Abstract
Spinal cord injury (SCI) leads to social and psychological problems in patients and requires costly treatment and care. In recent years, various pharmacological agents have been tested for acute SCI. Large scale, prospective, randomized, controlled clinical trials have failed to demonstrate marked neurological benefit in contrast to their success in the laboratory. Today, the most important problem is ineffectiveness of nonsurgical treatment choices in human SCI that showed neuroprotective effects in animal studies. Recently, attempted cellular therapy and transplantations are promising. A better understanding of the pathophysiology of SCI started in the early 1980s. Research had been looking at neuroprotection in the 1980s and the first half of 1990s and regeneration studies started in the second half of the 1990s. A number of studies on surgical timing suggest that early surgical intervention is safe and feasible, can improve clinical and neurological outcomes and reduce health care costs, and minimize the secondary damage caused by compression of the spinal cord after trauma. This article reviews current evidence for early surgical decompression and nonsurgical treatment options, including pharmacological and cellular therapy, as the treatment choices for SCI.
Keywords: Spinal cord injury, Treatment, Pharmacological treatment, Trauma, Cellular treatment, Management
Core tip: In recent years, various pharmacological agents have been tested for acute spinal cord injury (SCI). Today, the most important problem is ineffectiveness of nonsurgical treatment choices in human SCI that showed neuroprotective effects in animal studies. A number of studies on surgical timing suggest that early surgical intervention is safe and feasible, can improve clinical and neurological outcomes and reduce health care costs. This article reviews current evidence for early surgical decompression and nonsurgical treatment options, including pharmacological and cellular therapy, as the treatment choices for SCI.
INTRODUCTION
Currently, the management of patients with acute spinal cord injury (SCI) includes pharmacological agents, surgical intervention and cellular therapies. There is still no commonly accepted pharmacological agent used in the treatment of SCI but some clinical studies have been carried out to reveal an effective agent. The timing of surgery is another controversial issue. However, studies about cellular therapies give hope for the future. Various clinical studies using pharmacological agents, cellular therapies and surgical intervention for SCI are discussed and summarized in this review.
EPIDEMIOLOGY
The incidence of acute SCI has been reported as 15 to 40 in a million in the world[1]. The common causes of SCI are motor vehicle accidents, sport injuries, work-related accidents, assaults and falls[2]. It is more common in young men. The incidence of traumatic SCI was reported as 12.7 in a million in a study conducted in Turkey in 1992. The most common causes of these injuries are motor vehicle accidents (48.8%), falls (36.5%), cutting injuries (3.3%), gunshot wounds (1.9%) and jumping into the water (1.2%). Male/female ratio has been reported as 2.5:1[3]. The most common causes of non-traumatic SCIs are spinal vascular diseases (25%), tumors (25%), inflammatory diseases (20%) and spinal stenosis (19%)[4].
PATHOPHYSIOLOGY
The concept of a two-step mechanism for SCI was introduced in the early 1900s after progressive damage was shown in spinal cord injured animals by Allen[5]. It has been reported that the first step is primary mechanical damage that occurs within minutes as a result of mechanical SCI. The second step is the secondary injury triggered by the primary damage, resulting in microvascular damage, edema, demyelination, ischemia, excitotoxicity, electrolyte imbalances, free radical production, inflammation and late apoptotic cell death where many more factors are involved[6,7] (Table 1). The pathology behind these mechanisms includes ischemia arising from degenerative spinal cord perfusion and a cellular energy deficiency[8,9]. For this reason, in order to minimize the damage caused by spinal cord injuries, oxygen should be provided and blood pressure should be kept under control. Following an acute SCI, vascular injuries lead to a number of serious changes in the spinal cord which in turn result in a progressive spinal cord ischemia accompanied by a perfusion anomaly, ultimately causing both hemorrhagic and ischemic injuries[10,11]. The area around irreversible injury is the ischemic penumbra. If the ischemia exceeds beyond a critical level, the infarct area expands and irreversible injury occurs. Function can be restored in the case of regenerated blood flow before the beginning of injury[12] (Figure 1). SCIs may also lead to a petechial hemorrhage in the spinal cord following rupture of postcapillary venules or sulcal arteries. This rupture may result from a mechanical break triggered by the direct effect of the trauma or from an intravascular coagulation which is caused by venous stasis or distention[8,13].
Table 1.
Secondary ınjury mechanisms involved in the pathophysiology of spinal cord injury
| Systemic effects |
| Heart rate - brief increase then prolonged bradycardia |
| Blood pressure - brief hypertension then prolonged hypotension |
| Peripheral resistance - decreased |
| Cardiac output - decreased |
| Local vascular damage of the cord microcirculation |
| Mechanical disruption of capillaries and venules |
| Hemorrhage - especially gray matter |
| Loss of microcirculation - mechanical, thrombosis, vasospasm |
| Biomechanical changes |
| Excitotoxicity - glutamate |
| Neurotransmitter accumulation |
| Catecholamines - noradrenaline, dopamine |
| Arachidonic acid release |
| Free radical production |
| Eicosanoid production |
| Prostaglandins |
| Lipid peroxidation |
| Endogenous opioids |
| Cytokines |
| Electrolyte shifts |
| Increased intracellular calcium |
| Increased intracellular potassium |
| Increased intracellular sodium |
| Inflammatory response |
| Free radical generation |
| Macrophages |
| Axonal breakdown, removal of myelin debris |
| Release of cytokines |
| Glial cell activation |
| Cytotoxic effects on oligodendrocytes |
| Wallerian degeneration |
| Edema |
| Apoptosis |
| Loss of energy Metabolism |
| Decreased ATP production |
SCI: Spinal cord injury.
Figure 1.
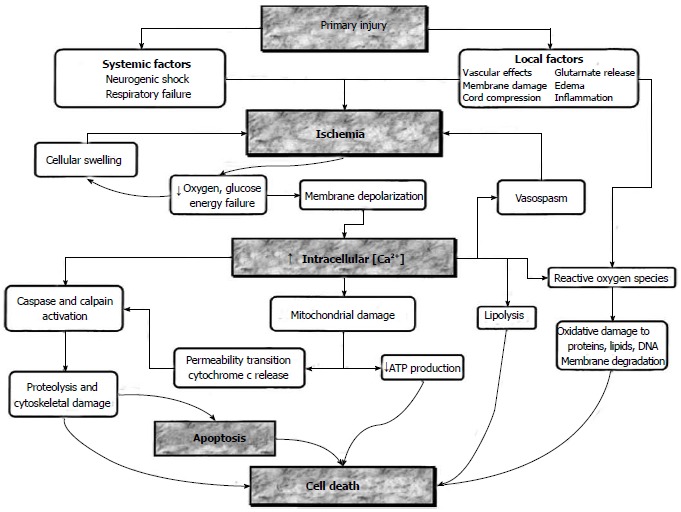
Major mechanisms of cell death are ischemia, intracellular calcium deposition, apoptosis. Pharmacological agents may intervene in these mechanisms at different stages shown in boxes (From Dumont RJ).
In spinal cord injuries, excessive free radicals lead to insufficient antioxidant systems as well as cell death[14]. These antioxidants are general occurrences in normal cells and their function is to keep harmful entities under control. However, the number of free radicals outdoes the number of these oxidants in severe pathological situations such as SCI. The free radicals may react to any cell constituent but lipids are the most delicate among the constituents. The destruction of the cell membrane that contains high amounts of polyunsaturated fatty acids is the very first step in the neuronal damage caused by free radicals[15]. Kaptanoglu et al[16-18] reported that melatonin, erythropoietin, thiopental and propofol can inhibit lipid peroxidation following SCI. SCI may also lead to the release of opioids as well as neurotransmitters. In turn, these opioids may obstruct the course of microcirculation by activating kappa opioid receptors. Therefore, studies focusing on the opioid receptors that have a selective effect on kappa receptors have yielded more successful results[8,19]. Following SCI, the lesions may contain a large amount of glutamate. In the early period, the glutamate receptor activation may increase intracellular sodium which in turn may lead to cytotoxic edema, intracellular acidosis and lysis[10]. Glutamate neurotoxicity triggers a chain of events which results in aggravated neuronal death and the development of reactive oxygen and nitrogen products[10].
Neuronal protection is highly important since the spinal neurons cannot achieve regeneration[20]. Apoptotic cell death is likely to happen in any cellular component of the spinal cord (neurons, astrocytes, oligodendrocytes and microglia). In conclusion, understanding the injuries secondary to neuronal death in SCIs remains the most vital issue for the implementation of advanced treatment methods[21] (Figure 2).
Figure 2.
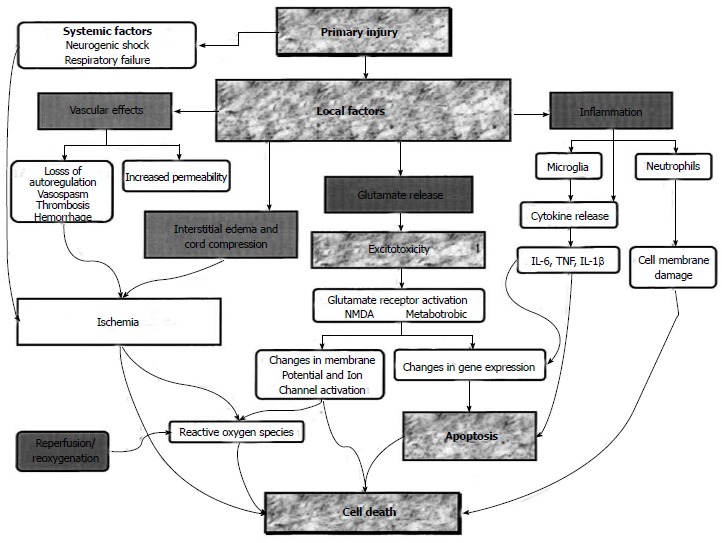
Role of vascular effects, inflammation, interstitial edema, glutamate release, cord compression and reperfusion which underlie the spinal cord injury are shown. Pharmacological agents may be useful at foci which are demonstrated in boxes (From Dumont RJ).
