Abstract
Human immunodeficiency virus (HIV)-exposed uninfected children (HEU) have an increased risk of morbidity and mortality compared with HIV-unexposed uninfected children (HUU); however, prior studies have not fully accounted for the role of both breastfeeding and age on this association. In this cohort of HEU and HUU in Uganda, non-breastfeeding HEU, from 6–11 months compared with non-breastfeeding HUU had a higher risk of hospitalizations [relative risk (RR): 10.1, 95% confidence interval (CI): 3.70–27.6], severe febrile illness (RR: 3.84, 95% CI: 2.06–7.17), severe diarrhea (RR: 6.37, 95% CI: 2.32–17.4) and severe malnutrition (RR: 18.4, 95% CI: 4.68–72.0). There were no differences between morbidity outcomes between breastfeeding HEU and HUU children, aged 6–11 months. In the 12–24 month age group, the only difference in morbidity outcomes among non-breast feeding children was an increased risk of severe malnutrition for HEU. These data suggest that the increased risk of morbidity among HEU aged 6–11 years is partially explained by early cessation of breastfeeding.
Keywords: Africa, HIV, HIV-exposed, Breastfeeding, Morbidity, Mortality
Introduction
Owing to the increased uptake of effective measures to prevent mother to child transmission of human immunodeficiency virus (HIV), there is a growing group of children born to HIV-infected mothers who remain uninfected with HIV. Studies in Sub-Saharan Africa have shown that these HIV-exposed uninfected children (HEU) have an increased risk of morbidity and mortality compared with HIV-unexposed uninfected children (HUU) [1–5]. The mechanisms underlying this relationship are thought to be mediated by direct immunologic effects, including impaired T cell immunity [6, 7] and decreased passively acquired humoral immunity [8, 9], and indirect factors such as increased parental mortality, exposure to maternal infections and poverty.
The effects of age and breastfeeding on the relationship between HIV-exposure and morbidity and mortality remain unclear. Few studies compare health outcomes of HEU and HUU children of more than 12 months of age. The majority of studies comparing HEU to HUU were completed under the previous World Health Organization (WHO) breastfeeding guidelines, which recommended early cessation for HIV-exposed children, as opposed to the current 2010 [10] recommendations which call for breastfeeding up to 12 months of age; this shift makes it difficult to determine the effect of longer periods of breastfeeding.
Despite limited data on the impact of breastfeeding on the risk of mortality of HEU compared with HUU, there is strong evidence of an independent association between early cessation of breastfeeding and mortality and morbidity in cohorts of HEU alone [4, 11–14]. Thus, as it has been established that breastfeeding has a protective effect on mortality in HUU [15] and, more recently, in cohorts of HEU [4, 12–14, 16], we hypothesized that early weaning may at least partially account for the excess risk of mortality and morbidity found among HEU in prior studies. Thus, we examined the relationships between breastfeeding, age, HIV-exposure and health outcomes in parallel cohorts of HEU and HUU infants in rural Uganda.
Methods
Study site and procedures
This study is a secondary analysis of data from the Prevention of Malaria and HIV disease (PROMOTE)-chemoprevention trial (Clinical Trial Registration: NCT00948896), a randomized controlled trial evaluating three regimens for the prevention of malaria among young children in Uganda. The trial was conducted in Tororo, a rural district characterized by subsistence farming and high rates of poverty and malaria transmission [17]. Convenience sampling was used to enroll 400 HIV-unexposed and 200 HIV-exposed children 4–5 months of age from the antenatal clinics in Tororo municipality, between June 2010 and July 2011. Inclusion criteria included living within 30 km of the study clinic with no intention of moving, confirmed HIV-status of the mother, agreement to present to the study clinic for fever. In addition, HEU were eligible if they were HIV-DNA polymerase chain reaction negative at the time of enrollment, and were breastfeeding. Exclusion criteria included having a chronic medical condition or active medical problems requiring in-patient evaluation at the time of screening.
