Abstract
Background: Docosahexaenoic acid–paclitaxel (DHA–paclitaxel, Taxoprexin®) is made by covalently conjugating the essential fatty acid DHA to the paclitaxel molecule. Preclinical studies of DHA–paclitaxel have demonstrated increased activity relative to paclitaxel and the potential for an improved therapeutic ratio. In the present study, the efficacy and toxicity profiles of DHA–paclitaxel were compared with those of dacarbazine.
Methods: In this study, 393 chemonaive patients with metastatic melanoma were randomly assigned to receive either DHA–paclitaxel at a starting dose of 900 mg/m2 IV on day 1 every 3 weeks or dacarbazine at a starting dose of 1000 mg/m2 IV on day 1 every 3 weeks. The primary end point of the study was the comparison of overall survival (OS).
Results: No significant difference in OS was noted between patients in the DHA–paclitaxel and dacarbazine arms. Similarly, there were no significant differences in response rate, duration of response, time to progression, and time to treatment failure between the two drugs. Safety results of the two drugs were as predicted from prior studies. Myelosuppression was more common with DHA–paclitaxel.
Conclusions: DHA–paclitaxel was not superior to dacarbazine. We conclude that further studies with the drug on an every 3-week schedule in melanoma are not warranted.
Keywords: chemotherapy, dacarbazine, DHA–paclitaxel, melanoma, phase III trial
The results of systemic treatment of metastatic melanoma remain unsatisfactory for most patients [1]. Dacarbazine was approved by the USA Food and Drug Administration specifically for the treatment of metastatic melanoma in 1974 and remains the standard chemotherapy for this cancer despite its limited efficacy with a response rate of 6%–8% based on recent studies that used the RECIST [2–4]. The use of interleukin 2 (also been approved for the treatment of metastatic melanoma) has remained limited because of the serious side-effects associated with it [5, 6]. Combination chemotherapy regimens, despite yielding response rates of 30%–40%, had no significant advantage over dacarbazine [7, 8]. Paclitaxel and docetaxel were evaluated for efficacy against melanoma over a decade ago. Ten of 90 chemotherapy-naive patients (11%) treated with single-agent paclitaxel in four trials responded, indicating a definite, although limited, activity against this cancer [9–12].
Docosahexaenoic acid–paclitaxel (DHA–paclitaxel, Taxoprexin®) is made by covalently conjugating the essential fatty acid DHA to the 2′-OH position of the paclitaxel molecule. Preclinical studies of DHA–paclitaxel (DHA-P) in immune-deficient nude mice and in other animals have demonstrated that it has increased activity relative to paclitaxel, indicating its potential for a superior therapeutic ratio [13].
Phase 1 clinical studies showed that the side-effect profile for DHA-P given once every 3 weeks at 900 mg/m2 or 1100 mg/m2 was reasonably well defined and manageable [14]. At the recommended phase 2 dose of 1100 mg/m2, DHA-P exhibited a small volume of distribution, a long terminal half-life, and a slow system clearance [14]. In a phase 2 study in patients with solid tumors, 36 assessable patients with metastatic melanoma were treated, 4 patients had partial response, and 13 had stabilization of disease [15]. The primary side-effects of DHA-P were neutropenia and febrile neutropenia. Its side-effect profile was notable for a lack of alopecia and relatively little peripheral neuropathy [16, 17]. The purpose of this phase 3 study was to compare the efficacy and toxicity profiles of DHA-P with those of dacarbazine.
patients and methods
This was a multicenter, open-label, prospective, randomized stratified study in patients with metastatic malignant melanoma (MMM). The protocol was designed in accordance with the general ethical principles outlined in the Declaration of Helsinki. Institutional review boards or ethics committees at each participating center approved the study protocol. All patients provided written informed consent. Patients meeting the enrollment criteria were randomly assigned in blocks within each country. Patients were stratified according to the American Joint Committee on Cancer (AJCC) staging system [18] and placed in one of the three groups, with balanced randomization between the groups (amendment 1, 30 October 2003). Group 1 consisted of patients with M1a disease. Group 2 consisted of patients with M1b disease. Group 3 consisted of patients with M1c disease. An elevated lactate dehydrogenase (LDH) level for staging and stratification purposes was defined as two separate elevated LDH results compared with the normal level obtained at least 24 h apart in the treating institution. Block size was set at four patients, with two patients randomly assigned to each arm. An independent contractor carried out randomization centrally and both the sponsor and the investigators were blinded to block information. Data from all randomized patients who received at least one dose of DHA-P or dacarbazine were included in the safety analyses. The response assessment was scheduled for the fourth week of the second cycle. Response was determined using RECIST [2] and adverse events were graded according to National Cancer Institute—Common Toxicity Criteria (NCI–CTC) version 2 (NCI–CTC, Version 3.0, 12 December 2003; http://ctep.cancer.gov/reporting/ctc.html). Toxic effects were assessed before each course.