TIMING OF SURGERY
Eventually, the ideal management of acute SCI is a combination of pharmacological therapy, early surgery, aggressive volume resuscitation and blood pressure elevation to maximize spinal cord perfusion, early rehabilitation and cellular therapies. A number of investigations were done before the 1970s to both clarify the secondary mechanisms of SCI and find evidence that early surgical decompression affords a better neurological outcome. However, the timing for surgery in spinal cord injuries is not clear yet in terms of neuronal recovery. Partial reversibility of complete cord injury is reported in a limited time interval[22]. A number of pre-clinical studies[1,23-25] suggest no benefit of early surgical intervention to achieve spinal cord decompression on outcomes; however, several others[13,26-28] indicate that longer spinal cord compression before surgery is associated with detrimental outcomes in animal SCI models. Animal models suggest that early decompression directly correlates with improved neurological outcome. Dimar et al[29] used a rat model with a different time range of extradural compression up to 72 h and demonstrated that animals with shorter compression times showed better neurological recovery. There are no class I clinical trials to guide the timing of surgery. Several class II and class III studies have been carried out; they demonstrate that early surgery (decompression/reconstruction) is safe and should be strongly considered in patients without life-threatening polytrauma and without major medical co-morbidities. Urgent surgical decompression should be carried out in patients with early neurological deterioration. It is important to avoid intra-operative hypotension to minimize the intraoperative risks with early intervention[30]. Many surgeons advocate early surgery for maximum restoration of neural tissues and rehabilitation and early mobilization of the spinal column. A number of authors defined appropriate early surgery in a range from 8 to 72 h[31-33]. Some authors also report that early surgery results in reduced medical complications and length of stay and cost[32-35].
In a systematic review, Furlan et al[36] evaluated 22 clinical studies examining either the feasibility and safety or efficacy of early surgical intervention to stabilize and align the spine and for decompression of the spinal cord. Some of these studies indicated that patients who undergo early surgical decompression can have similar outcomes to patients who received a delayed decompressive operation. However, there is evidence to suggest that early surgical intervention is safe and feasible and that it can improve clinical and neurological outcomes and reduce health care costs. In another systematic review of the current evidence for surgical decompression as a treatment for SCI, Cadotte et al[30] demonstrated emerging evidence and a growing consensus among surgeons who support early surgical intervention to help minimize the secondary damage caused by compression of the spinal cord after trauma. In a randomized controlled study by Cengiz et al[37], postoperative ASIA score (Table 2) significantly increased in the early surgery group and late surgery group compared to the pre-operative ASIA score. In addition to this finding, the post-operative ASIA score of the early surgery group was significantly better than the late surgery group. Patients in the early surgery group showed a 83.3% improvement in ASIA score, whereas the ASIA score of 26.6% patients in the late surgery group improved. Cadotte et al[30] suggested that early surgery is safe and strongly recommended in patients without life-threatening polytrauma and without major medical co-morbidities, according to findings in class II and class III studies. Urgent surgical decompression should be carried out in patients with deteriorating neurology.
Table 2.
American spinal ınjury association ımpairment scale
| A = Complete: No motor or sensory function is preserved in the sacral segments |
| B = Incomplete: Sensory but not motor function is preserved below the neurological level and includes sacral segments |
| C = Incomplete: Motor function preserved below the neurological level; more than half the key muscles below the neurological level have a muscle grade less than 3 |
| D = Incomplete: Motor function preserved below the neurological level; at least half the key muscles below the neurological level have a muscle grade of 3 or more |
| E = Normal: Motor and sensory function |
In addition, another level-2b evidence study suggested that compared to surgical intervention from 72 h to 5 d after thoracolumbar SCI, stabilization of spinal and cord surgical decompression in less than 8 h would result in better neurological outcome, shorter duration of hospitalization, shorter duration of stay in the intensive care unit and lower frequency of secondary complications[37].
No complications were seen in the early surgery group, whereas three cases of respiratory failure and one case of sepsis were seen in the late surgery group[37]. It was reported that early surgery results in reduced LOS, less secondary complications, early mobilization and transfer to rehabilitation and should be considered in all SCI patients.
Finally, the authors declare that as there is strong pre-clinical evidence for biological benefits of early surgical decompression in animal SCI models, surgical decompression of the injured spinal cord should be performed within 24 h when medically feasible. The optimal timing of surgical decompression in patients with a central cord injury remains unclear and there are clinical, neurological and functional benefits of early spinal cord decompression[36].
PHARMACOLOGICAL TREATMENT
A lot of pharmacological treatment methods have been studied by considering the pathophysiological mechanisms in SCI (Table 3). These methods are now mentioned.
Table 3.
Pharmacotherapy of acute spinal cord injury and mechanism(s) of action
| Methylprednisolone |
| Inhibition of lipid peroxidation/antioxidative/anti-inflammatory |
| Properties decrease ischemia, support energy metabolism, inhibit neurofilament degradation, decrease intracellular Ca, decrease PG F/ TxA, increase spinal neuron excitability, decrease cord edema |
| Ganglioside GM-1 |
| Stimulate neurite regrowth/regeneration |
| Opioid receptor antagonists |
| Antagonize the increase in endogenous opioid levels after SCI (opioid receptor activation can contribute to excitotoxicity) |
| TRH and its analogs |
| Antagonize endogenous opioids, platelet-activating factor, peptido- leukotrienes and excitatory amino acids |
| Nimodipine |
| Decrease intracellular Ca2+ accumulation, attenuate vasospasm |
| Gacyclidine (GK11) |
| Antagonism of glutamate receptors |
| Magnesium |
| Replace Mg2+ depletion that is common after SCI, diminish intracellular Ca2+ accumulation, block N-methyl-D-aspartate receptor ion channel, modulate binding of endogenous opioids |
| Hypothermia |
| Reduce extracellular glutamate, vasogenic edema, apoptosis, neutrophil and macrophage invasion and activation, and oxidative stress |
| Minocycline |
| Inhibition of microglial activation, inhibition of cytochrome c release |
| Erythropoietin |
| Reduced apoptosis and lipid peroxidation |
| Estrogen |
| Not clearly known |
| Progesterone |
| Reduce the production of inflammatory cytokines |
| Cyclooxygenase inhibitors |
| Prevents/antagonizes decreased blood flow/platelet aggregation from production of arachidonic acid metabolites |
| Riluzole |
| Blockade of voltage-sensitive sodium channels and antagonism of presynaptic calcium-dependent glutamate release |
| Atorvastatin |
| Prevents neuronal and oligodendrocytic apoptosis |
| Antioxidants |
| Antagonize deleterious effects of free radicals (lipid eroxidation, reperfusion injury, etc.) |
PG F: Prostaglandin F; SCI: Spinal cord injury.
Steroids
Corticosteroids have been used to reduce spinal cord edema in acute SCI for over 30 years due to their anti-inflammatory features[38]. Although the exact mechanisms of the neuroprotective effects of corticosteroids are not completely understood, it has been suggested that these include inhibition of lipid peroxidation, modulation of inflammatory and immune responses with inflammatory cytokines, the healing of the vascular perfusion and prevention of calcium entering into the cell[39,40].
Methylprednisolone
Methylprednisolone is a synthetic glucocorticoid and has been used in SCI and brain edema for a long time. Today, the widespread use of methylprednisolone results from three large-scale, prospective, randomized, double-blind, multi-center clinical studies called the National Acute Spinal Cord Injury Studies (NASCIS) I, II and III. In NASCIS I, the effects of ten day doses of 100 mg or 1000 mg of methylprednisolone started in patients with SCI within 48 h were evaluated[41]. No motor and sensory differences were found between the two regimes. As a result of animal experiments, it has been suggested that a 1000 mg dose is far below the required dose for effective neuroprotection and that after the initial dose of 30 to 40 mg/kg it would be more appropriate to continue with an intravenous maintenance dose[39].
Therefore, in the next NASCIS II trial, after an initial bolus of methylprednisolone 30 mg/kg, 5.4 mg/kg infusion per hour for 23 h was given[42]. All 487 patients in the study in the first 12 h after injury were randomized into one of the groups of methylprednisolone, naloxone or placebo. Statistically, significant sensory and motor improvements were reported when methylprednisolone was given in the first 8 h after injury in both full and partial SCI. NASCIS II verified that, besides being the first clinical study showing that methylprednisolone is an effective pharmacological agent for the treatment of SCI, it also provided the widespread use of it and confirmed its relationship with secondary damage and its effective pharmacological strength. Then, NASCIS III was performed to evaluate the efficacy of tirilazad mesylate as well as to compare methylprednisolone treatment in different time windows[43]. Because of the antioxidant properties, several complications of steroid use were intended to be avoided. Thirty milligrams per kilogram of methylprednisolone in the form of a bolus was given to all 499 patients in the study after the first 8 h after trauma and then either a 24 or 48 h infusion of methylprednisolone or 48 h of tirilazad mesylate were administered randomly. Of all treatment actions, the motor and sensory recovery was found to be similar in the first 3 h after trauma. In these patients, a 24 h infusion of methylprednisolone has been suggested to be sufficient. However, when methylprednisolone is started between 3 and 8 h, prolonging the infusion to 48 h has been proposed as more beneficial. Improvement in motor function was statistically significant at 6 mo and even after 1 year in the MP group compared with the controls (17.2 and 12.0 points improvement respectively, P = 0.030)[42].