At enrollment, all children were given an insecticide-treated bed net (ITN) and HEU were continued on or started on trimethoprim-sulfamethoxazole (TS) prophylaxis. HUU were randomized to malaria chemoprevention at 6 months of age and HEU were randomized after cessation of breastfeeding and when confirmed to be HIV-negative. Children were randomized 1:1:1:1 to either monthly sulfadoxine-pyrimethamine, daily TS, monthly dihydroartemisinin-piperaquine or no chemoprevention.
At the onset of the trial, HIV-infected mothers were counseled to stop breastfeeding at 6 months if there was a feasible and safe feeding alternative, per Uganda’s Ministry of Health (MOH) guidelines. Midway through the trial, MOH guidelines changed to reflect the 2010 WHO infant feeding guidelines [18], and HIV-infected mothers were counseled to breastfeed their children for 12 months. HIV-uninfected mothers were counseled to breastfeed for up to 2 years or beyond.
For the duration of the study, participants were seen in a dedicated study clinic for monthly routine visits and for any acute illness. Children were diagnosed and managed in accordance with standardized operating procedures and clinical case definitions based on the WHO’s Integrated Management of Childhood Illnesses [19]. Study clinicians also provided the primary clinical management of hospitalized children.
Statistical analysis
We restricted this secondary analysis to person-time accrued from 6 months of age until 24 months of age or earlier for those prematurely withdrawn from the study. Children who HIV sero-convertd were excluded. Children with sickle cell disease were also excluded because they had a disproportionately high rate of hospitalization and were unevenly distributed between HIV-exposed and unexposed groups.
Outcomes were (i) all-cause hospitalization; (ii) hospitalization not associated with malaria; (iii) severe febrile illness/pneumonia, a composite of sepsis syndrome (defined as fever and toxic appearance with or without hypotension, not due to malaria) and/or severe pneumonia (defined as pneumonia with severe respiratory distress); (iv) severe diarrhea (defined as bloody diarrhea or more than 7 stools per 24 hours, or requiring intravenous fluid replacement or hospitalization); (v) malaria (defined as a positive blood smear and fever); and (vi) malnutrition (defined as a hospitalization with a primary diagnosis of malnutrition or clinic visit with a recorded weight for height z-score of less than 3 standard deviations from the median WHO growth standards or a diagnosis of kwashiorkor or marasmus).
Exposure variables of interest included HIV-exposure and the following time-dependent variables: age, chemoprevention and breastfeeding status (any versus none). Breastfeeding status was assessed at each routine and acute visit, and the date of breastfeeding cessation was recorded as the last day a women ever breastfed her child.
We used the chi-squared test to compare proportions and the Wilcoxon rank-sum test to compare medians. Multivariate logistic regression models using generalized estimating equations with a log link, robust standard errors and adjustment for repeated measures in the same study participant were used to generate the daily relative risks of morbidity and mortality outcomes. Models examining associations between HIV-exposure status and both mortality and the risk of malaria were adjusted for a household wealth index at enrollment and the following time-dependent variables: age, malaria chemoprevention regimen and breastfeeding status. Given the strong association between HIV exposure status and age at breastfeeding cessation, models evaluating associations with morbidity outcomes used a composite measure of HIV-exposure and breastfeeding status, were stratified into two age groups (6–11 and 12–24 months), and adjusted for household wealth index, age and malaria chemoprevention regimen. The household wealth index was generated from number of household assets and categorized into lowest, middle and highest tertiles, as described previously [20]. Analyses were performed using STATA 12 (Stata Corporation, College Station, TX) and a p-value less than 0.05 was considered statistically significant.
Ethical considerations
This study was approved by the Makerere University School of Medicine Research and Ethics Committee, Uganda National Council for Science and Technology, and the University of California, San Francisco Committee on Human Research.
