Background: A positive correlation exists between sphingosine 1-phosphate (S1P) and LDL cholesterol.
Results: Hepatic LDL receptor overexpression decreased plasma S1P together with apoM in wild-type mice, but not in apoE-deficient mice.
Conclusion: LDL receptor is involved in the clearance of S1P, utilizing apoE as a ligand.
Significance: We propose the novel role of LDL receptor and apoE in the clearance of S1P.
Keywords: Apolipoprotein, Apolipoprotein E (ApoE), Lipoprotein Receptor, Low-density Lipoprotein (LDL), Sphingosine 1-Phosphate (S1P)
Abstract
Sphingosine 1-phosphate (S1P) is a vasoactive lipid mediator that is speculated to be involved in various aspects of atherosclerosis. About 70% of circulating plasma S1P is carried on HDL, and several pleiotropic properties of HDL have been ascribed to S1P. In the previous study with human subjects, however, LDL cholesterol or apoB, but not HDL cholesterol or apoA-I, had a significant positive correlation with the plasma S1P level, suggesting that the metabolic pathway for LDL might have some roles in the metabolism of S1P. In this study, we analyzed the association between LDL receptor, an important protein in the clearance of LDL, and circulating S1P. We observed that in LDL receptor-overexpressing mice, the plasma S1P levels as well as apolipoprotein M (apoM), a carrier of S1P, were decreased and that exogenously administered C17S1P bound to apoM-containing lipoproteins was cleared more rapidly. Unlike the situation in wild-type mice, LDL receptor overexpression in apoE-deficient mice did not reduce the plasma S1P or apoM levels, suggesting that apoE might be a ligand for the LDL receptor during the clearance of these factors. The present findings clarify the novel roles of the LDL receptor and apoE in the clearance of S1P, a multifunctional bioactive phospholipid.
Introduction
Atherosclerotic diseases remain a threat to all humanity all over the world despite currently available medicines such as statins, which can lower LDL cholesterol levels to almost the same levels as those in healthy subjects. To overcome this residual risk, HDL, especially the functionality of HDL, is thought to be the most promising target (1, 2). Although the main anti-atherosclerotic function of HDL can be attributed to its role in the reverse cholesterol transport system, HDL is also known to possess several pleiotropic properties, such as anti-inflammation and antioxidation (3), and these anti-atherosclerotic properties have been ascribed to many proteins and lipids carried on HDL. Among them, sphingosine 1-phosphate (S1P)2 has been demonstrated to contribute to many of the pleiotropic properties of HDL. S1P is a bioactive lipid mediator (4) that is carried mainly on HDL (∼70%) (5, 6), followed by albumin (∼30%), in the circulation. In the field of vascular biology, S1P has important roles because of its anti-atherosclerotic properties, such as anti-apoptosis (7), anti-inflammation (8, 9), vasorelaxation through the induction of eNOS (10, 11), and the preservation of vascular permeability (12–14). In agreement with these basic studies, the plasma S1P level and the HDL-linked S1P level have been shown to be reduced in subjects with coronary artery diseases (15, 16).
Although S1P is known to bind mainly to HDL, we have reported that in human subjects, the plasma S1P level is positively correlated with the levels of LDL cholesterol, triglycerides, apolipoprotein B (apoB), apolipoprotein C-II (apoC-II), and apolipoprotein C-III (apoC-III) as well as the red blood cell (RBC) count (a reservoir of S1P in the circulation) and the albumin level (the second carrier of S1P), but the plasma S1P level is not significantly correlated with the HDL cholesterol or apolipoprotein A-I (apoA-I) levels (17). Moreover, we further sought significant explanatory factors for these correlations using a multiple regression analysis that included sex, albumin level, RBC count, platelet count, the LDL cholesterol, HDL cholesterol, and TG levels and found that the LDL cholesterol level was a third explanatory variable for the plasma S1P level, following the albumin level and the RBC count. When we utilized the apolipoprotein level instead of LDL cholesterol, HDL cholesterol, and TG levels, apoB (and not apoA-I) was selected as a significant explanatory factor (Table 1). These results suggest the possibility that the metabolic pathway involved in the clearance of LDL, such as LDL receptor, might have some roles in the clearance of S1P.
TABLE 1.
Multiple regression analysis for plasma S1P
The independent effects of sex, RBC count, platelet count, and albumin, HDL-C, LDL-C, and TG levels (model A) or apoA-I, apolipoprotein A-II (apoA-II), apolipoprotein E (apoE), apoC-II, and apoC-III levels (model B) on the plasma Sph-1-P levels were evaluated using stepwise multiple regression analyses, using SPSS (Chicago, IL).
| B | β | p value | |
|---|---|---|---|
| Model A | |||
| RBC (104/μl) | 43.537 | 0.403 | 0.007 |
| Alb (g/dl) | 85.249 | 0.351 | 0.012 |
| LDL-C (mg/dl) | 0.456 | 0.301 | 0.022 |
| (not selected) | |||
| Sex | 0.987 | ||
| Platelet (104/μl) | 0.600 | ||
| HDL-C (mg/dl) | 0.059 | ||
| TG (mg/dl) | 0.999 | ||
| Model B | |||
| Alb (g/dl) | 88.679 | 0.365 | 0.010 |
| RBC (104/μl) | 40.530 | 0.375 | 0.018 |
| ApoB (mg/dl) | 0.607 | 0.288 | 0.039 |
| (not selected) | |||
| Sex | 0.696 | ||
| Platelet (104/μl) | 0.484 | ||
| ApoA-I (mg/dl) | 0.221 | ||
| ApoA-II (mg/dl) | 0.814 | ||
| ApoC-II (mg/dl) | 0.911 | ||
| ApoC-III (mg/dl) | 0.884 | ||
| ApoE (mg/dl) | 0.553 | ||
Recently, apolipoprotein M (apoM), an apolipoprotein carried mainly on HDL (18), has been proved to be a carrier of S1P (19). Interestingly, in several human studies, the apoM level exhibited a significant, positive correlation not only with the HDL cholesterol level, but also with the LDL cholesterol level (20), although most plasma apoM molecules exist on HDL (18, 21). In addition, the clearance of human apoM-containing HDL is reportedly retarded in LDLr-deficient mice, suggesting that apoM is cleared through the LDL receptor (22).
In view of the results from these reports, the LDL receptor might clear S1P as well as apoM-containing lipoproteins; if the LDL receptor clears S1P bound to apoM-containing lipoproteins, it is rational that plasma S1P levels are correlated with LDL cholesterol, because LDL receptor is established to clear LDL, and therefore S1P and LDL cholesterol undergo clearance through the same protein, LDL receptor. We have settled these problems by employing mice models and in vitro experiments.
EXPERIMENTAL PROCEDURES
Generation of Recombinant Adenoviruses
Human LDL receptor (LDLr) cDNA was cloned from a cDNA library for human liver (9505, TaKaRa Bio Inc., Shiga, Japan) using the following primers: forward primer, 5′-ttccagctaggacacagcaggtc-3′; and reverse primer, 5′-gaaatggaggtgtcatcctggt-3′. Human LDLr adenovirus (Ad-LDLr) was constructed using the AdEasy system (Stratagene, La Jolla, CA) and purified using CsCl gradient centrifugation. Adenovirus coding apoM (Ad-apoM) and its control blank adenovirus (Ad-null) (23) and adenovirus coding apoE (Ad-apoE) and its control LacZ expressing adenovirus (Ad-LacZ) (24) have been described previously.
Animal Experiments
C57BL/6 mice and apoE-deficient mice were obtained from CLEA Japan (Tokyo, Japan) and Sankyo Lab Service Co. (Tokyo, Japan), respectively. Ten-week-old C57BL/6 mice or apoE-deficient mice were injected with adenovirus via the tail vein at a dose of 2.5 × 108 pfu/g of body weight. The mouse experiments were performed on the fifth day after viral administration. All animal experiments were conducted in accordance with the guidelines for Animal Care and were approved by the animal committee of The University of Tokyo.
Analyses of Total Cholesterol Level in the Plasma
Five days after injection of the adenoviruses, the mice were subjected to a 6-h fast and blood samples were subsequently collected. The total cholesterol level was measured using enzymatic methods (439–17501, WAKO Pure Chemical Industries). To fractionate the lipoproteins, the plasma samples were pooled together and then separated using fast protein liquid chromatography (FPLC) utilizing a Superose 6 column.
