Abstract
Objective
The aim of this study is to compare the each clinical manifestation related with its mean survival time of Krukenberg tumors (KTs) of gastric origin versus with that of colorectal origin.
Methods
A total of 156 consecutive patients diagnosed with KTs of the ovary who underwent surgical treatment at a single institution between 2001 and 2010 were retrospectively evaluated. Each clinical manifestation related with its mean survival time compared two different groups. Survival analyses and comparisons were performed using the Kaplan-Meier method.
Results
Among the 156 patients with KT, 111 patients with KTs of gastric origin and 45 patients with KTs of colorectal origin were identified. For all patients with KTs, median survival time was 22.7 months. Each mean survival time among all patients with KTs of gastric origin and colorectal origin was 19.2 months and 27.3 months. The results showed that mean survival time of postmenopausal patients was 19.0 months compared with 32.5 months for premenopausal patients (P=0.015). Among all patients, mean survival time of those with metachronous cancer was longer than those with synchronous cancer (P=0.001). In all cases, especially when only one ovary was invaded, the mean survival time was relatively higher (P=0.001).
Conclusion
Patients with KTs of colorectal origin had a better prognosis than those of gastric origin. In all cases of KT, the mean survival time was significantly longer in postmenoposal patients, metachronous disease and unilateral ovarian involvement. Notably, synchronous, ascites positive, and ovary only metastasis showed more longer mean survival time in the KTs of colorectal origin than KTs of gastric origin.
Keywords: Krukenberg tumor, Metastatic ovarian cancer, Survival rate
Introduction
Krukenberg tumor (KT) refers to a malignancy in the ovary that metastasized from a primay site. It accounts for approximately 5% to 20% of metastatic ovarian cancer. It is named after Friedrich Ernst Krukenberg (1871-1946) who published five cases of "Krukenberg tumors" in 1896 when working as a student in a laboratory in Germany [1]. Literatures showed about 76% of KTs originate from stomach, 11% in colorectum, 4% from the breast, 3% from the biliary system, 3% from the appendix, and the remaining 3% from miscellaneous sites such as pancreas, uterine cervix, urinary bladder, or renal pelvis [2,3]. However, recent articles have shown a higher incidence of KT of colorectal origin compared with those of gastric origin [3,4]. Currently, the diagnosis of KT is based on diagnostic criteria of the World Health Organization based on the pathological description by Serov and Scully [5]. The following features should be present for the diagnosis of KT: the presence of stromal involvement, the presence of mucin-producing neoplastic signet ring cells, and ovarian stromal sarcomatoid proliferation. In Korea and many other countries, there are so many papers that analyze the clinical manifestations and mean survival time. However, study of survival analysis for primary site has not been conducted. Therefore we analyzed mean survival time of KT originated from gastrointestinal tract, further dividing into gastric and colorectal origin based on clinical manifestation. The current studies purpose on predicting the prognosis of KT and it is going to hopefully contribute in the future treatment.
Materials and methods
Between January 2001 and December 2010, analysis of electronic medical record was performed retrospectively on 160 patients diagnosed with metastatic tumor of ovary who underwent surgical treatment in our Department of Obstetrics and Gynecology. We excluded 2 patients who were operated on in other hospitals and those who did not have clear primary site identified in pathological examination. One patient had ovarian tumor which is originated in breast, another had it from vague site. We also exclude patients who did not have sufficient data in electronic medical record. We compared to the clinical manifestation of KTs of gastric origin and KTs of colorectal origin. We focused mainly on disparity in mean survival time s in patients by taking into account the following variables. We determined factors directly related to the prognosis by comparing the mean survival time of gastric origin and colorectal origin, premenopausal and postmenopausal, synchronous and metachronous, unilateral and bilateral ovarian invasion, presence and absence of ascites, and presence and absence of extraovarian metastasis all the patients. First mean survival time of gastric origin and colorectal origin were collected and compared, based on the following five factors: premenopausal or postmenopausal, synchronous or metachronous disease, unilateral or bilateral ovarian involvement, presence or absence of ascites, metastasis limited to ovary or metastasis to the other side. We used SPSS ver. 18.0 (SPSS Inc., Chicago, IL, USA) for statistical analysis by using Pearson chi-square test, independent t-test, and for one-way analysis by using variance test. Survival analyses and comparisons were performed using the Kaplan-Meier method. A P-value of <0.05 was considered to be statistically significant.