patient selection
Patients aged 18 years or older with histologically confirmed MMM and without prior systemic therapy for metastatic disease were eligible for this study. To be eligible, patients were required to have recurrent melanoma with measurable metastatic disease whose response was assessable by imaging or physical examination; an Eastern Cooperative Oncology Group (ECOG) performance status of zero to two; an expected survival of at least 3 months; at least one measurable indicator lesion; adequate renal and liver functions, defined as serum creatinine and total bilirubin levels no >1.5 times the institution’s upper normal limits (UNL), transaminase (i.e. aspartate aminotransferase or alanine aminotransferase ) and alkaline phosphatase levels no >2.5 times UNL, and serum albumin at least 2.5 g/dl; an absolute neutrophil count of ≥1500/μl; and a platelet count of ≥100 000/μl.
Patients were excluded from participation in the trial if they had received prior chemotherapy or if they had metastatic ocular melanoma. Patients with history of neoplasm other than melanoma were excluded, except for curatively treated nonmelanoma skin cancer or carcinoma in situ of the cervix or other cancers treated with a curative intent in patients with a disease-free survival of >5 years. Patients with untreated uncontrolled brain metastases and those requiring steroids for cerebral edema were ineligible. Patients who were pregnant or nursing and patients who were not practicing an acceptable method of birth control were excluded, as were women who reported that they might breast-feed during the study. Patients with current active infections requiring anti-infection treatment were ineligible. Patients with history of HIV disease or infection were excluded. Patients with peripheral neuropathy greater than grade 1 and patients with unstable or serious concurrent uncontrolled medical conditions were excluded. Patients with known hypersensitivity to polyethoxylated castor oil (Cremophor) were ineligible. Patients were ineligible if they had major surgery within 14 days, large field radiation therapy or endocrine therapy within 28 days, or biologic therapy within 42 days of their enrollment date. All patients gave their written consent to participate in this study in accordance with institutional and federal guidelines.
treatment
Patients received either DHA-P at a starting dose of 900 mg/m2 i.v. by 2-h infusion on day 1 every 3 weeks or dacarbazine at a starting dose of 1000 mg/m2 i.v. over at least 30 min on day 1 every 3 weeks. Treatment was to continue until tumor progression, intolerable toxicity, patient refusal to continue treatment, or the investigator’s decision that treatment should be discontinued.
pretreatment and during-treatment evaluation
Prior to randomization, patients received physical examinations, vital signs including blood pressure, heart rate, respiration rate, and temperature were checked, and pregnancy tests (urine or serum) were carried out for women of childbearing potential. Patients’ medical histories, height, weight, and performance status were also evaluated. Laboratory tests included completed blood count (CBC)/differential, platelet count, prothrombin time, activated partial thromboplastin time, and routine chemistry panel and urinalysis. Radiological work-up included chest radiography and computed tomography and/or magnetic resonance imaging scans of the brain, chest, abdomen, and pelvis. A baseline electrocardiogram was done before the start of therapy.
During treatment, patients had physical examinations, during which the vital signs, weight, and performance status were checked, before each course of drug administration. CBC and platelet counts and chemistry panel were carried out weekly. After the first course, hematology and chemistry studies were obtained on day 14 (±4 days) and within 72 h before each course. All measurable visceral tumors were measured on computed tomography scans taken every two courses. Disease status was assessed according to RECIST every 6 weeks. The method used for tumor assessment at baseline was to be used consistently for all evaluations throughout the study. Following the end of protocol treatment, no crossover was planned. All patients were to be followed until death.
efficacy and safety assessments
The primary efficacy parameter was overall survival (OS). The secondary efficacy parameters were objective tumor response determined using RECIST, duration of response, time to disease progression (TtP), and time to treatment failure (TTF). Survival data were collected monthly for the duration of follow-up.
