CNS HIVAnti-Retroviral Therapy Effects Research examined incidence and predictors of neurocognitive (NC) change in 436 human immunodeficiency virus (HIV)-infected adults over 4–7 semiannual visits; 22.7% evidenced NC decline and 16.5% NC improvement. These changes were predicted by HIV disease and treatment factors, demographics, and comorbid conditions.
Keywords: cognitive change, HIV, antiretroviral therapy, comorbidities
Abstract
Background. Human immunodeficiency virus (HIV)-associated neurocognitive disorders (HAND) can show variable clinical trajectories. Previous longitudinal studies of HAND typically have been brief, did not use adequate normative standards, or were conducted in the context of a clinical trial, thereby limiting our understanding of incident neurocognitive (NC) decline and recovery.
Methods. We investigated the incidence and predictors of NC change over 16–72 (mean, 35) months in 436 HIV-infected participants in the CNS HIV Anti-Retroviral Therapy Effects Research cohort. Comprehensive laboratory, neuromedical, and NC assessments were obtained every 6 months. Published, regression-based norms for NC change were used to generate overall change status (decline vs stable vs improved) at each study visit. Survival analysis was used to examine the predictors of time to NC change.
Results. Ninety-nine participants (22.7%) declined, 265 (60.8%) remained stable, and 72 (16.5%) improved. In multivariable analyses, predictors of NC improvements or declines included time-dependent treatment status and indicators of disease severity (current hematocrit, albumin, total protein, aspartate aminotransferase), and baseline demographics and estimated premorbid intelligence quotient, non-HIV-related comorbidities, current depressive symptoms, and lifetime psychiatric diagnoses (overall model P < .0001).
Conclusions. NC change is common in HIV infection and appears to be driven by a complex set of risk factors involving HIV disease, its treatment, and comorbid conditions.
(See the Editorial Commentary by Cysique on pages 481–2.)
Availability of combination antiretroviral therapy (cART) has substantially improved medical morbidity and life expectancy in human immunodeficiency virus (HIV)-infected (HIV+) individuals. Nevertheless, HIV-associated neurocognitive disorders (HAND) remain common [1–3]. Although the prevalence of the most severe form of HAND, HIV-associated dementia, has declined since the introduction of cART, milder forms of HAND persist and may be more prevalent in “earlier” disease stages that are maintained much longer than in the pre-cART era [2, 4, 5]. Recent reviews suggest modest neurocognitive (NC) improvement in HIV+ groups after beginning cART [6–8], but little is known about the incidence and predictors of NC change over time.
So far only 1 study has used formal NC “norms for change” to classify significant NC improvement or decline in individual participants [9]. One hundred ninety-two HIV+ and 101 HIV-uninfected (HIV–) Chinese former plasma donors were followed for 1 year, and results of the HIV– group were used to develop regression-based norms for change that adjusted for known factors that may affect follow-up results of medically stable people (eg, baseline level of performance, normal variability, and practice effects). Twenty-seven percent of the HIV+ group evidenced NC decline over 1 year; this change was predicted by baseline AIDS and lower CD4, and at follow-up was associated with lack of viral suppression on cART and lower current CD4.
Here we present the longitudinal CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) findings, including both baseline and time-dependent predictors of NC change. We used recently published regression-based norms for NC change, developed with a large US sample that was administered the CHARTER test battery over multiple visits [10].
METHODS
Subjects
Four hundred thirty-six CHARTER participants who underwent 4–7 study visits (16–72 months of follow-up; mean, 35 months) were identified from the longitudinal cohort (n = 699). This group had a total of 2680 visits, and its demographic, HIV disease, and treatment characteristics at baseline are summarized in Table 1.
Table 1.
Baseline Demographic, HIV Disease, and Treatment Characteristics of CHARTER Sample With 4–7 Study Visits (n = 436)
| Characteristic | Mean (SD), Median (IQR), or % |
|---|---|
| Age, y, mean (SD) | 43.9 (8.4) |
| Education, y, mean (SD) | 12.9 (2.5) |
| Sex, male | 80% |
| Race/ethnicity | |
| Non-Hispanic white | 43% |
| Non-Hispanic black | 44% |
| Hispanic | 11% |
| Other | 2% |
| Comorbidity status | |
| Incidental | 59% |
| Contributing | 29% |
| Confounding | 12% |
| AIDS | 60% |
| Nadir CD4 count, cells/µL, median (IQR) | 184 (49–320) |
| Current CD4 count, cells/µL, median (IQR) | 459 (289–644) |
| Currently on cART | 70% |
| Duration current regimen, mo, mean (SD) | 18.0 (21.2) |
| Prior cART only | 12% |
| ART naive | 18% |
| Undetectable HIV in plasma (n = 436) | 41% (58% if on ART) |
| Undetectable HIV in CSF (n = 395) | 66% (85% if on ART) |
| Neurocognitive impairment | 46% |
Abbreviations: ART, antiretroviral therapy; cART, combination antiretroviral therapy; CHARTER, CNS HIV Anti-Retroviral Therapy Effects Research; CNS, central nervous system; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; IQR, interquartile range; SD, standard deviation.
