Abstract
This review focuses on adipose tissue biology and introduces the concept of adipose tissue plasticity and expandability as key determinants of obesity-associated metabolic dysregulation. This concept is fundamental to our understanding of adipose tissue as a dynamic organ at the center of nutritional adaptation. Here, we summarize the current knowledge of the mechanisms by which adipose tissue can affect peripheral energy homeostasis, particularly in the context of overnutrition. Two mechanisms emerge that provide a molecular understanding for obesity-associated insulin resistance. These are a) the dysregulation of adipose tissue expandability and b) the abnormal production of adipokines. This knowledge has the potential to pave the way for novel therapeutic concepts and strategies for managing and/or correcting complications associated with obesity and the metabolic syndrome.
Keywords: obesity, adipokines, lipotoxicity, insulin resistance, Metabolic syndrome
VARIATIONS IN ADIPOSE TISSUE
Although changes in adipose mass is a familiar phenomenon to most healthy individuals, it only appears to become clinically relevant when abnormal fat accumulation is associated with health problems. For example, it is well known that overweight and obese individuals have a substantially greater risk of developing chronic diseases, such as cardiovascular disease (mainly ischemic heart disease and stroke), diabetes, musculoskeletal disorders (especially osteoarthritis), and some cancers (endometrial, breast, and colon). Furthermore, childhood obesity is associated with a greater chance of premature death and disability in adulthood. Hence, it is clear that a better understanding of the mechanisms linking adipose tissue development, function, and expansion is required to improve our chances of identifying the most successful therapeutic approaches.
In mammals, adipose tissue develops in many different sites throughout the body and generally occurs in areas of loose connective tissue, such as subcutaneous layers between muscle and dermis. However, adipose-specific depots also form around the heart, kidneys, and other internal organs. Recent studies indicate that the adipose tissue is not a homogeneous organ. In fact, new profiling technologies have revealed depot-specific differences in the metabolic profiles, which link depot-specific susceptibility to obesity and related disorders (e.g., intra-abdominal/visceral vs. subcutaneous) (see below). In addition, an early but popular classification of adipose tissues remains and refers not to its location but to its white or brown coloration [white adipose tissue (WAT) and brown adipose tissue (BAT), respectively]. Rodents have distinct depots to represent these two types of adipose tissue (e.g., epididymal WAT and interscapular BAT). The topographic distribution of BAT in humans is slightly different. Humans are born with BAT located mainly around the neck and large blood vessels of the thorax that it is subsequently replaced by WAT in adults. As more comparative studies are performed, it is becoming clear that additional differences exist between rodent and human adipose tissues (1). Hence, some caution should be exercised when extrapolating information from one species to another.
NORMAL PHYSIOLOGICAL FUNCTIONS OF ADIPOSE TISSUE
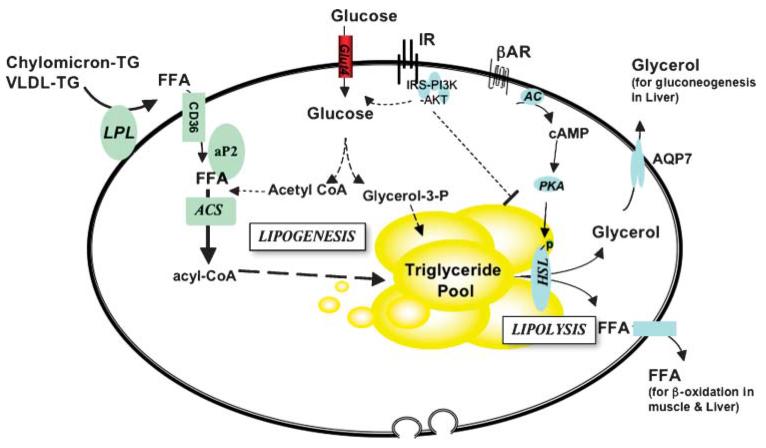
In addition to its roles in providing insulation and mechanical support, adipose tissues have traditionally been defined as the major sites for storage of surplus fuel. Indeed, during times of increased food intake and/or decreased energy expenditure, surplus energy is deposited efficiently in adipose tissue in the form of neutral triglycerides. This process is mediated by key lipogenic enzymes. However, when food is scarce and/or energy expenditure requirements increase, lipid reserves are released to provide fuel for energy generation. Therefore, adipocytes also contain "lipases" that break down triglycerides into glycerol and fatty acids that can then be transported in the blood to the liver, muscle, and BAT, where they are used in fatty acid oxidation (Fig. 1). There is also evidence that glycerol and FFA can be reesterified in adipocytes, thereby allowing FFA flux to be acutely regulated. Therefore, the two principal functions of WAT are to store excess energy as triglycerides, in large unilocular droplets, and to release it in the form of FFA. In contrast, BAT stores triglycerides in multilocular adipocytes as quick-access fuel for heat production through mitochondrial "uncoupling" of oxidative phosphorylation of FFA. This thermogenic process is vital in neonates exposed to the cold but may no longer be required and appears to be lost in adult humans, who have developed additional strategies to keep warm.
Fig. 1.
Lipid metabolism in adipocytes. Adipocytes are equipped with the biochemical machinery to function effectively as the body’s fuel store. To do this, it must mediate lipogenesis [conversion of FFA to triglycerides (TG) for storage] and lipolysis (breakdown of triglycerides to FFA and glycerol). It is also sensitive to changing nutritional cues. For example, it is insulin-sensitive [insulin stimulates glucose uptake and lipogenesis and inhibits lipolysis] and subject to adrenergic regulation [stimulates lipolysis and adaptive thermogenesis (brown adipose tissue)]. AC, adenylate cyclase; ACS, acyl-CoA synthase; AKT, AKR mouse thymoma viral proto-oncogene; AR, adrenergic receptor; HSL, hormone sensitive lipase; IR, insulin receptor; PI3K, phosphatidylinositol 3-kinase; PKA, protein kinase A.
It is unclear whether the locations of different adipose tissue depots in proximity to vital organs exert exclusively the function of mechanical support or, more likely, play a key metabolic role as a local source of emergency fuel. Nonetheless, given these functions, it is not surprising that the adipose tissue is exquisitely designed to respond to acute changes in nutritional cues. Indeed, at the molecular and biochemical levels, adipocytes are well equipped with the machinery to respond to both hormonal (e.g., insulin) and sympathetic (e.g., adrenergic) stimulation.
