Abstract
The electrostatic dust collector (EDC) is a passive dust sampling device for exposure assessment of airborne endotoxin and possibly allergens. EDCs consist of a non-conducting plastic folder holding two or four electrostatic cloths of defined area. The sampling time needed to achieve detectable and reproducible loading for bioaerosols has not been systematically evaluated. Thus, in 15 Iowa farm homes EDCs were deployed for 7-, 14-, and 28-day sampling periods to determine if endotoxin and allergens could be quantified and if loading rates were uniform over time, i.e. if loads doubled from 7 to 14 days or 14 to 28 days and quadrupled from 7 to 28 days. Loadings between left and right paired EDC cloths were not significantly different and were highly correlated for endotoxin, total protein, and cat (Fel d1), dog (Can f1), and mouse (Mus m1) allergens (P < 0.001). EDCs performed especially well for endotoxin sampling with close agreement between paired samples (Pearson r = 0.96, P < 0.001). Endotoxin loading of the EDCs doubled from 7- to 14-day deployments as hypothesized although the loading rate decreased from 14 to 28 days of sampling with only a 1.38-fold increase. Allergen exposure assessment using EDCs was overall less satisfactory. Although there was reasonable agreement between paired samples, only exposures to cat, dog, and mouse allergens were reliable and these only at the longer deployment times.
Keywords: allergens, asthma, bioaerosols, endotoxin, exposure assessment, house dust, passive sampling
INTRODUCTION
Endotoxin is a lipopolysaccharide, or lipooligosaccharide component of Gram-negative bacteria that acts as a microorganism-associated molecular pattern serving as a ligand to pattern recognition receptors of the innate immune system leading to airway inflammation, narrowing, and hyperreactivity (Hadina et al., 2008; Sigsgaard et al., 2008). Inhalation of endotoxin into the lungs is associated with clinical symptoms that include airway inflammation, toxic pneumonitis, mucous membrane irritation, and exacerbation of asthma (Brigham and Meyrick, 1986; Von Essen et al., 1990; Thorn and Rylander, 1998; Thorne et al., 2005a).
Exposure to indoor allergens, especially those generated from animals, arthropods, rodents, and molds, is associated with an increased risk of developing allergic sensitization and asthma among susceptible children (Sporik et al., 1999; Jacob et al., 2002; Salo et al., 2008). Allergen structures are most often proteins of biological origin such as mite, cat, mouse, and rat allergens (Douwes et al., 2003). Early-life exposure to dust containing endotoxin may have a protective effect against developing allergies. Thus, many epidemiology studies have sought to characterize exposures to both endotoxin and allergens in indoor environments.
Electrostatic dust collectors (EDCs) were developed for passive sampling of airborne particulate matter (PM). Unlike many active sampling methods, EDCs can be utilized inexpensively, without elaborate equipment and field staff, and collect integrated samples of airborne PM over long-time periods. Two types of EDCs are currently in use with 2-cloth and 4-cloth models. Electrostatic cloths in EDCs passively collect PM while resting on flat surfaces in homes, schools, office buildings, agricultural, and industrial facilities (Noss et al., 2008; Samadi et al., 2010; Liebers et al., 2012; Jacobs et al., 2013a). EDCs are ideal for large population studies because they can be mailed to, deployed by, and returned by study participants. This gives EDCs an advantage over other passive sampling methods including the pizza box dust settling method (Würtz et al., 2005) and the electrostatic cloth wiping method (Thorne et al., 2005b).
EDCs have been used to sample endotoxin, glucan, and allergens and have been compared to other types of sampling methods such as GSP samplers, impingement using BioSamplers, Harvard impactors, vacuum floor dust sampling and settled dust sampling of surfaces above 0.75 m using a vacuum (Noss et al., 2010; Frankel et al., 2012). EDCs have been used for allergen detection in homes, schools, and flooded homes undergoing remediation (Hoppe et al., 2012; Krop et al., 2014). Dog and mite antigen exposure has also been assessed in home and farming environments using EDCs (Zahradnik et al., 2011; Vredegoor et al., 2012). Dust from EDC cloths has even been extracted and cultured for fungi and bacteria (Madsen et al., 2012).
Determining the optimal sampling period and duplicate sampling capacity of EDCs is important for accurately sampling endotoxin and allergens. Depending on the levels of PM in a particular environment, different deployment times may be needed to efficiently sample that environment. Previous work (Noss et al., 2010) indicated that the endotoxin loads settled on EDCs from 2 to 4 weeks of sampling were similar and glucan amounts only increased by 51% despite the extra sampling time. This anecdotal finding suggested that the sampling rate of EDCs may decline over time either due to dissipation of electrostatic charge or loss of material from the dust-laden EDCs. This study was conducted in seven student homes and compared endotoxin and glucan over two sampling periods. Furthermore, the placement of these EDCs is crucial and it is unspecified whether or not the EDCs were deployed on bookshelves as a previous study recommended (Noss et al., 2008). Madsen et al. (2012) found that deployment of EDCs on a bookshelf compared to an open space significantly affected endotoxin concentrations. Other studies have deployed EDCs at differing time intervals including 8 weeks in schools and 1 month in 5 homes of study colleagues and acquaintances (Frankel et al., 2012; Jacobs et al., 2013b). EDCs have also been validated by sampling different cloths and glove types to determine the most suitable EDC materials (Thorne et al., 2005b).
