski C. B., Mailloux C. M., Ferrara T. M., Gowan K., Ben S., Jin Y., McFann K. K., Holland P. J., Fain P. R., Dinarello C. A., Spritz R. A. (2013) NLRP1 haplotypes associated with vitiligo and autoimmunity increase interleukin-1β processing via the NLRP1 inflammasome. Proc. Natl. Acad. Sci. U.S.A. 110, 2952–2956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cheng S. C., Joosten L. A., Kullberg B. J., Netea M. G. (2012) Interplay between Candida albicans and the mammalian innate host defense. Infect. Immun. 80, 1304–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chedid L., Parant M., Parant F., Lefrancher P., Choay J., Lederer E. (1977) Enhancement of nonspecific immunity to Klebsiella pneumoniae infection by a synthetic immunoadjuvant (N-acetylmuramyl-l-alanyl-d-isoglutamine) and several analogs. Proc. Natl. Acad. Sci. U.S.A. 74, 2089–2093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheng S. C., van de Veerdonk F. L., Lenardon M., Stoffels M., Plantinga T., Smeekens S., Rizzetto L., Mukaremera L., Preechasuth K., Cavalieri D., Kanneganti T. D., van der Meer J. W., Kullberg B. J., Joosten L. A., Gow N. A., Netea M. G. (2011) The dectin-1/inflammasome pathway is responsible for the induction of protective T-helper 17 responses that discriminate between yeasts and hyphae of Candida albicans. J. Leukoc. Biol. 90, 357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang H., Wei W., Shen Y. X., Dong C., Zhang L. L., Wang N. P., Yue L., Xu S. Y. (2004) Protective effect of melatonin against liver injury in mice induced by Bacillus Calmette-Guerin plus lipopolysaccharide. World J. Gastroenterol. 10, 2690–2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lundh M., Christensen D. P., Rasmussen D. N., Mascagni P., Dinarello C. A., Billestrup N., Grunnet L. G., Mandrup-Poulsen T. (2010) Lysine deacetylases are produced in pancreatic beta cells and are differentially regulated by proinflammatory cytokines. Diabetologia 53, 2569–2578 [DOI] [PubMed] [Google Scholar]
- 42. Andrei C., Margiocco P., Poggi A., Lotti L. V., Torrisi M. R., Rubartelli A. (2004) Phospholipases C and A2 control lysosome-mediated IL-1 β secretion: implications for inflammatory processes. Proc. Natl. Acad. Sci. U.S.A. 101, 9745–9750 [DOI] [PMC free article] [PubMed] [Google Scholar]