ADIPOSE TISSUE AS AN ENDOCRINE ORGAN
In 1994, the discovery of leptin, a satiety factor produced predominantly by adipose tissue, added a further dimension to our understanding of adipose tissue function (2). It demonstrated that this tissue was capable of emitting signals to regulate food intake and energy expenditure and thereby to orchestrate changes in energy balance and whole body nutritional status. Subsequent advances have identified many more adipose-derived secreted products (Table 1), which together with electron microscopic evaluations have reinforced this notion and led to the reclassification of adipose tissue as an endocrine organ. An important aspect of adipose tissue endocrinology is the recognition that numerous other cell types, in addition to adipocytes, are also present and play important roles in regulating adipose tissue function. The additional cell types present in the adipose tissue or its stroma-vascular fraction include pericytes and endothelial cells, monocytes, macrophages, and pluripotent stem cells (including preadipocytes). Interestingly, these nonadipocyte cells may also be the main source of some secreted factors.
TABLE 1.
Factors secreted by adipose tissue
| Lipid metabolism | Lipoprotein lipase (LPL), free fatty acids, glycerol, apolipoprotein E |
| Steroid hormones | Estrone, estradiol, testosterone |
| Growth factors and cytokines | IGF-1, nerve growth factor (NGF), vascular endothelial growth factor (VEGF), leptin, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6) |
| Vasoactive factors | Monobutyrin, angiotensinogen angiotensin II, atrial natriuretic peptide |
| Eicosanoids | Prostaglandins E2 (PGE2), prostaglandins F2a (PGF2a), prostacyclin (prostaglandin I2/PGI2) |
| Complement system | Factor B, factor C, C3, C1q, factor D [adipsin/acylation-stimulating protein (ASP/C3desARg)] |
| Binding proteins | Retinol BP, IGF-BPs, sTNFRs |
| Extracellular matrix proteins | Monocyte chemotactic protein-1 (MCP-1) |
| Others | Adiponectin (Acrp30/AdipoQ), cholesteryl ester transfer protein, plasminogen activator inhibitor 1, haptoglobin, LPA, lysophosphatidic acid, resistin, visfatin/PBEF, omentin, fasting-induced adipose factor, metallothionen, apelin |
Adapted from Ref. 45. BP, binding protein; IGF-BPs, insulin-like growth factor binding proteins; LPA, lysohosphatidic acid; PBEF, pre-B-cell colony-enhancing factor; sTNFRs, soluble tumor necrosis factor receptors.
Adipose tissue-derived secreted factors (particularly from WAT depots) can have effects on multiple biological systems, including energy homeostasis (lipid and carbohydrate metabolism, appetite, thermogenesis), the immune system, reproductive function, hemostasia, blood pressure, and angiogenesis (Table 1). As discussed below, some of these actions underscore the links between obesity and its related pathologies, such as insulin resistance, hypertension, hyperlipidemia, type 2 diabetes, and coronary heart disease.
ADIPOSE TISSUE DEVELOPMENT
In vivo adipose tissue development and expansion is controlled by the concerted actions of a number of factors that together form a highly regulated and integrated homeostatic network primarily designed to maintain energy homeostasis. These factors include a myriad of environmental, genetic, epigenetic, and pharmacological factors. Although some of these factors may be relevant to directly promoting adipose tissue development, most cause a state of "perceived" positive energy balance by either increasing appetite and/or reducing energy expenditure. This is a prerequisite for the development of obesity. However, the development of obesity also requires a healthy adipose tissue able to meet the storage demands derived from the positive energy balance. Hence, understanding the role of adipose tissue biology in vivo requires an assessment of whole body energy homeostasis. Indeed, an emerging theme in metabolic disease research is the network of signals that facilitate interorgan communication. Until recently, this aspect has been notoriously difficult to dissect.
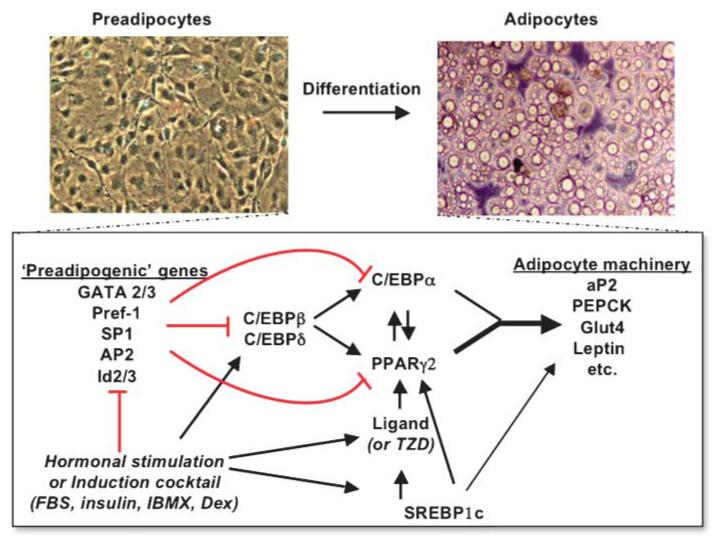
The study of adipocyte recruitment and differentiation has been further hampered by the diffuse nature of adipogenesis in vivo. This has made it difficult to dissect regions of committed, presumptive precursor cells from embryos and study their development ex vivo. Nonetheless, a number of strategies have emerged to create immortalized or primary cell lines that can be induced to differentiate in vitro. These have provided the possibility to identify and dissect the mechanisms that regulate adipocyte differentiation. Readers are directed to two recent excellent reviews that cover this topic in some detail (3, 4). In short, the most well-characterized aspect of adipocyte differentiation is the sequential cascade of transcriptional events that control adipogenesis (Fig. 2). The central players are peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT/enhancer binding protein a (C/EBPa), both of which are required to coordinate the expression of adipogenic genes (e.g., aP2, pyruvate carboxylate) (5). These genes characterize the phenotype of terminally differentiated adipocytes and, importantly, can be further regulated by additional transcription factors, such as sterol-regulatory element binding protein 1c (SREBP1c). However, the process of adipogenesis is far more complex, as indicated by the increasing number of novel regulators of preadipocyte differentiation. For instance, our laboratories have identified and characterized new transcription factors such as Eight-Twenty-One (ETO) (6) and Factor that Binds to Inducer of short transcripts (FBI) (7) as well as extracellular regulators such as Dickkopf proteins (Dkks) (8) and Insulin-like Growth Factor Binding Protein 2 (IGFBP2) (9). These are part of a growing list of factors able to stimulate or inhibit adipogenesis in vitro (Table 2). Extra layers of complexity in the adipogenic program derive not only from the number of factors but from the need to maintain a tightly regulated temporal sequence of intercellular and intracellular signaling events that occur before the stage of mature differentiated adipocytes. This suggests that expansion of adipose tissue is not just a passive process responsive to an excess of nutrients but is an energy-demanding program that is highly regulated and sophisticated.