The aim of this study was to evaluate EDCs as a sampling method for airborne PM for indoor, domestic environments. This validation included identifying an appropriate EDC sampling period and conducting a within-EDC comparison of sampled endotoxin, total protein, and allergens from farm homes.
METHODS
EDC assembly
EDC cloths were heated for 6h at 160°C to degrade preexisting endotoxin from packaging and the EDC folders were cleaned with a 70% alcohol solution. EDCs were assembled under dust-free and endotoxin-free conditions to prevent contamination prior to deployment. Field blank EDCs were prepared and handled similarly.
Deployment time study
EDC sampling was performed in fifteen farm homes in Northeastern Iowa within a 25 km radius of Maquoketa, IA, USA. A farm home was defined as a house with family or an individual farming livestock (usually cattle) or row crops (typically corn, soybeans, or both). A range of bioaerosol loads was desired, so households were selected with occupancy ranging from one to six family members. Three EDC samplers were deployed side-by-side in each home for 7, 14, and 28 days with a common deployment date and sequential retrieval. EDCs were placed in the living room/family room where occupants stated they spend the most time. Each home was equipped with three music stands (Hamilton KB95E Encore Symphonic) adjusted to a height of 135cm with the music rest set horizontal to hold one EDC. The stands were set ~2.5cm apart and away from ventilation and appliances. Each EDC was labeled with a sample ID and a designation of collection period (7, 14, or 28 days) and all EDCs were deployed and retrieved by one of the authors (B.K.B.) without touching the electrostatic cloths. Two blank EDCs were deployed for 7-, 14-, and 28-day periods (n = 6) and were assigned to six different participating homes. The blank EDCs were removed from their Ziploc® bags but the folder remained closed during the designated time period. They were placed on one of the music stands. After the assigned 7-, 14-, or 28-day sampling period, each EDC folder was closed and placed into its own Ziploc® bag for transport to the laboratory. In the laboratory, EDC cloths were removed from the EDC folder, transferred to sterile, endotoxin-free 50-ml polypropylene tubes and stored at −20°C until extraction and analysis. Endotoxin, total protein, and allergen assays were performed on all EDC cloths.
EDC extraction
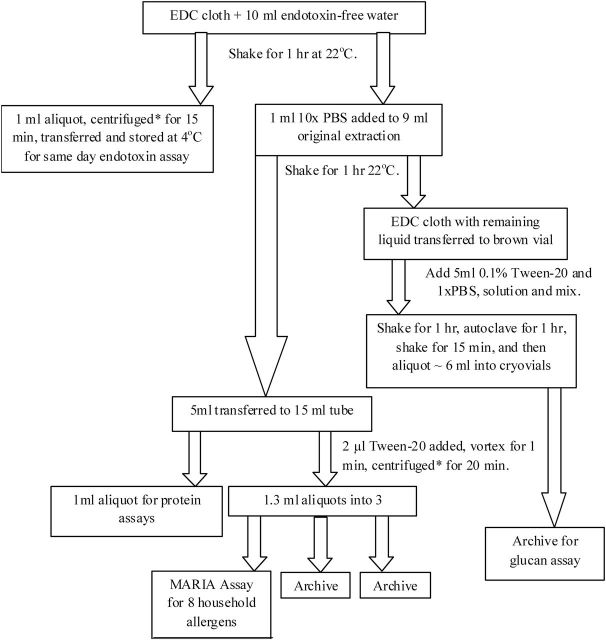
The study used the EDC serial extraction process illustrated in Fig. 1. Glassware was rendered endotoxin-free by heating overnight at 200°C prior to use. Endotoxin was extracted from each EDC cloth by elution into 10ml of sterile, endotoxin-free water (Lonza, Inc.). Cloths were shaken for 1h at 22°C, a 1ml aliquot was taken for same day endotoxin assay, and centrifuged for 15min at 600×g. To the remaining 9ml of extract, 1ml sterile 10× PBS was added and the extract was shaken for 1h at 22°C. Then 5ml of the elution was transferred to a centrifuge tube and 1ml aliquot was transferred to a cryovial and both were stored at −20°C. Tween 20 was added to the remaining 4ml using a wide-orifice pipette tip (no. RT-L250WS; Rainin), and the extract was vortexed and centrifuged for 15min at 600×g before being divided into three aliquots stored in endotoxin-free cryovials at −20°C for later allergen assay. The pellet was discarded.
1.
Scheme for electrostatic dust collector (EDC) serial extraction protocol for endotoxin, allergens, and protein determination. *Centrifugation at 600×g and 4°C.
Kinetic chromogenic Limulus Amebocyte Lysate (LAL) assay
EDC eluates were analysed using the kinetic chromogenic LAL assay (Kinetic-QCL; Lonza, Inc., Walkersville, MD, USA), as previously described (Thorne, 2000) but using water extraction without 0.05% Tween 20 and diluted using 4-fold serial dilutions of 1:1, 1:4, 1:16, and 1:64. Tween 20 was not added because it has been shown to interfere with the LAL assay up to a 50-fold dilution factor (Spaan et al., 2008). All LAL reagents were from the same lot (lot HL0476) and all samples and standard dilutions were prepared in heat-treated borosilicate glass tubes. A 12-point standard curve was generated using 2-fold serial dilutions of endotoxin standards (Escherichia coli E50:643; Lonza, Inc.; 13 EU ng–1) from 50.0 to 0.0244 EU ml–1. Eluates of sampled and blank EDCs were assayed in microtiter plates (Costar no. 3595; Corning, Inc.) using a microplate reader (SpectraMax 340, Molecular Devices, Inc.) with photometric measurements taken every 30 s for 90min at 405nm and 37°C. SoftMaxPro software (Ver 4.7.1, Molecular Devices, Inc.) was used to analyse the data. The minimum acceptable r 2 value for the standard curve was 0.995.
