Abstract
Purpose
Currently, there is no consensus concerning the possible beneficial colonic and systemic effects of prebiotic-containing infant formula. This study assesses whether the feeding of a galactooligosaccharides (GOS)-containing infant formula (0.44 g/dl of GOS) and the subsequent feeding of a GOS-containing follow-on formula (0.50 g/dl of GOS) have a prebiotic effect on intestinal microbiota that helps to decrease infections and allergy manifestations in healthy infants during the first year of life.
Methods
A multicentre, randomised, double-blind and placebo-controlled trial was carried out on 365 healthy term infants enrolled before 8 weeks of age and randomly assigned to a formula with or without GOS, until 12 months of age. The incidence of infections and allergy manifestations, the antibiotics prescribed and faecal characteristics were recorded up to 12 months of age, while faecal samples were collected up to 4 months for the measurement of secretory immunoglobulin A, short-chain fatty acids and microbiota.
Results
A prebiotic effect on the faecal analysis was observed at 4 months of life. The GOS group showed a lower faecal pH (P = 0.019), a lower decreasing trend in secretory immunoglobulin A (P = 0.078), lower butyric acid concentration (P = 0.040) and an increase in Bifidobacterium counts (P = 0.010). Changes in faecal characteristics involved greater frequency (P < 0.001) and softer consistency (P < 0.05). The incidence of infections or allergic manifestations during the first year of life was similar in both groups, with no statistical differences (P > 0.05).
Conclusions
The feeding of GOS-containing infant formula produced a definite prebiotic effect consisting of changes in faecal composition and microbiota, and in faecal consistency and the frequency of defaecation. No changes in the incidence of infection or allergic manifestation during the first year of life were observed.
Keywords: Clinical trial; Prebiotics; Infant nutrition; Infant formula; Randomised double-blind, placebo-controlled study
Introduction
Human milk oligosaccharides (HMOs), a complex mixture of glycan compounds, have been associated with the prevention of intestinal diseases, which are partially mediated by the modulation of the intestinal microbial ecology and immunological homeostasis [1, 2]. Prebiotics are non-digestible carbohydrates similar to HMOs. Because HMOs include a high quantity of galactose, galactooligosaccharides (GOS) are often the predominant prebiotic oligosaccharides used to supplement infant formulas. The prebiotic effect is defined as “the selective stimulation of growth and/or activity(ies) of one or a limited number of microbial genus(era)/species in the gut microbiota that confer(s) health benefits to the host” [3]. The main health benefits that intestinal microbiota confer are an improvement in faeces quality (pH, short-chain fatty acid content, frequency and consistency), a reduction in the risk of gastrointestinal infections, improved general well-being and a reduced incidence of allergic symptoms such as atopic eczema [3]. Infant formulas with added prebiotics are also associated with beneficial effects, but few studies have demonstrated an improvement in clinical outcomes such as infections and allergic manifestations [4–8]. Consequently, recent systematic reviews have concluded that further evidence is needed in order to recommend or not the routine addition of prebiotics to infant formulas to prevent infections [9] and allergy [9, 10]. The inclusion of GOS in infant formulas is believed to lead to an intestinal microbiota composition, with more bifidobacteria, fewer pathogens and a metabolic activity similar to that described in breastfed infants [11], resulting in beneficial effects on health through immune system regulation [3, 12]. No published studies have evaluated the effect of GOS-containing infant formulas on infections and allergy. Only prebiotic effects on the growth of Bifidobacterium and Lactobacillus genera have been evaluated [13, 14].
Our hypothesis was that the feeding of a GOS-containing formula would have a prebiotic effect on intestinal microbiota, which might help to reduce infections and allergic manifestations. To test this hypothesis, we conducted a prospective, randomised, double-blind and placebo-controlled trial to assess the effects of prebiotic supplementation on intestinal microbiota and some related parameters in the colon and on the incidence of infectious episodes and allergic manifestations during the first year of life.
Methods and materials
Study design and protocol
Healthy term infants of <2 months of age were recruited for a multicentre, randomised, double-blind and placebo-controlled trial in eight Spanish hospitals: Hospital Materno-Infantil (Málaga), Hospital Materno-Infantil (Granada), Hospital Virgen del Rocío (Sevilla), Hospital Virgen de la Arrixaca (Murcia), Hospital Infantil La Fe (Valencia), Hospital Doce de Octubre (Madrid), Hospital Clínico Universitario (Santiago de Compostela) and Hospital Infantil Vall d’Hebrón (Barcelona). The study was conducted according to the guidelines established in the Declaration of Helsinki. The protocol was approved by the ethics committees at each hospital, and written informed parental consent was obtained for each infant before inclusion.
Eligible infants had a gestational age of 37–42 weeks and a birth weight greater than 2,500 g and were exclusively formula fed for at least 15 days prior to enrolment. Infants that had previously consumed either prebiotics or probiotics and infants who themselves, or their mothers, had clinically significant diseases were excluded.
Infants were randomly assigned to receive an infant formula until 6 months of age and then the follow-on formula until 12 months of age either with GOS supplementation (GOS group) or without (control group). Enrolment and randomization were performed before or at 2 months of age. A separate randomization schedule in blocks of 10 was prepared for each study site by an external independent agent and provided in consecutively numbered and sealed envelopes. The introduction of complementary feeding was allowed at 4 months. Prebiotics and probiotics were not allowed during the feeding period. The contents of GOS were 0.44 g/dl in the study infant formula and 0.50 g/dl in the study follow-on formula (commercially available as Nutradefense® infant and follow-on formulas) (Table 1). Both infant and follow-on formulas were provided in powdered form, had identical sensorial characteristics and the same label and were designed, produced, codified (two numbers printed on cans) and provided by the Hero Group. Both the investigators and the infants’ parents were blind to the group allocation. The blinding was broken once the statistical analysis was completed.
Table 1.