Results
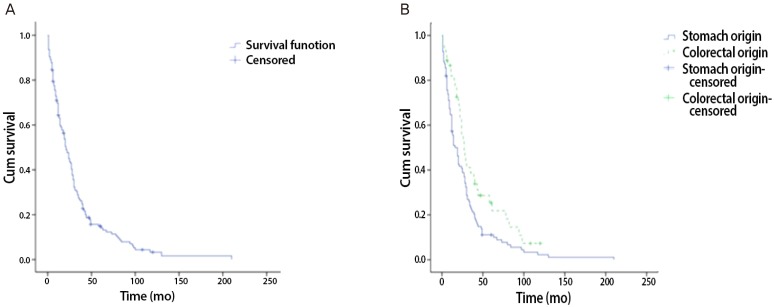
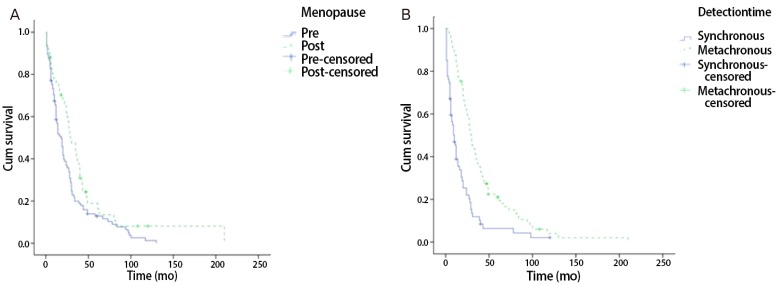
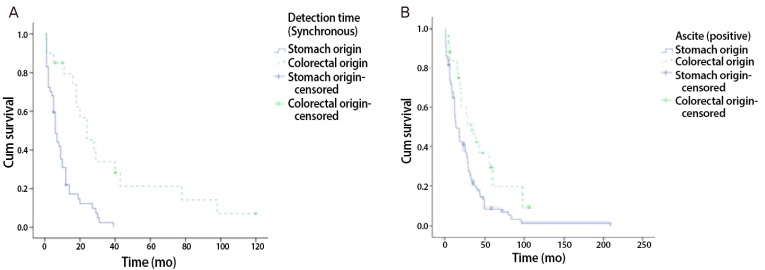
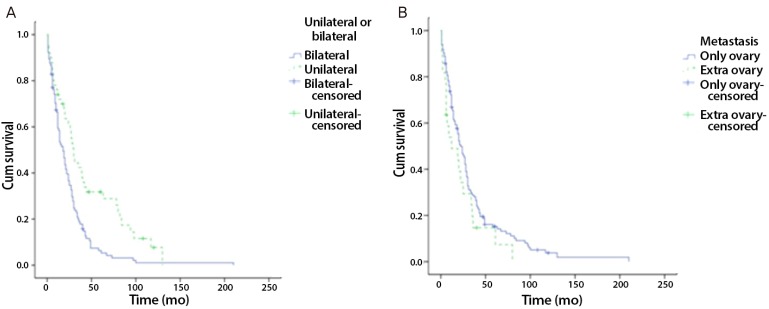
From January 2001 to December 2010, surgical treatments were performed on 160 patients with KT in our hospital. Among the 160 patients, 156 (93.3%) had KTs of gastrointestinal origin. Of this population, 111 patients (71.2%) had KTs of gastric origin, and 45 patients (28.8%) had KTs of colorectal origin. The remaining patients had KTs of breast origin and some did not have clear primary site identified in pathological examination. Our analysis involved primarily patients with KT of gastric origin. Clinical manifestations and mean survival time are summarized in Tables 1, 2, and 3. The age at which patients were diagnosed ranges from 19 to 82 years, with a mean age of 45.8 years. The average age of patients with KTs of gastric origin is 43.8 years (range, 24 to 76 years), and the average age of patients with KTs of colorectal origin is 50.4 years (range, 19 to 82 years) (Table 1). The average age of patients with