Allergen assays
A fluorescent multiplex array kit (MARIA, MRA-P8; Indoor Biotechnologies, Inc.) was used to measure multiple allergens following the manufacturer’s procedure. Sample extracts were thawed, vortexed, and centrifuged for 2min at 4°C at 16000×g. Each EDC sample was assayed at full strength and at a 1:5 dilution. A standard curve was prepared using a 12 point, 2-fold dilution of the Universal Allergen Standard (ST-UAS) starting at a dilution of 1:20. The plate was read on an xMAP instrument (Luminex1000, BioRad, Inc.). Each EDC sample was assayed for detection of cat (Fel d1), dog (Can f1), mouse (Mus m1), cockroach (Bla g2), dust mite (Der p1, Der f1, mite group 2), and rat (Rat n1) allergens.
Total protein assay
Samples for protein assay were thawed, vortexed, centrifuged (5min at 600×g), diluted into 5-fold serial dilutions, and added to a 96-well plate (product no. 12565591; Fisherbrand). The working reagents from the kit (QuantiPro™ BCA Assay kit, Sigma-Aldrich) were mixed according to the manufacturer’s instructions and 150 µl were added to the samples and incubated for 16h before measurement at 562nm (SpectraMax Plus 384, Molecular Devices, Inc.). The standard used was 1mg ml–1 bovine serum albumin diluted to a 0.5–30 µg ml–1 standard curve.
Statistical analysis
EDC concentrations of endotoxin, allergen, and protein were all log-normally distributed (Kolmogorov–Smirnov test). The extract concentrations were adjusted to account for the loss from the aliquots that were taken during serial extraction, with allergen and protein values multiplied by 1.11. This adjustment is reflective of removing 1ml for endotoxin analyses from the original 10ml extract and adding 1ml 10× PBS to the remaining 9ml of extract. Thus, the allergen and protein values are only reflective of 9ml of the original 10ml of extract and multiplying by a factor of 1.11 adjusts the values for the original 10ml extraction volume. All endotoxin samples were above the limit of detection (LOD). Eight of 12 blanks had non-detectable levels of endotoxin—only the blanks deployed in homes for 28 days had detectable endotoxin levels. As a result, 28-day endotoxin sample values were blank corrected by subtracting the mean of the four blanks (22.7 EU m–2). All allergen blanks were below the LOD, so the allergen samples were not blank corrected. Protein values were blank corrected by subtracting the mean of the 12 blanks (2940 µg m–2).
Agreement between left and right EDCs was assessed on log-transformed values for all analytes and displayed as Bland–Altman plots (Bland and Altman, 2010). Unpaired, equal variances and parametric two-sample t-tests were used to analyse each set of data for significance between left and right endotoxin, allergen, and total protein loads. Only pairs with both left and right cloths detectable were used for within-EDC comparisons for each analyte. For 7-, 14-, and 28-day comparisons of allergens, only homes with three or more of the six deployed EDC cloths above the LOD were evaluated. For endotoxin and total protein, data from both left and right EDC cloths were included in the calculation. For 7-, 14-, and 28-day allergen comparisons, all left and right pairs with both values below the LOD were excluded. Pairs for which both were detectable were averaged for each time period in each home. When one of the two values was below the LOD, a value for the undetectable value was imputed using LOD/√2.
To determine whether there was a significant difference in allergen and endotoxin loading between each time period, a linear mixed-model analysis was performed on log values. Endotoxin, cat, dog, and mouse allergens were each analysed in their own model. Linear mixed-model analyses were performed using a random effect to account for the repeated measures taken in each household with EDC side and sampling period as fixed effects. P < 0.05 were considered significant and those less than 0.01 were considered highly significant. The analyses were performed in SAS version 9.3 (SAS Institute, Inc., USA) using the SAS Mixed Procedure with a repeated statement for household and an unstructured covariance structure using restricted maximum likelihood estimation.
RESULTS
Table 1 shows descriptive statistics for endotoxin, allergen, and protein loading for 7, 14, and 28 days of deployment as well as comparisons between left and right EDC cloth values. While all EDCs had detectable endotoxin, fewer EDCs had detectable allergens and total protein. Results are not shown for dust mite and rat allergens since, as expected, few EDCs yielded detectable values. The large geometric standard deviations are indicative of the heterogeneity of bioaerosols in these households in terms of number of occupants and ownership of indoor pets.
Table 1.