Nutritional composition of the four formulas used in the study per dl
| Infant formula | Follow-on formula | |||
|---|---|---|---|---|
| Control | GOS | Control | GOS | |
| Energy (kcal) | 67 | 65 | 66 | 70 |
| Protein (g) | 1.4 | 1.4 | 1.6 | 1.6 |
| Casein–whey | 40:60 | 40:60 | 50:50 | 50:50 |
| Lactose (g) | 6.98 | 6.70 | 5.2 | 5.9 |
| Maltodextrins (g) | 0.72 | 0.80 | 3.2 | 3.2 |
| GOS (g) | – | 0.44 | – | 0.50 |
| Fat (g) | 3.5 | 3.5 | 2.9 | 2.9 |
| Linoleic acid (mg) | 442 | 442 | 433 | 353 |
| α-linolenic acid (mg) | 62 | 62 | 60.8 | 50.3 |
| Arachidonic acid (mg) | 6.9 | 6.9 | 2.7 | 2.7 |
| Docosahexaenoic acid (mg) | 6.9 | 6.9 | 2.7 | 2.7 |
| Nucleotides (mg) | 3.3 | 3.3 | 3.4 | 3.4 |
Study outcomes and data collection
Acute diarrhoea was defined as semiliquid or liquid faeces in three or more depositions per day for at least 3 days. Upper respiratory tract infections (URTI) were diagnosed by the primary care paediatrician based on specific symptoms. Recurrent URTI were defined as more than 3 episodes in 12 months. Any sign or symptom related to allergy, wheezing and atopic dermatitis (AD) and infection (fever, cough, rhinitis and diarrhoea) was recorded and closely supervised by the paediatrician. Atopic sensitisation to cow’s milk, egg and fish was evaluated by skin prick testing (SPT) at the age of 12 months.
For 5 days before each visit, parents recorded the frequency of defaecation, faecal consistency and intake of formula in daily records. Faecal consistency was rated on a 1–5 scale (1 = watery, 2 = loose, 3 = lumpy, 4 = soft, 5 = hard).
All parameters (URTI, wheezing, AD, fever, cough, rhinitis, diarrhoea, frequency of defaecation, faecal consistency and intake of formula) were recorded (reported by the parents and reviewed by a primary care paediatrician) during the visits at 3 (75–99 days), 4 (107–142 days), 6 (168–199 days), 9 (261–289 days) and 12 (352–375 days) months of life. Anthropometric measurements (weight, length and head circumference) were taken by the paediatrician at enrolment and during the visits.
To study the possible prebiotic effect, fresh faecal samples were collected from nappies in a plastic faeces container, frozen immediately after collection by the parents at home and stored until they were taken to a central collection site. Faeces were then kept at −80 °C until analysis. The faecal samples were collected in a subgroup of infants at enrolment and at 4 months of age, and pH, secretory immunoglobulin A (sIgA), short-chain fatty acids (SCFAs) and microbiota parameters were measured.
To measure faecal pH, an aliquot of 1 g of faeces was diluted tenfold in Milli-Q water. After dilution, the faecal pH was measured with a GLP21 pH meter (Crison, Barcelona, Spain). Faecal sIgA concentration was measured with the Immunodiagnostik K8870 (Bensheim, Germany) commercial enzyme-linked immunosorbent assay, according to the manufacturer’s instructions. SCFAs were detected and quantified using gas chromatography, as previously described [15].
Before bacterial quantification by real-time-polymerase chain reactions (PCRs), the genomic DNA in the faecal samples and the DNA from bacterial cultures used for calibration curves were extracted using a QIAamp DNA stool mini kit (Qiagen, Hilden, Germany) [16]. PCRs were conducted on a 25-μl final volume with 2–3 μl of template and 0.5 μM to 0.7 μM of primer pairs corresponding to each bacterium: Bifidobacterium [17], Lactobacillus [18], Bacteroides [19], Bifidobacterium species [20] and Clostridium difficile [21]. Bacterial counts were expressed as log10 colony-forming units (CFU) per gram of faecal samples.
Statistical analysis
The power of the study was calculated considering respiratory infections and Bifidobacterium population as primary outcomes. Sample size was estimated to detect a 20 % reduction in the incidence of respiratory infections, considering that each infant would have on average 6.3 episodes [mean 5.1, interquartile range (IQR) 3.3–7.8] of URTI in the first year of life [22]. Thus, with the power set at 85 % and the significance level at 0.05, the required sample size was about 156 infants per each cohort to show a difference of 20 % between the groups. Given that the variability of respiratory infections is greater than gastrointestinal infections, this sample size would also be sufficient for detecting differences in gastrointestinal infections. For the evaluation of the prebiotic effect, the minimum number of subjects, based on the faecal concentration of Bifidobacterium, was 20 subjects per group to achieve a mean difference of 30 % compared with the control formula [23] and taking into account 20 % of dropouts, with a probability of 80 % and a significance level of 0.05.
Continuous variables are reported as the mean and standard deviation (SD), or median and IQR, and categorical variables are reported as numbers or percentages. To detect differences at enrolment between the feeding groups in each quantitative variable, an unpaired student’s t test was used; for variables expressed as percentages, a Pearson Chi-square test was used. The results for the two groups were evaluated with an unpaired student’s t test for anthropometric measurements and for the incidence of diarrhoea, URTI and allergic manifestations. To compare the proportion of infants with URTI and acute diarrhoea incidences, as well as the antibiotic prescription rates of both groups, a Pearson Chi-square test was used. All parameters of the collected faecal samples were analysed with a repeated-measures analysis of variance. Pearson’s correlation coefficient was calculated among all parameters analysed in faeces. A general linear univariate model was used to analyse frequency of defaecation, and a Pearson Chi-square test was used to analyse faecal consistency.
All results with a significance level of P < 0.05 were considered statistically significant. Statistical analyses of data were performed using the Statistical Package for the Social Sciences (SPSS Version 18.0; Inc., Chicago, IL, USA).
Results
Subjects
A total of 365 infants were enrolled and randomized in the study, 177 in the control group and 188 in the GOS group (Fig. 1). The percentage of dropouts was similar in both feeding groups at around 27 %. One hundred and thirty-two infants in each group successfully completed the study (Fig. 1). At enrolment, there were no differences between the two groups in terms of gender, gestational age, type of delivery, birth weight, age at inclusion, number of days with exclusive breastfeeding before enrolment, family history of atopy, smoking parents and furry pets at home (Table 2).
Fig. 1.
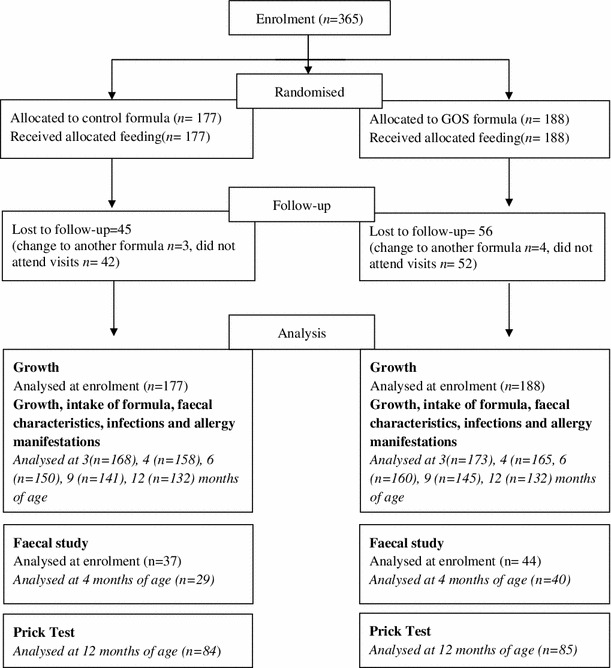
Flow chart of infants enrolled and disposition of the subjects throughout the study
Table 2.