Results
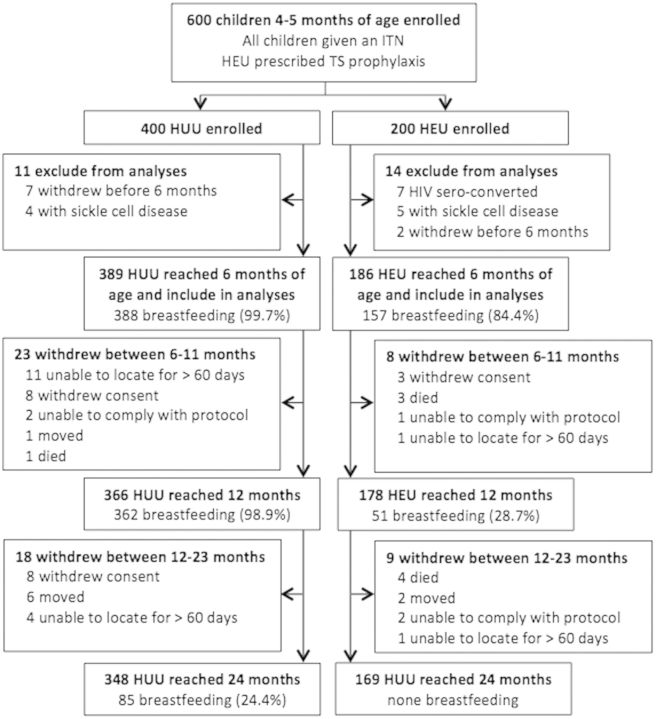
Of the 400 HUU enrolled, seven children withdrew before 6 months of age and 4 had sickle cell disease, leaving 389 children included in the analyses. A total of 348 HUU children remained in the study by 24 months, contributing 542 person-years (PY). Of the 200 HEU enrolled, two withdrew before 6 months, 7 HIV sero-converted after enrollment and five were excluded because of sickle cell disease, leaving 186 participants included in the analyses. A total of 159 HEU remained in the study by 24 months, contributing 169 PY (Fig. 1).
Fig. 1.
Study flow diagram. PY accrued from 6 to 24 months were included in this analysis. All PY from children with sickle cell disease or those who HIV sero-converted were excluded from the analysis. For children who withdrew, person-time was included up to study withdrawal date.
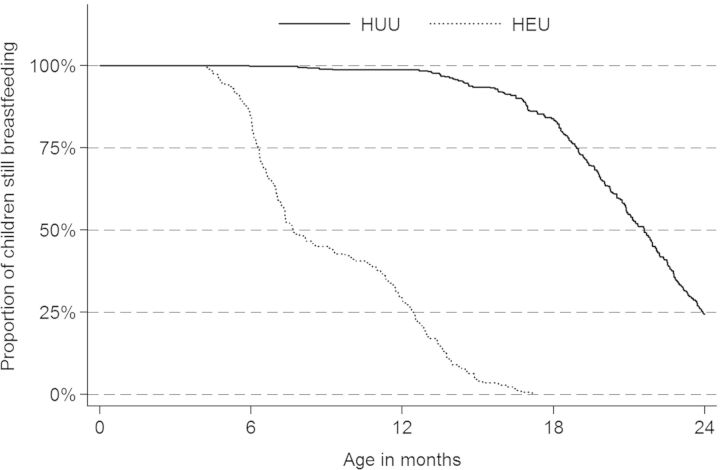
The proportion of children breastfeeding differed between HEU and HUU: at 6 months of age, 84.4 vs. 99.7%, (p < 0.0001); at 12 months of age, 28.7 vs. 98.9%, (p < 0.001); and at 24 months of age, 0 vs. 24.4% (p < 0.0001) (Fig. 2). At enrollment, we did not detect a difference in gender and household wealth index between HEU and HUU. HIV-infected mothers were older than HIV-uninfected mothers (median age was 24.5 vs. 30.0 years). A higher proportion of HIV-infected mothers reported their children had an ITN compared with HIV-uninfected mothers, but after enrollment all mothers and children were given an ITN and the reported use of ITNs among children increased to over 93%, and we did not detect a difference between HEU and HUU. At enrollment, 94.6% of HIV-infected mothers reported taking TS and 35.0% reported taking anti-retroviral therapy. The proportion of self-reported antiretroviral therapy (ART) use increased to 65.3% by the time the children reached 18 years of age (Table 1).