Endotoxin, allergen and protein loading displayed as geometric mean (GM) and geometric standard deviations (GSD) between different sampling periods (7, 14, and 28 days) and descriptive statistics for left and right electrostatic dust collector (EDC) cloths over all three sampling periods in 15 farm homes.
| n/N | GM | GSD | Median | Interquartile range | |
|---|---|---|---|---|---|
| Endotoxin (EU m–2) | |||||
| 7 | 30/30 | 934 | 3.2 | 605 | 403–2934 |
| 14 | 30/30 | 2007 | 2.8 | 1601 | 910–5163 |
| 28 | 30/30 | 2768 | 2.9 | 2162 | 1051–6842 |
| Left | 45/45 | 1633 | 3.2 | 1298 | 827–4590 |
| Right | 45/45 | 1835 | 3.3 | 1762 | 862–5280 |
| Allergens (ng m–2) | |||||
| Fel d1 | |||||
| 7 | 7/30 | 116 | 4.5 | 98 | 38–468 |
| 14 | 12/30 | 147 | 4.1 | 117 | 68–346 |
| 28 | 15/30 | 78 | 4.1 | 49 | 30–130 |
| Left | 15/45 | 126 | 3.7 | 114 | 54–224 |
| Right | 19/45 | 92 | 4.6 | 49 | 27–394 |
| Can f1 | |||||
| 7 | 11/30 | 286 | 7.0 | 141 | 92–454 |
| 14 | 12/30 | 491 | 5.9 | 313 | 136–1620 |
| 28 | 11/30 | 827 | 7.0 | 1692 | 182–3186 |
| Left | 18/45 | 336 | 5.2 | 203 | 110–1643 |
| Right | 16/45 | 744 | 8.0 | 431 | 134–3027 |
| Mus m1 | |||||
| 7 | 11/30 | 85 | 2.2 | 65 | 54–114 |
| 14 | 14/30 | 95 | 2.3 | 100 | 57–146 |
| 28 | 20/30 | 138 | 3.1 | 176 | 41–286 |
| Left | 22/45 | 119 | 2.8 | 133 | 54–232 |
| Right | 23/45 | 100 | 2.5 | 98 | 46–184 |
| Bla g2 | |||||
| 7 | 4/30 | 537 | 1.5 | 596 | 424–746 |
| 14 | 6/30 | 557 | 1.7 | 466 | 400–899 |
| 28 | 5/30 | 522 | 1.8 | 699 | 325–732 |
| Left | 7/30 | 495 | 1.7 | 461 | 325–759 |
| Right | 8/30 | 582 | 1.5 | 610 | 408–778 |
| Protein (µg m–2) | |||||
| 7 | 27/30 | 1647 | 3.9 | 1698 | 837–3243 |
| 14 | 30/30 | 3991 | 3.2 | 2302 | 1803–7404 |
| 28 | 29/30 | 3384 | 2.4 | 3454 | 1686–6098 |
| Left | 42/45 | 2996 | 3.2 | 2639 | 1537–4926 |
| Right | 44/45 | 2734 | 3.4 | 2399 | 1490–6142 |
Endotoxin, allergens, and total proteins were analysed to determine their consistency between EDC cloths within the same EDC folder. Figure 2A shows the relationship between left and right EDC endotoxin loads with 7, 14, and 28 days of sampling. The narrow difference in endotoxin loading between cloths indicates a high degree of sampling reproducibility. The relationship between left and right endotoxin loads also had a positive and highly significant Pearson correlation coefficient of 0.96 (P < 0.001). A two-sample t-test demonstrated no significant difference between left and right EDC cloths for endotoxin loading (P = 0.64).
2.
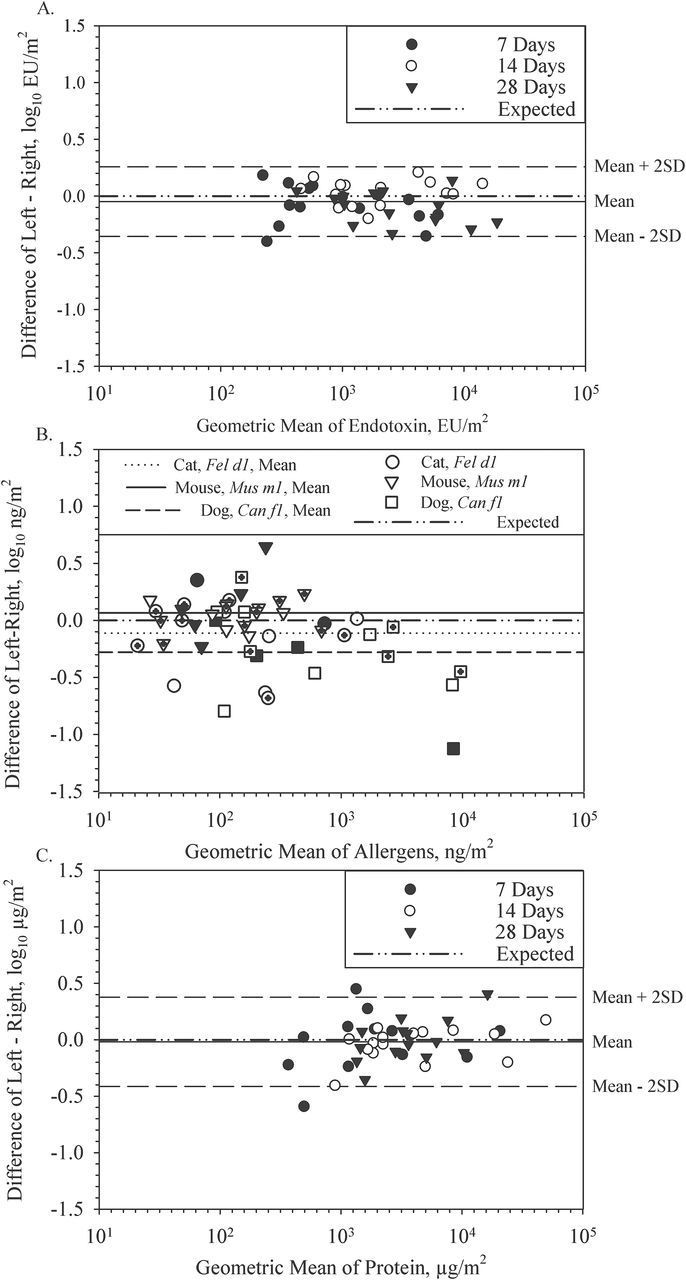
Bland–Altman plots showing within-electrostatic dust collector (EDC) left and right cloth comparisons of endotoxin (A), allergens (B), and protein loads (C) in each farm home for EDCs deployed for 7, 14, and 28 days of sampling. Allergen data for 7-day deployment are shown with a filled symbol, 14-day with an open symbol and 28-day with a hatched symbol.