Characteristics of infants enrolled in the study
| Control group (n = 177) | GOS group (n = 188) | P valuea | |
|---|---|---|---|
| Male/female | 96/81 | 107/81 | 0.673 |
| Gestational age (week) (mean ± SD) | 39.31 ± 1.20 | 39.13 ± 1.36 | 0.189 |
| Delivery (vaginal/caesarean) | 122/55 | 136/55 | 0.474 |
| Birth weight(g) (mean ± SD) | 3,249 ± 426 | 3,222 ± 488 | 0.570 |
| Age at enrolment (days) (mean ± SD) | 32.16 ± 18.21 | 29.38 ± 17.74 | 0.140 |
| Exclusive breastfeeding before enrolment (days) (mean ± SD) | 19.54 ± 15.44 | 16.82 ± 14.92 | 0.300 |
| Family history of atopy | |||
| Father (%) | 17 ± 10 | 20 ± 11 | 0.744 |
| Mother (%) | 25 ± 14 | 29 ± 15 | 0.726 |
| One brother (%) | 7 ± 4 | 13 ± 7 | 0.214 |
| Two brothers (%) | 4 ± 2 | 5 ± 3 | 0.586 |
| Smoking parents (%) | 60 ± 34 | 74 ± 39 | 0.279 |
| Furry pets at home (%) | 22 ± 12 | 34 ± 18 | 0.134 |
aDifference between the groups tested with unpaired Student’s t test for variables expressed as mean ± SD and a Pearson Chi-square for variables expressed as percentage
During the feeding period, all infants had adequate growth (e.g. the mean weight at 12 months was 9.94 ± 1.37 kg for the control group and 9.98 ± 1.10 kg for the GOS group) and the intake of formula appropriate for age, with no significant differences between the feeding groups at 3, 4, 6, 9 and 12 months of age (data not shown). No issues related to consumption of the formula (regurgitations, gassiness or rejections) were reported.
At enrolment, the faecal subsample included 81 infants (control group, 37; GOS group, 44); 12 were excluded due to parental decision, the introduction of a non-study formula, or improper faecal sample delivery or preservation. At 4 months, 69 participants were included in the subset analysis (control group, 29; GOS group, 40) (Fig. 1).
Faecal analysis
The results of the faecal parameters at enrolment and at 4 months of age in both feeding groups are shown in Tables 3, 4 and 5. No significant differences in faecal parameters were found between the groups at enrolment.
Table 3.
Results for faecal pH, sIgA and SCFAs, expressed as mean ± SD and median [IQR]
| Control group | GOS group | P valuea | |||
|---|---|---|---|---|---|
| Enrolment (n = 37) | 4 months (n = 29) | Enrolment (n = 44) | 4 months n = 40 | ||
| pH | 6.63 ± 0.89 | 7.09 ± 0.69 | 6.37 ± 0.94 | 6.57 ± 0.82 | 0.019 |
| sIgA (mg/g) | 3.45 ± 4.45 | 1.72 ± 1.46 | 4.35 ± 5.41 | 2.76 ± 2.06 | 0.078 |
| 1.84 [0.56–3.94] | 1.12 [0.63–2.68 | 2.36 [0.87–4.82] | 2.32 [1.31–3.54] | ||
| Total SCFAs (µmol/g) | 67.22 ± 26.46 | 98.91 ± 39.56 | 74.95 ± 40.13 | 93.36 ± 40.70 | 0.567 |
| Acetic acid (%)b | 78.13 ± 12.58 | 75.72 ± 9.76 | 78.48 ± 33.90 | 84.77 ± 8.52 | 0.005 |
| Propionic (%)b | 15.49 ± 10.14 | 15.27 ± 6.30 | 11.45 ± 8.91 | 11.53 ± 6.62 | 0.015 |
| Butyric acid (%)b | 4.01 ± 6.99 | 5.59 ± 4.06 | 4.73 ± 6.61 | 2.29 ± 2.29 | 0.040 |
aDifferences between the groups over time were analysed with a repeated-measures analysis of variance
bResults expressed as percentage of total SCFAs
Table 4.
Intestinal microbiota counts in faecal samples of infants (log10 CFU/g)
| Control group | GOS group | P valuea | |||
|---|---|---|---|---|---|
| Enrolment | 4 months | Enrolment | 4 months | ||
| Bifidobacterium spp | 8.22 ± 1.53 | 8.02 ± 1.66 | 7.78 ± 1.78 | 8.65 ± 1.31 | ΔI = 0.010 |
| Lactobacillus spp | 6.72 ± 0.70 | 6.87 ± 0.61 | 6.62 ± 0.75 | 7.11 ± 0.89 | ΔI = 0.151 |
| Bacteroides spp | 6.39 ± 2.27 | 6.83 ± 2.17 | 6.07 ± 2,10 | 7.12 ± 2.04 | ΔI = 0.187 |
| Clostridium difficile | 4.45 ± 1.43 | 5.64 ± 1.34 | 5.10 ± 0.84 | 5.79 ± 0.91 | ΔI = 0.065 |
Results are expressed as the mean ± SD
aDifferences between the change from enrolment to month 4 (ΔI) of both feeding groups tested with a unpaired Student’s t test
Table 5.
Bifidobacterium species at enrolment and at 4 months of age, as bacterial counts (log10 CFU/g fresh stools) and percentage of colonised infants
| Control group | GOS group | P valuea | |||
|---|---|---|---|---|---|
| Enrolment | 4 months | Enrolment | 4 months | ||
| B. bifidum |
6.48 ± 1.45 72.4 % |
7.16 ± 1.47 82.8 % |
6.99 ± 1.45 72.5 % |
7.94 ± 1.90 90.0 % |
0.082 |
| B. longum |
8.72 ± 0.96 75.9 % |
8.96 ± 0.86 75.9 % |
8.56 ± 1.39 77.5 % |
9.43 ± 0.57 77.5 % |
0.310 |
| B. adolescentis |
4.71 ± 0.57 58.6 % |
5.41 ± 1.19 62.1 % |
5.57 ± 1.46 50.0 % |
5.64 ± 1.51 52.5 % |
0.079 |
| B. breve |
5.36 ± 2.05 27.6 % |
4.93 ± 1.44 41.4 %b |
5.78 ± 1.59 32.5 % |
6.37 ± 1.57 57.5 %b |
0.055 |
| B. dentium |
4.30 ± 0.76 20.7 % |
4.99 ± 0.24 20.7 % |
4.95 ± 1.37 25.0 % |
5.68 ± 1.44 15.0 % |
0.410 |
| B. catenulatum |
6.26 ± 0.50 24.1 % |
6.70 ± 0.70 20.7 % |
6.75 ± 0.81 17.5 % |
7.09 ± 0.59 27.5 % |
0.094 |
Results expressed as the mean ± SD
aDifferences between the feeding groups using unpaired Student’s t test
bSignificant differences (P < 0.05) between the feeding groups at 4 months for percentage of colonised infants using Pearson Chi-square test
Faecal pH was significantly influenced by the formula received and was lower in the GOS group than in the control group (6.77 ± 0.82 vs. 7.09 ± 0.69, P = 0.019) (Table 3) at 4 months of age. The faecal sIgA concentration decreased during the feeding period in both groups (P = 0.046), although we observed no significant differences between the feeding groups at 4 months of age (control group, 1.72 ± 1.46 mg/g vs. GOS group, 2.76 ± 2.06 mg/g). A trend towards a lower reduction of faecal sIgA was observed for infants in the GOS group versus control group (P = 0.078) (Table 3).