Fig. 2.
Proportion of children breastfeeding (exclusive and partial) from 6 to 24 months, stratified by HIV-exposure status.
Table 1.
Demographics of study participants at enrollment
| Characteristic | HUU children |
HEU children |
p-value |
|---|---|---|---|
|
N = 389 |
N = 186 |
||
| % or median (IQR) | % or median (IQR) | ||
| Female child | 48.9 | 50.8 | 0.68 |
| Father alive | 99 | 92 | <0.001 |
| Mother alive | 100 | 100 | |
| Maternal age—median (IQR) | 24.5 (20.0–30.0) | 30.0 (28.0–35.0) | <0.001 |
| Mother reports using TS prophylaxis | N/A | 93.6 | |
| Mother reports using ART | N/A | 35.3 | |
| Mother reports receiving perinatal ART | N/A | 78.1 | |
| Mother reports child had an ITN | 46.9 | 62.6 | <0.001 |
| Household Wealth Tertile | |||
| Low | 32.8 | 31 | 0.25 |
| Middle | 36.1 | 31 | |
| High | 31.2 | 38 | |
Mortality
There were seven deaths among HEU compared with one death among HUU. The median age at death was 12.7 months, interquartile range (IQR) 9.5–16.5. Of the eight deaths, six occurred in children who were not breastfeeding. In children who died and were not breastfeeding, cessation of breastfeeding occurred over 3 months before death (Table 2). HEU had a 13.7 [95% confidence interval (CI): 1.12–167.3, p = 0.04] higher risk of death compared with HUU, when adjusting for age, malaria chemoprevention regimen, breastfeeding and wealth index.
Table 2.
Characteristics of HIV-exposed and unexposed children who died
| Primary cause of death | HIV-exposure status | Age at death (months) | Age of cessation of breastfeeding (months) |
|---|---|---|---|
| Pneumonia | Unexposed | 8 | No cessation of breastfeeding |
| Malnutrition | Exposed | 9 | 6 |
| Diarrhea | Exposed | 10 | 6 |
| Diarrhea | Exposed | 10 | 6 |
| Unknown | Exposed | 12 | No cessation of breastfeeding |
| Diarrhea | Exposed | 16 | 8 |
| Diarrhea | Exposed | 17 | 6 |
| Burkitt's Lymphoma | Exposed | 17 | 10 |
Morbidity outcomes
Breastfeeding and age moderated the association between HIV-exposure and non-malarial morbidity (Tables 3 and 4). Among children 6–11 months who were breastfeeding, there were no significant differences in morbidity outcomes between HUU and HEU. Non-breastfeeding HUU were excluded from the multivariate analysis due to an insufficient amount of person-time (1.8 PY). Non-breastfeeding HEU had a significantly higher risk of all non-malarial morbidity outcomes compared with breastfeeding HUU: hospitalizations [relative risk (RR): 10.1, 95% CI: 3.70–27.6, p < 0.0001], non-malarial hospitalizations (RR: 19.6, 95% CI: 4.95–77.3, p < 0.0001), severe febrile illness (RR: 3.84, 95% CI: 2.06–7.17, p < 0.0001), severe diarrhea (RR: 6.37, 95% CI: 2.32–17.4, p < 0.0001) and severe malnutrition (RR: 18.4, 95% CI: 4.68–72.0, p < 0.0001) (Table 3).
