Abstract
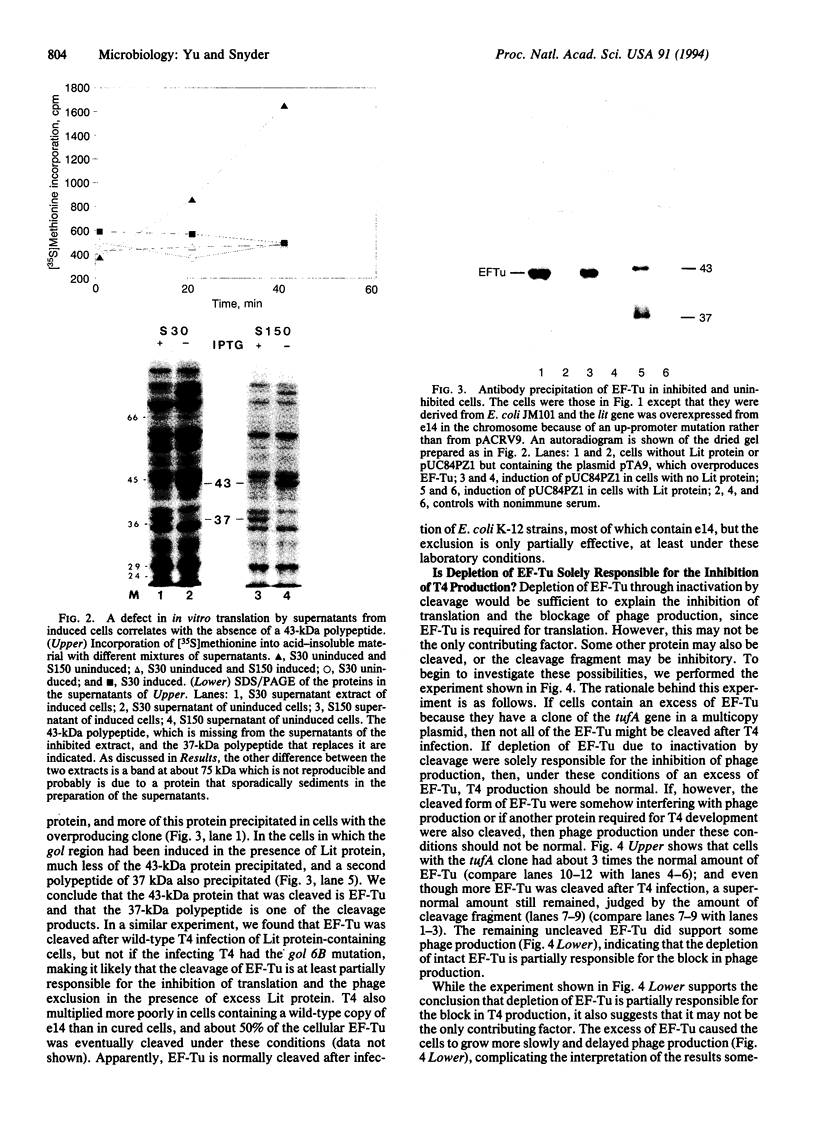
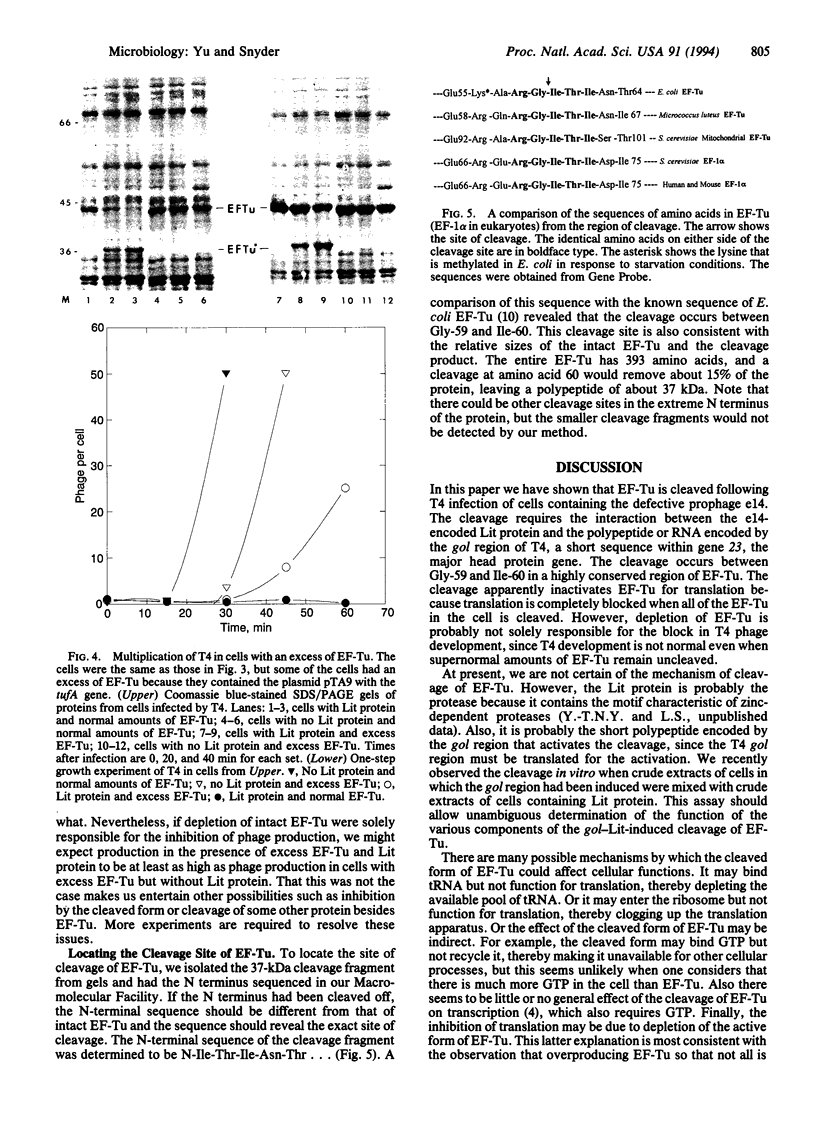
Bacteriophage T4 multiples poorly in Escherichia coli strains carrying the defective prophage, e14; the e14 prophage contains the lit gene for late inhibitor of T4 in E. coli. The exclusion is caused by the interaction of the e14-encoded protein, Lit, with a short RNA or polypeptide sequence encoded by gol from within the major head protein gene of T4. The interaction between Lit and the gol product causes a severe inhibition of all translation and prevents the transcription of genes downstream of the gol site in the same transcription unit. However, it does not inhibit most transcription, nor does it inhibit replication or affect intracellular levels of ATP. Here we show that the interaction of gol with Lit causes the cleavage of translation elongation factor Tu (EF-Tu) in a region highly conserved from bacteria to humans. The depletion of EF-Tu is at least partly responsible for the inhibition of translation and the phage exclusion. The only other phage-exclusion system to be understood in any detail also attacks a highly conserved cellular component, suggesting that phage-exclusion systems may yield important reagents for studying cellular processes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames G. F., Niakido K. In vivo methylation of prokaryotic elongation factor Tu. J Biol Chem. 1979 Oct 25;254(20):9947–9950. [PubMed] [Google Scholar]
- Arai K., Clark B. F., Duffy L., Jones M. D., Kaziro Y., Laursen R. A., L'Italien J., Miller D. L., Nagarkatti S., Nakamura S. Primary structure of elongation factor Tu from Escherichia coli. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1326–1330. doi: 10.1073/pnas.77.3.