A significant increase in total SCFAs (μmol/g) from enrolment through 4 months of age was observed for both feeding groups (P < 0.001) (Table 3). When analysed as a percentage of total SCFAs, significant group differences were observed for the three main fatty acids. The percentage of acid acetic was higher in the GOS group than in the control group (84.77 vs. 75.72 %, P = 0.005), while the percentages of propionic and butyric acids were lower in the GOS group compared with the control group (11.53 vs. 15.27 %, P = 0.015 and 2.29 vs. 5.59 %, P = 0.040, respectively) (Table 3). A negative correlation between pH and acetic acid (r = −0.630, P < 0.001) was observed.
For mean faecal bacterial counts (log10 CFU/g) from enrolment through 4 months of age, Bifidobacterium increased in infants in the GOS group compared with the control group (0.97 vs. −0.23; P = 0.010) (Table 4). No group differences were observed for Lactobacillus and Bacteroides. C. difficile significantly increased through 4 months of age in both feeding groups (P < 0.05), but was not influenced by the feeding type (P = 0.065) (Table 4). The percentage of infants with detectable C. difficile at enrolment was similar between the feeding groups (control group, 23.3 % vs. GOS group, 21.4 %). However, by 4 months of age, the percentage of infants in the GOS group with detectable C. difficile was significantly lower (GOS group, 45.2 % vs. control group, 63.3 %; P = 0.037).
The characterisation of Bifidobacterium species revealed that the percentage of infants colonised by B. breve and B. adolescentis increased from enrolment through 4 months of age. The percentage of B. breve-colonised infants at enrolment was similar between the feeding groups (control group, 27.5 % vs. GOS group, 32.5 %). However, at 4 months of age, percentage of infants in the GOS group with detectable B. breve was significantly higher (control group, 41.4 % vs. GOS group, 57.5 %, P < 0.05) (Table 5). Moreover, the B. breve count increased more strongly in the GOS group (6.37 ± 1.57 log10 CFU/g) than in the control group (4.93 ± 1.44 log10 CFU/g) (P = 0.055; Table 5).
The Pearson correlation was applied to determine the relationship between the microbiota and the SCFA concentration. A positive significant correlation was obtained between acetic acid and the Bifidobacterium population (r = 0.251; P = 0.002) and a negative correlation between butyric acid and Bifidobacterium (r = −0.235; P = 0.003).
Infections and allergy
The number of URTI episodes and the number of infants with recurrent URTI were similar in both groups [odds ratio (OR) = 1.057, CI = 0.550–2.032, P = 0.868] (Table 6). No significant differences were found for the number of episodes of diarrhoea per infant up to 12 months of age between the feeding groups (control group, 0.20 ± 0.52 vs. GOS group, 0.27 ± 0.67, P = 0.355) (Table 6). No significant differences between the feeding groups in the number of infants with at least one episode of diarrhoea (control group, 15.90 % vs. GOS group 18.18 %, P = 0.604) were observed (Table 6). Additionally, the prescription of antibiotics did not differ significantly between the groups (control group, 19.87 % vs.GOS group 17.79 %; P = 0.481) (Table 6).
Table 6.
Results of diarrhoea, URTI, antibiotic treatment, allergic manifestations and positive sensitisation skin prick test (SPT) during the first year
| Control group | GOS group | Odds ratio (95 % CI) | P valuea | |
|---|---|---|---|---|
| No. of episodes of diarrhoea/infant (mean ± SD) | 0.20 ± 0.52 | 0.27 ± 0.67 | 0.355 | |
| Infants with at least 1 episode of diarrhoea/year (%) | 15.9 | 18.2 | 1.185 (0.623–2.253) | 0.800 |
| No. of episodes of URTI/infant (mean ± SD) | 1.65 ± 1.83 | 1.84 ± 2.01 | 0.443 | |
| Infants with at least 3 episodes of URTI/year (%) | 15.9 | 16.7 | 1.057 (0.550–2.032) | 0.868 |
| Antibiotic treatment (%) | 19.8 | 17.8 | 0.873 (0.598–1.274) | 0.481 |
| Allergic manifestations (AD, wheezing, food allergy) | 28/132 | 39/132 | 1.558 (0.889–2.728) | 0.120 |
| Positive sensitisation SPT | 9/84 | 6/85 | 0.633 (0.215–1.864) | 0.403 |
aDifferences between the feeding groups. A Student’s t test was used for the number of diarrhoea episodes and the number of URTI episodes per infant. A Pearson Chi-square test was used to compare the proportion of infants with recurrent URTI and acute diarrhoea, as well as the rates of antibiotics used, allergic manifestations and ratio of positive sensitisation SPT in both groups
No group differences in allergic manifestations were observed up to 12 months of age (control group, 28; GOS group, 39; OR = 1.558, CI 0.889–2.728; P = 0.120) (Table 6). Skin prick testing was performed for 169 infants (control group, 84; GOS group, 85); 3 were positive in the control group (egg, 1; cow’s milk, 2) and 6 in the GOS group (egg, 6; cow’s milk, 2; fish, 1). No group differences in allergic sensitization were observed (OR = 0.633; CI 2.15–1.864; P = 0.403) (Table 6).
Faecal characteristics
Significant differences in the frequency of defaecation (no. of depositions/day) were observed between the feeding groups at 3 months of age (control group, 1.26 ± 0.83 vs. GOS group, 1.45 ± 0.97; P < 0.05) and at 4 months of age (control group, 1.26 ± 0.94 vs. GOS group, 1.50 ± 0.99; P < 0.05), while at 6, 9 and 12 months of age, the frequency of defaecation in both groups was similar. Moreover, statistically significant differences in faecal consistency were detected between the feeding groups, especially at 3, 4 and 6 months of age. In general, infants fed the GOS formula showed a higher percentage of lumpy and soft faeces and lower percentage of hard faeces compared with the infants fed the control formula (Table 7).