Three allergens with sufficient quantified values were assessed in left and right pairs for Fel d1 (n = 14), Can f1 (n = 15), and Mus m1 (n = 20). Figure 2B displays log-transformed allergen values between left and right EDC cloths, demonstrating a higher degree of variation within EDCs compared to endotoxin loads (Fig. 2B). Cat, dog, and mouse allergen comparisons between left and right EDC cloths demonstrated highly significant Pearson correlation coefficients ranging from 0.86 to 0.91 (P < 0.001). A two-sample t-test of each set of within-EDC cat, dog, and mouse allergen pairs yielded no significant difference between left and right values (P = 0.63, 0.34, and 0.57, respectively).
The relationship between left and right EDC total protein concentrations is shown in Fig. 2C for the 7-, 14-, or 28-day sampling periods. This plot illustrates a high degree of correlation (r = 0.92, P < 0.001) between the protein concentrations of the left and right EDC cloth. A two-sample t-test also indicated no significant difference between protein loads on the left and right EDC cloths (P = 0.86). Total protein was not analysed further for differences between 7 versus 14, 14 versus 28, or 7 versus 28 days because regression between the time periods was low ranging from <0.001 to 0.31.
The measured concentration of endotoxin in the same homes in overlapping 7-, 14-, and 28-day periods are shown in Fig. 3. The expected relationship assuming steady airborne concentration and no loss of sampling efficiency is indicated with a dashed line. Under these assumptions, the endotoxin concentrations would double from 7 to 14 and 14 to 28 days while quadrupling from 7 to 28 days of sampling. When a regression line was added to fit the points, the regression coefficients were found to decrease from 0.81 for 7 versus 14 days; 0.74 for 14 versus 28 days to 0.69 for 7 versus 28 days (Fig. 3A, B, and C). However, in Fig. 3B and C the regression lines were below the lines marking the expected relationships suggesting some loss of loading efficiency over the 28-day sampling period.
3.
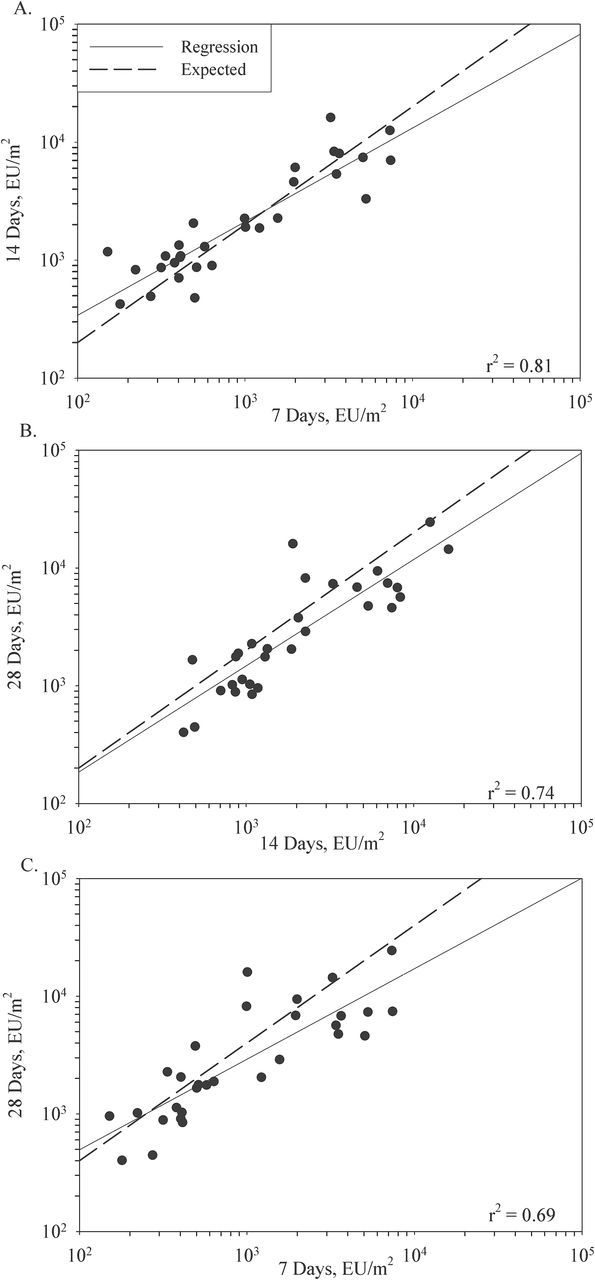
Relationships between 7-, 14-, and 28-day electrostatic dust collector (EDC) side-by-side sampled endotoxin loads from 15 farm homes. The dotted line is the expected (doubling or quadrupling) and the solid line is the regression line. (A) 7 days versus 14 days (B) 14 days versus 28 days and (C) 7 days versus 28 days.