Fig. 2.
The transcriptional control of adipogenesis involves the sequential activation of a transcription factor cascade. This begins with the transient expression of C/EBPβ and C/EBPδ, followed by C/EBPα, which in turn activates peroxisome proliferator-activated receptor γ 2 (PPARγ2). The latter pair is primarily responsible for switching on the broad program of adipogenesis. In addition, PPARγ exerts positive feedback on C/EBPα and acts synergistically to maintain the differentiated state. Sterol-regulatory element binding protein 1c (SREBP1c) is regulated by insulin and lipids and can activate PPARγ by inducing its expression as well as by promoting the production of an endogenous PPAR ligand. SREBP1c also activates the expression of many genes of the lipogenic program. Together, these factors contribute to the expression of genes that characterize the terminally differentiated phenotype. GATA, GATA binding protein; IBMX, 3-Isobutyl-1-methylxanthine; PEPCK, phosphoenolpyruvate carboxykinase; TZD, thiazolidinedione.
TABLE 2.
In vitro modulators of adipocyte differentiation
| Proadipogenic | Antiadipogenic |
|---|---|
| Insulina | Retinoic acid |
| IGF1 | Pref1 |
| Isobutylmethyl xanthine (IBMX)a |
Phorbal esters |
| Endothelin | |
| T3 | Dehydroepiandrosterone |
| Macrophage colony- stimulating factor (MCSF) |
DMSO |
| Sodium arsenate | |
| Polyunsaturated fatty acids Thiazolidinedione (TZD) |
Human immunodeficiency virus protease inhibitors |
| Indomethacin | 2,3,7,8-Tetrachlorodibenzo-p-dioxin |
| Prostacyclin (PGI2) | TGF-β |
| Angiotensin | TNF-α |
| Glucocorticoid | IL-1 |
| Dexamethasone (DEX)a | IL-6 |
| Growth hormone (GH) | IL-11 |
| Visfatin | Interferon γ (IFNγ) |
| Dkk1 | Leukemia inhibitory factor (LIF) |
| Resistin | |
| Mitotic inhibitors | |
| Prostaglandin F2α | |
| Wnt10b, Wnt3A, Wnt1 | |
| Resistin | |
| EGF | |
| PDGF | |
| IGFBP1/2 | |
| Growth hormone (GH) |
EGF, epidermal growth factor; IGFBP, insulin-like growth factor binding protein; PDGF, platelet-derived growth factor; TGF, transforming growth factor.
Used in standard adipogenic cocktail.
An example of the complexity is illustrated by the role played by the canonical Wnt signaling pathway, which was recently implicated in lineage determination and maintenance of the preadipocyte phenotype (10, 11). From our own work, we have also demonstrated that the Wnt antagonist Dickkopf1 (Dkk1) and its receptors (Krm1, LRP5, and LRP6) are coordinately regulated during early stages of human adipogenesis in vitro. Functionally, this results in reduced canonical Wnt signaling and promotes preadipocyte differentiation (8). We have also demonstrated that cytosolic accumulation of β-catenin is a prerequisite for the activation of TCF/LEF-dependent transcription in the nucleus (12). These studies demonstrate that a temporal regulation of adipogenic signals occurs at both the cell surface and in intracellular cytoplasmic compartments. Furthermore, we have shown that cross-talk exists between tumor necrosis factor-α (TNF-α) and Wnt signals, both of which can block adipogenesis (13). Therefore, it appears that cytokines, which are increased in obesity, may hijack canonical Wnt-signaling mechanisms to inhibit adipocyte differentiation (13). That some of these mechanisms may be relevant to human obesity is supported by the role played by Wnt10b mutants in human adipose tissue development (14); despite this fact, species differences also exist between the mouse and human Wnt networks (8).
ROLE OF ADIPOSE TISSUE IN OVERNUTRITION AND METABOLIC DYSREGULATION
In states of overnutrition, the mere presence of excess adipose tissue mass alone is unlikely to be responsible for obesity-associated peripheral insulin resistance and metabolic disruption. This is supported by the characterization of a number of rodent models of peripheral insulin resistance characterized by defective development of adipose tissue such as A-Zip (15). Indeed, the ablation of adipose depots exacerbates peripheral insulin resistance through hyperlipidemia-induced lipotoxicity and is reminiscent of the metabolic abnormalities associated with lipodystrophic disorders in lean individuals. These studies highlight the important requirement for a dedicated lipid storage organ even in the presence of normal nutritional loads.
From a thermodynamic point of view, no one will disagree with the concept that obesity is the result of a positive imbalance between food intake and energy expenditure. What is less intuitive is the concept that the expansion of the adipose tissue and its storage capacity may be prefixed or limited. The general belief is that normal adipose tissue should be able to expand passively to accommodate any excess of nutrients. However, in recent years, we have learned that the process of adipose tissue expansion (in the form of the development of new adipocytes and the growth of mature adipocytes) is a complex regulated process that requires energy. Under the current epidemic of obesity, the adipose tissue as a whole has never been under more pressure to expand to accommodate an unprecedented quantity of nutrients. Therefore, it is not surprising that specific genetic profiles that were able to maintain the storage competence of the adipose tissue now fail to cope with the excessive demands.
In addition to facilitating lipid storage, adipose tissue also plays an important part in systemic glucose homeostasis. This was elegantly demonstrated by metabolic phenotyping of the adipose-specific GLUT4 knockout mice. Here, disruption of insulin-stimulated glucose uptake specifically in adipocytes was sufficient to cause peripheral insulin resistance and glucose intolerance but without alterations in adipose tissue mass. Although the molecular basis for this remains elusive, it is clear that glucose uptake into adipose tissue plays a role in sensing and orchestrating adaptations in whole body carbohydrate metabolism (16). Intriguingly, other rodent models with adipocyte-specific genetic modifications when challenged with nutritional overload, can develop obesity but are protected from the associated metabolic dysregulation (insulin resistance, type 2 diabetes, fatty liver disease, and hypercholesterolemic atherosclerosis) (17-19). These observations have further fueled the understanding that both limited adipose tissue capacity and disrupted adipose function underlie the mechanisms linking obesity to insulin resistance.