Although NASCIS II and III have led to the establish-ment of clinical standard application of methylprednisolone in acute SCI in North America, there has been a lot of criticism regarding the results and comments of these studies recently. This situation has led to some centers giving up the application. Many researchers have published their in-depth analysis of NASCIS II and III trials[44,45]. It has been reported that especially the application of NASCIS III in 48 h had minimal effectiveness in neurological healing and increased wound infection rates, pulmonary embolism, severe pneumonia, sepsis and that it even increased secondary deaths due to respiratory complications with the use of steroids. The argument about whether to use this agent in acute SCI still continues[12].
Ganglioside GM-1
Gangliosides are glycosphingolipids that are in the outer lipid layer of the cell membrane and contain sialic acid. Potential effects in neuroprotective and neuronal function restoration were found in experimental studies[46]. By increasing cell regeneration in tissue, they reduce the neurotoxicity of the excitatory amino acids. Promising clinical results with GM1 were obtained in a single center prospective randomized clinical trial with 37 patients with SCI in 1991[47]. In the subsequent experimental studies of SCI with systemic administration of GM1, neuroprotective effects such as neurite outgrowth, plasticity strengthening, prevention of apoptosis and inhibition of excitotoxicity were obtained[47,48]. These positive results led to the realization of a multicenter randomized clinical trial published in 2001[49]. In this clinical trial between 1992 and 1997, over 750 patients were randomly divided into treatment arms, such as placebo, low-dose and high-dose GM1 ganglioside. In the 26th week, at least a two-degree increase was determined in the motor/sensory function of the patients who experienced a significant improvement in a modified Benzel classification with respect to the American Spinal Injury Association (ASIA) scores. Sensory and motor scores in patients treated with GM1 ganglioside and in many parameters including bowel and bladder function in partially paralyzed patients showed an improvement compared to placebo. However, there was no effect on the complete patients but the results of the study were promising for the incomplete patients.
Opioid receptor antagonists
After SCI, dynorphin A, an endogenous opioid, is allowed to flow and neurotoxic effects occur. Moreover, it decreases spinal cord blood flow with non-opioid mechanisms[50]. Naloxone is a nonspecific opiate receptor antagonist. In the experimental animal models of SCI, the application of naloxone leads to functional and electrophysiological improvement. Moreover, it reverses the spinal shock and improves the blood flow to the spinal cord[51,52]. It was extensively studied in the early 1980s and in the 1980s the opioid antagonist naloxone was examined in a Phase I SCI trial in humans[53-55]. However, beneficial effects of naloxone that were thought to be due to antagonization of the increase of the endogenous opiates observed after SCI were not confirmed. In NASCIS II, the first results obtained from the studies related to naloxone, one of three treatment arms that has not shown any significant neuroprotective benefit over placebo[41].
Thyrotropin releasing hormone and its analogs
Secondary injury mediators such as endogenous opioids, excitotoxic amino acids, leukotrienes and platelet activating factor have been shown to be antagonized by TRH. Functional improvement in rats after experimental SCI by TRH has been shown[56]. The only clinical trial which was ever performed with TRH in acute SCI was published in 1995. Pitts et al[57] showed that TRH is effective in increasing the blood flow, reducing lipid degradation, in ionic hemostasis and improving neurological function.
Nimodipine
It has been reported that calcium channel blockers improve the post-traumatic spinal cord blood flow with the regulation of microvasculature. Nimodipine has been shown to increase the blood flow of the spinal cord in experimental SCI[58]. In other animal experiments, however, no significant neurological improvement was observed with nimodipine treatment after spinal cord trauma or ischemia[59]. The SCI trial for humans was carried out in France in 1996[60]. The trial involved 100 patients in 4 treatment arms: nimodipine, MPSS (NASCIS II protocol), both agents and placebo. Although it is possible that the study was weak in showing a therapeutic effect, benefit over placebo was not shown in any treatment group. Because of the potential that systemic hypotension develops in impaired spinal cord blood flow autoregulation conditions, it may become detrimental so their usage causes concerns.
Gacyclidine (GK11)
Glutamate is the main excitatory amino acid in the central nervous system and plays an important role in the secondary SCI. Like gacyclidine (GK11), NMDA also has shown that receptor antagonists have significant neuroprotective effects after SCI in animal studies[61]. With the distribution of glutamate into each side of the central nervous system in humans, significant adverse effects of the systematic treatment may be seen. In previous studies, glutamate receptor antagonists had significant cognitive side effects, including agitation, sedation, hallucinations and memory deficits, even with competitive antagonists such as Selfotel[62]. Therefore, the development of clinical treatment of NMDA antagonists has become difficult. Besides considerably better tolerability than other N-methyl-D-aspartate antagonists, gacyclidine has improved function, histology and electrophysiology in a rat model[61,63].
Magnesium
Magnesium is a well known neuroprotective agent and plays a key role in free radical and glutamate damage in the vascular structure after SCI. Magnesium provides vasoprotection by reducing free radical generation in neural structures. It also stimulates the release of endothelial prostacyclin and provides dilation of the blood vessels supplying the spinal cord. It is believed that magnesium decreases lipid peroxidation by-products with the indirect effect arising from glutamate antagonism[64]. In a study conducted to demonstrate the vascular protection after SCI, Kaptanoglu et al[65] showed that magnesium reduced edema and vascular permeability in SCI ultrastructurally[65].
Hypothermia
Hypothermia has a neuroprotective effect with the reduction of brain edema and intracellular calcium, the increased release of gama aminobütirik asit (GABA) and the inhibition of glutamate release[66,67]. Additionally, moderate hypothermia has been reported to be effective in reducing apoptotic neuronal death[68]. Systemic cooling methods used to cool the spinal cord are intravenous fluid infusions and the local cooling is with a cold saline infusion through epidural or intrathecal catheters. To cool a long cord segment is technically difficult[69]. Clinical application of hypothermia in patients with SCI cannot be recommended to be used in neuroprotection because of complications, such as hypotension, bradycardia and infection, unless it becomes safe and applicable[69,70].
Minocycline
It has been shown that minocycline inhibits excitotoxicity, reduces apoptosis with caspase-1 and has neuroprotective effects in Parkinson's disease with possible inhibition of microglial activation and autoimmune encephalomyelitis, amyotrophic lateral sclerosis, ischemic brain injury models in adults and newborns[71-73]. After acute SCI, minocycline has been reported to reduce the size of the lesion. It has also been shown that minocycline can pass the blood-brain barrier easily and effectively reduces functional deficits and secondary spinal tissue loss in mitochondrial cytochrome c in experimental SCI[74].
Cethrin
This agent facilitated axonal growth and promoted functional recovery in a mouse model. The researchers observed an early neurological improvement and reduced apoptosis rates[75].
Erythropoietin
There have been many comprehensive studies for erythropoietin (EPO) in acute SCI. Erythropoietin and its derivatives are the endogenous cytokine mediators in the central nervous system with tissue protective effects. Kaptanoglu et al[17] showed that erythropoietin inhibits lipid peroxidation after SCI and provides ultrastructural neuroprotection. A dramatic decrease was shown in the volume of cavitation after rhEPO therapy according to the results of histological examination 7 d after spinal contusion. They contribute to inhibition of erythropoietin apoptosis, inflammation reduction, excitability modulation and proliferation and modulation of neuronal stem cells[76-78]. Improved white and grey matter sparing, reduced apoptosis and lipid peroxidation, reduced ERK phosphorylation, and decreased inflammatory cytokine release and neutrophil invasion were involved in non-behavioral results. The efficacy of EPO in acute SCI is not certain.
Estrogen
Laboratory evidence supports that female sex hormones may play a role in hormone-dependent neuroprotection. Estrogen-dependent neuroprotection takes place with increased expression of the antiapoptotic factor bcl-2 and by the activation of protein kinase pathways. Non-behavioral results involve reduced overall secondary tissue damage, reduced MPO activity, microglial/macrophage accumulation and reduced apoptosis.
Progesterone
Progesterone receptors are spread widely in the central nervous system. The effect of progesterone is shown by reducing the production of inflammatory cytokines increasing excitotoxicity in secondary neuronal injury. In the SCI model, it has been shown that progesterone can reduce the production of oxidants and free radicals and can provide stability of neurotrophins in the spinal cord[70]. More recently, it has also been shown that progesterone modifies the traditional neurotransmitter systems such as inhibitory GABA and excitatory amino acids in SSS[79]. Progesterone treatment was reported to be able to alter gene and protein expression, cell morphology and receptor and neurotransmitter expression in the injured spinal cord.