Procedures
At all study visits, subjects completed a venipuncture, neuromedical assessment, comprehensive NC testing, detailed substance use history, a fully structured psychiatric interview for lifetime and current (30-day) diagnoses of major depression and alcohol and other psychoactive substance use disorders, and a measure of mood symptoms in the previous 14 days. At visits where the participant consented (n = 2408), lumbar puncture was performed. Details of the CHARTER assessments, including assessment of comorbidities, are provided in prior publications [1, 2]. All procedures were approved by the human subjects protection committees of each participating institution. Written informed consent was obtained from all study participants.
Determination of Overall NC Change
To determine NC change, we generated a z score for each of 15 neuropsychological variables based on published normative data [10]. These z scores reflect how well or poorly the person performed at follow-up, relative to normal expectation for someone with the same baseline NC and other relevant characteristics (eg, age, education). The z scores were then averaged to provide a summary regression change score (sRCS). The top 5% of the sRCS distribution of the normative sample defined the “improve” range and the bottom 5% defined the “decline” range [10]. The remaining middle 90% was defined as “stable.” NC change status from baseline was generated for each follow-up visit. The individual visit change status for each participant was then merged into an overall change status: (1) decline: if a participant had at least 1 “decline” status and no “improve” status across visits; (2) improve: if a participant had at least 1 “improve” status and no “decline” status; (3) stable: if a participant had no “decline” or “improve” NC change status (all visits “stable”). Two participants out of an original cohort of 438 met criteria for both “improve” and “decline” during the follow-up period (at different visits) and were thus excluded from analyses.
The published normative standards for detecting NC change were derived from 172 HIV– controls and 124 HIV+ individuals who were selected based on strict criteria for clinical stability [10]. This provided a broader range of baseline NC performance in the total normative sample. The sRCS results were virtually identical for the HIV– and stable HIV+ subgroups (means across visits in z score units were 0.00 [standard deviation (SD), 0.32] for HIV+ and 0.01 [SD, 0.34] for HIV−; P = .93).
Statistical Methods
Participants classified by overall change status were compared on baseline characteristics using analysis of variance and Tukey honestly significant difference tests or χ2 tests. No other adjustments for multiple testing were applied. Initially, potential time-varying and static predictors of the first time when any NC change occurred were screened individually using Cox proportional hazards modeling. Variables univariably significant at a liberal 10% level were then combined into a Cox time-dependent multivariable model, and backward elimination with a minimal Akaike information criterion (AIC) was used to reduce the model. Because the resultant model was based only on visits having nonmissing data for all variables in the initial full model, the AIC-based modeling was repeated on reduced models, thus allowing inclusion of more visits. The procedure continued until no further variable reduction occurred. This method identifies a concise combination of predictors based on maximal available data and after eliminating variables that fail to contribute to a better fit if included in the model. The procedure includes variables contributing important information without regard to P values.
RESULTS
Over the total follow-up period, 99 participants (22.7%) were defined as NC “decliners,” 72 (16.5%) were “improvers,” and 265 (60.8%) were “stable” (Supplementary Digital Data Figure 1).
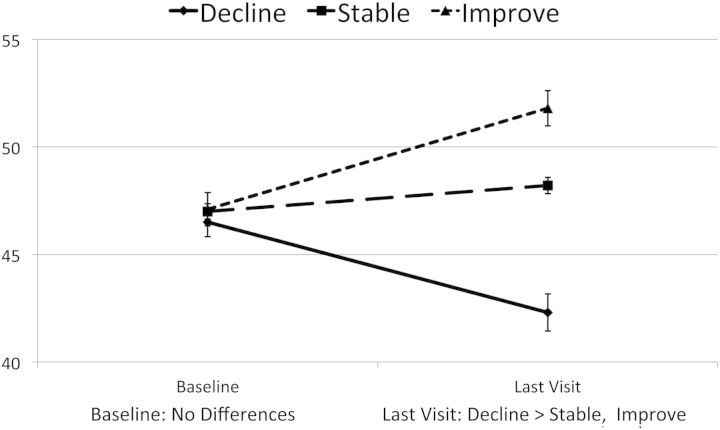
Decliners had a mean sRCS (z score units) of −0.52 compared with −0.01 for stable participants and 0.42 for improvers (P < .001); these mean z score differences approximate medium effect sizes. At the last visit, 61% of decliners were NC impaired, compared with 37% of the stable participants and 24% of the improvers (decline > stable > improve; P < .0001). Of the 99 decliners, 66 (66.7%) met criteria for symptomatic NC impairment (impairment with documented functional decline [1]) during their follow-up. This contrasts with 37.5% with symptomatic impairment for the improvers and 40.4% for stable participants (χ2df = 2 = 22.5, P < .0001). Figure 1 shows the 3 groups' mean changes in average, demographically corrected NC standard scores (T scores) from baseline to last visit. This demonstrates that, even though the visit where a change occurred usually was well before visit 7 (62% were before visit 4), the groups evidence very different trajectories across the entire follow-up period: Although NC performance was quite comparable at baseline, the 3 groups were very different at the final visit (F = 42.98; P < .0001). Importantly, however, although individuals within the total CHARTER group showed variable NC trajectories, the overall prevalence of HAND did not increase: 45.9% had NC impairment at baseline and 40.1% were impaired at their final visit.