Physical examinations, laboratory and tumor assessments, were carried out within 14 days of treatment cessation. Patients were observed for 30 days after the last drug administration for any adverse events. All drug-related toxic effects were monitored until they resolved.
statistical design
The initial protocol specified that an exponential survival model was assumed for the calculations of sample size and power. The number of deaths anticipated in the enrolled sample was based on the assumption that mean survival time for MMM patients was 180 days.
Two-sided testing of the difference in survival time between patients treated with DHA-P and patients treated with dacarbazine was planned. An increase of 30% in OS was anticipated. Enrollment of ∼575 patients (∼288 patients were randomly allocated to each treatment arm) was planned to allow for nonassessable patients and still meet the desired two-tailed α value of 0.05 with a power of 0.80.
As of amendment 3 (19 April 2006), the protocol was updated to provide for two interim analyses (when 25% and 50% of the anticipated deaths occurred) and to include both efficacy and futility stopping boundaries [19]. The O’Brien–Fleming boundary shape was used to define the efficacy boundary and the Pocock shape to define the futility boundary.
Using the randomization and survival data, each interim analysis calculated the log-rank statistic and compared it with the critical values calculated for the efficacy and futility stopping boundaries. If either interim analysis indicated that the trial had not reached the efficacy or futility boundary, the result would be that there was not sufficient evidence to stop the trial at that point. No other information from the interim analysis was to be conveyed to the sponsor. If either interim analysis indicated that the trial had crossed the efficacy or futility boundary, the sponsor would be contacted with the result. An independent Data Monitoring Committee reviewed the results of the interim analyses to determine whether the study should continue as originally designed, be changed, or be terminated as a result of these data.
The SAS procedure Proc Lifetest (SAS Institute, Inc., Cary, NC) was used to compute estimates of the survival function using the Kaplan–Meier method. This procedure produced report tables reflecting the median OS and the 95% confidence interval (CI), and the Kaplan–Meier curve for OS was presented in the report figures. OS curves were compared using the log-rank test in SAS. Censored values were included in the analysis as handled by this SAS procedure.
results
Fifty-six centers, 39 in the United States and 17 in Australia, enrolled study patients. Each study center enrolled from 1 to 41 patients. The first patient began treatment on 6 December 2002. The final patient went off study on 29 October 2007. Collection of survival data ceased as of November 2007. The intent-to-treat (ITT) population comprised 393 patients who were randomly allocated into either the DHA-P arm (n = 194) or the dacarbazine arm (n = 199). One of the patients randomized to the DHA-P arm and four patients were randomly allocated to the dacarbazine arm did not receive study treatment. Of these, three patients refused treatment (one in the DHA-P arm and two in the dacarbazine arm). Two patients in the dacarbazine arm were not eligible: one because of pretreatment thrombocytopenia and the second because of presence of brain metastasis. The remaining 193 patients in the DHA-P arm and 195 patients in the dacarbazine arm received were evaluable for safety.
Demographic characteristics and baseline ECOG performance status were similar between the two treatment arms (Table 1). The treatment arms had similar AJCC stage distribution. Rates of prior immunotherapy and response were similar between the two treatment arms, with <35% of all patients having received prior immunotherapy.
Table 1.
Demographic characteristics of the intent-to-treat group
| Characteristic | Treatment arma |
|
| DHA–paclitaxel (n = 194) | Dacarbazine (n = 199) | |
| No. of patients (%) | No. of patients (%) | |
| Age, years | ||
| Mean | 59.4 | 61.0 |
| Standard deviation | 13.76 | 13.53 |
| Median (range) | 61.0 (27–90) | 62.0 (21–87) |
| 18–64 | 120 (61.9) | 111 (55.8) |
| 65+ | 74 (38.1) | 88 (44.2) |
| Sex | ||
| Male | 122 (62.9) | 134 (67.3) |
| Female | 72 (37.1) | 65 (32.7) |
| Race/ethnicity | ||
| White | 186 (95.9) | 195 (98.0) |
| Hispanic | 3 (1.5) | 2 (1.0) |
| Black | 2 (1.0) | 2 (1.0) |
| Other | 3 (1.5) | 0 |
| ECOG performance status | ||
| 0 | 101 (52.1) | 100 (50.3) |
| 1 | 83 (42.8) | 87 (43.7) |
| 2 | 10 (5.2) | 11 (5.5) |
| 3 | 0 | 1 (0.5) |
| Stage | ||
| M1a | 13 (6.67 | 13 (6.53) |
| M1b | 42 (21.65 | 48 (24.12) |
| M1c | 139 (71.65) | 137 (68.84) |
| Unknown | 0 | 1 (0.50) |
Treatment assigned at randomization.