KTs of colorectal origin is significantly older than the others (P=0.002) (Table 1). All patients' mean follow-up period was 22.7 months, and differences observed during that follow-up period after metastasis diagnosis for gastric origin KT was 12.1 months and for colorectal origin KT were 24.0 months (P=0.001) (Table 1). Mean survival time of all patients with KT was 22.7 months (Fig. 1A). Mean survival time of the patients with colorectal origin was significantly longer than that of the patients with gastric origin (P=0.015) (Fig. 1B). Among all of the patients, 105 (67.3%) were premenopausal, and 51 (31.7%) were postmenopausal. In cases of gastric origin KT, there were more premenopausal patients (85 patients, 76.6%). However, in patients with colorectal origin KT, 25 patients (55.6%) were postmenopausal. Among all of the patients, the postmenopausal patients' mean survival time was 32.5 months, which is longer than that of the premenopausal patients' 19.0 months (P=0.021) (Fig. 2A). KT can also be classified according to the timeframe in which the metastatic ovarian cancer was diagnosed; synchronous vs. metachronous. Among all patient groups, 89 patients had metachronous cancer, with primary ovarian cancer discovered later. Out of all patients, those with metachronous cancer had longer mean survival time than those who had synchronous cancer (P=0.001) (Fig. 2B). In synchronous cancer group, KT of colorectal origin group showed longer mean survival time than KT of gastric origin group (P=0.001) (Fig. 3A). As in metachronous cancer, there was no difference between the two groups (P=0.536) (Table 3). Based on the ovaries involvements (unilateral vs. bilateral), KTs with bilateral involvement (105 cases, 67.3%) were more common (Table 1). The mean survival time in unilateral and bilateral involvement was 31.7 months and 19.3 months, respectively (P=0.001) (Fig. 4A). When comparing the unilateral with bilateral group, there was no significant difference in mean survival time between gastric origin and colorectal origin. Ninety-two total patients (59%) presented with ascites and 64 patients (41%) presented without ascites. However, the difference in mean