Cyclooxygenase inhibitors
These inflammatory prostaglandins have an important role in secondary injury. It has been shown that indomethacin reduces tissue damage and edema in SCI. Meclofenamate and ibuprofen are two non-steroidal anti-inflammatory agents used widely for spinal blood flow after SCI in cats[80]. In this study, the combination of a thromboxane inhibitor with a prostacyclin analogue was found to be similarly effective. It was observed that COX-2 expression increased after the damage of contusions in the SCI of a rat. With SC-236, a COX-2 inhibitor, neuroprotection was provided after SCI in rabbits and improvement was seen in behavioral deficits[81]. Although the application of COX-1 and COX-2 inhibitions in humans in SCI has not been reported, the widespread use of these in people has been disposed of because of many safety and pharmacokinetic issues.
Riluzole
Riluzole is a sodium channel blocker approved by the Food and Drug Administration for amyotrophic lateral sclerosis. It has been shown that riluzole has a neuroprotective effect and reduces the damage in gray and white matter after clip compression injury of spinal cord in a rat model. It also improves locomotor functions. Therefore, many pharmacokinetic and toxicity studies were carried out in humans for riluzole. There are no reports that dose response has an effect on the thoracic contusion SCI models. Kitzman et al[82] showed that signs of tail spasticity decreased with both 8 and 10 mg/kg doses but systemic side effects (lethargy, locomotor ataxia) were attributed to the higher dose in the 2009. It was demonstrated that there was a therapeutic neuroprotective efficacy with a postponement in intervention of 15 min[83] and 30 min[84].
Atorvastatin
Atorvastatin treatment provides protection against reactive gliosis, trauma-induced tissue necrosis and demyelination. It also prevents neuronal and oligodendrocytic apoptosis by reducing Inducible nitric oxide synthase, tumor necrosis factor-α and interleukin1-β expression from inflammatory cytokines[85].
Antioxidants
Free radicals increase significantly after spinal cord trauma in animals. Despite their different mechanisms, ascorbic acid and hypothermia with a synergistic effect reduce the production of free radicals and associated damage[21]. Melatonin[18], EPC-K1[86], vitamin E and selenium[6] free radical are scavenger agents and have been shown to be beneficial in SCI. Studies on spinal cord injuries which are related to nitric oxide synthase inhibitors[87], polyethylene glycol[88], lipopolysaccharide[89], anti-CD 11d antibodies[90], inosine[91] and pioglitazone[92] have been performed.
CELLULAR TRANSPLANTATION THERAPIES
As a repair strategy for SCI, the neural transplantation procedure has been studied over the past several decades in many animal models. The rationale for cell transplantation treatments are to provide the injured tissue with growth promoting factors, cell replacements, structural elements and myelinating units[93]. The aim of cell therapies is to provide functional recovery of deficit by an axonal regeneration and restoration (Table 4). Reconstructive and regenerative experimental cellular strategies containing embryonic or adult stem cells or tissue[94,95], genetically modified fibroblasts[96], Schwann cells (SCs)[97,98], olfactory ensheathing cells[93,99], bone marrow stromal cells[100,101], neural stem cells[102] and activated macrophages[103,104] have been reported with varying degrees of recovery in different models of SCI.
Table 4.
Cellular transplantatıon therapıes spinal cord injury
| Schwann cells | Secrete growth factors, reestablish microenvironment |
| Olfactory ensheathing cells | Promoting axonal regeneration |
| Bone marrow cells | Produce neuroprotective cytokines |
| Stimulated macrophages | Removal of myelin debris, release of cytokines |
| Oligodendrocyte progenitor cells | Achieve remyelination |
SCs
The Schwann cell is one of the most widely used cell types for repair of the spinal cord. In experimental models of SCI, SCs are the myelin-forming cells of the peripheral nervous system and have been shown not only to myelinate (remyelinate) axons after transplantation into the injured spinal cord, but also to form a permissive substrate for regenerating axons, as reported in many studies[98,105,106]. Schwann cell transplantation in a wide variety of SCI models, such as photochemical[93], transection[97] and subacute contusion[107], has resulted in improvements in locomotion as well as neurobiological indices of recovery. Oudega et al[97] demonstrated that SCs play a key role in peripheral nerve regeneration and also lead to release of various growth factors, creating a growth permissive feature for axonal regeneration. In addition, SCs can produce axon growth promoting substrates such as fibronectin and laminin[97]. On the other hand, SCs are able to myelinate both intact and regenerating central axons[108]. For this reason, it can be said that SC is one of the best cell types for cell transplant therapy SCI. Pre-clinical experiments regarding the survival and efficacy of human SCs in contusion models of SCI are needed.
Olfactory ensheathing cells
The olfactory mucosa contains multipotent progenitor cells capable of differentiating into both neural and non-neural cells[109]. Olfactory ensheathing cells (OECs) are capable of promoting axonal regeneration and remyelination after injury. As a possible source for autologous cells, the olfactory mucosa is capable of lifelong regeneration and is readily accessible with minimally invasive techniques. Adult neural stem progenitor cells from the subventricular zone of the brain and the spinal cord of rodents contain neuron precursors, oligodendrocytes and astroglia, some stem-like cells. Transplanting OECs into damaged spinal cord promotes axonal remyelination and regeneration, facilitating recovery of the SCI[110-112]. On the other hand, clinical studies showed that OEC transplantation is a safe method[113] with improved sensory-motor function of injured spinal cord[114,115].
Bone marrow cells
In recent years, some studies showed that bone marrow cells (BMCs) can be differentiated into glial cells or mature neurons under special experimental procedures[101,116]. BMCs grafting on SCI injury models have been studied and it has been observed that the transplanted BMCs improve neurological deficits by generating myelin producing cells or neural cells[117,118]. Furthermore, BMCs can produce neuroprotective cytokines, rescuing the neurons with impending cell death in case of injury[119,120]. Also, several clinical trials have explored the hypothesis that cell transplantation may enhance the recovery of neurological functions after SCI.
Stimulated macrophages
After an injury, macrophages and their associated cytokines invade the impaired tissue[121,122]. In the nervous system, macrophage-derived cytokines can induce regeneration-associated components such as nerve growth factor[123] and cell adhesion molecules. Stimulated macrophage implantation into transected rat spinal cord showed promoted tissue repair, including recovery of motor function, observed behaviorally and electrophysiologically[103]. On the other hand, in a study on sciatic nerve injury, it has been demonstrated that the blockage of macrophage invasion led to impairment of regeneration[104].
Oligodendrocyte progenitor cells
The oligodendrocyte progenitor cells (OPCs) and oligodendrocytes derived from OPC show great promise in CNS repair. They produce myelin in the CNS and originate from the neuroepithelial cells[124]. Whether OPCs could support the regeneration of injured axons is not yet clear but the promise of using OPCs in cell therapies lies in their ability to produce myelin on demyelinated axons. Demyelination due to oligodendrocyte death occurs in both contusive animal models of SCI[125] and humans[126]. After CNS disorders and traumas, demyelination of axons contributes to functional and physiological deficits. In addition, apoptosis plays a main role in oligodendrocyte death[127,128]. The remyelination of regenerated axons and demyelinated intact axons is a substantial repair strategy to accelerate functional recovery.
The high quality of the trials and the intense scrutiny of their design and interpretation of outcome measures play a critical role in shaping the next generation of trials. We propose the following recommendations for researchers for future trials: (1) statistical power needed for clinical trials; (2) injury severity and timing of experimental therapy administration; (3) appropriate clinical trial outcome measures; and (4) prospective clinical trial design. These recommendations will be helpful for the SCI community in its further clinical evaluation of novel therapies[129].
Measuring the success of the Walking Index for SCI might be used, which was revised recently and is an international attempt to make a complex, valid and reliable device for assessing walking independent of burden of care[130]. Later, a multinational collaboration, led by the Toronto SCI team and several centers in Canada, the United States and Europe, developed a novel outcome measure to quantitatively assess hand and upper extremity function in tetraplegic patients (the Graded Redefined Assessment of Strength, Sensibility and Prehension (GRASSP) outcome measure). There are two important parameters in the development of new outcome measures, one which establishes psychometric properties and the other that provides insights into functional and neurological impairment[131].
CONCLUSION
A number of studies suggest early surgical intervention. Recovery of loss of neurological function after acute SCI is one of the most important topics of the neurological sciences (Table 5). For many years, many researchers have tried to find a method to improve neurological function in acute SCI but regeneration of the spinal cord has not yet been demonstrated in humans. Although there are major developments in the pharmacological and surgical approaches, SCI continues to be a very complex medical problem.
Table 5.
Timing of surgery and nonsurgical treatment options of spinal cord injury including pharmacological and cellular therapy
| Timing of surgery |
| Early surgical intervention is safe and feasible which can improve clinical and neurological outcomes and reduce health care costs |
| Early surgical intervention helps minimize the secondary damage caused by compression of the spinal cord after trauma |
| Pharmacological and cellular therapy |
| There is still no accepted pharmacological treatment protocol in SCI |
| Methylprednisolone is the accepted agent used in SCI, however, some criticism has been reported by some authors. It might be used in young patients without accompanying diseases such as diabetes mellitus |
| Cellular treatment studies are continuing |
SCI: Spinal cord injury.
ACKNOWLEDGMENTS
Promotional and commercial use of Figure 1 and Figure 2 in printed paper, digital or mobile device format is prohibited without the permission from the publisher Lippincott Williams and Wilkins. Please contact journalpermissions@lww.com for further information.
