Abstract
An emerging view is that obesity causes metabolic problems when adipose tissue fails to meet the increased demands for fat storage. A study in this issue of Cell Metabolism (Waki et al., 2007) has identified harmine as a proadipogenic small molecule that promotes energy expenditure in white adipose tissue and delays the onset of obesity-associated diabetes.
Obesity is an important risk factor in a complex metabolic syndrome characterized by insulin resistance, dyslipidemia, and hypertension and is associated with diabetes and cardiovascular complications. Current pharmacological and behavioral therapeutic strategies have had limited success in controlling the prevalence of obesity. Given the urgency of the problem and the somber prospects ahead, new strategies are required to prevent the deleterious metabolic effects associated with obesity.
There is little doubt that obesity results from a positive imbalance between energy intake and energy expenditure. However, it is less clear why being obese leads to diabetes and cardiovascular complications. An emerging view is that obesity causes metabolic problems when adipose tissue fails to meet the increased demands for fat storage. Under these circumstances, the excess fat that cannot be efficiently and safely stored in adipose tissue accumulates in other metabolically relevant organs such as muscle, liver, β cells, and myocardium, causing lipotoxicity and associated metabolic complications.
Acceptance of the “lipotoxicity hypothesis” leads to the apparently paradoxical therapeutic approach of promoting adipogenesis in obese individuals as a strategy to improve the storage capacity of their adipose tissue, thereby preventing the development of complications associated with obesity. This rationale is explored by Waki et al. (2007) in this issue of Cell Metabolism. The authors performed a high-throughput screen to identify proadipogenic small molecules using preadipocytes engineered with a reporter system activated by PPARγ, a key adipogenic transcription factor. The idea was that small molecules that selectively enhance PPARγ would be identified through the activation of this reporter. This imaginative approach paid off with the identification of harmine.
Harmine, also known as telepathine and banisterine, is a naturally occurring β-carboline alkaloid found in numerous plants including the Middle Eastern plant harmal or Syrian rue (Peganum harmala), the South American vine Banisteriopsis caapi (“yage” or “ayahuasca”), tobacco, and passion flowers. Among its many biochemical actions is its ability to potently inhibit monoamine oxidase (MAO) and so prevent the breakdown of neurotransmitters (serotonin, dopamine, norepinephrine), hormones (melatonin), and also hallucinogenic monoamines (psilocybin, dimethyltryptamine, mescaline). In fact, harmine has been used for centuries in the Amazon and Orinoco River basins for magico-religious purposes as an active ingredient in Ayahuasca, a psychotropic tea. Intriguingly, Ayahuasca is still used and advertised today for “people with certain diseases or serious health problems like obesity, epilepsy, arthritis, kidney, liver, or stomach problems.” However, the scientific basis for this claim is unknown.
Tontonoz and colleagues (Waki et al., 2007) have discovered that harmine facilitates adipogenesis in vitro through selective upregulation of PPARγ expression and inhibition of Wnt signaling. These observations are important for several reasons. First, the identification of a small molecule that selectively increases the amount of PPARγ expression in preadipocytes and white adipose tissue (WAT) independently of ligand activation is novel. Furthermore, Waki et al. document that gene dosage and protein levels of PPARγ are themselves important factors controlling adipogenic potential independently and synergistically with ligand activation of PPARγ. The second interesting finding is the confirmatory observation that Wnt signaling can be regulated in line with adipose tissue expandability through its interactions with PPARγ. From this, it can be inferred that Wnt signaling may have a regulatory role controlling the degree and extent of PPARγ inducibility and adipose tissue expandability. How harmine inhibits Wnt signaling and enhances PPARγ is at present unclear. Harmine induction of PPARγ does not seem to be mediated by known regulators of PPARγ expression such as C/EBPs. However, the possibility remains that PPARγ promoters are repressed by Wnt target genes and/or that PPARγ expression itself inhibits Wnt signaling (Cawthorn et al., 2007; Moldes et al., 2003; Liu et al., 2006). Nonetheless, selective modulation of Wnt signaling in WAT may be a valid strategy to increase adipose tissue expandability and improve insulin sensitivity through its effects on PPARγ.