Figure 1.
Mean neurocognitive T score at baseline and last follow-up for 3 change groups.
Baseline Predictors of NC Change
Although decliners, improvers, and stable participants did not differ on most baseline demographics, disease, or treatment variables, there were a few differences (Table 2). Decliners were more likely than improvers to be female, Hispanic, and to have detectable virus in plasma and cerebrospinal fluid (CSF) (overall, and if on antiretroviral therapy [ART]); in all of these respects, the stable group was intermediate between decliners and improvers. Although decliners tended to have more severe non-HIV comorbidities than both other groups, the comparison was statistically significant only vs the stable group for confounding conditions.
Table 2.
Comparison of Neurocognitive Change Groups on Baseline Demographic, Treatment, and Disease Characteristics
| Characteristic | Decline | Stable | Improve | Group Differencesa P < .05 |
|---|---|---|---|---|
| No. of visits, mean (SD) | 6.2 (1.1) | 6.1 (1.2) | 6.2 (1.2) | |
| Age, y, mean (SD) | 42.7 (8.4) | 44.2 (8.7) | 43.8 (7.5) | |
| Education, mean (SD) | 12.5 (2.6) | 12.9 (2.4) | 13.3 (2.4) | |
| Male sex | 72% | 81% | 86% | D < I |
| Race/ethnicity | ||||
| Non-Hispanic white | 36% | 44% | 50% | |
| Non-Hispanic black | 39% | 46% | 44% | |
| Hispanic | 20% | 8% | 6% | D > I, S |
| Other | 5% | 2% | 0% | |
| WRAT-3 Reading subtest, mean (SD) | 92.8 (15.2) | 93.8 (14.8) | 96.6 (15.0) | |
| Comorbidity | ||||
| Incidental | 48% | 65% | 58% | |
| Contributing | 32% | 27% | 31% | |
| Confounding | 20% | 8% | 11% | D > S |
| NC impaired | 51% | 45% | 42% | |
| AIDS | 55% | 62% | 61% | |
| Nadir CD4, median (IQR) | 206 (60–354) | 180 (41–327) | 171 (26–303) | |
| Current CD4, median (IQR) | 432 (264–617) | 469 (295–659) | 432 (245–588) | |
| ART | 68% | 70% | 72% | |
| CPE scoreb | 7.8 (2.2) | 7.6 (1.9) | 7.8 (2.0) | |
| Detectable plasma | 66% | 59% | 50% | I < D |
| Detectable on ART | 50% | 44% | 31% | I < D |
| Detectable CSF | 47% | 40% | 28% | I < D, S |
| Detectable on ART | 30% | 24% | 8% | I < D, S |
| PI-based regimen | 60% | 56% | 46% | |
| HCV infected | 31% | 24% | 25% | |
Abbreviations: ART, antiretroviral therapy; CPE, central nervous system penetration effectiveness; CSF, cerebrospinal fluid; HCV, hepatitis C virus; IQR, interquartile range; NC, neurocognitive; PI, protease inhibitor; SD, standard deviation; WRAT-3, Wide Range Achievement Test, 3rd ed.
a D, decliner; I, improver; S, stable.
b Letendre et al [11].
Stability of Viral Suppression and NC Change
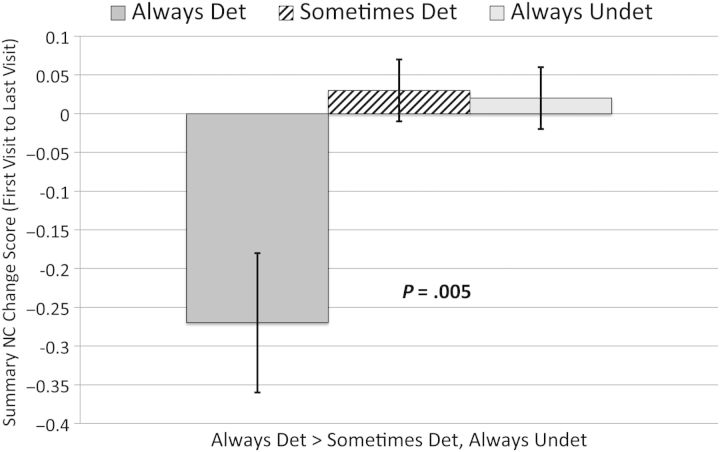
We classified participants as being “always undetectable” (plasma HIV RNA at all visits ≤50 copies/mL), “always detectable” (plasma HIV RNA at all visits >50 copies/mL), and “sometimes detectable” (plasma HIV RNA at least 1 visit ≤50 copies/mL, and at least 1 visit >50 copies/mL). We then compared these groups on their average sRCS across all visits. The “always detectable” group had a lower average sRCS score than both the “always undetectable” and “sometimes detectable” groups (−0.26 vs −0.06 and 0.003, respectively; P = .002), indicating greater NC decline in the “always detectable” group compared with the other groups (see Figure 2).
Figure 2.
Plasma viral load over time vs average neurocognitive (NC) change score. Abbreviations: Det, detectable; Undet, undetectable.