ECOG, Eastern Cooperative Oncology Group.
interim analyses
Planned interim analyses were carried out after ∼25% of the anticipated number of deaths occurred (115 events) and after ∼50% of the anticipated number of deaths (230 events). Results of the interim analyses were reviewed by an independent Data Monitoring Committee. The first interim analysis indicated that there was not sufficient evidence to stop the study because of efficacy or futility. However, the second interim analysis determined that the futility boundary had been crossed and there was no reasonable prospect that statistical significance would be achieved if patient accrual continued.
overall survival
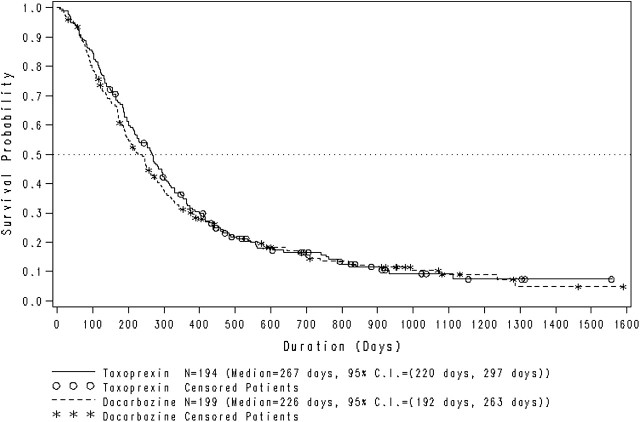
After termination of the study, the primary efficacy end point of OS was analyzed for the full ITT population, including the first 46 patients (stratified retrospectively), and for the prestratified patients (amendment 1, 30 October 2003) only (n = 347). OS by treatment arm is shown in Figure 1. In the full ITT population, the median OS was 267 days (95% CI 220–297) for the DHA-P arm and 226 days (95% CI 192–263) for the dacarbazine arm. Consistent with the interim analyses carried out in 2006 and 2007, no significant difference in OS was noted between patients in the two treatment arms. The survival rates were similar for patients in the two treatment arms at all time points from 1 to 36 months.
Figure 1.
Kaplan–Meier plot of overall survival time for the intent-to treat population (n = 393) of metastatic melanoma patients receiving DHA–paclitaxel or dacarbazine. CI, confidence interval.
secondary efficacy analyses
The secondary efficacy objectives analyzed included response rate, duration of response, TtP, and TTF. Response to treatment is summarized in Table 2. The overall best response rate (the sum of the complete response and partial response rates) was 5.2% for patients in the DHA-P arm and 5.5% for those in the dacarbazine arm. The percentages of patients with stable disease as their best response were 35.1% and 30.7% in the DHA-P and dacarbazine arms, respectively.
Table 2.
Summary of best-response data for the intent-to-treat group
| Best response | Treatment arma |
|
| DHA–paclitaxel (n = 194) | Dacarbazine (n = 199) | |
| No. of patients (%) | No. of patients (%) | |
| Overall responseb | 10 (5.2) | 11 (5.5) |
| Complete response | 1 (0.5) | 2 (1.0) |
| Partial responsec | 9 (4.6) | 9 (4.5) |
| Stable disease | 68 (35.1) | 61 (30.7) |
| Progressive disease | 110 (56.7) | 116 (58.3) |
| Not evaluable/not applicable | 6 (3.1) | 11 (5.5) |
Treatment assigned at randomization.
The number of patients achieving an overall response is the sum of the numbers of patients achieving complete and partial responses.
Two patients (one in the DHA–paclitaxel arm and one in the dacarbazine arm) did not have documentation of a confirmed partial response but were included on the basis of the investigator’s off-study assessment.
The median duration of response for patients in the DHA-P arm was 134 days (95% CI 77 to not estimated) and could not be estimated for the dacarbazine arm because of the censoring pattern. At 180 days, 30.5% of the DHA-P-arm responders and 58.3% of the dacarbazine-arm responders were progression free. Of the three patients with complete responses (one in the DHA-P arm and two in the dacarbazine arm), all maintained complete responses until their withdrawal from the study, with their survival data censored at 470, 203, and 324 days, respectively.