Table 2 contains the modeled geometric mean (GM) estimates for each unique linear mixed-model analysis. For endotoxin, preliminary models included sampling period (7, 14, and 28 days), EDC side (left, right), and the interaction between sampling period and EDC side. The interaction was not significant (P = 0.15) and was removed from the model. EDC side also fell out of the model (P = 0.98) leaving only sampling period and indicating that sampling period was highly significant (P < 0.001). The fold differences between GM estimates indicate that while there is a 2-fold increase from Day 7 to 14, there is a failure to double (1.38) from 14 to 28 days and a failure to quadruple from 7 to 28 days (2.96).
Table 2.
Linear mixed-models evaluation of log endotoxin (EU m–2) and allergen (ng m–2) sampling using electrostatic dust collectors (EDCs). Model estimates and 95% confidence interval (CI) of sampling 7, 14, and 28 days are shown.
| Effect | N | Modeled GM | 95% CI | Sampling period P-value | Fold differences between modeled GM | ||
|---|---|---|---|---|---|---|---|
| Endotoxin (EU m –2)a | |||||||
| Day 7 | 30 | 934 | 604–1450 | 7 versus 14 | 2.15 | ||
| Day 14 | 30 | 2010 | 1370–2950 | <0.0001 | 14 versus 28 | 1.38 | |
| Day 28 | 30 | 2770 | 1850–4140 | 7 versus 28 | 2.96 | ||
| Allergens (ng m –2) | |||||||
| Fel d1 b | |||||||
| Day 7 | 7 | 58.1 | 13.9–244 | 7 versus 14 | 1.87 | ||
| Day 14 | 7 | 109 | 24.7–479 | 0.40 | 14 versus 28 | 0.87 | |
| Day 28 | 7 | 95.1 | 25.9–349 | 7 versus 28 | 1.64 | ||
| Can f1 c | |||||||
| Day 7 | 6 | 276 | 27.7–2750 | 7 versus 14 | 2.00 | ||
| Day 14 | 6 | 554 | 84.7–3630 | 0.078 | 14 versus 28 | 1.69 | |
| Day 28 | 6 | 939 | 147–6020 | 7 versus 28 | 3.40 | ||
| Mus m1 d | Day 7 | 7 | 58.7 | 20.4–169 | 7 versus 14 | 1.59 | |
| Day 14 | 7 | 93.5 | 41.3–212 | 0.0085 | 14 versus 28 | 2.35 | |
| Day 28 | 7 | 220 | 87.7–552 | 7 versus 28 | 3.75 | ||
alogEndotoxin = 3.44–0.47(Day 7) − 0.14(Day 14) + ɛ
blogCatAllergen = 1.98–0.21(Day 7) + 0.058(Day 14) + ɛ
clogDogAllergen = 2.97–0.53(Day 7) − 0.23(Day 14) + ɛ
dlogMouseAllergen = 2.34–0.57(Day 7) − 0.37(Day 14) + ɛ
As with endotoxin, allergen levels were expected to exhibit a relatively constant airborne concentration and uniform loading rates over time. Due to low allergen loading and LOD issues, the data are sparser than for endotoxin. Similar to the endotoxin data analysis, for the allergens the interactions and EDC side fell out of the linear mixed-effects models leaving only sampling period. Model GM estimates and confidence intervals for each allergen are listed in Table 2. For cat allergen, values failed to double or quadruple from 14 to 28 and from 7 to 28, respectively, and the effect of sampling period was not significant (P = 0.40). Dog and mouse allergens both had EDC loadings that increased significantly over the sampling time (P = 0.078 {dog}, P = 0.0085 {mouse}).
DISCUSSION
For 7-, 14-, and 28-day sampling comparisons, left and right EDC cloths were compared for endotoxin, allergen, and total protein concentrations which all yielded a high degree of correlation and relatively low variation within EDC folders. Allergen was more variable than endotoxin concentrations within-EDC cloths. This variation may be due to differential settling of allergens on the cloth due to proximity of a pet to one side of the EDC compared to the other. It is also possible for a bolus of allergen from settling dog or cat hair to land on one cloth but not the other. These data show that deploying one EDC cloth is sufficient to accurately sample endotoxin exposures in a home allowing the other EDC cloth to be archived or utilized for a different analyte. Noss et al. (2008) came to a similar conclusion, but was limited to endotoxin over a 14-day sampling period. This study expands that scope to include the evaluation of within-EDC sampling consistency, analyses of protein and allergens, and the addition of 7- and 28-day time points. In this validation study, we used music stands for height consistency and consistency of location within each home, to eliminate exposure misclassification due to EDC placement. However, future studies using mailed EDCs and subject EDC deployment may have to use bookshelves or other available placements.
A previous study (Noss et al., 2010) which sampled in seven student homes indicated that 4 weeks of sampling only led to a 5% increase in endotoxin loading compared to 2 weeks. Thus, establishing an effective time period for endotoxin and allergen detection in farm homes utilizing EDCs as an accurate passive sampling method was considered essential to its validation. Our study differed considerably from Noss et al. (2010) and considered an additional time period of 7 days, allergens, and proteins, and farm homes instead of student homes. In this study, endotoxin loads from the three sampling durations were highly correlated. Linear mixed models indicated a highly significant effect of sampling period. Fold differences between GM estimates of endotoxin for 7 versus 14, 14 versus 28, and 7 versus 28 days of sampling were 2.15, 1.38, and 2.96, respectively, demonstrating an apparent reduction in sampling efficiency from Day 14 to Day 28 and 7 to 28 days. This reduction may be the result of cloth saturation of the electrostatic charge of the surface area or the deterioration of the cloth charge over time. Endotoxin concentrations have been shown to be influenced by a variety of factors including: pet ownership, smoking in the home, farm animal contact, family size, and socioeconomic status (Park et al., 2001; Thorne et al., 2009). Saturation may occur faster or slower in different homes depending on the presence of factors that influence endotoxin.