Analysis of Clearance of Lipoproteins Containing C17S1P and ApoM in Vivo
The conditional medium of apoM-overexpressing HepG2 cells (prepared as described previously (23)) or apoM-depleted HepG2 cells (prepared with siRNA against apoM (sc-61978, Santa Cruz Biotechnology)), the total volumes of which were 12 ml/dish, were concentrated to about 500 μl/dish by centrifugation and purification using Amicon Ultara-15 (UFC901008, Millipore Co., Bedford, MA) (25). Then, 160 μg of evaporated C17S1P (860641P, Avanti Polar Lipids, Alabaster, AL) was gently mixed with 2 ml of the condensed conditional medium. The mice were injected with 150 μl of C17S1P bound to apoM-containing lipoproteins through the tail vein. Plasma samples of the mice were collected at 30 min after administration.
Separation of ApoE-rich and ApoE-poor HDL
ApoE-rich and apoE-poor HDL were separated with heparin-Sepharose affinity chromatography (5-ml HiTrap heparin HP (GE Healthcare Bio-Science AB, Uppsala, Sweden)); HDL were isolated from the plasma using standard ultracentrifugation followed by dialysis against PBS and 2 mg of HDL was applied to the column. The column was washed with PBS containing 0.05 m NaCl (flow rate, 0.5 ml/min) and the eluted fraction was utilized as apoE-poor HDL, then washed with PBS containing 0.3 m NaCl (flow rate, 0.5 ml/min); the eluted fraction was utilized as apoE-rich HDL. They were again dialyzed against PBS before the use for the cell experiments. Total cholesterol and phospholipid levels of each HDL were measured with enzymatic methods (439–17501 and 433–36201, respectively, WAKO Pure Chemical Industries).
Cell Experiments
HepG2 cells were obtained from American Type Culture Collection (Manassas, VA). The cells were then cultured in DMEM (D5796, Sigma) supplemented with 10% fetal bovine serum (FBS; 10099-141, Invitrogen) and 1% penicillin/streptomycin (15070-063, Invitrogen).
To examine modulation of the medium apoM level by overexpression of the LDL receptor or apoE, HepG2 cells were infected with adenoviruses at an multiplicity of infection of 25. After 3 days, the medium was replaced with serum-free medium, and then 12 h later, the medium was collected and subjected to the analyses.
To examine the clearance of C17S1P on HepG2 cells, evaporated C17S1P was delivered on LDLr-overexpressing cells or control cells with the condensed conditional medium of apoM-overexpressing HepG2 cell or apoM-depleted HepG2 cell, PBS containing 0.4% fatty acid-free albumin, apoE-poor HDL, or apoE-rich HDL as the vehicle. The final concentration of C17S1P in the prepared medium was 5 μm for condensed conditional medium and albumin. For apoE-poor/rich HDL, the final concentration of 250 nm C17S1P was bound to 200 μg/ml (final concentration) of HDL. Then, C17S1P complexes were added to the cells. After 10 or 30 min, the medium and cells were collected and the C17S1P levels were measured.
To examine the effects of pitavastatin, we treated the cells with 10 μm pitavastatin (L-0250-022, Chemtech Laboratory, Inc., Tokyo, Japan) with or without 30 mm mevalonate (M4667, Sigma), as described previously (26). Evaporated C17S1P was delivered using the condensed conditional medium of apoM-overexpressing HepG2 cells. The final concentration of C17S1P in the prepared medium was 10 μm. After 10 min, the medium was collected and the C17S1P levels were measured.
Measurement of S1P and C17-S1P
The contents of S1P and C17-S1P in the plasma and medium were determined using two-step lipid extraction followed by HPLC separation, as described previously (27). Briefly, samples were sonicated in 3 ml of methanol/chloroform (2:1) with an internal standard for 30 min. After adding 2 ml of chloroform, 2.1 ml of 1 mm KCl, and 100 μl of 3 n NaOH, samples were centrifuged and the alkaline upper phase (3.8 ml) was collected to new tubes, to which 4 ml of chloroform and 200 μl of concentrated HCl were added. The resultant lower chloroform phases (3.5 ml) formed under these new acidic conditions were collected and evaporated under nitrogen gas and resolved in methanol, followed by HPLC separation with a TSKgel ODS-80TM column (0017202, Tosoh, Tokyo, Japan). For measurement of the S1P content, we used C17S1P (860641P, Avanti Polar Lipids) as an internal standard, whereas for C17S1P content, we used FTY720-phosphate (10006408, Cayman Chemical, Ann Arbor, MI).
Western Blotting
Cellular proteins and liver proteins were extracted using RIPA lysis buffer (25 mm Tris-HCl, pH 7.6, 1% Nonidet P-40, 0.1% SDS, 150 mm NaCl, 0.02% sodium deoxycholate, 1 mm orthovanadate, 1 mm PMSF, and protease inhibitor mixture (11836153001, Roche Applied Science). Membranous proteins were prepared as described previously (26). Western blotting was performed using 30 μg of the cellular or liver proteins according to a standard method. To analyze the proteins in the medium, the amount of medium subjected to Western blotting was adjusted according to the cellular protein level. For the plasma analysis, a volume corresponding to 0.5 μl of plasma was used for the Western blotting analysis. The following antibodies were used: anti-human apoM antiserum (developed in a previously reported paper (23)), anti-mouse apoM antibody (A00954, GenScript Co, Piscataway, NJ), anti-apoA-I antibody, anti-apoE antibody (AB740 and AB947, Chemicon International Inc., Temecula, CA), anti-apoB antibody, anti-mouse albumin antibody (sc-11795 and sc-46293, Santa Cruz Biotechnology), anti-LDL receptor antibody (10007665, Cayman Chemical Co.), anti-human albumin antibody (E80–129A, Bethyl Laboratories, Inc., Montgomery, TX), anti-pan cadherin antibody (RB-9036-PD, Thermo Fisher Scientific Inc., Fremont, CA), and anti-β-actin antibody (PM053, MBL, Nagoya, Japan). The intensities of the bands were measured by ImageJ (from the NIH).
Real Time PCR
Total RNAs extracted from murine livers with the GenElute Mammalian Total RNA Miniprep kit (Sigma) were subjected to reverse transcription with Superscript II enzyme (Invitrogen). Quantitative PCR was performed using an ABI 7300 Real-time PCR System (Applied Biosystems) for apoM (Mm00444525_m1), ABCA1 (Mm00442646_m1), ABCG1 (Mm00437390_m1), SR-BI (Mm00450234_m1), and β-actin (Mm00607939_s1). The expression levels of the genes of interest were adjusted to those of the endogenous β-actin mRNA as a control.
ELISA
The apoM level in the mice plasma was measured with mouse apolipoprotein M ELISA kit (CSB-EL001947MO, Cusabio Biotechnology Co., Wuhan, China), according to the manufacturer's protocol.
Statistical Analysis
All data were statistically analyzed using SPSS (Chicago, IL). The results were expressed as the mean ± S.E. Differences between two groups were evaluated using Student's t test, and differences among more than two groups were assessed using a one-way analysis of variance, followed by the Scheffe's test as a post hoc test. p values less than 0.05 were deemed statistically significant.
RESULTS
LDL Receptor Overexpression Decreased the Plasma Levels of S1P
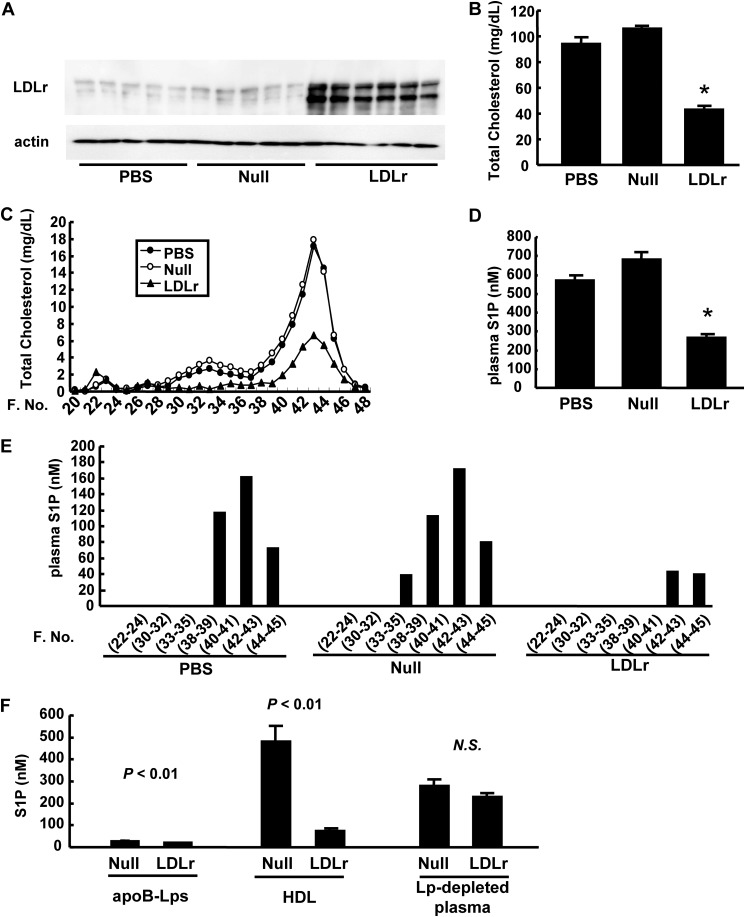
First, we overexpressed the LDL receptor in the liver of wild-type mice using adenoviral gene transfer (Fig. 1A) and examined the modulation of plasma S1P levels. As expected, the plasma total cholesterol level was reduced (Fig. 1B). FPLC analyses revealed that not only the LDL cholesterol level but also the HDL cholesterol level was reduced (Fig. 1C). As shown in Fig. 1D, the plasma S1P level was markedly reduced in LDLr-overexpressing mice. Among lipoprotein fractions, the amount of S1P carried on HDL was especially decreased (Fig. 1, E and F), whereas the amount of S1P in lipoprotein-depleted plasma was not significantly modulated in LDLr-overexpressing mice (Fig. 1F).