Footnotes
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: October 25, 2013
First decision: December 13, 2013
Article in press: April 29, 2014
P- Reviewer: Canavese F, Erkan S, Sewell M S- Editor: Ma YJ L- Editor: Roemmele A E- Editor: Wu HL
References
- 1.Aki T, Toya S. Experimental study on changes of the spinal-evoked potential and circulatory dynamics following spinal cord compression and decompression. Spine (Phila Pa 1976) 1984;9:800–809. doi: 10.1097/00007632-198411000-00006. [DOI] [PubMed] [Google Scholar]
- 2.Tator CH, Edmonds VE. Acute spinal cord injury: analysis of epidemiologic factors. Can J Surg. 1979;22:575–578. [PubMed] [Google Scholar]
- 3.Karacan I, Koyuncu H, Pekel O, Sümbüloglu G, Kirnap M, Dursun H, Kalkan A, Cengiz A, Yalinkiliç A, Unalan HI, et al. Traumatic spinal cord injuries in Turkey: a nation-wide epidemiological study. Spinal Cord. 2000;38:697–701. doi: 10.1038/sj.sc.3101064. [DOI] [PubMed] [Google Scholar]
- 4.Citterio A, Franceschini M, Spizzichino L, Reggio A, Rossi B, Stampacchia G. Nontraumatic spinal cord injury: an Italian survey. Arch Phys Med Rehabil. 2004;85:1483–1487. doi: 10.1016/j.apmr.2003.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Allen AR. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column. A preliminary report. JAMA. 1911;57:878–880. [Google Scholar]
- 6.Anderson DK, Means ED, Waters TR, Green ES. Microvascular perfusion and metabolism in injured spinal cord after methylprednisolone treatment. J Neurosurg. 1982;56:106–113. doi: 10.3171/jns.1982.56.1.0106. [DOI] [PubMed] [Google Scholar]
- 7.Tator CH, Fehlings MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;75:15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- 8.Amar AP, Levy ML. Pathogenesis and pharmacological strategies for mitigating secondary damage in acute spinal cord injury. Neurosurgery. 1999;44:1027–1039; discussion 1027-1039. doi: 10.1097/00006123-199905000-00052. [DOI] [PubMed] [Google Scholar]
- 9.Koyanagi I, Tator CH, Lea PJ. Three-dimensional analysis of the vascular system in the rat spinal cord with scanning electron microscopy of vascular corrosion casts. Part 2: Acute spinal cord injury. Neurosurgery. 1993;33:285–291; discussion 292. [PubMed] [Google Scholar]
- 10.Dumont RJ, Okonkwo DO, Verma S, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS. Acute spinal cord injury, part I: pathophysiologic mechanisms. Clin Neuropharmacol. 2001;24:254–264. doi: 10.1097/00002826-200109000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Fehlings MG, Tator CH, Linden RD. The effect of nimodipine and dextran on axonal function and blood flow following experimental spinal cord injury. J Neurosurg. 1989;71:403–416. doi: 10.3171/jns.1989.71.3.0403. [DOI] [PubMed] [Google Scholar]
- 12.Tator CH. Strategies for recovery and regeneration after brain and spinal cord injury. Inj Prev. 2002;8 Suppl 4:IV33–IV36. doi: 10.1136/ip.8.suppl_4.iv33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dolan EJ, Tator CH, Endrenyi L. The value of decompression for acute experimental spinal cord compression injury. J Neurosurg. 1980;53:749–755. doi: 10.3171/jns.1980.53.6.0749. [DOI] [PubMed] [Google Scholar]
- 14.Liau LM, Becker DP. A Comprehensive Reference Guide to the Diagnosis and Management of Neurological Problems. In: Youmans JR, eds , editors. Neurological Surgery, 4th ed. Philadelphia: Saunders; 1997. [Google Scholar]
- 15.Sakamoto A, Ohnishi ST, Ohnishi T, Ogawa R. Relationship between free radical production and lipid peroxidation during ischemia-reperfusion injury in the rat brain. Brain Res. 1991;554:186–192. doi: 10.1016/0006-8993(91)90187-z. [DOI] [PubMed] [Google Scholar]
- 16.Kaptanoglu E, Sen S, Beskonakli E, Surucu HS, Tuncel M, Kilinc K, Taskin Y. Antioxidant actions and early ultrastructural findings of thiopental and propofol in experimental spinal cord injury. J Neurosurg Anesthesiol. 2002;14:114–122. doi: 10.1097/00008506-200204000-00005. [DOI] [PubMed] [Google Scholar]
- 17.Kaptanoglu E, Solaroglu I, Okutan O, Surucu HS, Akbiyik F, Beskonakli E. Erythropoietin exerts neuroprotection after acute spinal cord injury in rats: effect on lipid peroxidation and early ultrastructural findings. Neurosurg Rev. 2004;27:113–120. doi: 10.1007/s10143-003-0300-y. [DOI] [PubMed] [Google Scholar]
- 18.Kaptanoglu E, Tuncel M, Palaoglu S, Konan A, Demirpençe E, Kilinç K. Comparison of the effects of melatonin and methylprednisolone in experimental spinal cord injury. J Neurosurg. 2000;93:77–84. doi: 10.3171/spi.2000.93.1.0077. [DOI] [PubMed] [Google Scholar]
- 19.Sharma HS, Olsson Y, Nyberg F. Influence of dynorphin A antibodies on the formation of edema and cell changes in spinal cord trauma. Prog Brain Res. 1995;104:401–416. doi: 10.1016/s0079-6123(08)61803-8. [DOI] [PubMed] [Google Scholar]
- 20.Li M, Ona VO, Chen M, Kaul M, Tenneti L, Zhang X, Stieg PE, Lipton SA, Friedlander RM. Functional role and therapeutic implications of neuronal caspase-1 and -3 in a mouse model of traumatic spinal cord injury. Neuroscience. 2000;99:333–342. doi: 10.1016/s0306-4522(00)00173-1. [DOI] [PubMed] [Google Scholar]
- 21.Lou J, Lenke LG, Ludwig FJ, O’Brien MF. Apoptosis as a mechanism of neuronal cell death following acute experimental spinal cord injury. Spinal Cord. 1998;36:683–690. doi: 10.1038/sj.sc.3100632. [DOI] [PubMed] [Google Scholar]
- 22.Fehlings MG, Tator CH. An evidence-based review of decompressive surgery in acute spinal cord injury: rationale, indications, and timing based on experimental and clinical studies. J Neurosurg. 1999;91:1–11. doi: 10.3171/spi.1999.91.1.0001. [DOI] [PubMed] [Google Scholar]
- 23.Croft TJ, Brodkey JS, Nulsen FE. Reversible spinal cord trauma: a model for electrical monitoring of spinal cord function. J Neurosurg. 1972;36:402–406. doi: 10.3171/jns.1972.36.4.0402. [DOI] [PubMed] [Google Scholar]
- 24.Thienprasit P, Bantli H, Bloedel JR, Chou SN. Effect of delayed local cooling on experimental spinal cord injury. J Neurosurg. 1975;42:150–154. doi: 10.3171/jns.1975.42.2.0150. [DOI] [PubMed] [Google Scholar]
- 25.Hejcl A, Urdzikova L, Sedy J, Lesny P, Pradny M, Michalek J, Burian M, Hajek M, Zamecnik J, Jendelova P, et al. Acute and delayed implantation of positively charged 2-hydroxyethyl methacrylate scaffolds in spinal cord injury in the rat. J Neurosurg Spine. 2008;8:67–73. doi: 10.3171/SPI-08/01/067. [DOI] [PubMed] [Google Scholar]
- 26.Delamarter RB, Sherman J, Carr JB. Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J Bone Joint Surg Am. 1995;77:1042–1049. doi: 10.2106/00004623-199507000-00010. [DOI] [PubMed] [Google Scholar]
- 27.Kobrine AI, Evans DE, Rizzoli HV. Experimental acute balloon compression of the spinal cord. Factors affecting disappearance and return of the spinal evoked response. J Neurosurg. 1979;51:841–845. doi: 10.3171/jns.1979.51.6.0841. [DOI] [PubMed] [Google Scholar]
- 28.Guha A, Tator CH, Endrenyi L, Piper I. Decompression of the spinal cord improves recovery after acute experimental spinal cord compression injury. Paraplegia. 1987;25:324–339. doi: 10.1038/sc.1987.61. [DOI] [PubMed] [Google Scholar]
- 29.Dimar JR, Glassman SD, Raque GH, Zhang YP, Shields CB. The influence of spinal canal narrowing and timing of decompression on neurologic recovery after spinal cord contusion in a rat model. Spine (Phila Pa 1976) 1999;24:1623–1633. doi: 10.1097/00007632-199908150-00002. [DOI] [PubMed] [Google Scholar]
- 30.Cadotte DW, Singh A, Fehlings MG. The timing of surgical decompression for spinal cord injury. F1000 Med Rep. 2010;2:67. doi: 10.3410/M2-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Glaser JA, Jaworski BA, Cuddy BG, Albert TJ, Hollowell JP, McLain RF, Bozzette SA. Variation in surgical opinion regarding management of selected cervical spine injuries. A preliminary study. Spine (Phila Pa 1976) 1998;23:975–982; discussion 983. doi: 10.1097/00007632-199805010-00002. [DOI] [PubMed] [Google Scholar]