Table 3.
Associations between HIV exposure and breastfeeding status with morbidity outcomes among infants 6–11 months of age
| Outcome | HUU breastfeedinga (189 PY of follow-up) |
HEU breastfeeding (43 PY of follow-up) |
HEU not breastfeeding (48 PY of follow-up) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events | Incidenceb | Events | Incidenceb | RR (95% CI)c | p | Events | Incidenceb | RR (95% CI)c | p | |
| All hospitalizations | 6 | 0.032 | 2 | 0.046 | 1.27 (0.22–7.39) | 0.79 | 17 | 0.354 | 10.1 (3.70–27.6) | <0.0001 |
| Non-malarial hospitalizations | 2 | 0.011 | 1 | 0.023 | 1.50 (0.14–16.4) | 0.74 | 12 | 0.250 | 19.6 (4.95–77.3) | <0.0001 |
| Severe febrile illnesses | 17 | 0.090 | 5 | 0.116 | 1.34 (0.44–4.07) | 0.61 | 13 | 0.271 | 3.84 (2.06–7.17) | <0.0001 |
| Severe diarrhea | 11 | 0.058 | 3 | 0.070 | 0.78 (0.17–3.58) | 0.75 | 22 | 0.458 | 6.37 (2.32–17.4) | <0.0001 |
| Severe malnutrition | 3 | 0.016 | 1 | 0.023 | 2.55 (0.18–35.5) | 0.49 | 15 | 0.312 | 18.4 (4.68–72.0) | <0.0001 |
HUYU, HIV-unexposed uninfected; HEU, HIV-exposed uninfected; PY, person-years; RR, relative risk; CI, confidence intervals.
aReference group.
bPer person-year.
cAdjusted for age, chemoprevention and wealth index.
Table 4.
Associations between HIV exposure and breastfeeding status with morbidity outcomes among infants 12–24 months of age
| Outcome | HUU not breastfeedinga (261 PY of follow-up) |
HEU not breastfeeding (166 PY of follow-up) |
HUU breastfeeding (92 PY of follow-up) |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events | Incidenceb | Events | Incidenceb | RR (95% CI)c | p | Events | Incidenceb | RR (95% CI)c | p | |
| All hospitalizations | 24 | 0.092 | 16 | 0.097 | 0.97 (0.54–2.43) | 0.94 | 11 | 0.119 | 1.14 (0.54–2.43) | 0.73 |
| Non-malarial hospitalizations | 14 | 0.053 | 6 | 0.036 | 0.70 (0.24–2.04) | 0.51 | 3 | 0.032 | 0.62 (0.16–2.46) | 0.50 |
| Severe febrile illnesses | 13 | 0.050 | 16 | 0.097 | 2.07 (0.93–4.64) | 0.08 | 2 | 0.022 | 0.53 (0.13–2.12) | 0.37 |
| Severe diarrhea | 8 | 0.031 | 9 | 0.054 | 2.21 (0.79–6.16) | 0.13 | 5 | 0.043 | 2.67 (0.64–12.8) | 0.17 |
| Severe malnutrition | 14 | 0.054 | 18 | 0.108 | 2.67 (1.01–7.05) | 0.05 | 4 | 0.043 | 2.36 (0.32–17.2) | 0.40 |
HUU, HIV-unexposed uninfected; HEU, HIV-exposed uninfected; PY, person-years; RR, relative risk; CI, confidence intervals.
aReference group.
bPer person-year.
cAdjusted for age, chemoprevention and wealth index.
Among non-breastfeeding children age 12–24 months, there was no detectable difference between the risk of hospitalizations, non-malarial hospitalizations or severe diarrhea between HUU and HEU. There was a trend toward an increased risk of severe febrile illness (RR: 2.07, 95% CI: 0.93–4.64, p = 0.08) and an increased risk of severe malnutrition (RR: 2.67, 95% 1.01–7.05, p = 0.05) among non-breastfeeding HEU compared with non-breastfeeding HUU (Table 4). Breastfeeding HEU were excluded from the multivariate analysis due to an insufficient amount of person-time (7.4 PY).