Predictors of Earlier NC Decline or Improvement
Univariable survival analyses using a combination of both time-invariant and time-dependent predictors were run to explore the effects of these predictors on incident NC change. “Time dependent” in this context means that the values of these variables are subject to change from visit to visit. Potential predictors univariably screened for subsequent multivariable analysis included demographic, disease, treatment, laboratory, and psychiatric variables. Table 3 displays the predictors identified as candidates for multivariable modeling (P < .10).
Table 3.
Univariable Predictors of Time to Neurocognitive Change (Decline or Improvement)
| Predictor | Decline |
Improvement |
||||||
|---|---|---|---|---|---|---|---|---|
| Risk | Reference | RR | P Value | Risk | Reference | RR | P Value* | |
| Age | Younger | 1 ya | 1.02 | .0937 | ||||
| Sex | Female | Male | 1.76 | .0153 | ||||
| Ethnicity | Hispanic | Non-Hispanic | 2.35 | .0018 | ||||
| Education | Higher | 1 yb | 1.10 | .0534 | ||||
| Premorbid IQc | Higher | 1 unitb | 1.02 | .0473 | ||||
| ART statusd | Off ART | On ART | 1.91 | .0038 | ||||
| CD4d | Lower | 100 cellsa | 1.14 | .0024 | ||||
| Nadir CD4 | Higher | 100 cellsb | 1.09 | .0833 | ||||
| Plasma VLd | Higher | 1 log10b | 1.26 | .0026 | Lower | 1 log10a | 1.27 | .0295 |
| Det/Undetd | Undet | Det | 1.53 | .0876 | ||||
| CSF VLd | Higher | 1 log10b | 1.26 | .0552 | Lower | 1 log10a | 1.47 | .0476 |
| Det/Undetd | Det | Undet | 1.50 | .0790 | Undet | Det | 1.73 | .0952 |
| ASTd | Lower | 1 unita | 1.01 | .0172 | ||||
| Protein totald | Lower | 1 unita | 1.96 | <.0001 | ||||
| Albumind | Lower | 1 unita | 2.36 | <.0001 | ||||
| HDLd | Lower | 1 unita | 1.01 | .0367 | ||||
| HCTd | Lower | 1 unita | 1.10 | <.0001 | Higher | 1 unitb | 1.06 | .0244 |
| Comorbiditye | Severe | Minimal | 2.47 | .0007 | ||||
| Utoxd | Positive | Negative | 1.58 | .0497 | ||||
| LT cannabis Dxd | No | Yes | 1.58 | .0863 | ||||
| LT methamphetamine Dxd | Yes | No | 1.81 | .0148 | ||||
| LT any substance Dxd | No | Yes | 1.63 | .0576 | ||||
| MDD (last 30 d)d | Yes | No | 1.68 | .0659 | ||||
| LT MDDd | Yes | No | 1.71 | .0118 | No | Yes | 1.63 | .0396 |
| Beck (total)d | Higher | 1 unitb | 1.03 | .0051 | ||||
Abbreviations: ART, antiretroviral therapy; AST, aspartate aminotransferase; Beck, Beck Depression Inventory II; CSF, cerebrospinal fluid; Det, detectable viral load; Dx, history of abuse or dependence diagnosis; HCT, hematocrit; HDL, high-density lipoprotein; IQ, intelligence quotient; LT, lifetime; MDD, major depressive disorder; RR, relative risk; SD, standard deviation; Undet, undetectable viral load; Utox, urine toxicology for drugs with central nervous system effects; VL, viral load.
a Higher/older.
b Lower.
c Measured using Wide Range Achievement Test, 3rd ed, Reading Standard Score (population mean = 100, SD = 15).
d Variable modeled in a time-dependent manner.
*P < .10 was considered significant.
Candidate predictors for multivariable modeling of either decline or improvement included time-dependent HIV treatment status (on/off ART) and disease severity indicators (degree of immunosuppression, HIV RNA load in plasma and CSF, serum total protein, albumin, hematocrit, high-density lipoprotein [HDL], and aspartate aminotransferase [AST]), all in the “expected” direction of worse medical management/status being associated with decline, and better management/status with improvement. In addition, overall severity of non-HIV comorbidities [2] and some specific (substance and mood related) psychiatric diagnoses as well as higher depressive symptoms were associated with earlier NC decline, whereas absence of lifetime major depression and substance use diagnoses were predictors of earlier improvement. Finally, younger age, female sex, and Hispanic ethnicity increased risk for decline, whereas higher education level and premorbid intelligence quotient (IQ) estimate (reading level) were associated with improvement.