FIGURE 1.
Effects of LDL receptor overexpression on plasma S1P levels. Ten-week-old C57BL6 mice were injected with PBS, Ad-LDLr, or Ad-null. Analyses were performed after subjecting the mice to a 6-h fast on the fifth day after infection (n = 5–6/group). A, hepatic LDL receptor expression; B, plasma total cholesterol levels; C, total cholesterol levels among FPLC fractions; D, plasma S1P levels, and E, the distribution of plasma S1P levels among FPLC fractions; F, S1P contents in apoB-containing lipoproteins (apoB-Lps), HDL, and lipoprotein-depleted plasma (Lp-depleted plasma), separated with standard ultracentrifugation (n = 4/group). *, p < 0.01 versus the other groups.
LDL Receptor Overexpression Decreased the Plasma Levels of ApoM
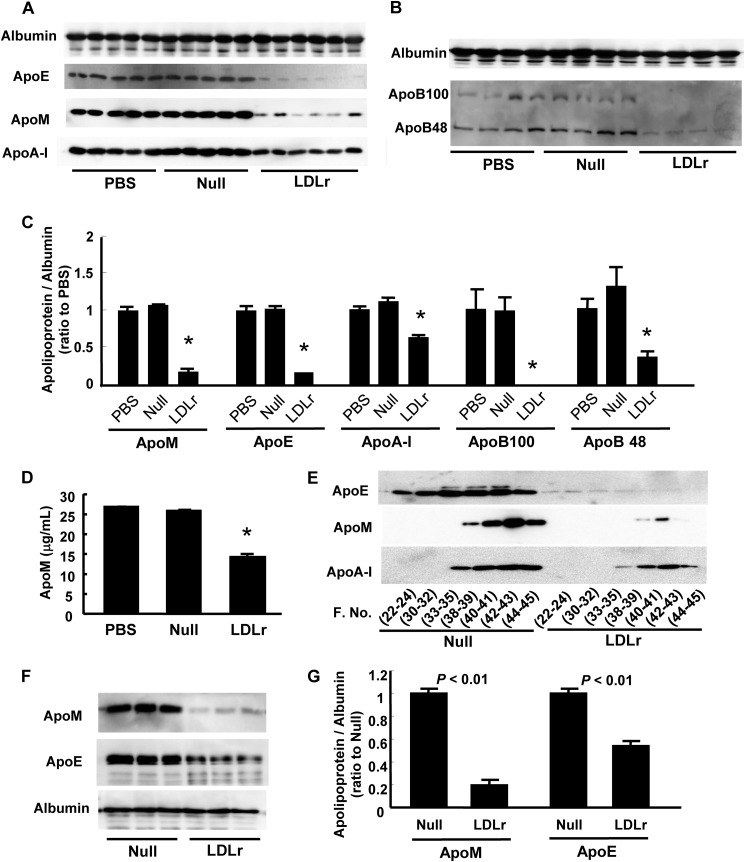
Because 70% of plasma S1P is distributed on HDL and apoM, which is a potent carrier of S1P (19), and apoM is reported to be cleared through LDL receptor (22), we next investigated the modulation of apoM by LDL receptor overexpression. As shown in Fig. 2, A, C, and D, the apoM level was significantly reduced, along with apoB and apoE levels (Fig. 2, A-C) in LDLr-overexpressing mice, which was concordant with the previous report (22). A decline in the apoM level, as well as in the apoE level, was also observed when the LDL receptor was overexpressed in HepG2 cells (Fig. 2, F and G). We further analyzed the apoM level in FPLC fractions and observed that the apoM level, which was distributed mainly within the HDL fraction in wild-type mice, was dramatically reduced in the HDL fraction (Fig. 2E), which was concordant with the modulation of S1P in FPLC fractions by LDL receptor overexpression (Fig. 1E). Regarding the apoE distribution, apoE levels in the LDL and HDL fractions were also reduced.
FIGURE 2.
Effects of LDL receptor overexpression on plasma apoM levels. A–E, modulation of plasma apolipoprotein levels in LDLr-overexpressing mice described in the legend to Fig. 1. A and B, plasma apolipoprotein levels determined with Western blot; C, the intensity were quantified with ImageJ, utilizing albumin as control; D, apoM levels measured with ELISA; and E, the distribution of apoE, apoM, and apoA-I among FPLC fractions, *, p < 0.01 versus the other groups. F and G, modulation of medium apolipoprotein levels in LDLr-overexpressing HepG2 cells. HepG2 cells were infected with Ad-LDLr or Ad-null. After 3 days, the medium was replaced with serum-free medium, and another 12 h later, the medium was collected and analyzed for apoM and apoE (n = 3/group). F, medium apolipoprotein levels determined with Western blot; G, the intensity were quantified with ImageJ, utilizing albumin as control.
LDL Receptor Is Involved in S1P Clearance in Vivo
The results obtained using LDLr-overexpressing mice prompted us to investigate whether the LDL receptor might also remove S1P as well as apoM from circulation, in addition to its role in LDL clearance. We next examined the clearance of C17S1P bound to apoM-containing lipoproteins in LDLr-overexpressing mice. We prepared apoM-containing lipoproteins as described under “Experimental Procedures,” in which evaporated C17S1P was dissolved. C17S1P was distributed among lipoproteins, especially in HDL fractions (fraction 37–45), which were abundant in apoM (data not shown).
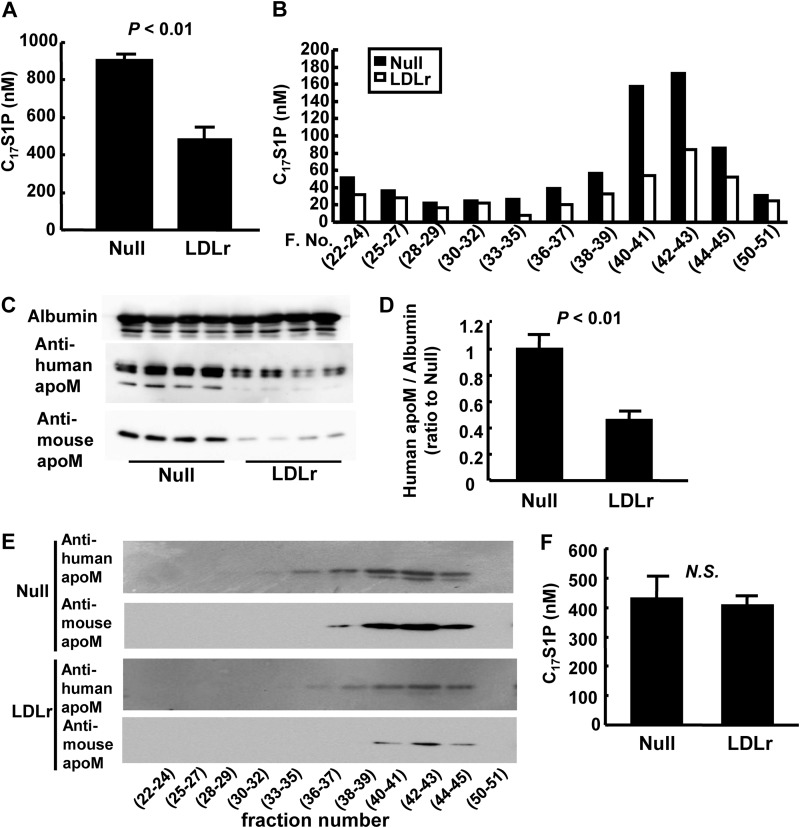
We injected C17S1P bound to apoM-containing lipoproteins into LDLr-overexpressing mice or control mice through the tail vein and found that at 30 min after administration, both C17S1P and human apoM levels were lower in the LDLr-overexpressing mice (Fig. 3, A, C, and D). In LDLr-overexpressing mice, the plasma C17S1P content was reduced in almost all the FPLC fractions; however, the C17S1P level in the HDL fraction was particularly reduced (Fig. 3B), along with a reduced amount of human apoM in the HDL fraction (Fig. 3E). When we injected C17S1P bound to apoM-depleted lipoproteins, prepared using siRNA against apoM, we observed no difference between mice infected with control virus and LDLr-overexpressing mice (Fig. 3F); this siRNA was confirmed to suppress the apoM protein level in the medium of HepG2 cells by 73%.