Table 7.
Faecal consistency and frequency of defaecation in both feeding groups up to 12 months of age
| 3 months | 4 months | 6 months | 9 months | 12 months | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | GOS | Control | GOS | Control | GOS | Control | GOS | Control | GOS | |
| Consistency number of cases (%) | ||||||||||
| Watery | 18 (3.3) | 10 (1.8) | 8 (1.2) | 29 (4.6)* | 7 (1.1) | 10 (1.5) | 9 (1.4) | 18 (2.8) | 8 (1.5) | 3 (0.5) |
| Loose | 73 (13.5) | 99 (17.7) | 60 (8.7) | 116 (18.4)* | 39 (6.0) | 62 (9.1)* | 34 (5.5) | 70 (10.9)* | 27 (5.0) | 29 (5.2) |
| Lumpy | 119 (21.9) | 199 (35.7)* | 117 (17.1) | 211 (33.5)* | 98 (15.1) | 140 (20.6)* | 86 (13.9) | 82 (12.8) | 61 (11.2) | 81 (14.5) |
| Soft | 293 (54.1) | 274 (49.2) | 306 (44.6) | 291 (46.2)* | 388 (54.7) | 395 (58.0) | 393 (63.5) | 381 (59.7) | 338 (62.6) | 355 (63.5) |
| Hard | 35 (6.4) | 11 (1.97)* | 53 (7.7) | 32 (5.1)* | 196 (30.2) | 156 (23.3)* | 198 (31.9) | 184 (28.8) | 167 (30.9) | 151 (27.0) |
|
No. of depositions/day Mean ± SD |
1.26 ± 0.83 | 1.45 ± 0.97* | 1.26 ± 0.94 | 1.50 ± 0.99* | 1.62 ± 0.96 | 1.64 ± 0.98 | 1.82 ± 0.92 | 1.84 ± 0.99 | 1.65 ± 0.88 | 1.73 ± 0.89 |
* P < 0.05 differences between the feeding groups tested with an unpaired Student’s t test for frequency of defaecation, and a Pearson Chi-squared test for faecal consistency
Discussion
This trial shows that supplementation of an infant/follow-on formula, with GOS as only the prebiotic, decreases intestinal pH and increases both acetic acid and the Bifidobacterium population. It also improves the faecal consistency and frequency of defaecation. The incidence of infections and allergy manifestations were not significantly affected.
Prebiotic substances modulate the beneficial and pathogenic intestinal microbiota and other related parameters. The clinical relevance for functional outcomes cannot be confirmed due to the low number of studies in the literature in which both intestinal and systemic changes have been studied. However, it is well established that intestinal microbiota plays a crucial role in immune system regulation [3]. The positive correlation between the Bifidobacterium population and the amount of intestinal sIgA is well known [24, 25]. Moreover, the intestinal pH modulates the intestinal environment, inhibiting or favouring the growth of different bacterial populations; more specifically, a decrease in intestinal pH results in a decrease in the amount of pathogenic microorganisms [26]. Consequently, it was unclear whether or not we would be able to detect the influence of the feeding of a GOS-containing infant formula on the immune system at systemic level as regard the incidence of infections and allergy manifestations through the modulation of intestinal microbiota and related biomarkers in healthy infants.
The current study demonstrated changes of several parameters in the colon. The faecal pH in the GOS group was lower than in the control group, as has been observed in other studies involving GOS [13] and GOS/fructooligosaccharides (FOS) [27, 28]. The main regulatory variable of the luminal pH is the production of SCFAs, which are fermentation products of anaerobic bacteria; in this respect, we obtained the expected negative correlation between pH and acetic acid.
The results of sIgA obtained were varied. During the study period, the sIgA concentration decreased in both groups, with a trend towards a lower decrease in the GOS group. However, we obtained higher values than those reported for studies with GOS-/FOS-containing formulas [29] and GOS-/polydextrose (PDX)-containing formulas [30]. This suggests a protective effect on the development of a gut mucosa immune response, given that the sIgA decrease was more stable over time in the infants who consumed the GOS-containing formula, reflecting a similar behaviour to that observed in breastfed infants [29, 30].
The SCFA profile is determined by the intestinal microbiota population and depends on the feeding patterns. However, in terms of quantity, acetic acid was always the main SCFA produced in both groups of the present clinical trial. This reflects the SCFA profile of infants of the same age and is consistent with the results of other studies with GOS-/FOS-containing formulas [23, 27, 28]. The influence of GOS supplementation was shown by the significant increase in the percentage of acetic acid and the decrease in butyric acid observed, a similar pattern to that described in breastfed infants [27, 28, 31]. The faecal acetic/butyric acid profile represents events that occur in the colon and is closely related to the composition of intestinal microbiota, especially the abundance of Bifidobacterium, as indicated by the positive correlation that we found between acetic acid and the Bifidobacterium population and the negative correlation between butyric acid and Bifidobacterium. On the other hand, the high propionic acid content of the faeces of the control group may indicate the presence of more complex microbiota, given that propionate and butyrate are produced by Bacteroides and Clostridium, but not by Bifidobacterium. Several animal and in vitro assays have shown that microbial products such as SCFAs interact directly with immune cells and enterocytes and modify their activity at cell receptor level [3]. We observed a greater increase in the Bifidobacterium genus count during the feeding period in the GOS group compared with the control group. Results were similar to previous reports of increased Bifidobacterium associated with GOS-containing [13, 14] and GOS-/FOS-containing [4, 28, 32] infant formulas. A greater number of Bifidobacterium may be necessary for post-natal maturation of the immune system and thus have protective effects against infections and allergy by stimulating the intestinal microbiota in early stages of life.
The current study demonstrated a significant increase in the percentage of infants colonised by B. adolescentis at 4 months of age, unlike previous studies in which no clear difference in faecal Bifidobacterium species between infants receiving formulas with or without prebiotics was demonstrated [20, 30]. Our study also described a trend towards increased counts in the GOS group, whereas previous studies have only reported a diversity of species similar to that observed in breastfed infants associated with the consumption of prebiotic-containing formulas. Consistent with previous reports of prebiotic-containing formulas [4, 19, 28], no significant changes in Lactobacillus were observed in the current study. Regarding C. difficile, in our study, the percentage of infants colonised was lower in the GOS group and the bacterial count increased significantly during the feeding period, with a higher concentration in the control group at 4 months of age. Scholtens et al. [32] also found a lower percentage of Clostridium spp. among infants fed a GOS-/FOS-containing formula for 26 weeks. However, these findings contrast with those reported in subsequent publications that found no differences in the counts between the formula-fed groups, although there were differences between these groups and breastfed infants [30, 33].