- 32.Vaccaro AR, Daugherty RJ, Sheehan TP, Dante SJ, Cotler JM, Balderston RA, Herbison GJ, Northrup BE. Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine (Phila Pa 1976) 1997;22:2609–2613. doi: 10.1097/00007632-199711150-00006. [DOI] [PubMed] [Google Scholar]
- 33.Vale FL, Burns J, Jackson AB, Hadley MN. Combined medical and surgical treatment after acute spinal cord injury: results of a prospective pilot study to assess the merits of aggressive medical resuscitation and blood pressure management. J Neurosurg. 1997;87:239–246. doi: 10.3171/jns.1997.87.2.0239. [DOI] [PubMed] [Google Scholar]
- 34.Chipman JG, Deuser WE, Beilman GJ. Early surgery for thoracolumbar spine injuries decreases complications. J Trauma. 2004;56:52–57. doi: 10.1097/01.TA.0000108630.34225.85. [DOI] [PubMed] [Google Scholar]
- 35.McKinley W, Meade MA, Kirshblum S, Barnard B. Outcomes of early surgical management versus late or no surgical intervention after acute spinal cord injury. Arch Phys Med Rehabil. 2004;85:1818–1825. doi: 10.1016/j.apmr.2004.04.032. [DOI] [PubMed] [Google Scholar]
- 36.Furlan JC, Noonan V, Cadotte DW, Fehlings MG. Timing of decompressive surgery of spinal cord after traumatic spinal cord injury: an evidence-based examination of pre-clinical and clinical studies. J Neurotrauma. 2011;28:1371–1399. doi: 10.1089/neu.2009.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cengiz SL, Kalkan E, Bayir A, Ilik K, Basefer A. Timing of thoracolomber spine stabilization in trauma patients; impact on neurological outcome and clinical course. A real prospective (rct) randomized controlled study. Arch Orthop Trauma Surg. 2008;128:959–966. doi: 10.1007/s00402-007-0518-1. [DOI] [PubMed] [Google Scholar]
- 38.Ducker TB, Hamit HF. Experimental treatments of acute spinal cord injury. J Neurosurg. 1969;30:693–697. doi: 10.3171/jns.1969.30.6.0693. [DOI] [PubMed] [Google Scholar]
- 39.Young W, DeCrescito V, Flamm ES, Blight AR, Gruner JA. Pharmacological therapy of acute spinal cord injury: studies of high dose methylprednisolone and naloxone. Clin Neurosurg. 1988;34:675–697. [PubMed] [Google Scholar]
- 40.Kokoszka JE, Coskun P, Esposito LA, Wallace DC. Increased mitochondrial oxidative stress in the Sod2 (+/-) mouse results in the age-related decline of mitochondrial function culminating in increased apoptosis. Proc Natl Acad Sci USA. 2001;98:2278–2283. doi: 10.1073/pnas.051627098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bracken MB, Collins WF, Freeman DF, Shepard MJ, Wagner FW, Silten RM, Hellenbrand KG, Ransohoff J, Hunt WE, Perot PL. Efficacy of methylprednisolone in acute spinal cord injury. JAMA. 1984;251:45–52. [PubMed] [Google Scholar]
- 42.Bracken MB, Shepard MJ, Collins WF, Holford TR, Young W, Baskin DS, Eisenberg HM, Flamm E, Leo-Summers L, Maroon J. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N Engl J Med. 1990;322:1405–1411. doi: 10.1056/NEJM199005173222001. [DOI] [PubMed] [Google Scholar]
- 43.Bracken MB, Shepard MJ, Holford TR, Leo-Summers L, Aldrich EF, Fazl M, Fehlings M, Herr DL, Hitchon PW, Marshall LF, et al. Administration of methylprednisolone for 24 or 48 hours or tirilazad mesylate for 48 hours in the treatment of acute spinal cord injury. Results of the Third National Acute Spinal Cord Injury Randomized Controlled Trial. National Acute Spinal Cord Injury Study. JAMA. 1997;277:1597–1604. [PubMed] [Google Scholar]
- 44.Coleman WP, Benzel D, Cahill DW, Ducker T, Geisler F, Green B, Gropper MR, Goffin J, Madsen PW, Maiman DJ, et al. A critical appraisal of the reporting of the National Acute Spinal Cord Injury Studies (II and III) of methylprednisolone in acute spinal cord injury. J Spinal Disord. 2000;13:185–199. doi: 10.1097/00002517-200006000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Hurlbert RJ. Methylprednisolone for acute spinal cord injury: an inappropriate standard of care. J Neurosurg. 2000;93:1–7. doi: 10.3171/spi.2000.93.1.0001. [DOI] [PubMed] [Google Scholar]
- 46.Bose B, Osterholm JL, Kalia M. Ganglioside-induced regeneration and reestablishment of axonal continuity in spinal cord-transected rats. Neurosci Lett. 1986;63:165–169. doi: 10.1016/0304-3940(86)90055-8. [DOI] [PubMed] [Google Scholar]
- 47.Geisler FH, Dorsey FC, Coleman WP. Recovery of motor function after spinal-cord injury--a randomized, placebo-controlled trial with GM-1 ganglioside. N Engl J Med. 1991;324:1829–1838. doi: 10.1056/NEJM199106273242601. [DOI] [PubMed] [Google Scholar]
- 48.Imanaka T, Hukuda S, Maeda T. The role of GM1-ganglioside in the injured spinal cord of rats: an immunohistochemical study using GM1-antisera. J Neurotrauma. 1996;13:163–170. doi: 10.1089/neu.1996.13.163. [DOI] [PubMed] [Google Scholar]
- 49.Geisler FH, Coleman WP, Grieco G, Poonian D. The Sygen multicenter acute spinal cord injury study. Spine (Phila Pa 1976) 2001;26:S87–S98. doi: 10.1097/00007632-200112151-00015. [DOI] [PubMed] [Google Scholar]
- 50.Long JB, Kinney RC, Malcolm DS, Graeber GM, Holaday JW. Intrathecal dynorphin A1-13 and dynorphin A3-13 reduce rat spinal cord blood flow by non-opioid mechanisms. Brain Res. 1987;436:374–379. doi: 10.1016/0006-8993(87)91683-0. [DOI] [PubMed] [Google Scholar]
- 51.Baskin DS, Simpson RK, Browning JL, Dudley AW, Rothenberg F, Bogue L. The effect of long-term high-dose naloxone infusion in experimental blunt spinal cord injury. J Spinal Disord. 1993;6:38–43. [PubMed] [Google Scholar]
- 52.Winkler T, Sharma HS, Stålberg E, Olsson Y, Nyberg F. Naloxone reduces alterations in evoked potentials and edema in trauma to the rat spinal cord. Acta Neurochir Suppl (Wien. ) 1994;60:511–515. doi: 10.1007/978-3-7091-9334-1_140. [DOI] [PubMed] [Google Scholar]
- 53.Flamm ES, Young W, Collins WF, Piepmeier J, Clifton GL, Fischer B. A phase I trial of naloxone treatment in acute spinal cord injury. J Neurosurg. 1985;63:390–397. doi: 10.3171/jns.1985.63.3.0390. [DOI] [PubMed] [Google Scholar]
- 54.Faden AI, Jacobs TP, Mougey E, Holaday JW. Endorphins in experimental spinal injury: therapeutic effect of naloxone. Ann Neurol. 1981;10:326–332. doi: 10.1002/ana.410100403. [DOI] [PubMed] [Google Scholar]
- 55.Holaday JW, Faden AI. Naloxone acts at central opiate receptors to reverse hypotension, hypothermia and hypoventilation in spinal shock. Brain Res. 1980;189:295–300. doi: 10.1016/0006-8993(80)90032-3. [DOI] [PubMed] [Google Scholar]
- 56.Hashimoto T, Fukuda N. Effect of thyrotropin-releasing hormone on the neurologic impairment in rats with spinal cord injury: treatment starting 24 h and 7 days after injury. Eur J Pharmacol. 1991;203:25–32. doi: 10.1016/0014-2999(91)90786-p. [DOI] [PubMed] [Google Scholar]
- 57.Pitts LH, Ross A, Chase GA, Faden AI. Treatment with thyrotropin-releasing hormone (TRH) in patients with traumatic spinal cord injuries. J Neurotrauma. 1995;12:235–243. doi: 10.1089/neu.1995.12.235. [DOI] [PubMed] [Google Scholar]
- 58.Guha A, Tator CH, Piper I. Effect of a calcium channel blocker on posttraumatic spinal cord blood flow. J Neurosurg. 1987;66:423–430. doi: 10.3171/jns.1987.66.3.0423. [DOI] [PubMed] [Google Scholar]
- 59.Ford RW, Malm DN. Failure of nimodipine to reverse acute experimental spinal cord injury. Cent Nerv Syst Trauma. 1985;2:9–17. doi: 10.1089/cns.1985.2.9. [DOI] [PubMed] [Google Scholar]
- 60.Petitjean ME, Pointillart V, Dixmerias F, Wiart L, Sztark F, Lassié P, Thicoïpé M, Dabadie P. Medical treatment of spinal cord injury in the acute stage. Ann Fr Anesth Reanim. 1998;17:114–122. doi: 10.1016/s0750-7658(98)80058-0. [DOI] [PubMed] [Google Scholar]