FIGURE 3.
Effects of LDL receptor overexpression on the clearance of S1P and apoM in vivo. Clearance of C17S1P bound to apoM-containing lipoproteins (A–E) and C17S1P bound to apoM-depleted lipoproteins (F) in vivo. C17S1P bound to apoM-containing lipoproteins or apoM-depleted lipoproteins was administered to mice infected with Ad-null or Ad-LDLr via the tail vein. The analyses were performed at 30 min after administration. A, plasma C17S1P levels; B, the distribution of C17S1P among FPLC fractions; C and D, plasma levels of exogenously administered human apoM and innate mouse apoM; and E, the distribution of exogenously administered human apoM among FPLC fractions, when mice were injected with C17S1P bound to apoM-containing lipoproteins (n = 4/group). F, plasma C17S1P levels when mice were injected with C17S1P bound to apoM-depleted lipoproteins (n = 5–6/group).
LDL Receptor Is Involved in S1P Clearance in Vitro
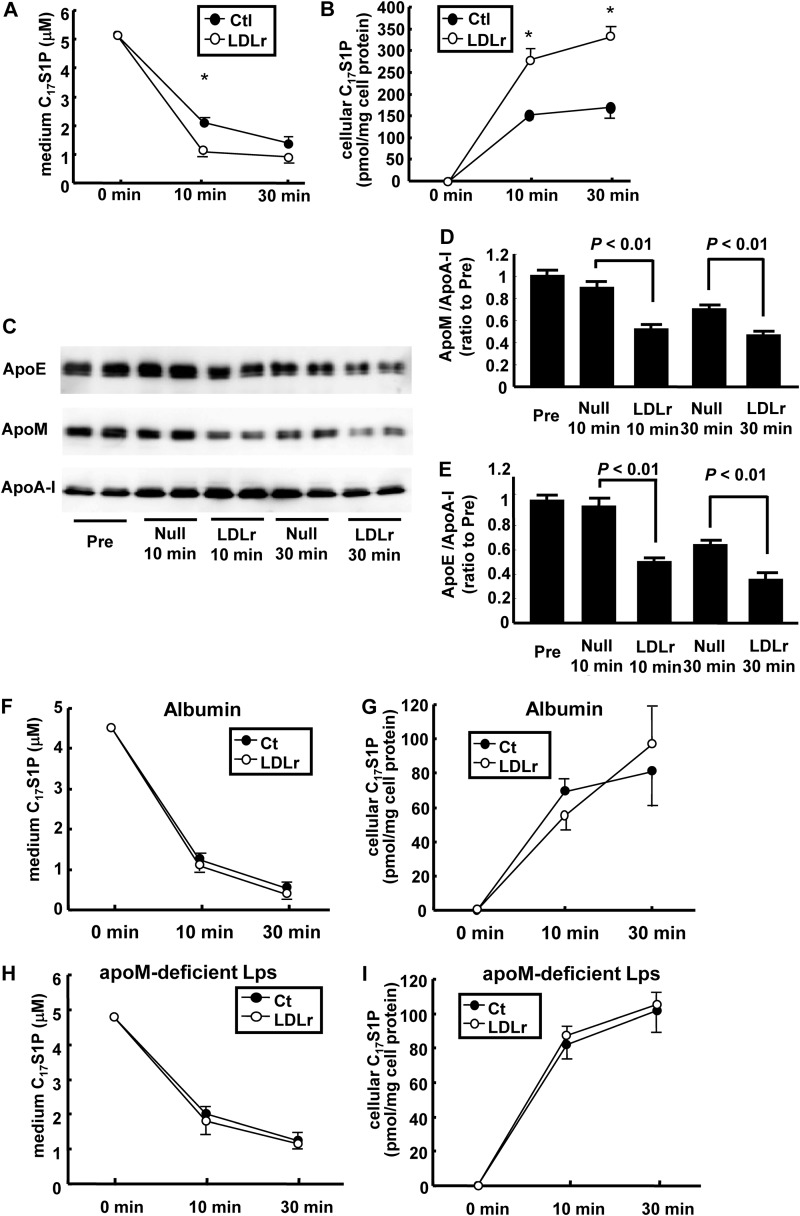
We also performed similar experiments in vitro; the clearance of C17S1P bound to apoM-containing lipoproteins on LDLr-overexpressing HepG2 cells or control cells was investigated. As shown in Fig. 4, A and B, C17S1P in the medium was cleared more rapidly on the LDLr-overexpressing HepG2 cells, whereas the cellular C17S1P content increased to a greater degree on the LDLr-overexpressing HepG2 cells. The medium levels of apoM and apoE also decreased faster on LDLr-overexpressing HepG2 cells than on the control cells (Fig. 4, C–E). In contrast, when C17S1P was bound to fatty acid-free albumin or apoM-depleted lipoproteins, no difference was observed in either the medium or cellular levels of C17S1P between the LDLr-overexpressing cells and on control cells (Fig. 4, F–I), demonstrating that the LDL receptor-mediated clearance of S1P might only be specific to S1P bound to apoM-containing lipoproteins.
FIGURE 4.
Effects of LDL receptor overexpression on the clearance of S1P and apoM in vitro. A–E, clearance of C17S1P bound to apoM-containing lipoproteins in vitro. In total, 5 μm (final concentration) of C17S1P bound to apoM-containing lipoproteins in serum-free DMEM was placed on HepG2 cells infected with Ad-LDLr (LDLr) or Ad-null (Ctl) (n = 4/group). A and B, time courses for the medium and cellular C17S1P contents adjusted according to the cellular protein level. *, p < 0.05 versus control. C–E, time courses for the medium apoM content and the apoE content. C, Western blot; D and E, apoM (D) and apoE (E) intensities were measured with ImageJ and adjusted to apoA-I levels. F–I, clearance of C17S1P bound to albumin or apoM-depleted lipoproteins on LDL receptor-overexpressing HepG2 cells. A final concentration of 5 μm C17S1P bound to fatty acid-free albumin (F and G) or apoM-depleted lipoproteins, prepared as described under ”Experimental Procedures,“ H and I, in serum-free DMEM was placed on LDLr-overexpressing HepG2 cells or HepG2 cells infected with blank adenovirus (n = 4/group). The panels represent the time course of the medium (F and H) and cellular C17S1P contents, adjusted according to the cellular protein level (G and I).
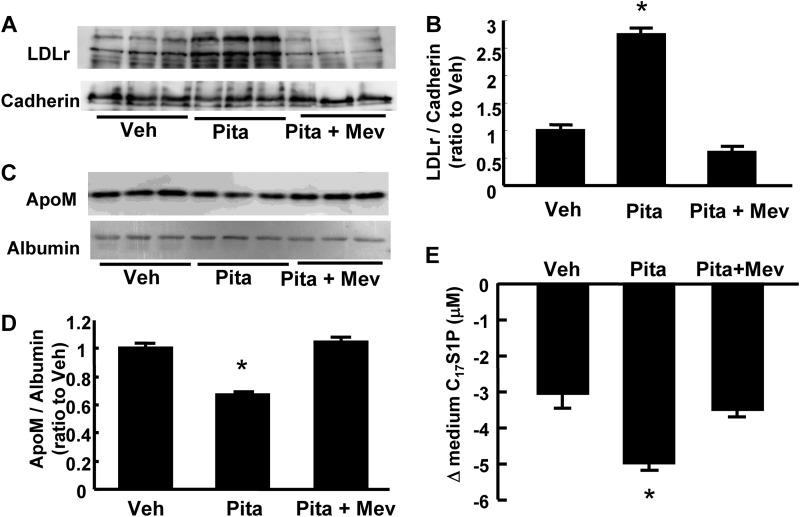
The LDL receptor is up-regulated by treatment with HMG-CoA reductase inhibitor. Therefore, we also investigated the effect of pitavastatin on the clearance of C17S1P bound to apoM-containing lipoproteins. As shown in Fig. 5, A and B, the LDLr protein level in the cellular membranous fractions was increased by treatment with pitavastatin and this up-regulation was reversed by mevalonate. Concordant with the results from LDLr-overexpressing HepG2 cells, the apoM level in the medium was reduced by treatment with pitavastatin, and this effect was reversed using mevalonate (Fig. 5, C and D). The clearance of C17S1P bound to apoM-containing lipoproteins was faster in the cells treated with pitavastatin (Fig. 5E).