HOW DOES ADIPOSE TISSUE RESPOND TO NUTRITIONAL OVERLOAD?
Two theories are emerging that provide a molecular understanding of obesity-related insulin resistance. First, in the face of a nutritional overload, there is a failure to accommodate all surplus nutrients. This results in leakage of nutrients that accumulate in other organs, such as liver and muscle. This inappropriate accumulation negatively affects the normal metabolic response or handling of this tissue. The second hypothesis suggests that excessive nutritional overload on the adipose tissue triggers qualitative and/or quantitative changes in adipokine production. These then act as modulators of insulin sensitivity and lead to both local and systemic insulin resistance. We next review each theory and outline the overlapping and distinct aspects that are now emerging.
STEATOSIS-INDUCED LIPOTOXICITY
What happens when the adipose tissue expandability becomes a limiting factor and nutrients cannot be stored safely in the adipocyte? These nutrients are redirected toward other organs, such as muscle, liver, heart, or pancreas. These tissues are not designed to store large amounts of lipids and therefore are more susceptible to the toxic effects of excess fat accumulation. Although the nature of the nutritional insult to these organs may be similar, the response to these potentially toxic species is specific to the targeted organ. Additionally, individual susceptibility may also be predetermined by the genetic background.
In our opinion, the simultaneous and chronic increase of glucose and lipid availability may result in a metabolic conflict in metabolically sensitive organs such as skeletal muscle and liver, leading to enhanced de novo lipogenesis and directing fatty acids toward reesterification. The nature of the lipotoxic insult to a specific organ will depend on three main factors: a) the magnitude and duration of the excess of nutrients; b) the functionality of the mechanisms of transport and storage of fatty acids into the tissue; and c) the oxidative capacity of the organ.
It is obvious that the greater the degree of overnutrition, the more severe the lipotoxic insult will be. However, not every organ will be affected similarly. The functionality of the transport system of fatty acids in diverse organs may condition the accessibility of nutrients and fatty acids to the tissue. In this respect, transcription factors such as SREBP1c may act as a gatekeeper for/against lipotoxicity in skeletal muscle and other peripheral organs. Over-activation of SREBP1 in these tissues may facilitate the deposition of triglycerides but also, in the long term, may facilitate the accumulation of more toxic long-chain fatty acyl-CoA, phosphatidate, diacylglycerols (DAGs), and ceramides, each with signaling and/or toxic properties. Also in the context of overnutrition, we should expect de novo induction of genetic programs attempting to respond to the excess of nutrients. For instance, we have shown that PPARγ2, an adipogenic transcription factor whose expression under physiological conditions is restricted to adipose tissue, can be induced ectopically in liver or muscle under conditions of excess nutrients (20). Induction of PPARγ2 in these organs facilitates the transport and storage of fatty acids, but this storage does not necessarily lead to lipid-induced toxicity. Therefore, it is not only the excess of nutrients but the limited storage capacity that determines the development of toxicity. The third factor to be considered is whether the oxidative capacity of the organ is able to clear the excess of nutrients. For this, the mitochondria are fundamental, and we have unpublished data indicating that genetic manipulations that induce defects in mitochondria biogenesis are also associated with the altered lipidomic composition that is seen in conditions of overnutrition. Therefore, a combination of these three variables determines the amount and quality of intracellular lipids available to regulate cell-signaling events and suggests new potential therapeutic approaches (see below).
There is debate about the specific type of lipid species that confers lipotoxicity. Traditionally, it has been suggested that ectopic accumulation of triglycerides in liver and muscle was a sign of lipotoxicity directly related to obesity-induced insulin resistance. However, our data indicate that ectopic deposition of triglycerides per se may not be the cause of lipotoxicity (20). Instead, we consider the accumulation of triglycerides in peripheral organs observed under conditions of positive energy balance to reflect the nutrient buffer capacity of the organ. We recently showed that in ob/ob mice there is a correlation between the hepatic increase in triglycerides and reactive lipid species (21). In this respect, measuring triglycerides may be a good marker of excess nutrient entering the liver. Although intuitively it may be considered logical to prevent ectopic triglyceride deposition to prevent lipotoxicity, our data suggest that strategies directed to selectively inhibit triglyceride deposition may induce a more toxic state determined by the accumulation of other more reactive lipid species (20). This is observed in the genetic ablation of PPARγ2 in liver of ob/ob mice (20). Impaired deposition of triglycerides in the context of positive energy balance causes a state of severe insulin resistance and precipitates a global metabolic failure.
The intracellular mediators of lipid-induced insulin resistance have also been the subject of intense investigations and could provide clues to the nature of the toxic lipid species. In the circulation, NEFAs from lipolysis are good candidates for inducing insulin resistance and type 2 diabetes, particularly in obesity and hyperlipidemic conditions. Indeed, lipid infusions in rodents promote gluconeogenesis and glucose output from the liver (22), and chronically increased levels decrease insulin secretion from β cells, possibly through lipotoxic mechanisms (23). In adipose tissue and muscle, increased NEFAs inhibit insulin sensitivity and insulin-stimulated glucose uptake. The Randle hypothesis originally provided a mechanism by which substrate competition led to reduced glucose uptake. However, this has since been challenged and an alternative signaling-based mechanism proposed (24). Here, NEFA treatment is postulated to result in altered membrane lipid composition and to increase the availability of reactive lipids such as DAGs and ceramides. These then activate serine kinase signaling cascades that are DAG-and/or ceramide-sensitive. The kinase cascades include IRS-1 serine kinases such as PKCs and stress kinases such as IKKβ and JNK. Hence, NEFA treatment can increase IRS-1 serine phosphorylation, a posttranslational modification observed in insulin-resistant tissues.
In agreement with this, we recently demonstrated that phosphorylation of a single residue in IRS-1 (i.e., serine 24) can be induced by the DAG mimic phorbol ester and impairs lipid-protein interactions of the IRS-1 pleckstrin homology domain. This alters intracellular localization of IRS1 and reduces its normal function in insulin signaling. Intriguingly, this event is not stimulated by C2-ceramides (25). However, in studies addressing the putative role of increased ceramides in insulin resistance, we provide evidence that ceramide derivatives such as membrane-associated gangliosides are likely to be important mediators of lipid-induced insulin resistance. Indeed, by specifically inhibiting the activity of glucosylceramide synthase, a key enzyme in the synthesis of glycosphingolipids, we can reverse insulin resistance in numerous models of obesity while retaining increased ceramide levels (26). Although some evidence already exists implicating GM3 in altering receptor tyrosine kinase signal transduction (27, 28), future lipidomic investigations should help elucidate the identities and mechanisms by which specific glycosphingolipid(s) mediate insulin resistance.