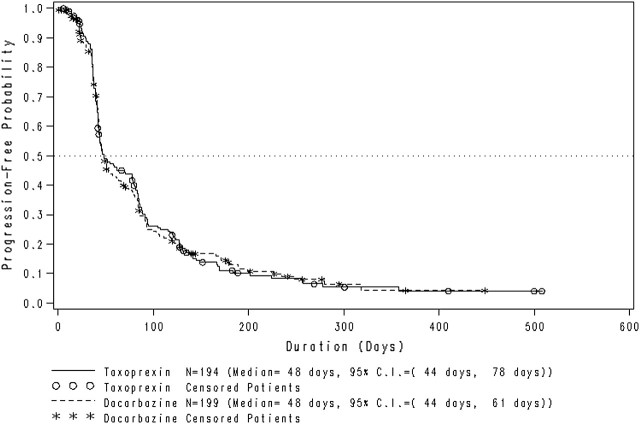
The TtP for all patients in the ITT population by treatment arm is shown in Figure 2. The median TtP for patients in the DHA-P arm was 48 days (95% CI 44–78), and for patients in the dacarbazine group, it was also 48 days (95% CI 44–61). The progression-free survival at 180 days was 11.1% for the DHA-P arm and 13.1% for the dacarbazine arm.
Figure 2.
Time to disease progression in the intent-to-treat population (n = 393) of metastatic melanoma patients receiving DHA–paclitaxel or dacarbazine. CI, confidence interval.
The median TTF for patients in the Taxoprexin® group was 47 days (95% CI 43–70 days) and for patients in the dacarbazine group was 48 days (95% CI 44–61 days). TTF rates were similar for the two groups at 1 month, 1 year, and all intervening time points.
therapy administered
In the DHA-P arm, 193 patients received a total of 749 courses of the drug, with a median of 2 courses and with 74.6% of patients receiving 80%–100% of their prescribed dose. In the dacarbazine arm, 195 patients received a total of 698 courses with a median of 2 courses with 79.5% of patients receiving 80%–100% of their prescribed dose.
Patients were removed from the study because of progressive disease in 83.0% on the DHA-P arm and 79.4% on the dacarbazine arm. The reasons for going off-study were similar between the two arms and included adverse event; investigator’s decision; patient’s request; and rarely because of death, intercurrent illness, noncompliance, or protocol violation.
therapy modifications
Dosing delays resulting from toxicity were reported for 23 patients (11.9%) in the DHA-P arm and for 56 patients (28.7%) in the dacarbazine arm. Dose reductions for toxicity were reported for 25 patients (13.0%) in the DHA-P arm and for 42 patients (21.5%) in dacarbazine arm.
adverse events
Table 3 presents the adverse events reported for ≥10% of patients in either arm, regardless of severity or drug relationship. The events reported for more than half the patients in either arm were limited to neutropenia and fatigue. Nonhematologic adverse events were similar between the two treatment arms, with the exception of rash, which was reported more often by patients in the DHA-P arm than in the dacarbazine arm.
Table 3.
Adverse events (regardless of relationship) reported for ≥10% of safety-evaluable patients in either treatment arm
| Adverse events by system organ class | Treatment arma |
|
| DHA–paclitaxel (n = 193) | Dacarbazine (n = 195) | |
| No. of patients (%) | No. of patients (%) | |
| Patients with adverse events | 192 (99.5) | 192 (98.5) |
| General disorders | ||
| Fatigue | 105 (54.4) | 110 (56.4) |
| Pyrexia | 37 (19.2) | 31 (15.9) |
| Edema peripheral | 25 (13.0) | 20 (10.3) |
| Blood and lymphatic system disorders | ||
| Neutropenia | 127 (65.8) | 57 (29.2) |
| Leukopenia NOS | 47 (24.4) | 30 (15.4) |
| Anemia NOS | 26 (13.5) | 35 (17.9) |
| Thrombocytopenia | 1 (0.5) | 33 (16.9) |
| Gastrointestinal disorders | ||
| Nausea | 75 (38.9) | 95 (48.7) |
| Constipation | 48 (24.9) | 72 (36.9) |
| Vomiting NOS | 41 (21.2) | 42 (21.5) |
| Diarrhea NOS | 34 (17.6) | 25 (12.8) |
| Abdominal pain NOS | 25 (13.0) | 15 (7.7) |
| Nervous system disorders | ||
| Headache NOS | 23 (11.9) | 25 (12.8) |
| Skin and subcutaneous tissue disorders | ||
| Rash NOS | 46 (23.8) | 13 (6.7) |
| Musculoskeletal and connective tissue disorders | ||
| Pain in limb | 22 (11.4) | 20 (10.3) |
| Back pain | 20 (10.4) | 28 (14.4) |
| Arthralgia | 19 (9.8) | 22 (11.3) |
| Respiratory, thoracic, and mediastinal disorders | ||
| Cough | 35 (18.1) | 21 (10.8) |
| Dyspnea NOS | 32 (16.6) | 17 (8.7) |
| Metabolism and nutrition disorders | ||
| Anorexia | 25 (13.0) | 31 (15.9) |
| Disorders noted during investigations | ||
| Weight decreased | 23 (11.9) | 24 (12.3) |
| Psychiatric disorders | ||
| Insomnia | 22 (11.4) | 22 (11.3) |
Treatment assigned at randomization.