survival time between these subgroups was not statistically significant. However, when comparing patients with ascites, the mean survival time of patients in colorectal origin (33.1 months) was better than that of patients in gastric origin (19.7 months), and the difference was statistically significant (P=0.012) (Fig. 3B). At the time of diagnosis, 134 patients (85.9%) had metastasis only to the ovary, while the remaining 22 patients (14.1%) had extra-ovarian metastasis. There was no significant difference in mean survival time based on this criterion (P=0.122) (Fig. 4B). However, in the ovarian-only group, mean survival time of colorectal origin (30.3 months) was longer than that of gastric origin (21.0 months), which was statistically significant (P=0.052) (Table 3).
Table 1.
All patient characteristics
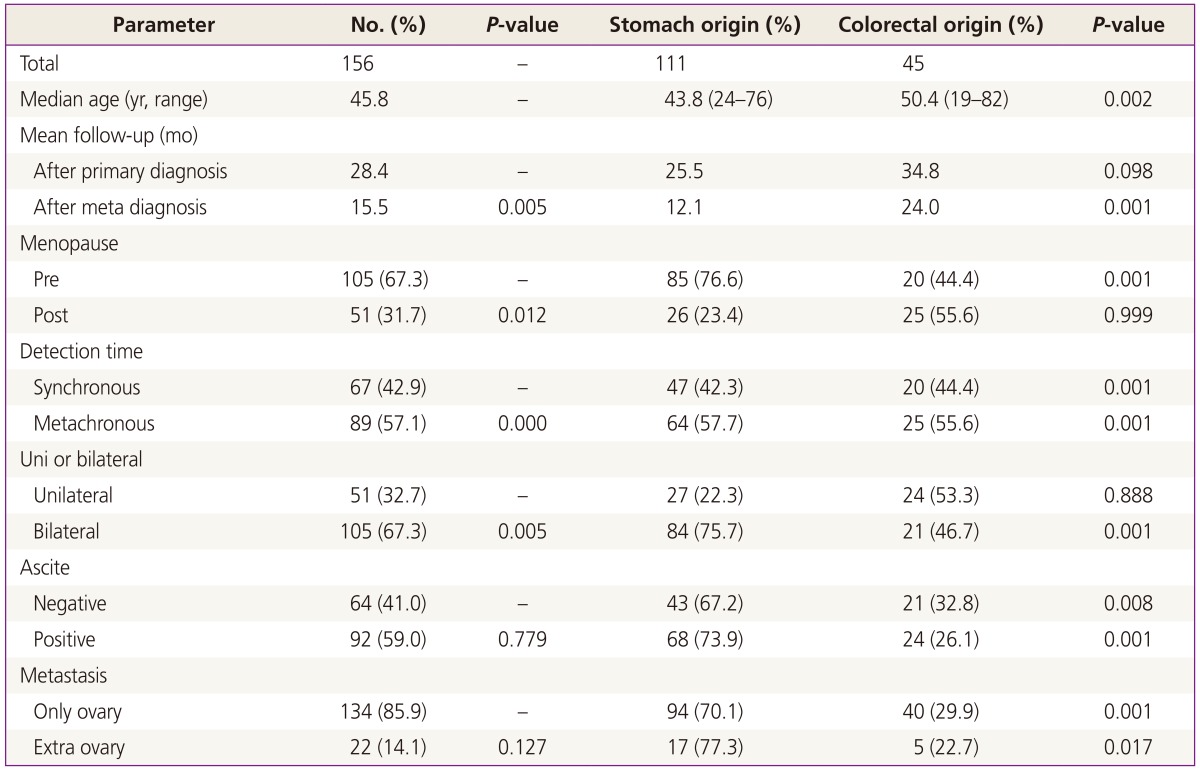
Table 2.
Comparison between mean survival time of all patients
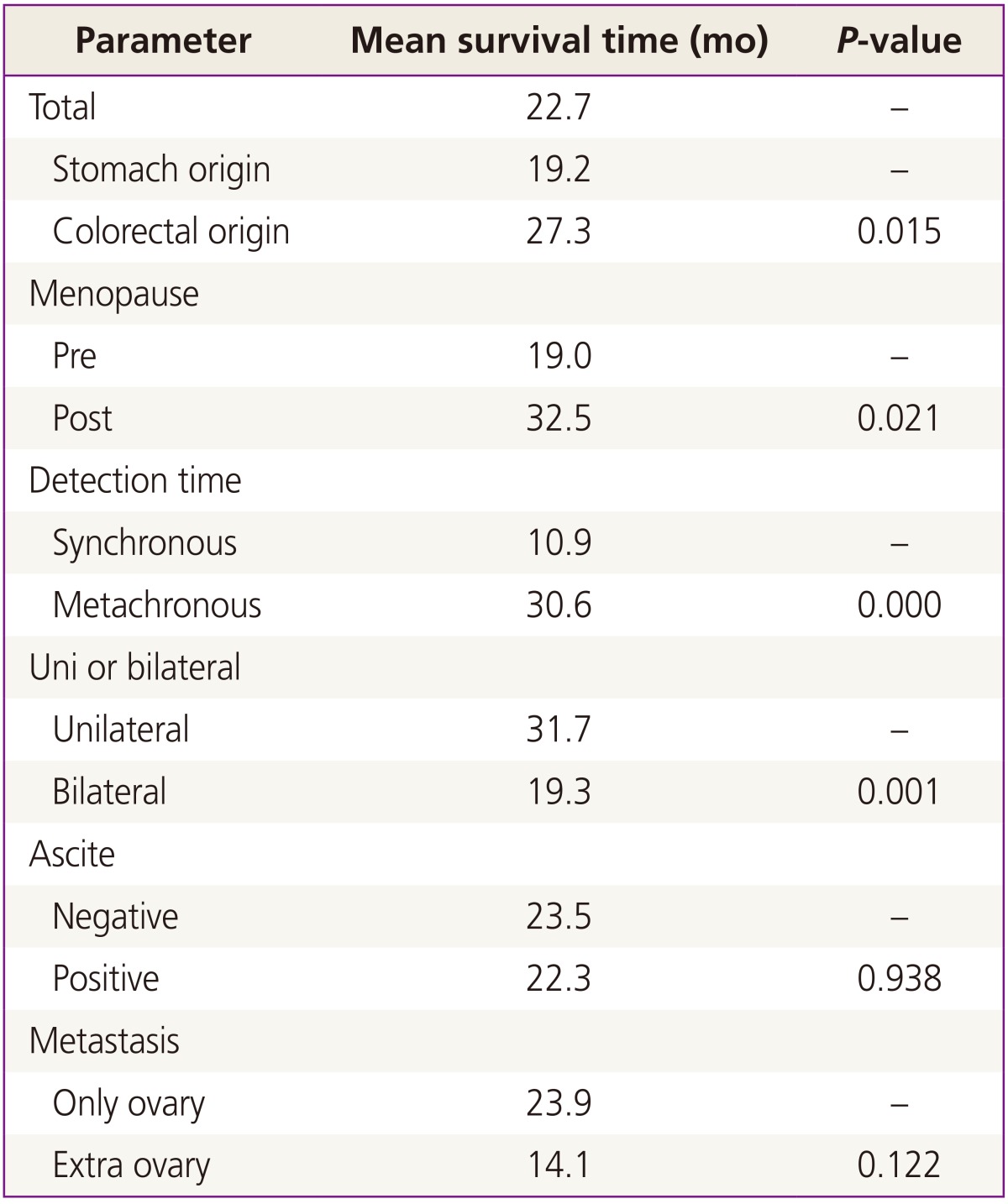
Table 3.
Comparison mean survival time of the patients with gastric and colorectal origin
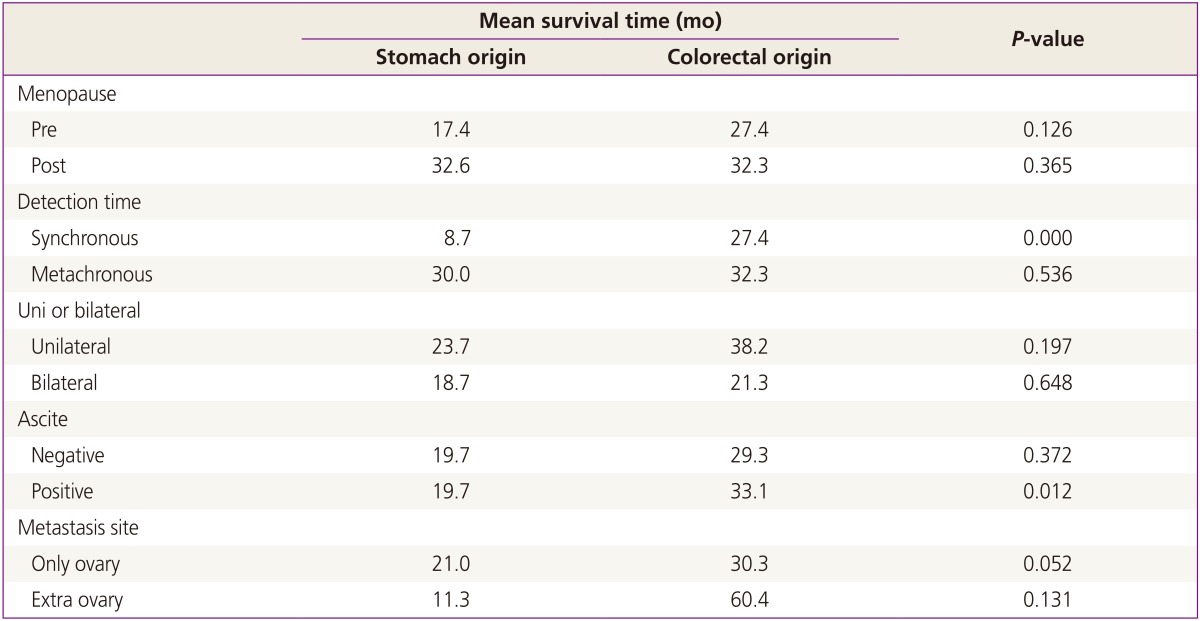
Fig. 1.