In the final multivariable model, Hispanic ethnicity (vs non-Hispanic: relative risk [RR], 2.16 [95% confidence interval {CI}, 1.29–3.61]); confounded comorbidity status (vs incidental: RR, 2.12 [95% CI, 1.22–3.67]); being off ART (vs on ART: RR, 1.94 [95% CI, 1.26–3.00]); having low albumin (vs 1 unit higher: RR, 1.58 [95% CI, .99–2.52]) and low hematocrit (vs 1 unit higher: RR, 1.08 [95% CI, 1.03–1.13]); and having a lifetime methamphetamine use diagnosis (vs none: RR, 1.87 [95% CI, 1.16–3.02]) and more depressive symptoms (vs 1 unit lower: RR, 1.02 [95% CI, 1.00–1.04]) were associated with earlier time to NC decline (model P < .0001). The multivariable combination of predictors of improvement included higher premorbid IQ estimate (vs 1 unit less: RR, 1.02 [95% CI, 1.00–1.04]); lower total protein (vs 1 unit higher: RR, 1.85 [95% CI, 1.30–2.63]); lower AST (vs 1 unit higher: RR, 1.01 [95% CI, 1.00–1.03]); and no lifetime major depressive disorder (vs positive history: RR, 2.09 [95% CI, 1.27–3.45]) (model P < .0001).
DISCUSSION
Whereas previous cross-sectional and longitudinal research assessed clinical correlates of NC impairment in HIV-infected individuals (ie, HAND), our focus was on the incidence, nature, and predictors of NC change. Importantly, we employed recently published, regression-based norms that allowed us to detect significant NC change in individual participants, while controlling for normal test–retest variability, practice effects, and statistical artifacts. We found that almost 40% of subjects showed NC change, with 23% declining and 17% improving. The clinical significance of “decliner” status, in particular, is supported by significantly higher rates of symptomatic NC impairment in this group compared with those who were stable or improved.
We identified many univariable candidate predictors of NC decline or improvement when time-dependent clinical and laboratory findings were considered. These included several predictors specific to HIV and its treatment (ART status, immunosuppression, and plasma and CSF HIV RNA load), as well as others reflecting more general health status (AST, serum protein, albumin, HDL, and hematocrit). The multivariable model predicting time to decline reflected significant combined effects of 5 time-dependent variables (being off ART and having a lower hematocrit, lower albumin, a lifetime methamphetamine use diagnosis, and more depressive symptoms) and 2 static predictors (more significant non-HIV risks for NC impairment and Hispanic ethnicity). By contrast, a significant combination of multivariable predictors of NC improvement included 3 time-dependent variables (lower serum protein, lower AST, and absence of any lifetime history of major depressive disorder) and 1 static predictor (higher estimated premorbid IQ).
As an observational study, CHARTER is unable to demonstrate ART effects. Nevertheless, beneficial effects of cART on VL and immune function are well established [6–8], so the joint, time-dependent links of ART status and associated HIV biomarkers with both positive and negative NC outcomes are potentially important. Specifically, our findings suggest that being off ART uniquely increases risk for NC decline, and current virologic control and degree of immunocompetence were univariable predictors of both types of NC change. Also consistent with the CHARTER findings, in the 1 prior observational study that used regression-based NC norms for change to identify significant NC decline, such decline was associated with lower follow-up CD4 and lack of viral suppression in a large HIV+ Chinese sample [9].
Considered together, the current and previously published findings suggest that protection of the central nervous system (CNS) and favorable NC outcomes may be achieved by instituting cART early [12] and monitoring patients carefully to ensure maintenance of viral suppression and immunocompetence. In practice, however, these treatment goals often are not fully realized: Only 79 of the 436 (18.1%) longitudinal participants in CHARTER had undetectable virus in plasma during all study visits. Furthermore, sustained viral suppression does not in itself preclude persisting or even incident HAND [7]. The mechanisms of NC decline in virally suppressed patients are uncertain, but ART toxicity [13] and other non-HIV-related factors may be involved in some cases.
In addition to HIV disease and treatment predictors, we identified several other participant characteristics that may influence NC outcomes over time (Table 3). Factors that appeared beneficial include the combination of higher education level and reading-based estimate of premorbid IQ. These 2 variables are indicators of “cognitive reserve” [14], a concept used to explain individual differences in the threshold of CNS insult required to produce symptomatic neurologic disease. In particular, our finding that higher premorbid IQ was a unique predictor of NC improvement extends prior cross-sectional [15, 16] and longitudinal [17] studies supporting positive effects of cognitive reserve in HIV.
As noted in the CHARTER baseline report, this HIV+ population had many and diverse non-HIV-related comorbid conditions that may confer increased risks for NC impairment [2]. All participants were classified at baseline into 1 of the 3 specified comorbidity levels [1], and each successive level had more of such comorbidities (averaging 1.5, 3.2, and 4.2 conditions, respectively; P < .01 for all comparisons). In addition, successive groups had more severe comorbidities, as well as higher rates of NC impairment (40%, 59%, 83%). Past research has tended to exclude people with the highest level of comorbidities (those that represent “confounds”), and we are not aware of any previous, systematic attempt to relate comorbidity level to NC change over time. Here we found that the rate of NC decline was much higher in the participants who were classified as confounded at baseline (38.5%, vs 24.6% for those with “contributing” and 18.3% for those with “incidental” comorbidities). The specific reasons for these differences undoubtedly are as varied and complex as the patterns of comorbid conditions involved, and could not be established here with any certainty. Nevertheless, the published comorbidity classification system [1] has shown good interrater reliability in CHARTER [2], and successive levels of comorbidity appear to confer increased risk not only for cross-sectional NC impairment, but also for NC decline over time. These associations may justify more frequent or intensive medical monitoring and support for HIV+ patients who have higher comorbidity burdens.