EDCs have previously been deployed in schools to determine endotoxin concentrations over an 8-week period obtaining endotoxin levels well over 20000 EU m–2 in some schools (Jacobs et al., 2013b), much higher than even the 28-day interquartile range in this study of 1051–6842 EU m–2. However, this environment is remarkably different than homes. Children attend regular school hours and tend to have weekends and holidays off. Schools house many active children who contribute to the endotoxin load (Thorne et al., 2009). Because of the differences between homes and schools, one should be cautious about generalizing these results.
Another EDC validation study was recently published that studied EDCs deployed in the living rooms of 27 Danish flats whose residents were above 50 years of age (Madsen et al., 2012). EDCs were deployed in two seasons for 12–15 days. The median value of 1560 EU m–2 (range: 145–12919 EU m–2) was comparable to our median value of 1601 EU m–2 for 14 days of sampling (range: 423–16145 EU m–2). We might have expected higher levels in Iowa farm homes. Insufficient information is provided by Madsen et al. (2012) to speculate as to the source of endotoxin in the Danish study.
If EDCs are also being utilized for allergen exposure assessment, 14-day sampling may be preferred as some allergen values were below the LOD after 7 days of deployment. The farm homes hosting EDCs in this study had minimal allergens overall, especially for mites, cockroaches, and rats. Thus, a more extensive study of allergen sampling in problem homes using EDCs may be essential to further establish the optimal deployment period. We would not expect much airborne mite allergen in living rooms since it is generally associated with the bedroom area and particles ranging from 10 to 30 µm in size that are not very mobile (Tovey et al., 1981). Rats are very rarely observed in Iowa homes and in previous studies, we have found little evidence of rat allergen (Hoppe et al., 2012). Even in a study of 63 flooded Iowa homes undergoing remediation, only five EDCs had detectable rat allergen (Hoppe et al., 2012).
A companion animal hospital was the location of another EDC deployment study that analysed endotoxin and Fel d1 and Can f1 allergens (Samadi et al., 2010). The GM and range [GM (range)] of allergen values from our study for Fel d1 [106ng m–2 (10–1372ng m–2)] and Can f1 [488ng m–2 (38–30674ng m–2)] were comparable to Fel d1 in the examination room [158ng m–2 (123–246ng m–2)] and Can f1 in the office [668ng m–2 (228–1781ng m–2)] in the companion hospital. However, the results from that study may not be generalized, since one companion hospital was sampled. In addition, the ranges for our study were broader and this variability may be attributable to multiple homes sampled, the difference in home hygiene practices or the number of pets in a farm home when compared to a companion hospital whose hygiene practices should be standardized for better hygiene. In our study, pet keeping was related to the presence of allergens in the home. Cat allergen was lower than expected at 28 days of sampling, suggesting a decreased EDC capture efficiency for cat allergen after 14 days. However, a similar reduction for dog allergen was less pronounced. Thus, presence and proximity of the animal to the EDCs and reduction of sampling efficiency after Day 14 were presumed to impact allergen concentrations.
CONCLUSION
The advantage of this study was systematically investigating EDC sampling duration in combination with endotoxin and allergen concentrations. This study confirmed that one EDC can be used for multiple analyses including protein, endotoxin, and allergens allowing more flexibility in study design because samples collected side-by-side within the same EDC agree well. Establishing a sampling period was particularly important in order to ensure that EDCs have sufficient bioaerosol loading but are not oversampled such that the capture efficiency is diminished. Results of this study would support the use of an EDC sampling period of 14 days in homes for endotoxin loading.
FUNDING
University of Iowa, Environmental Health Sciences Research Center (NIH P30 ES005605).
ACKNOWLEDGEMENTS
We acknowledge Sarah S. Perry for her assistance with the statistical analyses. The authors thank the occupants of the 15 farm homes for their cooperation and hospitality.