The bowel habit is a useful marker of intestinal function, especially in the colon. It can be defined by frequency of defaecation, faecal consistency and weight and form of faeces. The typical higher frequency of defaecation and softer faeces pattern observed in breastfed infants is considered the standard of reference for comparison with formula-fed infants. In the current study, the frequency of defaecation and faecal consistency were significantly influenced by the GOS-containing formulas. Other previous studies involving prebiotic-containing infant formula found no difference in the frequency of defaecation, but faecal consistency was significantly improved, implying that the faeces became softer [30, 33]. In agreement with several previous reports with GOS-/FOS-containing formulas [34, 35], we also found a higher frequency of defaecation, especially up to the sixth month, mainly due to the introduction of complementary feeding. In addition, infants fed the GOS-containing formula showed higher prevalence of loose faeces and fewer formed/soft faeces than the infants in the control group. Other researchers have also described the softening of faeces in infants fed formulas containing GOS [13], GOS/FOS [4, 35], GOS/FOS/pectin acidic-oligosaccharides (pAOS) [36] or GOS/PDX [30, 37]. This effect is potentially beneficial in reducing the hard faeces of formula-fed infants and is probably a result of the osmotic stimulation caused by SCFAs during the fermentation of GOS by colonic bacteria.
Regarding the clinical relevance of the results, especially concerning infections and allergy, only three studies with GOS-/FOS-containing formulas have reported a reduction in infectious episodes [4–7]. In these studies, the feeding time was only 6 months, the infant formula used was hydrolysed, the infants involved had a high risk of atopy in two studies and one study was not blinded, with a wide inclusion age. In our study, infections and the number of episodes of diarrhoea and URTI showed no reduction. In fact, a systematic review has concluded that the data available in relation to the use of prebiotics in infant formulas for the prevention of infections remain inconclusive [9]. Concerning the use of antibiotics, we found no differences between the feeding groups, unlike the two previous studies with hydrolysed infant formulas [6, 7].
Neither the age of enrolment nor the GOS dose seemed to affect the differences found. In the study carried out by Arslanoglu et al. [5], the formula contained 0.8 g/dl of GOS/FOS and the enrolment age was 13 days. The authors stated that early feeding intervention had a more noticeable influence on the immune system, unlike in our study, which used lower doses (0.44 g/dl of GOS) in which the infants were older at enrolment (30 days). However, Bruzzese et al. [7] described the protective effects of a GOS-/FOS-containing formula (0.4 g/dl of GOS/FOS) when infants were even older at enrolment (54 days of age). In contrast, in a study using a GOS-/PDX-containing formula provided to infants aged 9–48 months for 108 days, Ribeiro et al. [37] observed no difference in the incidence of diarrhoea or URTI, despite recording softer faeces and a greater number of depositions in the supplemented group, as was the case in our study. Therefore, the effects of doses and age of inclusion are not conclusive and need further research.
Recent studies have described how feeding interventions that enhance microbial diversity in early life may provide an effective way of preventing eczema in high-risk infants [38]. Moro et al. [4] reported a significant reduction in eczema in infants up to 6 months at high risk of atopy and fed a hydrolysed GOS-/FOS-containing formula. The same working group confirmed the protective effect beyond the feeding period, over the first 2 years of life, in a study with 134 infants [6]. Grüber et al. [8] enrolled infants under 8 weeks and with low risk of atopy who were allocated to a control or prebiotic groups with a GOS-/FOS-/pAOS-containing formula. The researchers reported a significant reduction in AD in the prebiotic group up to the first year. However, the severity of AD was not significantly affected, and there were no statistically significant differences in allergic sensitisation to hen’s egg or cow’s milk between the groups [8]. Further evidence supporting the use of prebiotics in infant formulas to prevent allergy is required, according to two recent systematic reviews [9, 10].
The infants in the present study were all healthy, term infants who lived in a modern environment with a low risk of infectious disease. Moreover, both groups were fed a formula containing nucleotides and long-chain polyunsaturated fatty acids, substances that have also been said to be involved in immune system regulation [39, 40], the only difference being the inclusion (or not) of GOS. Despite the absence of a significant difference between the two groups in terms of clinical manifestations of either infections or allergies, differences in key leading indicators of immunity were found in association with a demonstrated prebiotic effect. In the present study, infections and allergy manifestations were infrequent both in the GOS group and in the control group, making it difficult to demonstrate a reduction from an already low baseline incidence. Further studies in populations with a higher incidence of infections, perhaps in developing countries, and on the effects on allergy manifestation in infants with a high risk of atopy are needed.
Our study did not include a group of breastfed infants. Nevertheless, our results were compared with the data currently available in the scientific literature on breastfed outcomes.
We conclude that the feeding of a GOS-containing infant formula produced a definite prebiotic effect, consisting of changes in faecal composition and microbiota, and in faecal consistency and frequency of defaecation. We observed no reduction in the incidence of infections or allergic manifestations during the first year of life. The critical association between prebiotic supplementation and improved immunological function in healthy human infants is still to be fully defined. Further trials, potentially in at-risk groups, are needed to verify whether adding prebiotics to infant formulas has a preventive effect on the infant immune system, as well as to elucidate the specific mechanisms of action.
Acknowledgments
We would like to acknowledge the help of the primary care paediatricians who actively collaborated in recruiting and monitoring infants. We would also like to thank our volunteers for participating in this study. This trial was supported by the Hero Group.
Conflict of interest
María-José Bernal, Rosario Martínez, Inmaculada Ortuño and María-Luisa Vidal are members of the Research & Development Department, Hero, Spain, and S.A. María-Isabel Vasallo was also a member during the study. The other authors declare no conflict of interest.