- 61.Gaviria M, Privat A, d’Arbigny P, Kamenka J, Haton H, Ohanna F. Neuroprotective effects of a novel NMDA anta-gonist, Gacyclidine, after experimental contusive spinal cord injury in adult rats. Brain Res. 2000;874:200–209. doi: 10.1016/s0006-8993(00)02581-6. [DOI] [PubMed] [Google Scholar]
- 62.Davis SM, Albers GW, Diener HC, Lees KR, Norris J. Termination of Acute Stroke Studies Involving Selfotel Treatment. ASSIST Steering Committed. Lancet. 1997;349:32. doi: 10.1016/s0140-6736(05)62166-6. [DOI] [PubMed] [Google Scholar]
- 63.Hirbec H, Gaviria M, Vignon J. Gacyclidine: a new neuroprotective agent acting at the N-methyl-D-aspartate receptor. CNS Drug Rev. 2001;7:172–198. doi: 10.1111/j.1527-3458.2001.tb00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaptanoglu E, Beskonakli E, Solaroglu I, Kilinc A, Taskin Y. Magnesium sulfate treatment in experimental spinal cord injury: emphasis on vascular changes and early clinical results. Neurosurg Rev. 2003;26:283–287. doi: 10.1007/s10143-003-0272-y. [DOI] [PubMed] [Google Scholar]
- 65.Kaptanoglu E, Beskonakli E, Okutan O, Selcuk Surucu H, Taskin Y. Effect of magnesium sulphate in experimental spinal cord injury: evaluation with ultrastructural findings and early clinical results. J Clin Neurosci. 2003;10:329–334. doi: 10.1016/s0967-5868(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 66.Tüzgen S, Kaynar MY, Güner A, Gümüştaş K, Belce A, Etuş V, Ozyurt E. The effect of epidural cooling on lipid peroxidation after experimental spinal cord injury. Spinal Cord. 1998;36:654–657. doi: 10.1038/sj.sc.3100660. [DOI] [PubMed] [Google Scholar]
- 67.Yu CG, Jimenez O, Marcillo AE, Weider B, Bangerter K, Dietrich WD, Castro S, Yezierski RP. Beneficial effects of modest systemic hypothermia on locomotor function and histopathological damage following contusion-induced spinal cord injury in rats. J Neurosurg. 2000;93:85–93. doi: 10.3171/spi.2000.93.1.0085. [DOI] [PubMed] [Google Scholar]
- 68.Xu RX, Nakamura T, Nagao S, Miyamoto O, Jin L, Toyoshima T, Itano T. Specific inhibition of apoptosis after cold-induced brain injury by moderate postinjury hypothermia. Neurosurgery. 1998;43:107–114; discussion 114-115. doi: 10.1097/00006123-199807000-00070. [DOI] [PubMed] [Google Scholar]
- 69.Vanický I, Marsala M, Gálik J, Marsala J. Epidural perfusion cooling protection against protracted spinal cord ischemia in rabbits. J Neurosurg. 1993;79:736–741. doi: 10.3171/jns.1993.79.5.0736. [DOI] [PubMed] [Google Scholar]
- 70.Fu ES, Tummala RP. Neuroprotection in brain and spinal cord trauma. Curr Opin Anaesthesiol. 2005;18:181–187. doi: 10.1097/01.aco.0000162838.56344.88. [DOI] [PubMed] [Google Scholar]
- 71.Lee SM, Yune TY, Kim SJ, Park DW, Lee YK, Kim YC, Oh YJ, Markelonis GJ, Oh TH. Minocycline reduces cell death and improves functional recovery after traumatic spinal cord injury in the rat. J Neurotrauma. 2003;20:1017–1027. doi: 10.1089/089771503770195867. [DOI] [PubMed] [Google Scholar]
- 72.Stirling DP, Khodarahmi K, Liu J, McPhail LT, McBride CB, Steeves JD, Ramer MS, Tetzlaff W. Minocycline treatment reduces delayed oligodendrocyte death, attenuates axonal dieback, and improves functional outcome after spinal cord injury. J Neurosci. 2004;24:2182–2190. doi: 10.1523/JNEUROSCI.5275-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wells JE, Hurlbert RJ, Fehlings MG, Yong VW. Neuroprotection by minocycline facilitates significant recovery from spinal cord injury in mice. Brain. 2003;126:1628–1637. doi: 10.1093/brain/awg178. [DOI] [PubMed] [Google Scholar]
- 74.Teng YD, Choi H, Onario RC, Zhu S, Desilets FC, Lan S, Woodard EJ, Snyder EY, Eichler ME, Friedlander RM. Minocycline inhibits contusion-triggered mitochondrial cytochrome c release and mitigates functional deficits after spinal cord injury. Proc Natl Acad Sci USA. 2004;101:3071–3076. doi: 10.1073/pnas.0306239101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dergham P, Ellezam B, Essagian C, Avedissian H, Lubell WD, McKerracher L. Rho signaling pathway targeted to promote spinal cord repair. J Neurosci. 2002;22:6570–6577. doi: 10.1523/JNEUROSCI.22-15-06570.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Arishima Y, Setoguchi T, Yamaura I, Yone K, Komiya S. Preventive effect of erythropoietin on spinal cord cell apoptosis following acute traumatic injury in rats. Spine (Phila Pa 1976) 2006;31:2432–2438. doi: 10.1097/01.brs.0000239124.41410.7a. [DOI] [PubMed] [Google Scholar]
- 77.Gorio A, Gokmen N, Erbayraktar S, Yilmaz O, Madaschi L, Cichetti C, Di Giulio AM, Vardar E, Cerami A, Brines M. Recombinant human erythropoietin counteracts secondary injury and markedly enhances neurological recovery from experimental spinal cord trauma. Proc Natl Acad Sci USA. 2002;99:9450–9455. doi: 10.1073/pnas.142287899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cetin A, Nas K, Büyükbayram H, Ceviz A, Olmez G. The effects of systemically administered methylprednisolone and recombinant human erythropoietin after acute spinal cord compressive injury in rats. Eur Spine J. 2006;15:1539–1544. doi: 10.1007/s00586-006-0091-2. [DOI] [PubMed] [Google Scholar]
- 79.Thomas AJ, Nockels RP, Pan HQ, Shaffrey CI, Chopp M. Progesterone is neuroprotective after acute experimental spinal cord trauma in rats. Spine (Phila Pa 1976) 1999;24:2134–2138. doi: 10.1097/00007632-199910150-00013. [DOI] [PubMed] [Google Scholar]
- 80.Hall ED, Wolf DL. A pharmacological analysis of the pathophysiological mechanisms of posttraumatic spinal cord ischemia. J Neurosurg. 1986;64:951–961. doi: 10.3171/jns.1986.64.6.0951. [DOI] [PubMed] [Google Scholar]
- 81.Dumont RJ, Verma S, Okonkwo DO, Hurlbert RJ, Boulos PT, Ellegala DB, Dumont AS. Acute spinal cord injury, part II: contemporary pharmacotherapy. Clin Neuropharmacol. 2001;24:265–279. doi: 10.1097/00002826-200109000-00003. [DOI] [PubMed] [Google Scholar]
- 82.Kitzman PH. Effectiveness of riluzole in suppressing spasticity in the spinal cord injured rat. Neurosci Lett. 2009;455:150–153. doi: 10.1016/j.neulet.2009.03.016. [DOI] [PubMed] [Google Scholar]
- 83.Springer JE, Azbill RD, Kennedy SE, George J, Geddes JW. Rapid calpain I activation and cytoskeletal protein degradation following traumatic spinal cord injury: attenuation with riluzole pretreatment. J Neurochem. 1997;69:1592–1600. doi: 10.1046/j.1471-4159.1997.69041592.x. [DOI] [PubMed] [Google Scholar]
- 84.Stutzmann JM, Pratt J, Boraud T, Gross C. The effect of riluzole on post-traumatic spinal cord injury in the rat. Neuroreport. 1996;7:387–392. doi: 10.1097/00001756-199601310-00003. [DOI] [PubMed] [Google Scholar]
- 85.Pannu R, Barbosa E, Singh AK, Singh I. Attenuation of acute inflammatory response by atorvastatin after spinal cord injury in rats. J Neurosci Res. 2005;79:340–350. doi: 10.1002/jnr.20345. [DOI] [PubMed] [Google Scholar]
- 86.Fujimoto T, Nakamura T, Ikeda T, Taoka Y, Takagi K. Effects of EPC-K1 on lipid peroxidation in experimental spinal cord injury. Spine (Phila Pa 1976) 2000;25:24–29. doi: 10.1097/00007632-200001010-00006. [DOI] [PubMed] [Google Scholar]
- 87.Sharma HS, Badgaiyan RD, Alm P, Mohanty S, Wiklund L. Neuroprotective effects of nitric oxide synthase inhibitors in spinal cord injury-induced pathophysiology and motor functions: an experimental study in the rat. Ann N Y Acad Sci. 2005;1053:422–434. doi: 10.1111/j.1749-6632.2005.tb00051.x. [DOI] [PubMed] [Google Scholar]
- 88.Baptiste DC, Austin JW, Zhao W, Nahirny A, Sugita S, Fehlings MG. Systemic polyethylene glycol promotes neurological recovery and tissue sparing in rats after cervical spinal cord injury. J Neuropathol Exp Neurol. 2009;68:661–676. doi: 10.1097/NEN.0b013e3181a72605. [DOI] [PubMed] [Google Scholar]