ALTERED PRODUCTION OF ADIPOKINES
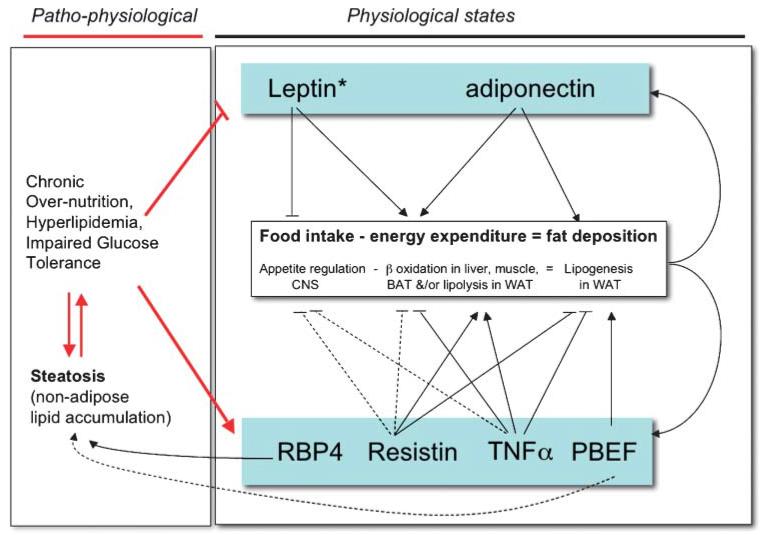
The second equally relevant response of adipose tissue to excessive nutritional loads is to alter their adipokine production. The term "adipokine" here refers to a secreted protein from adipose tissue whose production is altered in obese states and may affect obesity-associated metabolic complications. Much attention has focused on the endocrine actions of these adipokines, particularly with respect to their potential/putative roles in regulating whole body glucose homeostasis (Table 3). To avoid repetition, we have summarized the current information in Table 3 and we refer readers to recent reviews that discuss this aspect of adipokine action in some detail (19, 29, 30). However, a less well-characterized aspect of adipokine action is their potential to mediate paracrine/autocrine events regulating adipose tissue development, expansion, and/or plasticity. Indeed, many of the same adipokines that are implicated in obesity-related insulin resistance can also affect lipid metabolism/flux and thereby contribute to altered fat distribution (Table 4). Therefore, in addition to their endocrine actions on food intake and/or energy expenditure, adipokines are likely to act locally, exerting autocrine or paracrine effects that can impair adipose tissue functions and compromise further WAT expansion. For these reasons, it is clear that adipokine production and lipotoxicity are not mutually exclusive mechanisms in obesity-related insulin resistance. However, one aspect that may differentiate the two mechanisms is the possibility that adipokines are likely to play a significant role in normal physiological responses to fluctuations in nutritional status. These responses are then dysregulated and/or no longer sufficient in the face of overnutrition. In contrast, steatosis-induced lipotoxicity is almost entirely associated with pathological states (Fig. 3).
TABLE 3.
Tissue-specific effects of adipokines on glucose homeostasis
| Adipokine | Effect on Food/Fat Intake or Central Nervous System | Hepatic Glucose Production | Glucose Disposal/Tolerance in Muscle and/or Adipose | Beta Cell Survival and Insulin Production |
|---|---|---|---|---|
| Leptin | Anorexigenic | Enhances insulin sensitivity via AMPK | Enhances insulin sensitivity in muscle via AMPK and decreases intracellular lipid levels | As part of “adipo-insular” feedback loop, inhibits insulin release by inhibiting proinsulin synthesis and insulin secretion; may have a role in β-cell survival; in obesity, leptin resistance may impair the protective mechanism |
| Adiponectin | No effect on food intake, but ICV leads to increased energy expenditure; present in human cerebrospinal fluid | Enhances insulin sensitivity via AMPK | Mixed results; increases insulin action to no effect at all | No effect on insulin synthesis and secretion in healthy subjects but may improve insulin secretion in HFD models |
| TNF-α | Anorexigenic | Reduces insulin signaling in rodents; no direct effect in healthy humans infused with TNF-α | Reduces insulin-stimulated glucose uptake by white adipose tissue and muscle; multiple mechanisms include serine phosphorylation of IRS-1 and AS160, activation of several serine kinases including JNK and AMPK, NF-κB activation, SOCS3 expression, Glut4 suppression, and ROS production | Multiple effects in vitro from impairing glucose-stimulated insulin secretion to apoptosis; in vivo role in obesity unclear; may play a role in β-cell failure; however, the expression of TNF-α in the pancreas of transgenic mice without obesity resulted in insulitis, not diabetes |
| IL-6 | Anorexigenic | Reduces insulin sensitivity by inhibition of insulin receptor signal transduction, at least in part by expression of SOCS-3 | Conflicting data: both promoting glucose uptake and inhibitory with similar action to TNF-α | Role in obesity unclear; may play a protective role in β-cell hyperplasia, in that expression of IL-6 in the pancreas of transgenic mice resulted in islet hyperplasia and insulitis |
| Resistin | Potentially anorexigenic; present in human cerebrospinal fluid | Reduces insulin sensitivity; increases glucose output–primary effect | Reduces glucose uptake but not as dramatic as action on liver | Impairs glucose-stimulated insulin secretion in vitro |
| RBP4 | Unknown | Reduces insulin sensitivity; is gluconeogenic via PEPCK activation | Impaired insulin action in muscle mechanism not clear | Unknown |
| PBEF/visfatin | Unknown | Unclear | Enhances via insulin receptor | Potential role in β-cell survival, as circulating visfatin is increased with progressive β-cell deterioration |
| Omentin (humans, not mice) | Unknown | Unknown | Enhances insulin-stimulated signals and glucose uptake but not insulin-mimetic | Unknown |
AMPK, adenosine mono phosphate-activated protein kinase; HFD, high fat diet; ICV, intracerebroventricular; PEPCK, phosphoenolpyruvate carboxykinase; SOCS-3, suppressor of cytokine signalling-3.
TABLE 4.