Malaria
The incidence of malaria from 6–24 months of age in both HUU and HEU was 4.5 per PY and 2.76 per PY, respectively, p < 0.0001. HEU had a 37% decreased risk of malaria compared with HUU (RR 0.63, 95% CI: 0.53–0.73, p < 0.0001), after adjusting for age, breastfeeding, household wealth index and chemoprevention. There were no interactions between breastfeeding and HIV-exposure in the models used to assess the risk of malaria.
Discussion
In this study, we compared the health outcomes of a cohort of HEU and HUU children and found a 13-fold higher risk of mortality and a higher risk of non-malarial morbidity among HEU children compared with HUU children. Cessation of breastfeeding and younger age moderate the association between non-malarial morbidity outcomes and HIV-exposure. In children 6–11 months of age, non-breastfeeding HEU had a 4–20-fold increase risk of non-malarial morbidity outcomes, compared with breastfeeding HUU. Our findings are in agreement with prior studies on cohorts of HEU, which have shown an association between lack of breastfeeding and poor health outcomes such as pneumonia [4, 12], hospitalization [4, 11, 14], malnutrition [14, 21–23], diarrhea [4] and death [4, 11]. However, this study is among the first to directly compare outcomes of HEU with HUU while controlling for differences in duration of breastfeeding between HEU and HUU. The profound difference in health outcomes between non-breastfeeding HEU and breastfeeding HUU, in addition to the lack of difference detected between breastfeeding HEU and HUU, supports prior findings that early cessation of breastfeeding is an independent predictor of morbidity among HEU and suggests that it at least partially accounts of the excess risk of non-malarial morbidity of HEU compared with HUU children between the ages of 6 and 11 months.
The association between HIV-exposure and the risk of non-malarial morbidity outcomes declined among children 12–24 months of age. Only the risk of severe malnutrition was significantly higher in non-breastfeeding HEU compared with both non-breastfeeding and breastfeeding HUU children. There are few studies that assess the duration of the risk of detailed morbidity outcomes between HEU and HUU children beyond 12 months. Our data suggest that there is no difference in the risk of infectious morbidity, aside from malaria and malnutrition, among HEU and HUU children from 12 to 24 months. We hypothesize that the waning association with age may be due to maturation of the immune system of HEU and less dependence on the passive immunity provided by breastfeeding to fight infections.
The persistent increased risk of malnutrition seen in our data has also been described in cohorts of HEU in Uganda followed for the first 5 years of life [22]. Additionally, breastfeeding has been found to mitigate the risk of severe malnutrition for up to 15 months in a trial among HEU in Zambia [23]. In the current study, the virtual absence of breastfeeding HEU from 12 to 24 months precluded analyses to isolate the effect of breastfeeding on the association of HIV-exposure and malnutrition. However, among non-breastfeeding children from ages 12 to 24 months, HEU had almost a 3-fold increase in severe-malnutrition compared with HUU. This difference may be due to the long-term sequelae of early weaning and increased incidence of severe malnutrition established before 1 year of age among HEU. Overall, the increased risk of severe-malnutrition in HEU compared with HUU from 6 to 24 months underscores the importance of extended breastfeeding, growth monitoring and early nutritional interventions for HEU.
An additional secondary finding of this study was a lower risk of malaria in HEU compared with HUU children, even when controlling for covariates associated with malaria incidence and chemoprevention. We hypothesize that in this study the difference in malaria incidence may be attributable to differences in adherence of chemoprevention in HEU. A total of 94% of HIV-infected women reported taking TS at enrollment, and they may be more likely to adhere to their child’s chemoprevention regimen, as they are accustomed to taking medications themselves and may perceive their child’s risk of malaria to be higher.