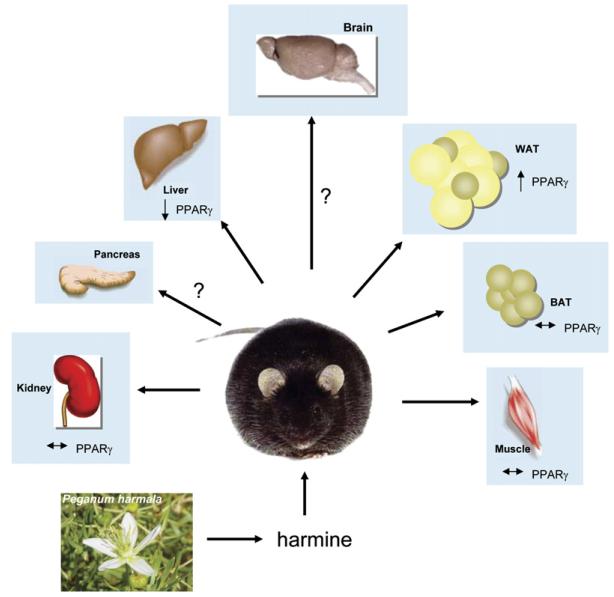
Waki et al. (2007) then went on to validate the proadipogenic effect of harmine in vivo. An unexpected finding was that, despite its proadipogenic effects in vitro, a high dose (30 mg/kg twice per day) of harmine was required to increase PPARγ levels in WAT of lean and obese mice. Interestingly, no differences in body weight, adipose mass, or food intake were observed. However, despite this lack of effect on adipose tissue mass, treatment with harmine delayed the onset of diabetes in obese mice. Harmine-treated mice exhibited increased energy expenditure and improved insulin and glucose tolerance. This was accompanied by increased circulating adiponectin levels and decreased lipid and inflammatory profiles. The mechanism behind this apparent paradox is that the effect of harmine on adipose tissue function in vivo is to promote increased fatty acid oxidation selectively in WAT (Figure 1) rather than to promote lipid storage. Mechanistically, this twist was not completely unexpected since it is known that PPARγ can, in the presence of specific co-activators such as PGC-1α and/or appropriate sympathetic stimuli, induce the genetic program required for mitochondrial biogenesis and thermogenesis characteristic of brown adipose tissue (BAT) in vivo. Nonetheless, the tissue-specific response identified by this pharmacological treatment highlights the potential for therapeutically improving carbohydrate metabolism through the modulation of adipocyte PPARγ and circumvents the hepatic and renal side effects previously associated with PPARγ activation.
Figure 1. Effects of Harmine on PPARγ Expression in db/db Mice.
Harmine is a naturally occurring β-carboline alkaloid found in Syrian rue (Peganum harmala) and other plants. A small-molecule library screen (Waki et al., 2007) has identified it as a proadipogenic molecule that acts by inducing PPARγ expression. Obese (db/db) mice treated with harmine show a delay in the onset of diabetes, coincident with increased oxygen consumption and thermogenesis. A 2-fold increase in PPARγ levels was selectively seen in white adipose tissue, while there was a 50% decrease in PPARγ levels in the liver and no change in muscle, brown adipose tissue, or kidney. The effect of harmine on PPARγ expression in the brain and pancreas is currently unknown. Peganum harmala image reproduced from http://commons.wikimedia.org/.
Based on all of these observations, several inferences can be made. The first is a cautionary reminder about the pharmacological translatability in vivo of the results from in vitro screening strategies. Although the original hypothesis was that strategies promoting adipose tissue expansion might prevent the metabolic syndrome, strictly speaking, what this report shows is that increased fat oxidation may be sufficient. This observation does not invalidate proadipocyte screens for identifying lead compounds, nor does it contradict the lipotoxic theory, since an alternative strategy for decreasing the lipotoxic insult is to eliminate the excess of lipids through their oxidation. In this context, it could be argued that harmine not only increases the adipogenic potential but, more importantly, decreases the demands for lipid storage.