NOS, not otherwise specified.
Table 4 shows the incidence of severe (greater than or equal to grade 3) adverse events that were considered to be possibly, probably, or definitely related to study drugs and were reported for ≥10% of patients. In the DHA-P arm, 73.6% of patients were reported to have at least one severe adverse event including neutropenia. In the dacarbazine arm, 34.9% of patients were reported to have at least one severe adverse event that included neutropenia, thrombocytopenia, and lymphopenia. Adverse events that led to the discontinuation of the study therapy were reported for 34 (17.6%) patients in the DHA-P arm and for 31 (15.9%) patients in the dacarbazine arm; the most common being peripheral neuropathy (3 patients) in case of DHA-P, thrombocytopenia (5 patients) in case of dacarbazine. Three patients (1.6%) had adverse events leading to death that were considered to be related to the study therapy in the DHA-paclitaxel arm. Of these patients, one had congestive heart failure, one had cardiopulmonary arrest, and one had congestive heart failure, pneumonia, and renal failure. While in the dacarbazine arm, two patients (1.0%) had adverse events at the time of death; one had severe fatigue and the other had sepsis and renal failure.
Table 4.
Severe (greater than or equal to grade 3) and drug-related adverse events reported for two or more patients (≥1.0%) in either treatment arm
| Adverse events by system organ class | Treatment arma |
|
| DHA–paclitaxel (n = 193) | Dacarbazine (n = 195) | |
| No. of patients (%) | No. of patients (%) | |
| Patients with severe and drug-related adverse eventsb | 142 (73.6) | 68 (34.9) |
| General disorders and administration site conditions | ||
| Fatigue | 7 (3.6) | 4 (2.1) |
| Blood and lymphatic system disorders | ||
| Neutropenia | 118 (61.1) | 43 (22.1) |
| Leukopenia NOS | 37 (19.2) | 27 (13.8) |
| Febrile neutropenia | 14 (7.3) | 3 (1.5) |
| Lymphopenia | 6 (3.1) | 13 (6.7) |
| Anemia NOS | 3 (1.6) | 7 (3.6) |
| Thrombocytopenia | 1 (0.5) | 16 (8.2) |
| Gastrointestinal disorders | ||
| Nausea | 4 (2.1) | 2 (1.0) |
| Vomiting NOS | 3 (1.6) | 1 (0.5) |
| Metabolism and nutrition disorders | ||
| Hyperglycemia NOS | 5 (2.6) | 1 (0.5) |
| Hyponatremia | 4 (2.1) | 2 (1.0) |
| Dehydration | 0 | 2 (1.0) |
| Infections and infestations | ||
| Pneumonia NOS | 3 (1.6) | 1 (0.5) |
| Infection NOS | 2 (1.0) | 1 (0.5) |
| Skin and subcutaneous tissue disorders | ||
| Rash NOS | 2 (1.0) | 0 |
| Nervous system disorders | ||
| Peripheral neuropathy NOS | 2 (1.0) | 0 |
| Musculoskeletal and connective tissue disorders | ||
| Myalgia | 2 (1.0) | 0 |
| Pain in limb | 2 (1.0) | 0 |
| Arthralgia | 2 (1.0) | 0 |
| Immune system disorders | ||
| Hypersensitivity NOS | 2 (1.0) | 2 (1.0) |
| Drug hypersensitivity | 2 (1.0) | 0 |
| Cardiac disorders | ||
| Congestive heart failure | 2 (1.0) | 0 |
| Hepatobiliary disorders | ||
| Hyperbilirubinemia | 2 (1.0) | 0 |
| Respiratory, thoracic, and mediastinal disorders | ||
| Dyspnea NOS | 1 (0.5) | 2 (1.0) |
Treatment assigned at randomization.