Tissue-specific effects of adipokines on lipid metabolism and flux
| Adipokine | Food Intake (Energy Intake) | Fat Utilization, β-Oxidation, Thermogenesis (Energy Expenditure) | Fat Storage, Adipogenesis, Nonadipose Lipogenesis (Excess Energy Storage Capacity) | Levels in Common Forms of Obesity |
|---|---|---|---|---|
| Leptin | Anorexigenic | Enhances β-oxidation via AMPK activation in muscle and liver; reduces intracellular lipids; promotes thermogenesis in BAT | Antiadipogenic in vitro, promotes adipose lipolysis in vivo via the sympathetic nervous system | High (but evidence of leptin resistance) |
| Adiponectin | No effect on food intake, but ICV leads to increased energy expenditure; present in human cerebrospinal fluid | Enhances via AMPK activation in muscle and liver; reduces intracellular lipids | Promotes lipogenesis in adipocytes in vitro | Low |
| TNF-α | Anorexigenic | Inhibits muscle AMPK; increased FA incorporation into diacylglycerol, and increase in intracellular ceramide levels; reduces BAT function and promotes BAT atrophy | Antiadipogenic and stimulates lipolysis via TNFR1-mediated transcriptional signals; increases hepatic de novo synthesis of fatty acid and triglycerides | High (but with autocrine/paracrine activity) |
| IL-6 | Anorexigenic | Stimulates fat oxidation via AMPK activation | Stimulates adipose tissue lipolysis; increases hepatic de novo synthesis of fatty acid and cholesterol | High |
| Resistin | Potentially anorexigenic; present in human cerebrospinal fluid | Decreases fatty acid uptake and metabolism in muscle in vitro | Antiadipogenic and stimulates lipolysis in adipocytes | High |
| RBP4 | Unknown | Unknown | Promotes liver steatosis | High |
| PBEF/visfatin | Unknown | Unknown | Promotes lipogenesis in adipocytes | Controversial: may be higher in visceral fat |
| Omentin (humans, not mice) | Unknown | Unknown | Unknown | Increased in human visceral fat |
BAT, brown adipose tissue.
Fig. 3.
Impact of adipokines on thermodynamic balance during physiological states and states of overnutrition. Potential actions in normal physiology are indicated for each adipokine. However, in pathophysiological states associated with overnutrition, these may be separated into two functional groups: adipokines whose levels or effective activity (*) are decreased (upper panel) and adipokines whose levels and/or activity are increased (lower panel). Solid lines indicate evidence for direct action. Dashed lines are indicative of putative or potential for indirect mechanisms involving other adipokines or extracellular mediators. CNS, central nervous system; TNF, tumor necrosis factor; WAT, white adipose tissue. PBEF, pre-B-cell colony enhancing factor; RBP-4, retinol binding protein-4.
CYTOKINE ACTION, LIPID FLUX, AND ADIPOSE TISSUE PLASTICITY
Proinflammatory cytokines play an important role in both immunity and energy metabolism. In obesity, the inappropriate but chronic increase in proinflammatory cytokine production in adipose tissue can have a profound impact on whole body energy balance (31, 32). The negative action of the prototypical cytokine TNF-α on insulin sensitivity is well documented (33) (Table 3). However, TNF-α also inhibits adipocyte differentiation, promotes lipid mobilization in mature adipocytes, and impairs BAT function (13, 33-35). Many of these actions are mediated by the same TNF receptor 1, suggesting that some signaling specificity exists (13, 34, 36). Together, these actions effectively limit adipose tissue expandability and decrease its lipid storage capacity. In this way, local TNF action in adipose tissue can significantly contribute to obesity-associated hyperlipidemia and lipotoxicity in other organs, such as muscle, liver, and β-cells. Moreover, TNF can regulate the production of other adipokines (including leptin, adiponectin, visfatin, and other proinflammatory cytokines) and thereby further mediate and/or amplify its effects on peripheral organs. Whether the same actions are true for all adipocytokines, for example interleukin-6, remains to be established (Table 4) (37). Nonetheless, in nonobese situations, the cytokine-mediated interplay between the immune system and adipose tissue biology appears to be a valuable network whereby surplus fuel can be made readily available for use by activated immune cells during infection and/or inflammation.
OTHER INSULIN RESISTANCE-INDUCING ADIPOKINES AND ADIPOSE TISSUE PLASTICITY
As with TNF-α, other adipokines that impair insulin action (e.g., resistin, RB4) are also increased in obese states and affect lipid flux (38, 39). We speculate that these adipokines are likely to impair adipogenesis and adipocyte lipogenesis and/or to promote adipose lipolysis. In so doing, they reduce adipose tissue expandability and thereby contribute to altering lipid flux. In agreement with this, an intriguing recent report suggests that RPB4 may play a role in body fat distribution (40) and liver fat deposition (41). Furthermore, resistin is reported to exhibit antiadipogenic and lipolysis actions (42).
INSULIN-SENSITIZING ADIPOKINES AND ADIPOSE TISSUE PLASTICITY
The actions of the insulin-sensitizing adipokines, such as adiponectin and leptin, are also relevant to impairing adipose tissue function and plasticity. By promoting insulin-stimulated lipogenesis, they can promote triglyceride accumulation and adipose expandability. In addition, their peripheral activities promote fuel oxidation/energy expenditure in muscle and protect nonadipose tissues from accumulating lipids (Table 4). However, in obese insulin-resistant states, the activities of both adiponectin and leptin are reduced substantially, either through decreased levels or decreased activity, respectively. Hence, intracellular lipids accumulate in nonadipose tissues while adipose expansion is impaired. Finally, the recently discovered adipokine visfatin/PBEF has been reported to have lipogenic properties; therefore, it too has a potential role in regulating lipid metabolism (43, 44).
TREATMENT OF OBESITY-RELATED PATHOLOGIES
From the arguments presented here and considering that overnutrition-induced lipotoxicity is a major player in obesity-associated metabolic complications, we envisage several unorthodox therapeutic strategies. The most logical approach will be to decrease the excess of nutrients in the system through strategies aimed at decreasing food intake, increasing energy expenditure, or, more imaginatively, by promoting the elimination of nutrients through unconventional routes such as the urine. However, to date, these approaches have been unsuccessful in most patients. Hence, other less conventional strategies to prevent the deleterious effects of obesity-induced lipotoxicity are required. These may include increasing the nutrient buffer capacity of adipose tissue and other organs by promoting the deposition of neutral triglycerides instead of more toxic reactive lipid species. This may be accomplished at the level of the adipose tissue by increasing adipocyte recruitment and facilitating the development of hyper-plastic forms of adipose tissue. In fact, this strategy is currently available through the use of thiazolidinediones, activators of the proadipogenic transcription factor PPARγ. Alternatively, it is conceivable that strategies directed at modifying the coupling between adipocyte growth and the recruitment of new adipocytes will have the added value of posing some metabolic advantages. However, the most promising strategy will be to increase the pro-oxidative capacity of adipose tissue and/or other organs through its transformation into BAT. Another potential strategy could be the modulation of organ-specific lipid metabolism networks to redirect nutrients toward organs with increased capacity to successfully deal with surplus nutrients. However, for this approach to be feasible, it would be necessary to increase substantially our knowledge of lipid metabolic networks operating in specific organs under physiological and pathophysiological conditions.