The results from Waki et al. (2007) also suggest that levels of PPARγ may be an important determinant of the white versus brown phenotype. This agrees with a recent observation that mice expressing a dominant-negative mutant of PPARγ maintain normal white adipocytes in WAT but have fewer brown adipocytes in this depot (Gray et al., 2006). It is unclear by what mechanism a BAT-like phenotype is induced in WAT of harmine-treated animals and whether this is sufficient to explain the improved metabolic profiles. Nevertheless, given that harmine is a known inhibitor of MAO and cyclin-dependent kinases and also binds imidazoline and 5-hydroxytryptamine receptors, numerous additional mechanisms may also be contributing to its beneficial in vivo effects. For example, chronic MAO inhibition could increase sympathetic tone, while imidazoline receptor binding and activation may enhance insulin secretion by β cells (Cooper et al., 2003; Morgan et al., 2003). Additionally, it is noteworthy that the tissue-specific actions of harmine are most likely determined by a combination of the differential expression of these binding proteins and the tissue-specific uptake and inactivation of harmine. In any case, it would be informative to know if PPARγ is also induced in the brain of these animals and accounts for some of the neurological phenotypes of harmine (Inestrosa et al., 2005). The adipose tissue specificity of harmine-induced effects on PPARγ requires further clarification. The fact that harmine induces changes in Wnt target genes in other organs without affecting PPARγ levels suggests tissue specificity but warns of possible important side effects of harmine or other potential drugs using a similar mechanism of action.
In the twists and turns that follow proadipogenic screenings of small molecules, unexpected in vivo results can open up exciting new avenues for metabolic research. Although harmine itself is not a suitable antiobesity or antidiabetic drug, identification of its WAT-specific pro-oxidative effects may lead to novel therapeutic strategies to counteract the deleterious metabolic effects associated with obesity.
REFERENCES
- Cawthorn WP, Heyd F, Hegyi K, Sethi JK. Cell Death Differ. 2007 doi: 10.1038/sj.cdd.4402127. Published online April 20, 2007. 10.1038/sj.cdd.4402127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EJ, Hudson AL, Parker CA, Morgan NG. Eur. J. Pharmacol. 2003;482:189–196. doi: 10.1016/j.ejphar.2003.09.039. [DOI] [PubMed] [Google Scholar]
- Gray SL, Dalla Nora E, Backlund EC, Manieri M, Virtue S, Noland RC, O’Rahilly S, Cortright RN, Cinti S, Cannon B, Vidal-Puig A. Endocrinology. 2006;147:5708–5714. doi: 10.1210/en.2006-0684. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Godoy JA, Quintanilla RA, Koenig CS, Bronfman M. Exp. Cell Res. 2005;304:91–104. doi: 10.1016/j.yexcr.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang H, Zuo Y, Farmer SR. Mol. Cell. Biol. 2006;26:5827–5837. doi: 10.1128/MCB.00441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldes M, Zuo Y, Morrison RF, Silva D, Park BH, Liu J, Farmer SR. Biochem. J. 2003;376:607–613. doi: 10.1042/BJ20030426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan NG, Cooper EJ, Squires PE, Hills CE, Parker CA, Hudson AL. Ann. N Y Acad. Sci. 2003;1009:167–174. doi: 10.1196/annals.1304.019. [DOI] [PubMed] [Google Scholar]
- Waki H, Park KW, Mitro N, Pei L, Damoiseaux R, Wilpitz DC, Reue K, Saez E, Tontonoz P. Cell Metab. 2007;5:357–370. doi: 10.1016/j.cmet.2007.03.010. this issue. [DOI] [PubMed] [Google Scholar]