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardwell J. C., Régnier P., Chen S. M., Nakamura Y., Grunberg-Manago M., Court D. L. Autoregulation of RNase III operon by mRNA processing. EMBO J. 1989 Nov;8(11):3401–3407. doi: 10.1002/j.1460-2075.1989.tb08504.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergsland K. J., Kao C., Yu Y. T., Gulati R., Snyder L. A site in the T4 bacteriophage major head protein gene that can promote the inhibition of all translation in Escherichia coli. J Mol Biol. 1990 Jun 5;213(3):477–494. doi: 10.1016/S0022-2836(05)80209-8. [DOI] [PubMed] [Google Scholar]
- Bourgaize D. B., Fournier M. J. Initiation of translation is impaired in E. coli cells deficient in 4.5S RNA. Nature. 1987 Jan 15;325(6101):281–284. doi: 10.1038/325281a0. [DOI] [PubMed] [Google Scholar]
- Brody H., Greener A., Hill C. W. Excision and reintegration of the Escherichia coli K-12 chromosomal element e14. J Bacteriol. 1985 Mar;161(3):1112–1117. doi: 10.1128/jb.161.3.1112-1117.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulewicz K., Faulhammer H. G., Sprinzl M. Properties of native and nicked elongation factor Tu from Thermus thermophilus HB 8. Eur J Biochem. 1981 Dec;121(1):155–162. doi: 10.1111/j.1432-1033.1981.tb06444.x. [DOI] [PubMed] [Google Scholar]
- Hill C. W., Gray J. A., Brody H. Use of the isocitrate dehydrogenase structural gene for attachment of e14 in Escherichia coli K-12. J Bacteriol. 1989 Jul;171(7):4083–4084. doi: 10.1128/jb.171.7.4083-4084.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwabe N., Kuma K., Hasegawa M., Osawa S., Miyata T. Evolutionary relationship of archaebacteria, eubacteria, and eukaryotes inferred from phylogenetic trees of duplicated genes. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9355–9359. doi: 10.1073/pnas.86.23.9355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurnak F. Structure of the GDP domain of EF-Tu and location of the amino acids homologous to ras oncogene proteins. Science. 1985 Oct 4;230(4721):32–36. doi: 10.1126/science.3898365. [DOI] [PubMed] [Google Scholar]