Kaplan-Meier survival curve. (A) Overall survival in all patients (n=156). (B) Comparing overall survival gastric origin Krukenberg tumor to colorectal origin Krukenberg tumor.
Fig. 2.
Kaplan-Meier survival curve. (A) Comparing overall survival premenopause patients to postmenopause patients. (B) Comparing overall survival synchronous Krukenberg tumor to metachronous Krukenberg tumor.
Fig. 3.
Kaplan-Meier survival curve. (A) In synchronous Krukenberg tumor (KT) patients, comparing overall survival gastric origin KT to colorectal origin KT. (B) In all patients with ascites, comparing overall survival gastric origin to colorectal origin.
Fig. 4.
Kaplan-Meier survival curve. (A) Comparing overall survival unilateral ovary invasion patients to bilateral ovary invasion patients. (B) Comparing overall survival only ovary metastasis patients to extraovary metastasis patients.
Discussion
The term "Krukenberg tumor" is used either as a broad designation to cover all metastatic tumors of the ovary or in a narrow sense to describe metastatic tumors from the tract [6,7]. In some cases, it is not easy to differentiate primary ovarian tumor and metastatic ovarian tumor. Notably, metastatic ovarian tumor from colon is more difficult to differentiate. The misdiagnosis rate is 45% in 1980s, but accuracy of the diagnosis is increasing with the development of diagnostic techniques [8]. The most common origins of KTs are gastrointestinal cancers, and 93.3% tumors in present series were from either gastric or colorectal cancers. Several investigators have suggested that stomach cancer is the most frequent source of ovarian metastasis [6,7]. But others have reported colon cancer as the most common source [8,9]. We found that most of KTs arise from either primary gastric cancer (66.4%) or colorectal cancer (26.9%). In general, KTs are rare in Western countries, account only 3% to 4%.. Somehow, KTs are more common in Korea than Western countries because of high incidence of gastric cancer [10]. Most of the journals regarding KTs describe the diagnosis or the clinical manifestation. In our study, we analyzed mean survival time and focused on the prognosis of two cancers. Age at diagnosis ranges from 13 to 81 years, but average occured age is from 42 to 50.4. The mean age of the patients at the time of diagnosis with non-genital cancer that metastasized to the ovary was 45.8 years (premenopausal patients accounted for 67.3% of the patients), it is younger than that of primary ovarian cancer (mid-50s) [9]. Prevalence of KT is higher in premenopausal patients than postmenopausal patients. Otherwise, mean survival time of postmenopausal patients is higher than premenopausal patients. The onset ages of KTs are 10 years younger than primary ovarian cancers. Because young women get more stomach cancer than older and colon cancers have opposite result. In this study, the mean survival time after resection of the metastatic ovarian tumors from gastrointestinal organs was 22.7 months which is similar to other studies. We found that the mean survival time with KTs from colorectal cancer was significantly longer than from gastric cancer (P=0.015). KTs often have some symptoms: abdominal or pelvic pain, bloating, ascites, and pain during sexual intercourse. Sometimes KTs can cause a reaction of the ovarian stroma, and then provoke hormone production that results in vaginal bleeding, menstrual habits change, hirsuitism and occasionally virilization as a main symptom [11,12]. Several mechanisms have been suggested to explain the progression and recurrence pathway of the cancers such as lymphatic spread, hematogenous spread, direct invasion, peritoneal seeding. Although KT also may be induced by complex mechanisms, lymph node metastasis is considered to be the most potent risk factor in recurrence [13]. It was reported that patients who had KTs were younger than those had primary ovarian cancers, and the functioning ovary was prone to metastatic disease because the rich ovarian blood supply leads to hematogenous metastasis [14]. KTs which involved in bilateral ovaries are more common than in unilateral ovary. Moreover, in unilateral KTs, right ovary is more often involved than left one. In this study, 105 