References
- 1.Boehm G, Moro G. Structural and functional aspects of prebiotics used in infant nutrition. J Nutr. 2008;138:1818S–1828S. doi: 10.1093/jn/138.9.1818S. [DOI] [PubMed] [Google Scholar]
- 2.Penders J, Thijs C, Vink C, Stelma FF, Snijders B, Kummeling I, van den Brandt PA, Stobberingh EE. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics. 2006;118:511–521. doi: 10.1542/peds.2005-2824. [DOI] [PubMed] [Google Scholar]
- 3.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I, Wolvers D, Watzl B, Szajewska H, Stahl B, Guarner F, Respondek F, Whelan K, Coxam V, Davicco MJ, Léotoing L, Wittrant Y, Delzenne NM, Cani PD, Neyrinck AM, Meheust A. Prebiotic effects: metabolic and health benefits. Br J Nutr. 2010;104:S1–S63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 4.Moro G, Arslanoglu S, Stahl B, Jelinek J, Wahn U, Boehm G. A mixture of prebiotic oligosaccharides reduces the incidence of atopic dermatitis during the first six months of age. Arch Dis Child. 2006;91:814–819. doi: 10.1136/adc.2006.098251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arslanoglu S, Moro GE, Boehm G. Early supplementation of prebiotic oligosaccharides protects formula-fed infants against infections during the first 6 months of life. J Nutr. 2007;137:2420–2424. doi: 10.1093/jn/137.11.2420. [DOI] [PubMed] [Google Scholar]
- 6.Arslanoglu S, Moro GE, Schmitt J, Tandoi L, Rizzardi S, Boehm G. Early dietary intervention with a mixture of prebiotic oligosaccharides reduces the incidence of allergic manifestations and infections during the first two years of life. J Nutr. 2008;138:1091–1095. doi: 10.1093/jn/138.6.1091. [DOI] [PubMed] [Google Scholar]
- 7.Bruzzese E, Volpicelli M, Squeglia V, Bruzzese D, Salvini F, Bisceglia M, Lionetti P, Cinquetti M, Iacono G, Amarri S, Guarino A. A formula containing galacto- and fructo-oligosaccharides prevents intestinal and extra-intestinal infections: an observational study. Clin Nutr. 2009;28(2):156–161. doi: 10.1016/j.clnu.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Grüber C, van Stuijveberg M, Mosca F, Moro G, Chirico G, Braegger CP, Riedler J, Boehm G, Wahn U. Reduced occurrence of early atopic dermatitis because of immunoactive prebiotics among low-atopy-risk infants. J Allergy Clin Immunol. 2010;126(4):791–797. doi: 10.1016/j.jaci.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Braegger C, Chmielewska A, Decsi T, Kolacek S, Mihatsch W, Moreno L, ESPGHAN Committee on Nutrition et al. Supplementation of infant formula with probiotics and/or prebiotics: a systematic review and comment by the ESPGHAN Committee on Nutrition. J Pediatr Gastroenterol Nutr. 2011;52:238–250. doi: 10.1097/MPG.0b013e3181fb9e80. [DOI] [PubMed] [Google Scholar]
- 10.Osborn DA, Sinn JK (2013) Prebiotics in infants for prevention of allergy. Cochrane Database Syst Rev 3:CD006474. doi:10.1002/14651858.CD006474.pub3 [DOI] [PubMed]
- 11.Schmelzle H, Wirth S, Skopnik H, Radke M, Knol J, Böckler HM, Brönstrup A, Wells J, Fusch C. Randomized double-blind study of the nutritional efficacy and bifidogenicity of a new infant formula containing partially hydrolyzed protein, a high beta-palmitic acid level, and nondigestible oligosaccharides. J Pediatr Gastroenterol Nutr. 2003;36(3):343–351. doi: 10.1097/00005176-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura N, Gaskins HR, Collier CT, Nava GM, Rai D, Petschow B, Russell WM, Harris C, Mackie RI, Wampler JL, Walker DC. Molecular ecological analysis of faecal bacterial populations from term infants fed formula supplemented with selected blends of prebiotics. Appl Environ Microbiol. 2009;75:1121–1128. doi: 10.1128/AEM.02359-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ben XM, Zhou XY, Zhao WH, Yu WL, Pan W, Zhang WL, Wu SM, Van Beusekom CM, Schaafsma A. Supplementation of milk formula with galacto-oligosaccharides improves intestinal micro-flora and fermentation in term infants. Chin Med J (Engl) 2004;117:927–931. [PubMed] [Google Scholar]
- 14.Fanaro S, Marten B, Bagna R, Vigi V, Fabris C, Peña-Quintana L, Argüelles F, Scholz-Ahrens KE, Sawatzki G, Zelenka R, Schrezenmeir J, de Vrese M, Bertino E. Galactooligosaccharides are bifidogenic and safe at weaning: a double-blind randomized multicenter study. J Pediatr Gastroenterol Nutr. 2008;48:82–88. doi: 10.1097/MPG.0b013e31817b6dd2. [DOI] [PubMed] [Google Scholar]
- 15.Girard-Pipau F, Pompei A, Schneider S, Nano JL, Hebuterne X, Boquet P, Rampal P. Intestinal microflora, short chain and cellular fatty acids, influence of a probiotic Saccharomyces boulardii. Microb Ecol Health Dis. 2002;14:220–227. [Google Scholar]
- 16.Gueimonde M, Tölkko S, Korpimäki T, Salminen S. New real-time quantitative PCR procedure for quantification of bifidobacteria in human faecal samples. Appl Environ Microbiol. 2004;70:4165–4169. doi: 10.1128/AEM.70.7.4165-4169.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuki T, Watanabe K, Fujimoto J, Takada T, Tanaka R. Use of 16S rRNA gene-targeted group-specific primers for real-time PCR analysis of predominant bacteria in human feces. Appl Environ Microbiol. 2004;70:7220–7228. doi: 10.1128/AEM.70.12.7220-7228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haarman M, Knol J. Quantitative real-time PCR analysis of faecal Lactobacillus species in infant receiving a prebiotic infant formula. Appl Environ Microbiol. 2006;72:2359–2365. doi: 10.1128/AEM.72.4.2359-2365.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Layton A, McKay L, Williams D, Garrett V, Gentry R, Sayler G. Development of bacteroides 16S rRNA Gene TaqMan-based real-time PCR assays for estimation of total, human, and bovine faecal pollution in water. Appl Environ Microbiol. 2006;72:4214–4224. doi: 10.1128/AEM.01036-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rinne MM, Gueimonde M, Kalliomäki M, Hoppu U, Salminen SJ, Isolauri E. Similar bifidogenic effects of prebiotic-supplemented partially hydrolyzed infant formula and breastfeeding on infant gut microbiota. FEMS Immunol Med Microbiol. 2005;43:59–65. doi: 10.1016/j.femsim.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 21.Penders J, Vink C, Driessen C, et al. Quantification of Bifidobacterium spp., Escherichia coli and Clostridium difficile in faecal samples of breast-fed and formula-fed infants by real-time PCR. FEMS Microbiol Lett. 2005;243:141–147. doi: 10.1016/j.femsle.2004.11.052. [DOI] [PubMed] [Google Scholar]