Our results should be interpreted within the limitations of the study design. This study likely underestimates the risk of poor health outcomes of both HEU and HUU in routine settings because children in our cohort had more access to medications and health care compared with the general population in rural Uganda. However, the controlled study environment is also a strength, as we had detailed data on breastfeeding, standardized definitions of health outcomes and potential confounders. Finally, as a secondary analysis, sample sizes were not powered to test the hypothesis that HIV-exposure increases the risk of morbidity and mortality. This may have limited our ability to detect small differences in morbidity between HEU and HUU in the 12–24 month age group. The low number of deaths in HUU limited our ability to stratify mortality by age and HIV-exposure and breastfeeding groups
In conclusion, we found an increased risk of non-malaria morbidity among HIV-exposed children from 6 to 11 months; however, aside from malnutrition we did not detect this relationship in children ages 12–24 months. Among children aged 6–11 months, some of the association between HIV-exposure and morbidity could be explained by earlier cessation of breastfeeding among HEU. Programmatic interventions that seek to increase uptake of the 2010 WHO Infant Feeding Guidelines [17] and Option B+ [10], which recommend breastfeeding HEU for 12 months, should improve the health outcomes of the growing population of HEU children.
Funding
The study was funded by the National Institute of Child Health and Human Development (P01HD059454 and K236045901:TDR) and the National Institutional of Allergy and Infectious Diseases (A1060530 and A151982) at the National Institutes of Health. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Acknowledgements
We thank the study participants, parents and guardians of study participants and study staff for their support and participation in the study.
References
- 1.Filteau S. The HIV-exposed, uninfected African child. Trop Med Int Health. 2009;14:276–87. doi: 10.1111/j.1365-3156.2009.02220.x. [DOI] [PubMed] [Google Scholar]
- 2.Nakiyingi JS, Bracher M, Whitworth JA, et al. Child survival in relation to mother’s HIV infection and survival: evidence from a Ugandan cohort study. AIDS. 2003;17:1827–34. doi: 10.1097/00002030-200308150-00012. [DOI] [PubMed] [Google Scholar]
- 3.Ng’weshemi J, Urassa M, Isingo R, et al. HIV impact on mother and child mortality in rural Tanzania. J Acquir Immune Defic Syndr. 2003;33:393–404. doi: 10.1097/00126334-200307010-00015. [DOI] [PubMed] [Google Scholar]
- 4.Shapiro RL, Lockman S, Kim S, et al. Infant morbidity, mortality, and breast milk immunologic profiles among breast-feeding HIV-infected and HIV-uninfected women in Botswana. J Infect Dis. 2007;196:562–9. doi: 10.1086/519847. [DOI] [PubMed] [Google Scholar]
- 5.Landes M, van Lettow M, Chan AK, et al. Mortality and health outcomes of HIV-exposed and unexposed children in a PMTCT cohort in Malawi. PloS One. 2012;7:e47337. doi: 10.1371/journal.pone.0047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clerici M, Saresella M, Colombo F, et al. T-lymphocyte maturation abnormalities in uninfected newborns and children with vertical exposure to HIV. Blood. 2000;96:3866–71. [PubMed] [Google Scholar]
- 7.Mazzola TN, da Silva MTN, Abramczuk BM, et al. Impaired bacillus calmette-guérin cellular immune response in HIV-exposed, uninfected infants. AIDS. 2011;25:2079–87. doi: 10.1097/QAD.0b013e32834bba0a. [DOI] [PubMed] [Google Scholar]