We propose that, in univariable analyses, lower age and higher nadir CD4 appeared as marginal (P < .10) predictors of time to NC decline only because of their associations with other participant characteristics in this cohort. In fact, among our NC decliners, lower age was significantly associated not only with shorter duration of HIV infection and higher nadir CD4 counts, but also with higher likelihood of coinfection with hepatitis C virus, higher AST, and increased likelihood of a lifetime methamphetamine use diagnosis. As noted, neither age nor nadir CD4 cell count was identified as a contributor to the multivariable prediction models.
We did not anticipate a higher rate of NC decline to be associated with Hispanic ethnicity, but there are multiple factors that may be contributing to worse outcomes in this group. In general, Hispanics in the United States tend to have lower access to healthcare than non-Hispanic whites [18, 19]. They are much less likely to have health insurance coverage [18] or a usual source of healthcare [19]. HIV+ Hispanics tend to be late to be tested for the virus [20, 21], to obtain medical care, and to initiate therapy after diagnosis [22]. They also may be more likely to receive suboptimal HIV care [23]. Thus, not surprisingly, they have been observed to have worse HIV disease characteristics, including lower CD4 counts, higher plasma viral loads, more opportunistic infections, and higher rates of AIDS [24, 25]. Among HIV+ adults, reduced life expectancy from late initiation of therapy and from early discontinuation of therapy are greatest for Hispanics [26]. Few studies have examined the prevalence and pattern of NC impairment among HIV+ Hispanics. The limited data available are cross-sectional and typically include selected groups with small numbers [27–30]. Although there have been inconsistent findings [22], most studies have found Hispanic ethnicity to be associated with worse NC status [27, 29, 30].
Future research should attempt to clarify factors within the HIV+ Hispanic population that may influence disease outcomes in general, and NC decline in particular. CHARTER was not designed for this goal. Even though all CHARTER participants were receiving care during this study, it is of interest that, compared to non-Hispanic whites, Hispanics in CHARTER had a higher rate of AIDS (74% vs 58%, P = .03) and lower nadir CD4 cell count (median of 96 vs 190, P = .04). Other factors that may be relevant to consider in future research include immigration status, country of origin, time living in the United States, housing, employment, nutrition, acculturation, language barriers, and health literacy, to name a few (see [31]).
A major limitation of the current study is that it is observational, without control (other than statistical adjustments) over the many treatments, diseases, and other factors that may affect NC outcomes over time. Causation is difficult to assign in observational research. However, our finding of significant, time-dependent clinical and biological predictors of NC change, and the fact that many such associations are consistent with those in prior longitudinal studies, suggests that the observed associations may be clinically meaningful. Furthermore, they suggest that consistent use of ART to maintain virologic control and avoid serious immunosuppression may have beneficial long-term effects in protecting the CNS and improving NC outcomes. Finally, increased comorbidity burden and factors associated with Hispanic ethnicity deserve more attention in clinical care of HIV+ individuals.
Supplementary Material
Notes
Acknowledgments. The CNS HIV Anti-Retroviral Therapy Effects Research group is supported by the National Institutes of Health (award N01 MH22005).
Disclaimer. The views expressed in this article are those of the authors and do not reflect the official policy or position of the United States government.
Potential conflicts of interest. S. L. has received support for research projects from Abbott, Merck, Tibotec, and GlaxoSmithKline; has consulted for Gilead Sciences, GlaxoSmithKline, Merck, and Tibotec; and has received lecture honoraria from Abbott and Boehringer-Ingelheim. R. J. E. has received consultant fees from NeurogesX. F. V. has received research support from the Precision Photonics Corporation, and has served on a data safety and management board for Ardea Biosciences, Inc. J. H. A. is a consultant for Eli Lilly Pharmaceuticals. J. A. M. authors chapters on human immunodeficiency virus for the Merck Manual. A. C. C. has received research support from Boehringer-Ingelheim, Gilead Sciences, Merck & Co, Roche Molecular Systems, Schering-Plough, and Tibotec-Virco; is a member of a data safety monitoring board for a Merck-sponsored study; participated on an advisory board for Pfizer in 2009; and (along with an immediate family member) previously owned stock in Abbott Laboratories, Bristol-Myers Squibb, Johnson & Johnson, and Pfizer. C. M. M. receives royalties from Lippincott Williams & Wilkins and UptoDate. D. B. C. is supported by the Alzheimer Association; has also received research support from Lilly, Roche, Pfizer, Bavarian Nordic, and Biogen; and has been a scientific advisor or consultant to Amgen, Biogen Idec, Drinker, Biddle & Reath (PML Consortium Scientific Advisory Board), Quintiles, Roche, Genentech, Novartis, GlaxoSmithKline, Millennium, Bristol-Myers Squibb, Genzyme, and Pfizer. D. M. S. has provided consultancy to GlaxoSmithKline and Gilead. C. F.-N. serves as an Associate Editor for the Journal of Alzheimer's Disease (2012). I. G. has received honoraria from Abbott Pharmaceuticals as part of their Educational Speaker Program. All other authors report no potential conflicts of interest.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