REFERENCES
- Bland JM, Altman DG. (2010). Statistical methods for assessing agreement between two methods of clinical measurement. Int J Nurs Stud; 47(8): 931–6. [PubMed] [Google Scholar]
- Brigham KL, Meyrick B. (1986). Endotoxins and lung injury. Am Rev Respir Dis; 133: 913–27. [PubMed] [Google Scholar]
- Douwes J, Thorne P, Pearce N, et al. (2003). Bioaerosol health effects and exposure assessment: progress and prospects. Ann Occup Hyg; 47(3): 187–200. [DOI] [PubMed] [Google Scholar]
- Frankel M, Timm M, Hansen EW, et al. (2012). Comparison of sampling methods for assessment of indoor microbial exposure. Indoor Air; 22: 405–14. [DOI] [PubMed] [Google Scholar]
- Hadina S, Weiss JP, McCray PB, Jr, et al. (2008). MD-2-dependent pulmonary immune responses to inhaled lipooligosaccharides: effect of acylation state. Am J Respir Cell Mol Biol; 38(6): 647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppe KA, Metwali N, Perry SS, et al. (2012). Assessment of airborne exposures and health in flooded homes undergoing renovation. Indoor Air; 22(6): 446–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob B, Ritz B, Gehring U, et al. (2002). Indoor exposure to molds and allergic sensitization. Environ Health Perspect; 110(7): 647–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JH, Krop EJM, de Wind S, et al. (2013a) Endotoxin levels in homes and classrooms of Dutch school children and respiratory health. Euro Respir J; 42(2): 314–22. [DOI] [PubMed] [Google Scholar]
- Jacobs JH, Krop EJM, Borras-Santos A, et al. (2013b) Endotoxin levels in settled airborne dust in European schools: the HITEA school study. Indoor Air; 24(2): 148–57. [DOI] [PubMed] [Google Scholar]
- Krop EJM, Jacobs JH, Sander I, et al. (2014). Allergens and β-glucans in Dutch homes and schools: characterizing airborne levels. PloS One; 9(2): e88871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebers V, van Kampen V, Bünger J, et al. (2012). Assessment of airborne exposure to endotoxin and pyrogenic active dust using electrostatic dustfall collectors (EDCs). J Toxicol Environ Health; 75(8–10): 501–7. [DOI] [PubMed] [Google Scholar]
- Madsen AM, Matthiesen CB, Frederiksen MW, et al. (2012). Sampling, extraction and measurement of bacteria, endotoxin, fungi and inflammatory potential of settling indoor dust. J Environ Monit; 14: 3230–9. [DOI] [PubMed] [Google Scholar]
- Noss I, Doekes G, Sander I, et al. (2010). Passive airborne dust sampling with the electrostatic dustfall collector: optimization of storage and extraction procedures for endotoxin and glucan measurement. Ann Occup Hyp; 54(6): 651–8. [DOI] [PubMed] [Google Scholar]
- Noss I, Wouters IM, Visser M, et al. (2008). Evaluation of a low-cost electrostatic dust fall collector for indoor air endotoxin exposure assessment. Appl Environ Microbiol; 74(18): 5621–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JH, Spiegelman DL, Gold DR, et al. (2001). Predictors of airborne endotoxin in the home. Environ Health Perspect; 109(8): 859–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salo PM, Arbes SJ, Jr, Crockett PW, et al. (2008). Exposure to multiple indoor allergens in US homes and its relationship to asthma. J Allergy Clin Immunol; 121(3): 678–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samadi S, Heederik D JJ, Krop EJM, et al. (2010). Allergen and endotoxin exposure in a companion animal hospital. Occup Environ Med; 67(7): 486–92. [DOI] [PubMed] [Google Scholar]
- Sigsgaard T, Hoffmann HJ, Thorne PS. (2008). The role of innate immunity in occupational allergy: recent findings. Curr Opinion in Allergy & Clin Immunol; 8(2): 120–5. [DOI] [PubMed] [Google Scholar]
- Spaan S, Doekes G, Heederik D, et al. (2008). Effect of extraction and assay media on analysis of airborne endotoxin. Appl Environ Microbiol; 74(12): 3804–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporik R, Squillace SP, Ingram JM, et al. (1999). Mite, cat, and cockroach exposure, allergen sensitisation, and asthma in children: a case-control study of three schools. Thorax; 54(8): 675–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne PS. (2000). Inhalation toxicology models of endotoxin- and bioaerosol-induced inflammation. Toxicology; 152(1–3): 13–23. [DOI] [PubMed] [Google Scholar]
- Thorne PS, Cohn RD, Deepak M, et al. (2009). Predictors of endotoxin levels in U.S. housing. Environ Health Perspect; 117(5): 763–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne PS, Kulhankova K, Yin M, et al. (2005a) Endotoxin exposure is a risk factor for asthma: the National Survey of Endotoxin in United States housing, Am J Respir Crit Care Med; 172(11): 1371–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne PS, Metwali N, Avol E, et al. (2005b) Surface sampling for endotoxin assessment using electrostatic wiping cloths. Ann Occup Hyg; 49(5): 401–6. [DOI] [PubMed] [Google Scholar]
- Thorn J, Rylander R. (1998). Inflammatory response after inhalation of bacterial endotoxin assessed by the induced sputum technique. Thorax; 53(12): 1047–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovey ER, Chapman MD, Platts-Mills TAE. (1981). Mite faeces are a major source of house dust allergens. Nature; 289: 592–3. [DOI] [PubMed] [Google Scholar]
- Von Essen S, Robbins RA, Thompson AB, et al. (1990). Organic dust toxic syndrome: an acute febrile reaction to organic dust exposure distinct from hypersensitivity pneumonitis. Clin Toxicol; 28(4): 389–420. [DOI] [PubMed] [Google Scholar]
- Vredegoor DW, Willemse T, Chapman MD, et al. (2012). Can f 1 levels in hair and homes of different dog breeds: lack of evidence to describe any dog breed as hypoallergenic. J Allergy Clin Immunol; 130(4): 904–9. [DOI] [PubMed] [Google Scholar]
- Würtz H, Sigsgaard T, Valbjorn O, et al. (2005). The dustfall collector—a simple passive tool for long-term collection of airborne dust: a project under the Danish Mould in Buildings Program (DAMIB). Indoor Air; 15(Suppl. 9): 33–40. [DOI] [PubMed] [Google Scholar]
- Zahradnik E, Sander I, Kendzia B, et al. (2011). Passive airborne dust sampling to assess mite antigen exposure in farming environments. J Environ Monit; 13: 2638–44. [DOI] [PubMed] [Google Scholar]