The conclusion from these reflections is that although it is unquestionable that the development of obesity requires a situation of positive energy balance, the capacity for expansion of the adipose tissue is another variable that could modify the final degree of obesity and associated complications. In fact, it could be suggested that it may not be the absolute amount of fat accumulated but the mismatch between energy surplus and storage capacity, and the ectopic deposition of the surplus of energy in nonadipose tissue organs, that could be the main determinant of obesity-induced metabolic complications.
Acknowledgments
The authors are grateful to past and present members of their groups who have contributed to the studies referred to in this review. The authors apologize for the omission of many relevant references due to space limitations. Their research is supported by Diabetes UK, Medical Research Council, the Biotechnology and Biological Science Research Council, the Welcome Trust, British Heart Foundation, and the European Union Sixth Framework Programme on Hepatic and Adipose tissue and functions in the Metabolic Syndrome (EU-FP6 HEPADIP).
REFERENCES
- 1.Casteilla L, Penicaud L, Cousin B, Calise D. Choosing an adipose tissue depot for sampling. Factors in selection and depot specificity. Methods Mol. Biol. 2001;155:1–19. doi: 10.1385/1-59259-231-7:001. [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- 3.Rosen ED, MacDougald OA. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 4.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jitrapakdee S, Slawik M, Medina-Gomez G, Campbell M, Wallace JC, Sethi JK, O’Rahilly S, Vidal-Puig AJ. The peroxisome proliferator-activated receptor-gamma regulates murine pyruvate carboxylase gene expression in vivo and in vitro. J. Biol. Chem. 2005;280:27466–27476. doi: 10.1074/jbc.M503836200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rochford JJ, Semple RK, Laudes M, Boyle KB, Christodoulides C, Mulligan C, Lelliott CJ, Schinner S, Hadaschik D, Mahadevan M, et al. ETO/MTG8 is an inhibitor of C/EBPbeta activity and a regulator of early adipogenesis. Mol. Cell. Biol. 2004;24:9863–9872. doi: 10.1128/MCB.24.22.9863-9872.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laudes M, Christodoulides C, Sewter C, Rochford JJ, Considine RV, Sethi JK, Vidal-Puig A, O’Rahilly S. Role of the POZ zinc finger transcription factor FBI-1 in human and murine adipogenesis. J. Biol. Chem. 2004;279:11711–11718. doi: 10.1074/jbc.M310240200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Christodoulides C, Laudes M, Cawthorn WP, Schinner S, Soos M, O’Rahilly S, Sethi JK, Vidal-Puig A. The Wnt antagonist Dickkopf-1 and its receptors are coordinately regulated during early human adipogenesis. J. Cell Sci. 2006;119:2613–2620. doi: 10.1242/jcs.02975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wheatcroft SB, Kearney MT, Shah AM, Ezzat VA, Miell JR, Modo M, Williams SC, Cawthorn WP, Medina-Gomez G, Vidal-Puig A, et al. IGF-binding protein-2 protects against the development of obesity and insulin resistance. Diabetes. 2007;56:285–294. doi: 10.2337/db06-0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- 11.Bennett CN, Ross SE, Longo KA, Bajnok L, Hemati N, Johnson KW, Harrison SD, MacDougald OA. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 12.Hagen T, Sethi JK, Foxwell N, Vidal-Puig A. Signalling activity of beta-catenin targeted to different subcellular compartments. Biochem. J. 2004;379:471–477. doi: 10.1042/BJ20031749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cawthorn W, Heyd F, Hegyi K, Sethi J. Tumour necrosis factor-alpha inhibits adipogenesis via a beta-catenin/TCF4 (TCF7L2)-dependent pathway. Cell Death Differ. 2007 Apr 20; doi: 10.1038/sj.cdd.4402127. Epub ahead of print. doi:10.1038/sj.cdd.4402127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christodoulides C, Scarda A, Granzotto M, Milan G, Dalla Nora E, Keogh J, De Pergola G, Stirling H, Pannacciulli N, Sethi JK, et al. WNT10B mutations in human obesity. Diabetologia. 2006;49:678–684. doi: 10.1007/s00125-006-0144-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim JK, Gavrilova O, Chen Y, Reitman ML, Shulman GI. Mechanism of insulin resistance in A-ZIP/F-1 fatless mice. J. Biol. Chem. 2000;275:8456–8460. doi: 10.1074/jbc.275.12.8456. [DOI] [PubMed] [Google Scholar]
- 16.Herman MA, Kahn BB. Glucose transport and sensing in the maintenance of glucose homeostasis and metabolic harmony. J. Clin. Invest. 2006;116:1767–1775. doi: 10.1172/JCI29027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Makowski L, Hotamisligil GS. The role of fatty acid binding proteins in metabolic syndrome and atherosclerosis. Curr. Opin. Lipidol. 2005;16:543–548. doi: 10.1097/01.mol.0000180166.08196.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hertzel AV, Smith LA, Berg AH, Cline GW, Shulman GI, Scherer PE, Bernlohr DA. Lipid metabolism and adipokine levels in fatty acid-binding protein null and transgenic mice. Am. J. Physiol. Endocrinol. Metab. 2006;290:E814–E823. doi: 10.1152/ajpendo.00465.2005. [DOI] [PubMed] [Google Scholar]