patients (67.3%) presented with bilateral involvement which was significantly more frequent than 51 patients (32.7%) that presented unilaterally. However patients who had KTs from colorectal cancers had unilateral presentations more frequently (24 patients, 53.3%). Prognosis of unilateral involvement is better than bilateral, which can influence overall prognosis. Synchronous diagnosis means finding ovarian metastasis and original cancer at the same time. Metachronous KTs have longer mean survival time than synchronous KTs. Because metachronous KTs include some interval before it was recurred. In recent study, Most of the KTs are diagnosed later to primary cancer or diagnosed at the same time with primary cancer which shows no significant differences in survival rate [15]. In this case, however, metachronous was 30.6 months, and it is longer than mean survival time of synchronous that was 10.9 months (P=0.000). Synchronous KTs originated in colorectal cancers had a significantly longer survival time than that in gastric cancers (P=0.001). In this study, the metastatic ovarian cancers were present at diagnosis of the primary cancer in 67 of 156 cases. In general, the presence of ascites in ovarian cancer is a poor prognostic sign. In our case, 92 patients have ascites (59.0%); however, the mean survival time of patients who have ascites was not shorter than that of patients who do not have ascites. In this study, 134 patients (85.9%) showed solitary metastasis to the ovary. Since KTs are secondary (metastatic) tumor, management might be logically driven by identifying and treating the primary cancer. Some studies have opinions that the role of surgical resection has not been adequately addressed, but if metastasis is limited to the ovaries, surgery may improve survival rate [15,16,17]. In operation for KTs, T stage must be considered. Based on T stages, the tumor cells could scatter more easily into the peritoneal cavity, rather than metastasize. Therefore, the importance of transcoelomic metastasis to ovaries from advanced gastrointestinal carcinomas should not be overlooked. Thus, T stage of the primary carcinomas is the most important predictor of KTs. Timing of operation for KTs was also analyzed. Whether the surgery should be performed synchronously or metachronously had a significant impact on mean survival time. However, the optimal treatment of KTs is unclear [16]. Metastatic ovarian tumors from other primary sites are a manifestation of advanced disease and therefore the prognosis is generally poor. In one study, the overall five-year survival rate of 357 patients with KTs was just 5.4% [8]. In another study, five-year survival rate of KTs after resection of primary lesion in the stomach, colon and rectum, breast were 0%, 20.7%, 22.2%, respectively [18]. Some prior studies have suggested that presence of ascites and bilateral invasion of ovary did not significantly impact on the survival rate. Controversially, for patients with ascites, mean survival time of patients with colorectal origin was 33.1 months, which is longer than that of synchronous 19.7 months (P=0.012). In all patients, there was no survival rate difference between extra-ovarian metastasis and ovarian-only metastasis. In case of ovarian-only involvement, mean survival time of KTs from colorectal cancers was 30.3 months, which is longer than that of KTs from gastric cancers (21.0 months, P=0.052). This study concluded that the clinical manifestation and overall survival time of patients are distinctly based on origin of the primary lesion (gastric lesion vs. colorectal lesion). In this study, regarding some factors are common. The factors are premenopausal, metachronous, bilateral ovarian involvement, presence of ascites, and ovarian-only metastasis. Comparing of all patient's mean survival time, menopause, metachronous, and unilateral involvement appeared statistically significant differences in mean survival time. Furthermore, individual factors: synchronous diagnosis, presence of ascites, and ovarian-only metastasis appeared a significant longer mean survival time in KTs from colorectal cancers than KTs from gastric cancers. These results will predict the overall prognosis of patients with KT.