- 22.Von Linstow ML, Holst KK, Larsen K, Koch A, Andersen PK, Hogh B. Acute respiratory symptoms and general illness during the first year of life: a population-based birth cohort study. Pediatr Pulmonol. 2008;43(6):584–593. doi: 10.1002/ppul.20828. [DOI] [PubMed] [Google Scholar]
- 23.Knol J, Scholtens P, Kafka C, Steenbakkers J, Gro S, Helm K, Klarczyk M, Schöpfer H, Böckler HM, Wells J. Colon microflora in infants fed formula with galacto- and fructo-oligosaccharides: more like breast-fed infants. J Pediatr Gastroenterol Nutr. 2005;40:36–42. doi: 10.1097/00005176-200501000-00007. [DOI] [PubMed] [Google Scholar]
- 24.Fukushima Y, Kawata Y, Harac H, Teradac A, Mitsuoka T. Effect of a probiotic formula on intestinal immunoglobulin A production in healthy children. Int J Food Microbiol. 1998;42:39–44. doi: 10.1016/S0168-1605(98)00056-7. [DOI] [PubMed] [Google Scholar]
- 25.Yasui H, Nagaoka N, Mike A, Hayakawa K, Ohwaki M. Detection of Bifidobacterium strains that induce large quantities of IgA. Microb Ecol Health Dis. 1992;5:155–162. doi: 10.3109/08910609209141310. [DOI] [Google Scholar]
- 26.Topping DL, Cliftton PM. Short-chain fatty acids and human colonic function: roles of resistant starch and non-starch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 27.Bakker-Zierikzee AM, Alles MS, Knol J, Kok FJ, Tolboom JJ, Bindels JG. Effects of infant formula containing a mixture of galacto- and fructo-oligosaccharides or viable Bifidobacterium animalis on the intestinal microflora during the first 4 months of life. Br J Nutr. 2005;94:783–790. doi: 10.1079/BJN20051451. [DOI] [PubMed] [Google Scholar]
- 28.Holscher HD, Faust KL, Czerkies LA, Litov R, Ziegler EE, Lessin H, Hatch T, Sun S, Tappenden KA. Effects of prebiotic-containing infant formula on gastrointestinal tolerance and fecal microbiota in a randomized controlled trial. J Parenter Enter Nutr. 2012;36(1 Suppl):95S–105S. doi: 10.1177/0148607111430087. [DOI] [PubMed] [Google Scholar]
- 29.Bakker-Zierikzee AM, van Tol EAF, Kroes H, Alles MS, Kok FJ, Bindels JG. Faecal IgA secretion in infants fed on pre- or probiotic infant formula. Pediatr Allergy Immunol. 2006;17:134–140. doi: 10.1111/j.1399-3038.2005.00370.x. [DOI] [PubMed] [Google Scholar]
- 30.Scalabrin DMF, Mitmesser SH, Welling GW, Harris CL, Marunycz JD, Walker DC, Bos NA, Salminen S, Vanderhoof JA. New prebiotic blend of polydextrose and galacto-oligosaccharides has a bifidogenic effect in young infants. J Pediatr Gastroenterol Nutr. 2012;54:343–352. doi: 10.1097/MPG.0b013e318237ed95. [DOI] [PubMed] [Google Scholar]
- 31.Edwards CA, Parrett AM, Balmer SE, Wharton BA. Faecal short chain fatty acids in breast-fed and formula-fed babies. Acta Paediatr. 1994;83:459–462. doi: 10.1111/j.1651-2227.1994.tb13059.x. [DOI] [PubMed] [Google Scholar]
- 32.Scholtens PA, Alliet P, Raes M, Alles MS, Kroes H, Boehm G, Knippels LM, Knol J, Vandenplas Y. Faecal secretory immunoglobulin A is increased in healthy infants who receive a formula with short-chain galacto-oligosaccharides and long-chain fructo-oligosaccharides. J Nutr. 2008;138:1141–1147. doi: 10.1093/jn/138.6.1141. [DOI] [PubMed] [Google Scholar]
- 33.Xia Q, Williams T, Hustead D, Price P, Morrison M, Yu Z. Quantitative analysis of intestinal bacterial populations from term infants fed formula supplemented with fructo-oligosaccharides. J Pediatr Gastroenterol Nutr. 2012;55:314–320. doi: 10.1097/MPG.0b013e3182523254. [DOI] [PubMed] [Google Scholar]
- 34.Veereman-Wauters G, Staelens S, Van de Broek H, Plaskie K, Westing F, Roger LG, McCartney AL, Assam P. Physiological and bifidogenic effects of prebiotic supplements in infant formulae. J Pediatr Gastroenterol Nutr. 2011;52:763–771. doi: 10.1097/MPG.0b013e3182139f39. [DOI] [PubMed] [Google Scholar]
- 35.Costalos C, Kapiki A, Apostolou M, Papathoma E. The effect of a prebiotic supplemented formula on growth and stool microbiology of term infants. Early Hum Dev. 2008;84:45–49. doi: 10.1016/j.earlhumdev.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Fanaro S, Jelinek J, Stahl B, Boehm G, Kock R, Vigi V. Acidic oligosaccharides from pectin hydrolysate as new component for infant formulae: effect on intestinal flora, stool characteristics, and pH. J Pediatr Gastroenterol Nutr. 2005;41:186–190. doi: 10.1097/01.mpg.0000172747.64103.d7. [DOI] [PubMed] [Google Scholar]
- 37.Ribeiro TCM, Costa-Ribeiro H, Almeida PS, Pontes MV, Leite M, Filadelfo L, Khoury JC, Bean JA, Mitmesser SH, Vanderhoof JA, Scalabrin DM. Stool pattern changes in toddlers consuming a follow-on formula supplemented with polydextrose and galactooligosaccharides. J Pediatr Gastroenterol Nutr. 2012;54:288–290. doi: 10.1097/MPG.0b013e31823a8a4c. [DOI] [PubMed] [Google Scholar]
- 38.Ismail IH, Oppedisano F, Joseph SJ, Boyle RJ, Licciardi PV, Robins-Browne RM, Tang ML. Reduced gut microbial diversity in early life is associated with later development of eczema but not atopy in high-risk infants. Pediatr Allergy Immunol. 2012;23(7):674–681. doi: 10.1111/j.1399-3038.2012.01328.x. [DOI] [PubMed] [Google Scholar]
- 39.Singhal A, Macfarlane G, Macfarlane S, et al. Dietary nucleotides and faecal microbiota in formula-fed infants: a randomized controlled trial. Am J Clin Nutr. 2008;87:1785–1792. doi: 10.1093/ajcn/87.6.1785. [DOI] [PubMed] [Google Scholar]
- 40.Field CJ, Van Aerde JE, Robinson LE, Clandinin MT. Effect of providing a formula supplemented with long-chain polyunsaturated fatty acids on immunity in full-term neonates. Br J Nutr. 2008;99:91–99. doi: 10.1017/S0007114507791845. [DOI] [PubMed] [Google Scholar]