FIGURE 5.
Effects of HMG-CoA reductase inhibitor on the medium levels of S1P and apoM in vitro. HepG2 cells were incubated in serum-free DMEM containing 10 μm pitavastatin with or without 300 mm mevalonate for 24 h. A and B, membranous protein levels of LDL receptor. Cadherin was utilized as control (n = 3/group). C and D, medium apoM levels adjusted according to albumin level (n = 3/group). E, changes in the medium C17S1P contents at 10 min after the medium replacement with serum-free medium containing 10 μm (a final concentration) C17S1P bound to apoM-containing lipoproteins (n = 4 group). *, p < 0.01 versus other groups.
ApoE Overexpression Decreased the Plasma Levels of S1P and ApoM
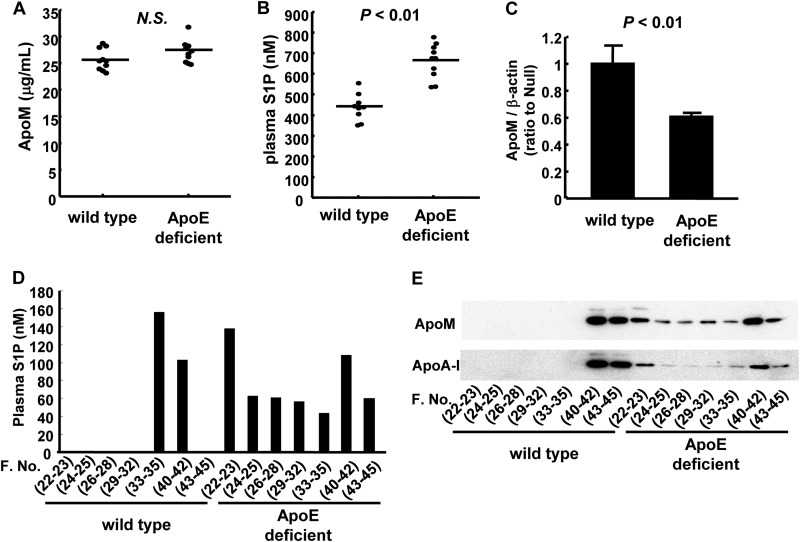
Next, we investigated how the LDL receptor clears S1P bound to apoM-containing lipoproteins. As shown in Figs. 2, A, C, F, and G, and 4, C and E, the apoE level was also reduced, along with the apoM level, in LDLr-overexpressing mice or cells. Considering that although apoB100 is known to be a ligand for the LDL receptor, apoE can also serve as a ligand for the LDL receptor on both TG-rich lipoproteins (28, 29) and apoE-rich HDL (30, 31), we next investigated the effects of apoE on the plasma S1P and apoM levels. First, we examined the plasma S1P and apoM levels in apoE-deficient and wild-type mice. As shown in Fig. 6, A and B, the plasma S1P level was higher in apoE-deficient mice than in the wild-type mice, whereas the plasma apoM level was not significantly modulated in the apoE-deficient mice. ApoE-deficient mice are mice with an extremely different lipoprotein metabolism including S1P and apoM from wild-type mice; in apoE-deficient mice, S1P and apoM are distributed not only to HDL, but also to VLDL/LDL (Fig. 6, D and E) and the hepatic expression of apoM is lower in apoE-deficient mice (Fig. 6C), whereas the expression levels of the key enzymes involved in S1P metabolism were not modulated (data not shown). Therefore, these results did not rule out the possibility that apoE might influence the metabolism of S1P bound to apoM-containing lipoproteins.
FIGURE 6.
Plasma apoM and S1P levels in apoE-deficient mice and wild-type mice. Ten-week-old C57BL6 mice (wild-type mice) and apoE-deficient mice after subjecting the mice to a 6-h fast were analyzed for plasma apoM and S1P levels (n = 10/group). A, the plasma apoM levels determined with ELISA; B, the plasma S1P levels measured with HPLC; C, the hepatic apoM mRNA levels determined with real-time PCR, utilizing β-actin as control; D and E, the distribution of the S1P level (D) and the apoM and apoA-I levels (E) among FPLC fractions.
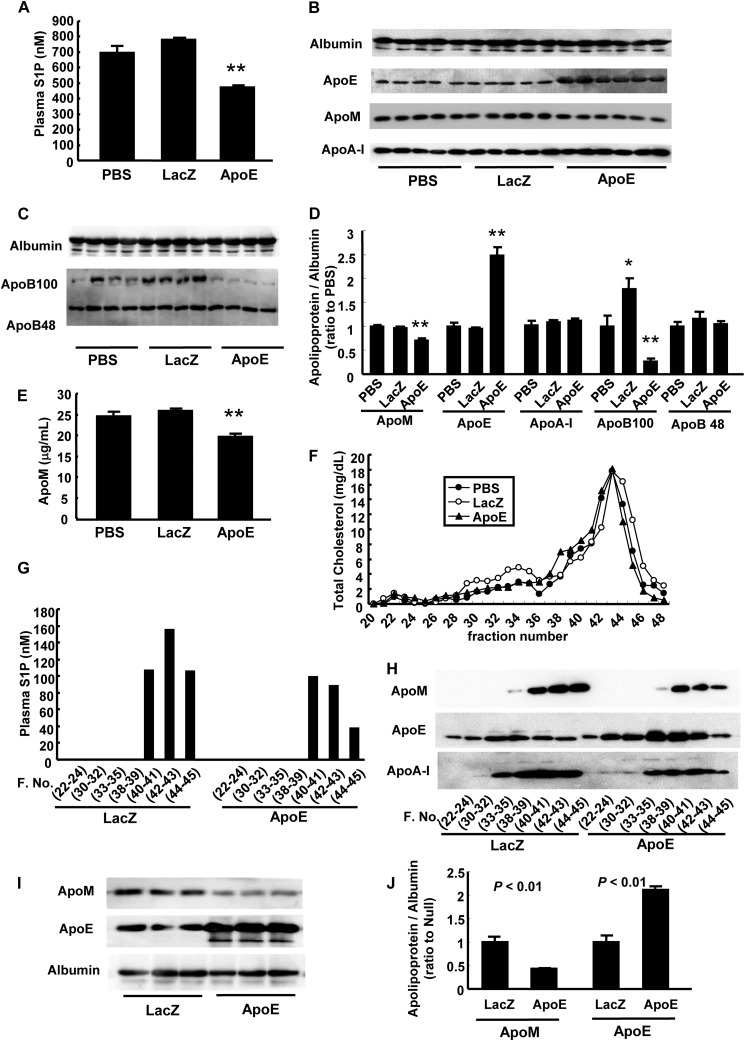
Therefore, we next investigated the effects of apoE overexpression on plasma S1P and apoM levels and found that the plasma S1P and apoM levels were significantly reduced in the apoE-overexpressing mice (Fig. 7, A, B, D, and E), together with a reduced cholesterol level of LDL and apoB (Fig. 7, C and F). The plasma S1P and apoM levels were reduced in the HDL fraction, although the apoE level was increased in almost all FPLC fractions (Fig. 7, G and H). This decrease in the apoM level during apoE overexpression was also observed in apoE-overexpressing HepG2 cells (Fig. 7, I and J). These effects of the overexpression of apoE on the plasma S1P and apoM levels suggest that apoE might have some roles in accelerating the clearance of S1P bound to apoM-containing lipoproteins.
FIGURE 7.
ApoE overexpression decreased the plasma levels of S1P and apoM. A–H, 10-week-old C57BL6 mice were injected with PBS, Ad-apoE, or Ad-LacZ (as a control). The analyses were performed after a 6-h fast on the fifth day after infection (n = 5–6/group). A, plasma S1P levels; B-D, plasma apolipoprotein levels determined with Western blot (B and C) and the intensity were quantified with ImageJ, utilizing albumin as control (D); E, plasma apoM levels measured with ELISA; F, the distribution of total cholesterol among FPLC fractions; G, the S1P level among the FPLC fractions; and H, the apolipoproteins among FPLC fractions. *, p < 0.05 versus PBS; **, p < 0.01 versus the other groups. I and J, modulation of medium apolipoproteins level in apoE-overexpressing HepG2 cells. HepG2 cells were infected with Ad-apoE or Ad-LacZ. After 3 days, the medium was replaced with serum-free medium and another 12 h later, the medium was collected and analyzed for apoM and apoE (n = 3/group). I, medium apolipoprotein levels determined with Western blot, and J, the intensity were quantified with ImageJ, utilizing albumin as control.