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Young RH. From Krukenberg to today: the ever present problems posed by metastatic tumors in the ovary. Part I: historical perspective, general principles, mucinous tumors including the Krukenberg tumor. Adv Anat Pathol. 2006;13:205–227. doi: 10.1097/01.pap.0000213038.85704.e4. [DOI] [PubMed] [Google Scholar]
- 2.Yada-Hashimoto N, Yamamoto T, Kamiura S, Seino H, Ohira H, Sawai K, et al. Metastatic ovarian tumors: a review of 64 cases. Gynecol Oncol. 2003;89:314–317. doi: 10.1016/s0090-8258(03)00075-1. [DOI] [PubMed] [Google Scholar]
- 3.Man M, Cazacu M, Oniu T. Krukenberg tumors of gastric origin versus Krukenberg tumors of colorectal origin. Chirurgia (Bucur) 2007;102:407–410. [PubMed] [Google Scholar]
- 4.McGill F, Ritter DB, Rickard C, Kaleya RN, Wadler S, Greston WM. Management of Krukenberg tumors: an 11-year experience and review of the literature. Prim Care Update Ob Gyns. 1998;5:157–158. doi: 10.1016/s1068-607x(98)00047-x. [DOI] [PubMed] [Google Scholar]
- 5.Serov SF, Scully RE. Histologic typing of ovarian tumors. Vol. 9 Geneva: World Health Organization; 1973. [Google Scholar]
- 6.Petru E, Pickel H, Heydarfadai M, Lahousen M, Haas J, Schaider H, et al. Nongenital cancers metastatic to the ovary. Gynecol Oncol. 1992;44:83–86. doi: 10.1016/0090-8258(92)90017-d. [DOI] [PubMed] [Google Scholar]
- 7.Hale RW. Krukenberg tumor of the ovaries: a review of 81 records. Obstet Gynecol. 1968;32:221–225. [PubMed] [Google Scholar]
- 8.Webb MJ, Decker DG, Mussey E. Cancer metastatic to the ovary: factors influencing survival. Obstet Gynecol. 1975;45:391–396. [PubMed] [Google Scholar]
- 9.Moore RG, Chung M, Granai CO, Gajewski W, Steinhoff MM. Incidence of metastasis to the ovaries from nongenital tract primary tumors. Gynecol Oncol. 2004;93:87–91. doi: 10.1016/j.ygyno.2003.12.039. [DOI] [PubMed] [Google Scholar]
- 10.Park CH, Kim YH, Lee SK, Rew JH, Park JY, Hong SY, et al. Two cases of Krukenberg tumors. Korean J Gynecol Oncol Colposc. 1996;7:68–75. [Google Scholar]
- 11.Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: a clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol. 2006;30:277–299. doi: 10.1097/01.pas.0000190787.85024.cb. [DOI] [PubMed] [Google Scholar]
- 12.Papakonstantinou E, Liapis A, Kairi-Vassilatou E, Iavazzo C, Kleanthis CK, Kondi-Pafiti A. Virilizing ovarian Krukenberg tumor in a 27-year-old pregnant woman: a case report and literature review. Eur J Gynaecol Oncol. 2011;32:331–333. [PubMed] [Google Scholar]
- 13.Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87:236–242. doi: 10.1046/j.1365-2168.2000.01360.x. [DOI] [PubMed] [Google Scholar]
- 14.La Fianza A, Alberici E, Pistorio A, Generoso P. Differential diagnosis of Krukenberg tumors using multivariate analysis. Tumori. 2002;88:284–287. doi: 10.1177/030089160208800408. [DOI] [PubMed] [Google Scholar]
- 15.Cheong JH, Hyung WJ, Chen J, Kim J, Choi SH, Noh SH. Survival benefit of metastasectomy for Krukenberg tumors from gastric cancer. Gynecol Oncol. 2004;94:477–482. doi: 10.1016/j.ygyno.2004.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Al-Agha OM, Nicastri AD. An in-depth look at Krukenberg tumor: an overview. Arch Pathol Lab Med. 2006;130:1725–1730. doi: 10.5858/2006-130-1725-AILAKT. [DOI] [PubMed] [Google Scholar]
- 17.Kim WY, Kim TJ, Kim SE, Lee JW, Lee JH, Kim BG, et al. The role of cytoreductive surgery for non-genital tract metastatic tumors to the ovaries. Eur J Obstet Gynecol Reprod Biol. 2010;149:97–101. doi: 10.1016/j.ejogrb.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Jiang R, Tang J, Cheng X, Zang RY. Surgical treatment for patients with different origins of Krukenberg tumors: outcomes and prognostic factors. Eur J Surg Oncol. 2009;35:92–97. doi: 10.1016/j.ejso.2008.05.006. [DOI] [PubMed] [Google Scholar]