APPENDIX
The CNS HIV Anti-Retroviral Therapy Effects Research (CHARTER) group is affiliated with the Johns Hopkins University; Mount Sinai School of Medicine; University of California, San Diego; University of Texas Medical Branch, Galveston; University of Washington, Seattle; and Washington University, St Louis; and is headquartered at the University of California, San Diego. Members are as follows. Director: Igor Grant, MD; Co-directors: J. Allen McCutchan, MD, Ronald J. Ellis, MD, PhD, Thomas D. Marcotte, PhD; Center Manager: Donald Franklin Jr; Neuromedical Component: Ronald J. Ellis, MD, PhD (Principal Investigator [PI]), J. Allen McCutchan, MD, Terry Alexander, RN; Laboratory, Pharmacology and Immunology Component: Scott Letendre, MD (PI), Edmund Capparelli, PharmD; Neurobehavioral Component: Robert K. Heaton, PhD (PI), J. Hampton Atkinson, MD, Steven Paul Woods, PsyD, Matthew Dawson; Virology Component: David M. Smith, MD (PI); Imaging Component: Christine Fennema-Notestine, PhD (Co-PI), Michael J. Taylor, PhD (Co-PI), Rebecca Theilmann, PhD; Data Management Unit: Anthony C. Gamst, PhD (PI), Clint Cushman; Statistics Unit: Ian Abramson, PhD (PI), Florin Vaida, PhD; Protocol Coordinating Component: Thomas D. Marcotte, PhD (PI), Jennifer Marquie-Beck, MPH; Johns Hopkins University site: Justin McArthur (PI), Vincent Rogalski, RN; Icahn School of Medicine at Mount Sinai site: Susan Morgello, MD (Co-PI) and David Simpson, MD (Co-PI), Letty Mintz, NP; University of California, San Diego site: J. Allen McCutchan, MD (PI), Will Toperoff, NP; University of Washington site: Ann Collier, MD (Co-PI) and Christina Marra, MD (Co-PI), Trudy Jones, MN, ARNP; University of Texas site: Benjamin Gelman, MD, PhD (PI), Eleanor Head, RN, BSN; and Washington University site: David Clifford, MD (PI), Muhammad Al-Lozi, MD, Mengesha Teshome, MD.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
References
- 1.Antinori A, Arendt G, Becker JT, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99. doi: 10.1212/01.WNL.0000287431.88658.8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heaton RK, Clifford DB, Franklin DR, Jr, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sevigny JJ, Albert SM, McDermott MP, et al. Evaluation of HIV RNA and markers of immune activation as predictors of HIV-associated dementia. Neurology. 2004;63:2084–90. doi: 10.1212/01.wnl.0000145763.68284.15. [DOI] [PubMed] [Google Scholar]
- 4.Heaton RK, Franklin DR, Ellis RJ, et al. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol. 2011;17:3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McArthur JC, Brew BJ. HIV-associated neurocognitive disorders: is there a hidden epidemic? AIDS. 2010;24:1367–70. doi: 10.1097/QAD.0b013e3283391d56. [DOI] [PubMed] [Google Scholar]
- 6.Cysique LA, Brew BJ. Neuropsychological functioning and antiretroviral treatment in HIV/AIDS: a review. Neuropsychol Rev. 2009;19:169–85. doi: 10.1007/s11065-009-9092-3. [DOI] [PubMed] [Google Scholar]
- 7.Tozzi V, Balestra P, Bellagamba R, et al. Persistence of neuropsychologic deficits despite long-term highly active antiretroviral therapy in patients with HIV-related neurocognitive impairment: prevalence and risk factors. J Acquir Immune Defic Syndr. 2007;45:174–82. doi: 10.1097/QAI.0b013e318042e1ee. [DOI] [PubMed] [Google Scholar]
- 8.Al-Khindi T, Zakzanis KK, van Gorp WG. Does antiretroviral therapy improve HIV-associated cognitive impairment? A quantitative review of the literature. J Int Neuropsychol Soc. 2011;17:956–69. doi: 10.1017/S1355617711000968. [DOI] [PubMed] [Google Scholar]
- 9.Cysique LA, Letendre SL, Ake C, et al. Incidence and nature of cognitive decline over 1 year among HIV-infected former plasma donors in China. AIDS. 2010;24:983–90. doi: 10.1097/QAD.0b013e32833336c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cysique LA, Franklin D, Jr, Abramson I, et al. Normative data and validation of a regression based summary score for assessing meaningful neuropsychological change. J Clin Exp Neuropsychol. 2011;33:505–22. doi: 10.1080/13803395.2010.535504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Letendre S, Marquie-Beck J, Capparelli E, et al. Validation of the CNS penetration-effectiveness rank for quantifying antiretroviral penetration into the central nervous system. Arch Neurol. 2008;65:65–70. doi: 10.1001/archneurol.2007.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcotte TD, Deutsch R, McCutchan JA, et al. Prediction of incident neurocognitive impairment by plasma HIV RNA and CD4 levels early after HIV seroconversion. Arch Neurol. 2003;60:1406–12. doi: 10.1001/archneur.60.10.1406. [DOI] [PubMed] [Google Scholar]