Considered to be possibly, probably, or definitely related to the study drug.
NOS, not otherwise specified.
discussion
DHA-P administered at single dose every 3-week schedule was not demonstrated to be superior to dacarbazine with respect to OS in patients with MMM. The OS observed with DHA-P was within the ranges reported for single-agent use of paclitaxel [9, 10, 12, 20–23] and dacarbazine [3, 4, 7, 8, 24, 25] in melanoma clinical studies. Similarly, there were no significant differences in tumor response rates, TtP, or OS between the two study drugs. There were no unusual safety concerns noted in either arm. Single dose, every 3-week schedule, as used in this trial, might not have been the most dose-intensive dose schedule of DHA-P. Further study of DHA-P in melanoma using single dose every 3-week dose schedule is not recommended.
In a more recent phase 2 study [26], chemotherapy-naive patients with melanoma were treated with DHA-P at 500 mg/m2 by 1-h i.v. infusion weekly for 5 weeks of every 6-week cycle. Three patients (10%) had partial responses lasting between 4 and 5.6 months and 15 patients (50%) had stable disease lasting 2.8 and 8.9 months. The median OS was 14.8 months. The toxicity profile was acceptable. For future studies with DHA-P in solid tumors, weekly administration of the drug appears to be a better choice than single dose every 3-week schedule.
funding
Luitpold Pharmaceuticals, Inc.
disclosure
The authors declared no conflict of interest.
References
- 1.Anderson CM, Buzaid AC, Legha SS. Systemic treatments for advanced cutaneous melanoma. Oncology (Williston Park) 1995;9:1149–1158. [PubMed] [Google Scholar]
- 2.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 3.Avril MF, Aamdal S, Grob JJ, et al. Fotemustine compared with dacarbazine in patients with disseminated malignant melanoma: a phase III study. J Clin Oncol. 2004;22:1118–1125. doi: 10.1200/JCO.2004.04.165. [DOI] [PubMed] [Google Scholar]
- 4.Bedikian AY, Millward M, Pehamberger H, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738–4745. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 5.Atkins MB, Lotze MT, Dutcher JP, et al. High-dose recombinant interleukin-2 therapy for patients with metastatic melanoma: analysis of 270 patients treated between 1985 and 1993. J Clin Oncol. 1999;17:2105–2116. doi: 10.1200/JCO.1999.17.7.2105. [DOI] [PubMed] [Google Scholar]
- 6.Allen IE, Kupelnick B, Kumashiro M, et al. Efficacy of interleukin-2 in the treatment of metastatic melanoma. Cancer Ther. 1998;1:168–173. [Google Scholar]
- 7.Buzaid AC, Legha SS, Winn R, et al. Cisplatin (C), vinblastine (V), DTIC (D) (CVD) versus DTIC alone in metastatic melanoma: preliminary results phase III cancer community oncology program (CCOP) Proc Am Soc Clin Oncol. 1993;12 389a (Abstr 1328) [Google Scholar]
- 8.Chapman PB, Einhorn LH, Meyers ML, et al. Phase III multi-center randomized trial of the Dartmouth regimen versus DTIC in patients with metastatic melanoma. J Clin Oncol. 1999;17:2745–2751. doi: 10.1200/JCO.1999.17.9.2745. [DOI] [PubMed] [Google Scholar]
- 9.Legha SS, Ring S, Papadopoulos N, et al. A phase II trial of taxol in metastatic melanoma. Cancer. 1990;65:2478–2481. doi: 10.1002/1097-0142(19900601)65:11<2478::aid-cncr2820651114>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Einzig AI, Hochster H, Wiernik PH, et al. A phase II study of taxol in patients with malignant melanoma. Invest New Drugs. 1991;9:59–64. doi: 10.1007/BF00194546. [DOI] [PubMed] [Google Scholar]
- 11.Einzig AI, Wiernik PH, Wadler S, et al. Phase I study of paclitaxel (taxol) and granulocyte colony stimulating factor (G-CSF) in patients with unresectable malignancy. Invest New Drugs. 1998;16:29–36. doi: 10.1023/a:1006004809169. [DOI] [PubMed] [Google Scholar]