- 19.Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr. Rev. 2006;27:762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- 20.Medina-Gomez G, Gray S, Yetukuri L, Shimomura K, Virtue S, Campbell M, Curtis K, Jimenez-Linan M, Blount M, Yeo GSH, et al. PPARγ2 prevents lipotoxicity by controlling adipose tissue expandability and peripheral lipid metabolism. PLoS Genet. 2007 Apr 1; doi: 10.1371/journal.pgen.0030064. Epub ahead of print. doi:10.1371/journal.pgen.0030064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yetukuri L, Katajama K, Medina-Gomez G, Seppanen-Laakso T, Vidal-Puig A, Oresic M. Bioinformatics strategies for lipidomics analysis: characterization of obesity related hepatic steatosis. BMC Syst. Biol. 2007;1:12. doi: 10.1186/1752-0509-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bergman RN, Ader M. Free fatty acids and pathogenesis of type 2 diabetes mellitus. Trends Endocrinol. Metab. 2000;11:351–356. doi: 10.1016/s1043-2760(00)00323-4. [DOI] [PubMed] [Google Scholar]
- 23.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 24.Shulman GI. Cellular mechanisms of insulin resistance. J. Clin. Invest. 2000;106:171–176. doi: 10.1172/JCI10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nawaratne R, Gray A, Jorgensen CH, Downes CP, Siddle K, Sethi JK. Regulation of insulin receptor substrate 1 pleckstrin homology domain by protein kinase C: role of serine 24 phosphorylation. Mol. Endocrinol. 2006;20:1838–1852. doi: 10.1210/me.2005-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aerts JM, Ottenhoff R, Powlson AS, Grefhorst A, van Eijk M, Dubbelhuis PF, Kuipers F, Serlie MJ, Wennekes T, Overkleeft HS, et al. Pharmacological inhibition of glucosylceramide synthase enhances insulin sensitivity. Diabetes. 2007 Feb 7; doi: 10.2337/db06-1619. Epub ahead of press. doi:10.2337/db06-1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tagami S, Inokuchi Ji J, Kabayama K, Yoshimura H, Kitamura F, Uemura S, Ogawa C, Ishii A, Saito M, Ohtsuka Y, et al. Ganglioside GM3 participates in the pathological conditions of insulin resistance. J. Biol. Chem. 2002;277:3085–3092. doi: 10.1074/jbc.M103705200. [DOI] [PubMed] [Google Scholar]
- 28.Yamashita T, Hashiramoto A, Haluzik M, Mizukami H, Beck S, Norton A, Kono M, Tsuji S, Daniotti JL, Werth N, et al. Enhanced insulin sensitivity in mice lacking ganglioside GM3. Proc. Natl. Acad. Sci. USA. 2003;100:3445–3449. doi: 10.1073/pnas.0635898100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Eldor R, Raz I. Lipotoxicity versus adipotoxicity— the deleterious effects of adipose tissue on beta cells in the pathogenesis of type 2 diabetes. Diabetes Res. Clin. Pract. 2006;74(Suppl. 1):3–8. [Google Scholar]
- 31.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 32.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J. Clin. Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sethi JK, Hotamisligil GS. The role of TNF alpha in adipocyte metabolism. Semin. Cell Dev. Biol. 1999;10:19–29. doi: 10.1006/scdb.1998.0273. [DOI] [PubMed] [Google Scholar]
- 34.Sethi JK, Xu H, Uysal KT, Wiesbrock SM, Scheja L, Hotamisligil GS. Characterisation of receptor-specific TNFalpha functions in adipocyte cell lines lacking type 1 and 2 TNF receptors. FEBS Lett. 2000;469:77–82. doi: 10.1016/s0014-5793(00)01250-3. [DOI] [PubMed] [Google Scholar]
- 35.Nisoli E, Briscini L, Giordano A, Tonello C, Wiesbrock SM, Uysal KT, Cinti S, Carruba MO, Hotamisligil GS. Tumor necrosis factor alpha mediates apoptosis of brown adipocytes and defective brown adipocyte function in obesity. Proc. Natl. Acad. Sci. USA. 2000;97:8033–8038. doi: 10.1073/pnas.97.14.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu H, Sethi JK, Hotamisligil GS. Transmembrane tumor necrosis factor (TNF)-alpha inhibits adipocyte differentiation by selectively activating TNF receptor 1. J. Biol. Chem. 1999;274:26287–26295. doi: 10.1074/jbc.274.37.26287. [DOI] [PubMed] [Google Scholar]
- 37.Spangenburg EE. Point:counterpoint. Interleukin-6 does/does not have a beneficial role in insulin sensitivity and glucose homeostasis. J. Appl. Physiol. 2007;102:820. doi: 10.1152/japplphysiol.01353.2006. author reply 825. [DOI] [PubMed] [Google Scholar]
- 38.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 39.Steppan CM, Lazar MA. The current biology of resistin. J. Intern. Med. 2004;255:439–447. doi: 10.1111/j.1365-2796.2004.01306.x. [DOI] [PubMed] [Google Scholar]
- 40.Gavi S, Stuart LM, Kelly P, Melendez MM, Mynarcik DC, Gelato MC, McNurlan MA. Retinol-binding protein 4 is associated with insulin resistance and body fat distribution in non-obese subjects without type 2 diabetes. J. Clin. Endocrinol. Metab. 2007 Feb 13; doi: 10.1210/jc.2006-1815. Epub ahead of print. doi:10.1210/jc.2006-1815. [DOI] [PubMed] [Google Scholar]
- 41.Stefan N, Hennige AM, Staiger H, Machann J, Schick F, Schleicher E, Fritsche A, Haring HU. High circulating retinol-binding protein 4 is associated with elevated liver fat, but not with total-, subcutaneous-, visceral-, or intramyocellular fat in humans. Diabetes Care. 2007 Jan 26; doi: 10.2337/dc06-2342. Epub ahead of print. doi:10.2337/dc06-2342. [DOI] [PubMed] [Google Scholar]
- 42.Ort T, Arjona AA, MacDougall JR, Nelson PJ, Rothenberg ME, Wu F, Eisen A, Halvorsen YD. Recombinant human FIZZ3/resistin stimulates lipolysis in cultured human adipocytes, mouse adipose explants, and normal mice. Endocrinology. 2005;146:2200–2209. doi: 10.1210/en.2004-1421. [DOI] [PubMed] [Google Scholar]
- 43.Sethi JK. Is PBEF/Visfatin/Nampt an authentic adipokine relevant to metabolic syndrome? Curr. Hypertens. Rep. 2007;9:33–38. doi: 10.1007/s11906-007-0007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sethi JK, Vidal-Puig A. Visfatin: the missing link between intra-abdominal obesity and diabetes? Trends Mol. Med. 2005;11:344–347. doi: 10.1016/j.molmed.2005.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vernon RG, Denis RG, Sorensen A. Signals of adiposity. Domest. Anim. Endocrinol. 2001;21:197–214. doi: 10.1016/s0739-7240(01)00121-7. [DOI] [PubMed] [Google Scholar]