LDL Receptor Overexpression Did Not Affect the Plasma Levels of S1P or ApoM in ApoE-deficient Mice
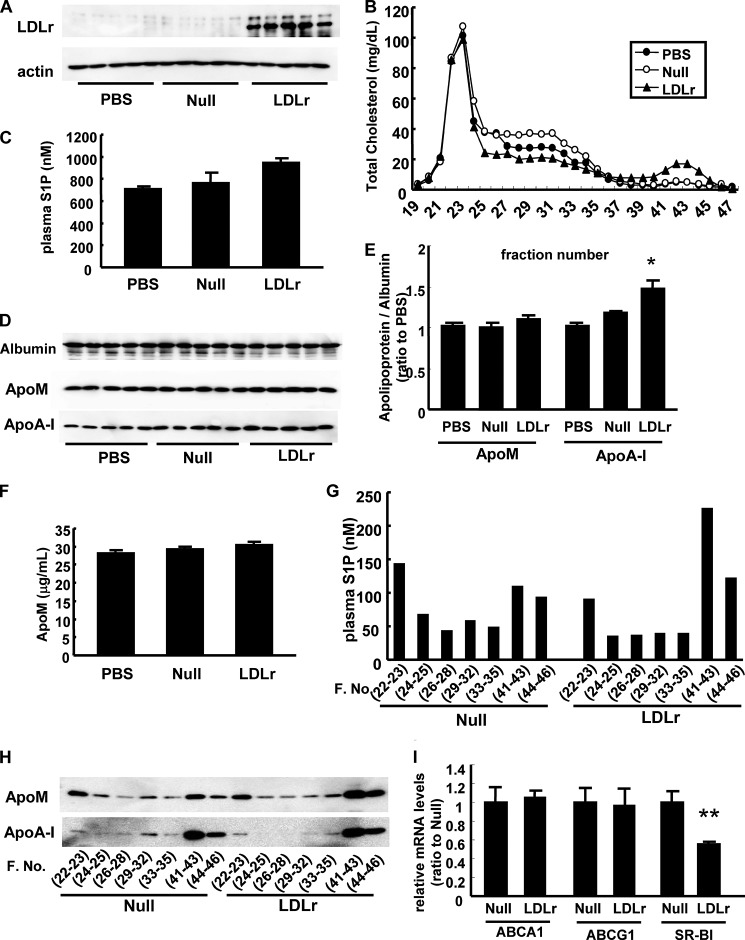
Considering the results of our experiments, apoE on apoM-containing lipoproteins, which carry S1P, might serve as a ligand for the LDL receptor. To confirm this hypothesis, we overexpressed the LDL receptor in apoE-deficient mice (Fig. 8A). Overexpression of the LDL receptor reduced the IDL/LDL cholesterol levels (Fig. 8B); however, the plasma apoM and S1P levels were not reduced in LDLr-overexpressing apoE-deficient mice (Fig. 8, C–F). Interestingly, overexpression of the LDL receptor in apoE-deficient mice tended to result in increase in the plasma S1P and apoM levels especially in HDL fractions (Fig. 8, G and H) along with the increase in HDL (Fig. 8B) and apoA-I (Fig. 8, D and E) amounts. Although the mechanism behind has not been elucidated, one possible explanation is that overexpression of the LDL receptor may decrease the expression level of hepatic scavenger receptor class B, type I (SR-BI), a receptor for HDL (Fig. 8I). Therefore, apoE on apoM-containing lipoproteins is essential for the clearance of apoM and S1P through the LDL receptor, and apoE may serve as a ligand for the LDL receptor.
FIGURE 8.
LDL receptor overexpression did not affect the plasma S1P or apoM level in apoE-deficient mice. Ten-week-old apoE-deficient mice were injected with PBS, Ad-LDLr or Ad-null; 5 days thereafter, the mice were subjected to a 6-h fast and the analyses were subsequently performed (n = 5/group). A, hepatic expression of the LDL receptor; B, the distribution of total cholesterol among FPLC fractions; C, plasma S1P level; D and E, plasma apolipoprotein levels determined with Western blot (D) and the intensity were quantified with ImageJ, utilizing albumin as control (E); F, plasma apoM levels measured with ELISA; G, the distribution of the S1P levels among the FPLC fractions; H, distribution of apoM and apoA-I levels among FPLC fractions; and I, hepatic mRNA levels of ABCA1, ABCG1, and SR-BI determined with real-time PCR, utilizing β-actin as control. *, p < 0.05 versus the other groups; **, p < 0.01 versus the other groups.
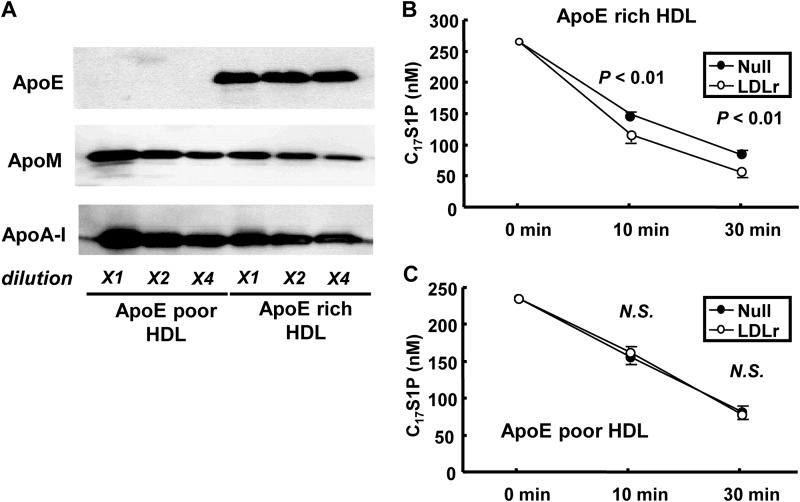
LDL Receptor Is Involved in the Clearance of S1P Bound to ApoE-rich HDL, but Not to ApoE-poor HDL
Finally, we separated apoE-rich HDL with heparin-Sepharose affinity chromatography as described under “Experimental Procedures.” The S1P and other lipid contents in apoE-rich HDL and apoE-poor HDL are shown in Table 2. We investigated the clearance of C17S1P bound to apoE-rich HDL and C17S1P bound to apoE-poor HDL on LDLr-overexpressing HepG2 cells or control cells. Although apoM was rather rich in apoE-poor HDL as shown in Fig. 7A, the clearance of C17S1P was faster on LDLr-overexpressing HepG2 cells only when C17S1P was bound to apoE-rich HDL (Fig. 7, B and C). These results also support that apoE has a crucial role in the clearance of S1P bound to apoM-containing lipoproteins through the LDL receptor.
TABLE 2.
S1P contents in apoE-poor HDL and apoE-rich HDL
One mg of HDL prepared from healthy subjects (n = 4) was separated into apoE-poor HDL and apoE-rich HDL, as described undere “Experimental Procedures.” S1P, total cholesterol (TC), and total phospholipid (PL) contents of whole HDL, ApoE-poor HDL, and ApoE-rich HDL were determined. Data were expressed as the mean ± S.E.
| Whole HDL | ApoE-poor HDL | ApoE-rich HDL | |
|---|---|---|---|
| S1P (ng) | 89.5 ± 8.1 | 68.8 ± 9.5 | 19.6 ± 2.9 |
| TC (μg) | 133.3 ± 7.7 | 83.8 ± 4.8 | 23.3 ± 3.5 |
| PL (μg) | 398.5 ± 25.7 | 335.6 ± 32.9 | 34.1 ± 4.5 |
| S1P/PL (ng/mg) | 224.8 ± 15.0 | 205.6 ± 20.6 | 588.9 ± 71.2 |
| TC/PL (μg/mg) | 335.4 ± 10.4 | 254.0 ± 17.7 | 682.1 ± 19.9 |
DISCUSSION
S1P is mainly carried on HDL (∼70%), followed by albumin (∼30%), in circulation, and several anti-atherosclerotic properties of HDL have been ascribed to S1P carried on HDL. Contrary to this established distribution of S1P among lipoproteins, we previously reported that the plasma S1P level is not correlated with the HDL cholesterol or apoA-I levels (17). Interestingly, the plasma S1P level is significantly and positively correlated with LDL cholesterol and apoB levels. The similar correlation between plasma S1P and LDL cholesterol level is also recently reported from other group (32). These observations suggest that the metabolic pathway involved in the clearance of LDL, such as LDL receptor, might have some roles in the clearance of S1P.