- 13.Al-Khindi T, Zakzanis KK, van Gorp W. Does antiretroviral therapy improve HIV-associated cognitive impairment? J Int Neuropsychol Soc. 2011;17:956–69. doi: 10.1017/S1355617711000968. [DOI] [PubMed] [Google Scholar]
- 14.Stern Y. Cognitive reserve. Neuropsychologia. 2009;47:2015–28. doi: 10.1016/j.neuropsychologia.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morgan EE, Woods SP, Smith C, Weber E, Scott JC, Grant I. Lower cognitive reserve among individuals with syndromic HIV-associated neurocognitive disorders (HAND) AIDS Behav. 16:2279–85. doi: 10.1007/s10461-012-0229-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thames AD, Foley JM, Panos SE, Singer EJ, El-Saden S, Hinkin C. Cognitive reserve masks neurobehavioral expression of human immunodeficiency virus associated neurological disorder in older patients. Neurobehav HIV Med. 2011;3:87–93. [Google Scholar]
- 17.Basso MR, Bornstein RA. Estimated premorbid intelligence mediates neurobehavioral change in individuals with HIV across 12 months. J Clin Exp Neuropsychol. 2000;22:208–18. doi: 10.1076/1380-3395(200004)22:2;1-1;FT208. [DOI] [PubMed] [Google Scholar]
- 18.Adams PF, Kirzinger WK, Martinez ME. Summary health statistics for the US population: National Health Interview Survey, 2011. National Center for Health Statistics. Vital Health Stat. 2012;10:48–9. [PubMed] [Google Scholar]
- 19.National Center for Health Statistics, National Center for Health Statistics. Health, United States, 2012: with special feature on emergency care. Hyattsville, MD:: 2013. [PubMed] [Google Scholar]
- 20.Chen NE, Gallant JE, Page KR. A systematic review of HIV/AIDS survival and delayed diagnosis among Hispanics in the United States. J Immigr Minor Health. 2012;14:65–81. doi: 10.1007/s10903-011-9497-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dennis AM, Napravnik S, Sena AC, Eron JJ. Late entry to HIV care among Latinos compared with non-Latinos in a southeastern US cohort. Clin Infect Dis. 2011;53:480–7. doi: 10.1093/cid/cir434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner BJ, Cunningham WE, Duan N, et al. Delayed medical care after diagnosis in a US national probability sample of persons infected with human immunodeficiency virus. Arch Intern Med. 2000;160:2614–22. doi: 10.1001/archinte.160.17.2614. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro MF, Morton SC, McCaffrey DF, et al. Variations in the care of HIV-infected adults in the United States: results from the HIV Cost and Services Utilization Study. JAMA. 1999;281:2305–15. doi: 10.1001/jama.281.24.2305. [DOI] [PubMed] [Google Scholar]
- 24.Giordano TP, Bartsch G, Zhang Y, et al. Disparities in outcomes for African American and Latino subjects in the Flexible Initial Retrovirus Suppressive Therapies (FIRST) trial. AIDS Patient Care STDs. 2010;24:287–95. doi: 10.1089/apc.2009.0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swindells S, Cobos DG, Lee N, et al. Racial/ethnic differences in CD4T cell count and viral load at presentation for medical care and in follow-up after HIV-1 infection. AIDS. 2002;16:1832–4. doi: 10.1097/00002030-200209060-00020. [DOI] [PubMed] [Google Scholar]
- 26.Losina E, Schackman BR, Sadownik SN, et al. Racial and sex disparities in life expectancy losses among HIV-infected persons in the United States: Impact of risk behavior, late initiation, and early discontinuation of antiretroviral therapy. Clin Infect Dis. 2009;49:1570–8. doi: 10.1086/644772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Durvasula RS, Miller EN, Myers HF, Wyatt GE. Predictors of neuropsychological performance in HIV positive women. J Clin Exp Neuropsychol. 2001;23:149–63. doi: 10.1076/jcen.23.2.149.1211. [DOI] [PubMed] [Google Scholar]
- 28.Levin B, Berger JR, Didona T, Duncan R. Cognitive function in asymptomatic HIV-1 Infection: the effects of age, education, ethnicity, and depression. Neuropsychology. 1992;6:303–13. [Google Scholar]
- 29.Mindt MR, Byrd D, Ryan EL, et al. Characterization and sociocultural predictors of neuropsychological test performance in HIV+ Hispanic individuals. Cultur Divers Ethnic Minor Psychol. 2008;14:315–25. doi: 10.1037/a0012615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wojna V, Skolasky RL, Hechavarria R, et al. Prevalence of human immunodeficiency virus-associated cognitive impairment in a group of Hispanic women at risk for neurological impairment. J Neurovirol. 2006;12:356–64. doi: 10.1080/13550280600964576. [DOI] [PubMed] [Google Scholar]
- 31.Escarce JJ, Kanika K. Access to and quality of health care. In: Mitchell MT, Mitchell F, editors. Hispanics and the future of America. Washington, DC: National Academies Press; 2006. National Research Council. Panel on Hispanics in the United States. Committee on Population, Division of Behavioral and Social Sciences and Education. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.