- 12.Powderly J, Khan K, Richards J, et al. A 2-stage controlled phase 1/2 study of STA-4783 in combination with paclitaxel in patients with advanced metastatic melanoma abstract. J Clin Oncol. 2005;23(Suppl 16):725. (Abstr 7561) [Google Scholar]
- 13.Wolff AC, Donehower RC, Carducci MK, et al. Phase I study of DHA-paclitaxel, a taxane-fatty acid conjugate with a unique pharmacology and toxicity profile. Clin Cancer Res. 2003;9:3589–3597. [PubMed] [Google Scholar]
- 14. Data On File. Shirley, NY: Luitpold Pharmaceuticals, Inc. [Google Scholar]
- 15.Bradley MO, Webb NL, Anthony FH, et al. Tumor targeting by covalent conjugation of a natural fatty acid to paclitaxel. Clin Cancer Res. 2001;7:3229–3238. [PubMed] [Google Scholar]
- 16.Payne M, Ellis P, Dunlop D, et al. DHA-paclitaxel (Taxoprexin) as first-line treatment in patients with stage IIIB or IV non-small cell lung cancer: report of a phase II open-label multicenter trial. J Thorac Oncol. 2006;1(9):984–990. [PubMed] [Google Scholar]
- 17.Jones RJ, Hawkins RE, Eatock MM, et al. A phase II open-label study of DHA-paclitaxel (Taxoprexin) by 2-h intravenous infusion in previously untreated patients with locally advanced or metastatic gastric or oesophageal adenocarcinoma. Cancer Chemother Pharmacol. 2008;61(3):435–441. doi: 10.1007/s00280-007-0486-8. [DOI] [PubMed] [Google Scholar]
- 18.Balch CM, Buzaid AC, Soong SJ, et al. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J Clin Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 19.Burington BE, Emerson SS. Flexible implementations of group sequential stopping rules using constrained boundaries. Biometrics. 2003;59:770–777. doi: 10.1111/j.0006-341x.2003.00090.x. [DOI] [PubMed] [Google Scholar]
- 20.Zimpfer-Rechner C, Hofmann U, Figl R, et al. Randomized phase /I study of weekly paclitaxel versus paclitaxel and carboplatin as second-line therapy in disseminated melanoma: a multicentre trial of the Dermatologic Co-operative Oncology Group (DeCOG) Melanoma Res. 2003;13(5):531–536. doi: 10.1097/00008390-200310000-00012. [DOI] [PubMed] [Google Scholar]
- 21.Bedikian AY, Plager C, Papadopoulos N, et al. Phase I evaluation of paclitaxel by short intravenous infusion in metastatic melanoma. Melanoma Res. 2004;14:63–66. doi: 10.1097/00008390-200402000-00010. [DOI] [PubMed] [Google Scholar]
- 22.Walker L, Schalch H, King DM, et al. Phase I trial of weekly paclitaxel in patients with advanced melanoma. Melanoma Res. 2005;15:453–459. doi: 10.1097/00008390-200510000-00015. [DOI] [PubMed] [Google Scholar]
- 23.Zonder JA, LoRusso P, Heilbrun L, et al. Phase II trial of weekly paclitaxel as 2nd line treatment for metastatic melanoma (MMM) Proc Am Soc Clin Oncol. 2000;19:571a. (Abstr #2249) [Google Scholar]
- 24.Middleton MR, Grob JJ, Aaronson N, et al. Randomized phase III study of temozolomide versus dacarbazine in the treatment of patients with advanced metastatic malignant melanoma. doi: 10.1200/JCO.2000.18.1.158. [published correction appears in J Clin Oncol. 2000;18:2351]. J Clin Oncol 2000; 18: 158–166. [DOI] [PubMed] [Google Scholar]
- 25.Patel PM, Suciu L, Mortier L, et al. Extended schedule, escalated dose temozolomide versus dacarbazine in stage IV malignant melanoma: final results of the randomized phase III study (EORTC 18032). 33rd European Society of Medical Oncology (ESMO) Congress 2008 (AbstLBA8). 1 December 2008. [Google Scholar]
- 26.Homsi J, Bedikian AY, Kim KB, et al. Phase 2 open-label study of weekly docosahexaenoic acid-paclitaxel in cutaneous and mucosal metastatic melanoma patients. Melanoma Res. 2009;19:238–242. doi: 10.1097/CMR.0b013e32832a1e2f. [DOI] [PubMed] [Google Scholar]