In this study, we overexpressed LDL receptor and observed that the plasma S1P level, especially in HDL fractions, was markedly decreased. Recently, apoM, an apolipoprotein distributed mainly on HDL, has been elucidated to be a carrier of S1P (19), and the previous reports demonstrated that apoM was cleared through LDL receptor (22, 33). In our models, we also observed that apoM was decreased in LDLr-overexpressing mice, as well as S1P (Figs. 2, 3, and 4, A–E), suggesting that S1P bound to apoM might be cleared through the LDL receptor. The main function of the LDL receptor is the uptake of LDL from the circulation into the liver; therefore, it seems reasonable that the plasma S1P level should be positively related to the LDL cholesterol level. Actually, the treatment of HepG2 cells with an HMG-CoA reductase inhibitor, statin, accelerated the clearance of C17S1P bound to apoM-containing lipoproteins (Fig. 5), which is consistent with the results from a previous study demonstrating that statin treatment decreased the apoM level in human subjects (34). This result may suggest a limitation of the clinical benefit of statin: namely, the problem of residual risk. Statin lowers the LDL cholesterol level to almost the same level as that seen in healthy subjects and prevents cardiovascular diseases; however, statin cannot completely eliminate ischemic heart diseases (35). In in vitro experiments, S1P exhibited anti-atherosclerotic properties and attributes of the pleiotropic properties of HDL. Therefore, S1P might be a useful target for overcoming the issue of residual risk.
In this study, we also demonstrated that both the plasma S1P and apoM levels decreased in apoE-overexpressing mice (Fig. 7). These results suggested that apoE plays some role in accelerating the clearance of plasma S1P and apoM. Both apoB100 and apoE reportedly function as ligands for the LDL receptor. Although apoE on VLDL reportedly serves as a ligand for the LDL receptor (28, 29), several reports have demonstrated the possibility that apoE and the LDL receptor are involved in the clearance of HDL from the circulation (30, 31). Because apoM and S1P exist mainly on HDL and minimally on apoB-containing lipoproteins (5, 18), apoE on apoM-containing lipoproteins might serve as a ligand for the LDL receptor. Actually, LDL receptor overexpression in apoE-deficient mice did not decrease either the plasma apoM or S1P levels (Fig. 8). The result that the clearance of C17S1P bound to apoE-rich HDL, but not that bound to apoE-poor HDL, was influenced by LDL receptor also supports the crucial role of apoE in the clearance of S1P bound to apoM (Fig. 9). Regarding LDL receptor-mediated apoM clearance, Christoffersen et al. (22) have suggested the other mechanism in the previous paper; apoM is shuttled from HDL to LDL and the apoM is cleared with LDL particles. The pathway proposed in the previous article may also exist in the present study; S1P levels in IDL/LDL fractions (fraction 26–35) were decreased in LDL receptor overexpressing apoE-deficient mice (Fig. 8G), although the total plasma S1P level and S1P levels in HDL fractions (fraction 41–46) were rather increased in LDLr-overexpressing apoE-deficient mice.
FIGURE 9.
LDL receptor overexpression accelerated the clearance of C17S1P bound to apoE-rich HDL, but not C17S1P bound to apoE-poor HDL. Clearance of C17S1P bound to apoE-rich HDL or apoE-poor HDL in vitro. A, 1 (X1), 0.5 (X2), and 0.25 μg (X4) of separated apoE-rich HDL (right) and apoE-poor HDL (left) were subjected to Western blot. B and C, in total, 250 nm (final concentration) of C17S1P bound to 200 μg/ml (final concentration) of apoE-rich HDL (B) or apoE-poor HDL (C) in serum-free DMEM was placed on LDLr-overexpressing HepG2 cells or HepG2 cells infected with a blank adenovirus (n = 6/group). Time courses for the medium C17S1P contents are shown.
Although the in vivo experiments with apoE-overexpressing mice and LDLr-overexpressing apoE-deficient mice and in vitro experiments using apoE-poor and apoE-rich HDL supported that the LDL receptor might utilize apoE as a ligand in the clearance of S1P bound to apoM-containing lipoproteins, the result from the experiment comparing apoE-deficient mice with wild-type mice seemed inconsistent with this hypothesis. One possible explanation is that the hepatic apoM expression level in apoE-deficient mice was lower than that in wild-type mice, suggesting that lower apoM production blurred retarded clearance of apoM in apoE-deficient mice (Fig. 6C). Another possibility is that in apoE-deficient mice, apoM and S1P are distributed not only to HDL but also to VLDL/IDL fractions, whereas most of them are distributed to HDL fractions in wild-type mice (Fig. 6, D and E). Because VLDL/IDL, especially that of apoE-deficient mice, possesses different structures and undergoes a different metabolism from HDL, the modulations of total plasma apoM and S1P might be somewhat different in apoE-deficient mice. Although the distributions of apoM and S1P were different in apoE-deficient mice, the result that the S1P and apoM levels in HDL fractions were not decreased in LDLr-overexpressing apoE-deficient mice (Fig. 8, G and H) supported that apoE is necessary for the LDL receptor to clear S1P bound to apoM-containing HDL.
Another interesting observation in the present study was that overexpression of the LDL receptor in apoE-deficient mice tended to increase HDL cholesterol as well as S1P and apoM in HDL fractions (Fig. 8, G and H). Although the mechanisms for this are not yet elucidated, hepatic expression of SR-BI, an HDL receptor, was found to be significantly decreased in LDLr-overexpressing apoE-deficient mice (Fig. 8I), which might increase HDL cholesterol as well as apoM and S1P in HDL fractions. These results suggest that SR-BI may also have some important roles in the metabolism of S1P bound to apoM-containing lipoproteins. Further studies are needed to elucidate this possibility. In conclusion, the LDL receptor is involved in the clearance of S1P bound to apoM-containing lipoproteins, possibly utilizing apoE as a ligand, which may be related to the paradoxical correlation between the plasma S1P level and apoB-containing lipoproteins.
This work was supported by Japan Society for the Promotion of Science KAKENHI Grants 22249017 and 25253040 (to Y. Y.) and 25860740 (to M. K.) and CREST from the Japan Science and Technology Agency.
- S1P
- sphingosine 1-phosphate
- Ad-apoE
- adenovirus coding apoE
- Ad-apoM
- adenovirus coding apoM
- Ad-LacZ
- control LacZ expressing adenovirus
- Ad-LDLr
- adenovirus coding LDL receptor
- Ad-null
- control blank adenovirus
- LDLr
- LDL receptor
- SR-BI: scavenger receptor class B
- type I.