- Kao C., Gumbs E., Snyder L. Cloning and characterization of the Escherichia coli lit gene, which blocks bacteriophage T4 late gene expression. J Bacteriol. 1987 Mar;169(3):1232–1238. doi: 10.1128/jb.169.3.1232-1238.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C., Snyder L. The lit gene product which blocks bacteriophage T4 late gene expression is a membrane protein encoded by a cryptic DNA element, e14. J Bacteriol. 1988 May;170(5):2056–2062. doi: 10.1128/jb.170.5.2056-2062.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levitz R., Chapman D., Amitsur M., Green R., Snyder L., Kaufmann G. The optional E. coli prr locus encodes a latent form of phage T4-induced anticodon nuclease. EMBO J. 1990 May;9(5):1383–1389. doi: 10.1002/j.1460-2075.1990.tb08253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molineux I. J. Host-parasite interactions: recent developments in the genetics of abortive phage infections. New Biol. 1991 Mar;3(3):230–236. [PubMed] [Google Scholar]
- Riis B., Rattan S. I., Clark B. F., Merrick W. C. Eukaryotic protein elongation factors. Trends Biochem Sci. 1990 Nov;15(11):420–424. doi: 10.1016/0968-0004(90)90279-k. [DOI] [PubMed] [Google Scholar]
- Wittinghofer A., Frank R., Leberman R. Composition and properties of trypsin-cleaved elongation factor Tu. Eur J Biochem. 1980 Jul;108(2):423–431. doi: 10.1111/j.1432-1033.1980.tb04738.x. [DOI] [PubMed] [Google Scholar]
- Young C. C., Bernlohr R. W. Elongation factor Tu is methylated in response to nutrient deprivation in Escherichia coli. J Bacteriol. 1991 May;173(10):3096–3100. doi: 10.1128/jb.173.10.3096-3100.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]