- 8.Farquhar C, Nduati R, Haigwood N, et al. High maternal HIV-1 viral load during pregnancy is associated with reduced placental transfer of measles IgG antibody. J Acquir Immune Defic Syndr. 2005;40:494–7. doi: 10.1097/01.qai.0000168179.68781.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones CE, Naidoo S, De Beer C, et al. Maternal HIV infection and antibody responses against vaccine-preventable diseases in uninfected infants. JAMA. 2011;305:576–84. doi: 10.1001/jama.2011.100. doi:10.1001/jama.2011.100. [DOI] [PubMed] [Google Scholar]
- 10.World Health Organization. Programmatic Update: Use of Antiretroviral Drugs for Treating Pregnant Women and Preventing HIV Infection in Infants. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 11.Homsy J, Moore D, Barasa A, et al. Breastfeeding, mother-to-child hiv transmission, and mortality among infants born to hiv-infected women on highly active antiretroviral therapy in rural uganda. J Acquir Immune Defic Syndr. 2010;53:28–35. doi: 10.1097/QAI.0b013e3181bdf65a. [DOI] [PubMed] [Google Scholar]
- 12.Asbjörnsdóttir KH, Slyker JA, Weiss NS, et al. Breastfeeding is associated with decreased pneumonia incidence among HIV-exposed, uninfected Kenyan infants. AIDS. 2013;27:2809–15. doi: 10.1097/01.aids.0000432540.59786.6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kourtis AP, Wiener J, Kayira D, et al. Health outcomes of HIV-exposed uninfected African infants. AIDS. 2013;27:749–59. doi: 10.1097/QAD.0b013e32835ca29f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taha TE, Hoover DR, Chen S, et al. Effects of cessation of breastfeeding in HIV-1–exposed, uninfected children in Malawi. Clin Infect Dis. 2011;53:388–95. doi: 10.1093/cid/cir413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Effect of breastfeeding on infant and child mortality due to infectious diseases in less developed countries: a pooled analysis. WHO Collaborative Study Team on the Role of Breastfeeding on the Prevention of Infant Mortality. Lancet. 2000;355:451–5. [PubMed] [Google Scholar]
- 16.Kuhn L, Sinkala M, Semrau K, et al. Elevations in mortality associated with weaning persist into the second year of life among uninfected children born to HIV-infected mothers. Clin Infect Dis. 2010;50:437–44. doi: 10.1086/649886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okello PE, Van Bortel W, Byaruhanga AM, et al. Variation in malaria transmission intensity in seven sites throughout Uganda. Am J Trop Med Hyg. 2006;75:219–25. [PubMed] [Google Scholar]
- 18. WHO | Guidelines on HIV and infant feeding 2010. WHO. http://www.who.int/maternal_child_adolescent/documents/9789241599535/en/. (9 December 2013, date last accessed)
- 19. WHO | Recommendations for management of common childhood conditions. WHO. http://www.who.int/maternal_child_adolescent/documents/management_childhood_conditions/en/index.html. (18 February 2014, date last accessed)
- 20.Osterbauer B, Kapisi J, Bigira V, et al. Factors associated with malaria parasitaemia, malnutrition, and anaemia among HIV-exposed and unexposed Ugandan infants: a cross-sectional survey. Malar J. 2012;11:432. doi: 10.1186/1475-2875-11-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taha TE, Dadabhai SS, Rahman MH, et al. Trends in birth weight and gestational age for infants born to HIV-infected, antiretroviral treatment-naive women in Malawi. Pediatr Infect Dis J. 2012;31:481–6. doi: 10.1097/INF.0b013e31824d9bd9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Owor M, Mwatha A, Donnell D, et al. Long-term follow-up of children in the HIVNET 012 perinatal HIV prevention trial: five-year growth and survival. J Acquir Immune Defic Syndr. 2013;64:464–71. doi: 10.1097/QAI.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arpadi S, Fawzy A, Aldrovandi GM, et al. Growth faltering due to breastfeeding cessation in uninfected children born to HIV-infected mothers in Zambia. Am J Clin Nutr. 2009;90:344–53. doi: 10.3945/ajcn.2009.27745. [DOI] [PMC free article] [PubMed] [Google Scholar]