REFERENCES
- 1. Iacob A. O., Choudhury R. P. (2012) Targeting HDL-cholesterol to reduce residual cardiovascular risk. Curr. Opin. Lipidol. 23, 172–174 [DOI] [PubMed] [Google Scholar]
- 2. Schofield J. D., France M., Ammori B., Liu Y., Soran H. (2013) High-density lipoprotein cholesterol raising: does it matter? Curr. Opin. Cardiol. 28, 464–474 [DOI] [PubMed] [Google Scholar]
- 3. Brewer H. B., Jr. (2011) Clinical review: The evolving role of HDL in the treatment of high-risk patients with cardiovascular disease. J. Clin. Endocrinol. Metab. 96, 1246–1257 [DOI] [PubMed] [Google Scholar]
- 4. Yatomi Y. (2006) Sphingosine 1-phosphate in vascular biology: possible therapeutic strategies to control vascular diseases. Curr. Pharm. Des. 12, 575–587 [DOI] [PubMed] [Google Scholar]
- 5. Okajima F. (2002) Plasma lipoproteins behave as carriers of extracellular sphingosine 1-phosphate: is this an atherogenic mediator or an anti-atherogenic mediator? Biochim. Biophys. Acta 1582, 132–137 [DOI] [PubMed] [Google Scholar]
- 6. Rodríguez C., González-Díez M., Badimon L., Martínez-González J. (2009) Sphingosine-1-phosphate: a bioactive lipid that confers high-density lipoprotein with vasculoprotection mediated by nitric oxide and prostacyclin. Thromb. Haemost. 101, 665–673 [PubMed] [Google Scholar]
- 7. Goetzl E. J. (2001) Pleiotypic mechanisms of cellular responses to biologically active lysophospholipids. Prostaglandins 64, 11–20 [DOI] [PubMed] [Google Scholar]
- 8. Kimura T., Tomura H., Mogi C., Kuwabara A., Damirin A., Ishizuka T., Sekiguchi A., Ishiwara M., Im D. S., Sato K., Murakami M., Okajima F. (2006) Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J. Biol. Chem. 281, 37457–37467 [DOI] [PubMed] [Google Scholar]
- 9. Argraves K. M., Argraves W. S. (2007) HDL serves as a S1P signaling platform mediating a multitude of cardiovascular effects. J. Lipid Res. 48, 2325–2333 [DOI] [PubMed] [Google Scholar]
- 10. Igarashi J., Bernier S. G., Michel T. (2001) Sphingosine 1-phosphate and activation of endothelial nitric-oxide synthase: differential regulation of Akt and MAP kinase pathways by EDG and bradykinin receptors in vascular endothelial cells. J. Biol. Chem. 276, 12420–12426 [DOI] [PubMed] [Google Scholar]
- 11. Nofer J. R., van der Giet M., Tölle M., Wolinska I., von Wnuck Lipinski K., Baba H. A., Tietge U. J., Gödecke A., Ishii I., Kleuser B., Schäfers M., Fobker M., Zidek W., Assmann G., Chun J., Levkau B. (2004) HDL induces NO-dependent vasorelaxation via the lysophospholipid receptor S1P3. J. Clin. Invest. 113, 569–581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Garcia J. G., Liu F., Verin A. D., Birukova A., Dechert M. A., Gerthoffer W. T., Bamberg J. R., English D. (2001) Sphingosine 1-phosphate promotes endothelial cell barrier integrity by Edg-dependent cytoskeletal rearrangement. J. Clin. Invest. 108, 689–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sanchez T., Estrada-Hernandez T., Paik J. H., Wu M. T., Venkataraman K., Brinkmann V., Claffey K., Hla T. (2003) Phosphorylation and action of the immunomodulator FTY720 inhibits vascular endothelial cell growth factor-induced vascular permeability. J. Biol. Chem. 278, 47281–47290 [DOI] [PubMed] [Google Scholar]
- 14. Sanchez T., Hla T. (2004) Structural and functional characteristics of S1P receptors. J. Cell. Biochem. 92, 913–922 [DOI] [PubMed] [Google Scholar]
- 15. Sattler K. J., Elbasan S., Keul P., Elter-Schulz M., Bode C., Gräler M. H., Bröcker-Preuss M., Budde T., Erbel R., Heusch G., Levkau B. (2010) Sphingosine 1-phosphate levels in plasma and HDL are altered in coronary artery disease. Basic Res. Cardiol. 105, 821–832 [DOI] [PubMed] [Google Scholar]
- 16. Argraves K. M., Sethi A. A., Gazzolo P. J., Wilkerson B. A., Remaley A. T., Tybjaerg-Hansen A., Nordestgaard B. G., Yeatts S. D., Nicholas K. S., Barth J. L., Argraves W. S. (2011) S1P, dihydro-S1P and C24:1-ceramide levels in the HDL-containing fraction of serum inversely correlate with occurrence of ischemic heart disease. Lipids Health Dis. 10, 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ohkawa R., Nakamura K., Okubo S., Hosogaya S., Ozaki Y., Tozuka M., Osima N., Yokota H., Ikeda H., Yatomi Y. (2008) Plasma sphingosine-1-phosphate measurement in healthy subjects: close correlation with red blood cell parameters. Ann. Clin. Biochem. 45, 356–363 [DOI] [PubMed] [Google Scholar]
- 18. Xu N., Dahlbäck B. (1999) A novel human apolipoprotein (apoM). J. Biol. Chem. 274, 31286–31290 [DOI] [PubMed] [Google Scholar]
- 19. Christoffersen C., Obinata H., Kumaraswamy S. B., Galvani S., Ahnström J., Sevvana M., Egerer-Sieber C., Muller Y. A., Hla T., Nielsen L. B., Dahlbäck B. (2011) Endothelium-protective sphingosine-1-phosphate provided by HDL-associated apolipoprotein M. Proc. Natl. Acad. Sci. U.S.A. 108, 9613–9618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Axler O., Ahnström J., Dahlbäck B. (2007) An ELISA for apolipoprotein M reveals a strong correlation to total cholesterol in human plasma. J. Lipid Res. 48, 1772–1780 [DOI] [PubMed] [Google Scholar]
- 21. Christoffersen C., Nielsen L. B., Axler O., Andersson A., Johnsen A. H., Dahlbäck B. (2006) Isolation and characterization of human apolipoprotein M-containing lipoproteins. J. Lipid Res. 47, 1833–1843 [DOI] [PubMed] [Google Scholar]
- 22. Christoffersen C., Benn M., Christensen P. M., Gordts P. L., Roebroek A. J., Frikke-Schmidt R., Tybjaerg-Hansen A., Dahlbäck B., Nielsen L. B. (2012) The plasma concentration of HDL-associated apoM is influenced by LDL receptor-mediated clearance of apoB-containing particles. J. Lipid Res. 53, 2198–2204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kurano M., Tsukamoto K., Ohkawa R., Hara M., Iino J., Kageyama Y., Ikeda H., Yatomi Y. (2013) Liver involvement in sphingosine 1-phosphate dynamism revealed by adenoviral hepatic overexpression of apolipoprotein M. Atherosclerosis 229, 102–109 [DOI] [PubMed] [Google Scholar]
- 24. Hara M., Matsushima T., Satoh H., Iso-o N., Noto H., Togo M., Kimura S., Hashimoto Y., Tsukamoto K. (2003) Isoform-dependent cholesterol efflux from macrophages by apolipoprotein E is modulated by cell surface proteoglycans. Arterioscler. Thromb. Vasc. Biol. 23, 269–274 [DOI] [PubMed] [Google Scholar]
- 25. Kurano M., Iso-O. N., Hara M., Ishizaka N., Moriya K., Koike K., Tsukamoto K. (2011) LXR agonist increases apoE secretion from HepG2 spheroid, together with an increased production of VLDL and apoE-rich large HDL. Lipids Health Dis. 10, 134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kurano M., Hara M., Tsuneyama K., Okamoto K., Iso-O. N., Matsushima T., Koike K., Tsukamoto K. (2012) Modulation of lipid metabolism with the overexpression of NPC1L1 in mouse liver. J. Lipid Res. 53, 2275–2285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yatomi Y. (2008) Plasma sphingosine 1-phosphate metabolism and analysis. Biochim. Biophys. Acta 1780, 606–611 [DOI] [PubMed] [Google Scholar]
- 28. Innerarity T. L., Mahley R. W. (1978) Enhanced binding by cultured human fibroblasts of apo-E-containing lipoproteins as compared with low density lipoproteins. Biochemistry 17, 1440–1447 [DOI] [PubMed] [Google Scholar]
- 29. Fisher C., Abdul-Aziz D., Blacklow S. C. (2004) A two-module region of the low-density lipoprotein receptor sufficient for formation of complexes with apolipoprotein E ligands. Biochemistry 43, 1037–1044 [DOI] [PubMed] [Google Scholar]
- 30. Stewart J. E., Skinner E. R., Best P. V. (1998) Receptor binding of an apolipoprotein E-rich subfraction of high density lipoprotein to rat and human brain membranes. Int. J. Biochem. Cell Biol. 30, 407–415 [DOI] [PubMed] [Google Scholar]
- 31. Rosales C., Tang D., Gillard B. K., Courtney H. S., Pownall H. J. (2011) Apolipoprotein E mediates enhanced plasma high-density lipoprotein cholesterol clearance by low-dose streptococcal serum opacity factor via hepatic low-density lipoprotein receptors in vivo. Arterioscler. Thromb. Vasc. Biol. 31, 1834–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kowalski G. M., Carey A. L., Selathurai A., Kingwell B. A., Bruce C. R. (2013) Plasma sphingosine-1-phosphate is elevated in obesity. PLoS One 8, e72449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Christoffersen C., Pedersen T. X., Gordts P. L., Roebroek A. J., Dahlbäck B., Nielsen L. B. (2010) Opposing effects of apolipoprotein M on catabolism of apolipoprotein B-containing lipoproteins and atherosclerosis. Circ. Res. 106, 1624–1634 [DOI] [PubMed] [Google Scholar]
- 34. Kappelle P. J., Ahnström J., Dikkeschei B. D., de Vries R., Sluiter W. J., Wolffenbuttel B. H., van Tol A., Nielsen L. B., Dahlbäck B., Dullaart R. P. (2010) Plasma apolipoprotein M responses to statin and fibrate administration in type 2 diabetes mellitus. Atherosclerosis 213, 247–250 [DOI] [PubMed] [Google Scholar]
- 35. Sampson U. K., Fazio S., Linton M. F. (2012) Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr. Atheroscler Rep. 14, 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]