- 89.Davis AE, Campbell SJ, Wilainam P, Anthony DC. Post-conditioning with lipopolysaccharide reduces the inflammatory infiltrate to the injured brain and spinal cord: a potential neuroprotective treatment. Eur J Neurosci. 2005;22:2441–2450. doi: 10.1111/j.1460-9568.2005.04447.x. [DOI] [PubMed] [Google Scholar]
- 90.Ditor DS, Bao F, Chen Y, Dekaban GA, Weaver LC. A therapeutic time window for anti-CD 11d monoclonal antibody treatment yielding reduced secondary tissue damage and enhanced behavioral recovery following severe spinal cord injury. J Neurosurg Spine. 2006;5:343–352. doi: 10.3171/spi.2006.5.4.343. [DOI] [PubMed] [Google Scholar]
- 91.Liu F, You SW, Yao LP, Liu HL, Jiao XY, Shi M, Zhao QB, Ju G. Secondary degeneration reduced by inosine after spinal cord injury in rats. Spinal Cord. 2006;44:421–426. doi: 10.1038/sj.sc.3101878. [DOI] [PubMed] [Google Scholar]
- 92.McTigue DM, Tripathi R, Wei P, Lash AT. The PPAR gamma agonist Pioglitazone improves anatomical and locomotor recovery after rodent spinal cord injury. Exp Neurol. 2007;205:396–406. doi: 10.1016/j.expneurol.2007.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.García-Alías G, López-Vales R, Forés J, Navarro X, Verdú E. Acute transplantation of olfactory ensheathing cells or Schwann cells promotes recovery after spinal cord injury in the rat. J Neurosci Res. 2004;75:632–641. doi: 10.1002/jnr.20029. [DOI] [PubMed] [Google Scholar]
- 94.McDonald JW, Liu XZ, Qu Y, Liu S, Mickey SK, Turetsky D, Gottlieb DI, Choi DW. Transplanted embryonic stem cells survive, differentiate and promote recovery in injured rat spinal cord. Nat Med. 1999;5:1410–1412. doi: 10.1038/70986. [DOI] [PubMed] [Google Scholar]
- 95.Akiyama Y, Honmou O, Kato T, Uede T, Hashi K, Kocsis JD. Transplantation of clonal neural precursor cells derived from adult human brain establishes functional peripheral myelin in the rat spinal cord. Exp Neurol. 2001;167:27–39. doi: 10.1006/exnr.2000.7539. [DOI] [PubMed] [Google Scholar]
- 96.Liu Y, Himes BT, Murray M, Tessler A, Fischer I. Grafts of BDNF-producing fibroblasts rescue axotomized rubrospinal neurons and prevent their atrophy. Exp Neurol. 2002;178:150–164. doi: 10.1006/exnr.2002.7977. [DOI] [PubMed] [Google Scholar]
- 97.Oudega M, Xu XM. Schwann cell transplantation for repair of the adult spinal cord. J Neurotrauma. 2006;23:453–467. doi: 10.1089/neu.2006.23.453. [DOI] [PubMed] [Google Scholar]
- 98.Beattie MS, Bresnahan JC, Komon J, Tovar CA, Van Meter M, Anderson DK, Faden AI, Hsu CY, Noble LJ, Salzman S, et al. Endogenous repair after spinal cord contusion injuries in the rat. Exp Neurol. 1997;148:453–463. doi: 10.1006/exnr.1997.6695. [DOI] [PubMed] [Google Scholar]
- 99.Barakat DJ, Gaglani SM, Neravetla SR, Sanchez AR, Andrade CM, Pressman Y, Puzis R, Garg MS, Bunge MB, Pearse DD. Survival, integration, and axon growth support of glia transplanted into the chronically contused spinal cord. Cell Transplant. 2005;14:225–240. doi: 10.3727/000000005783983106. [DOI] [PubMed] [Google Scholar]
- 100.Baptiste DC, Fehlings MG. Update on the treatment of spinal cord injury. Prog Brain Res. 2007;161:217–233. doi: 10.1016/S0079-6123(06)61015-7. [DOI] [PubMed] [Google Scholar]
- 101.Muñoz-Elías G, Woodbury D, Black IB. Marrow stromal cells, mitosis, and neuronal differentiation: stem cell and precursor functions. Stem Cells. 2003;21:437–448. doi: 10.1634/stemcells.21-4-437. [DOI] [PubMed] [Google Scholar]
- 102.Cummings BJ, Uchida N, Tamaki SJ, Salazar DL, Hooshmand M, Summers R, Gage FH, Anderson AJ. Human neural stem cells differentiate and promote locomotor recovery in spinal cord-injured mice. Proc Natl Acad Sci USA. 2005;102:14069–14074. doi: 10.1073/pnas.0507063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rapalino O, Lazarov-Spiegler O, Agranov E, Velan GJ, Yoles E, Fraidakis M, Solomon A, Gepstein R, Katz A, Belkin M, et al. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med. 1998;4:814–821. doi: 10.1038/nm0798-814. [DOI] [PubMed] [Google Scholar]
- 104.Perry VH, Brown MC, Gordon S. The macrophage response to central and peripheral nerve injury. A possible role for macrophages in regeneration. J Exp Med. 1987;165:1218–1223. doi: 10.1084/jem.165.4.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takami T, Oudega M, Bates ML, Wood PM, Kleitman N, Bunge MB. Schwann cell but not olfactory ensheathing glia transplants improve hindlimb locomotor performance in the moderately contused adult rat thoracic spinal cord. J Neurosci. 2002;22:6670–6681. doi: 10.1523/JNEUROSCI.22-15-06670.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Blight AR, Young W. Central axons in injured cat spinal cord recover electrophysiological function following remyelination by Schwann cells. J Neurol Sci. 1989;91:15–34. doi: 10.1016/0022-510x(89)90073-7. [DOI] [PubMed] [Google Scholar]
- 107.Firouzi M, Moshayedi P, Saberi H, Mobasheri H, Abolhassani F, Jahanzad I, Raza M. Transplantation of Schwann cells to subarachnoid space induces repair in contused rat spinal cord. Neurosci Lett. 2006;402:66–70. doi: 10.1016/j.neulet.2006.03.070. [DOI] [PubMed] [Google Scholar]
- 108.Gilmore SA. Autoradiographic studies of intramedullary Schwann cells in irradiated spinal cords of immature rats. Anat Rec. 1971;171:517–528. doi: 10.1002/ar.1091710408. [DOI] [PubMed] [Google Scholar]
- 109.Huard JM, Youngentob SL, Goldstein BJ, Luskin MB, Schwob JE. Adult olfactory epithelium contains multipotent progenitors that give rise to neurons and non-neural cells. J Comp Neurol. 1998;400:469–486. [PubMed] [Google Scholar]
- 110.Boyd JG, Doucette R, Kawaja MD. Defining the role of olfactory ensheathing cells in facilitating axon remyelination following damage to the spinal cord. FASEB J. 2005;19:694–703. doi: 10.1096/fj.04-2833rev. [DOI] [PubMed] [Google Scholar]
- 111.Boyd JG, Skihar V, Kawaja M, Doucette R. Olfactory ensheathing cells: historical perspective and therapeutic potential. Anat Rec B New Anat. 2003;271:49–60. doi: 10.1002/ar.b.10011. [DOI] [PubMed] [Google Scholar]
- 112.Keyvan-Fouladi N, Li Y, Raisman G. How do transplanted olfactory ensheathing cells restore function? Brain Res Brain Res Rev. 2002;40:325–327. doi: 10.1016/s0165-0173(02)00215-1. [DOI] [PubMed] [Google Scholar]
- 113.Mackay-Sim A, Féron F, Cochrane J, Bassingthwaighte L, Bayliss C, Davies W, Fronek P, Gray C, Kerr G, Licina P, et al. Autologous olfactory ensheathing cell transplantation in human paraplegia: a 3-year clinical trial. Brain. 2008;131:2376–2386. doi: 10.1093/brain/awn173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Guest J, Herrera LP, Qian T. Rapid recovery of segmental neurological function in a tetraplegic patient following transplantation of fetal olfactory bulb-derived cells. Spinal Cord. 2006;44:135–142. doi: 10.1038/sj.sc.3101820. [DOI] [PubMed] [Google Scholar]
- 115.Lima C, Pratas-Vital J, Escada P, Hasse-Ferreira A, Capucho C, Peduzzi JD. Olfactory mucosa autografts in human spinal cord injury: a pilot clinical study. J Spinal Cord Med. 2006;29:191–203; discussion 204-206. doi: 10.1080/10790268.2006.11753874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Sanchez-Ramos J, Song S, Cardozo-Pelaez F, Hazzi C, Stedeford T, Willing A, Freeman TB, Saporta S, Janssen W, Patel N, et al. Adult bone marrow stromal cells differentiate into neural cells in vitro. Exp Neurol. 2000;164:247–256. doi: 10.1006/exnr.2000.7389. [DOI] [PubMed] [Google Scholar]
- 117.Chopp M, Zhang XH, Li Y, Wang L, Chen J, Lu D, Lu M, Rosenblum M. Spinal cord injury in rat: treatment with bone marrow stromal cell transplantation. Neuroreport. 2000;11:3001–3005. doi: 10.1097/00001756-200009110-00035. [DOI] [PubMed] [Google Scholar]
- 118.Akiyama Y, Radtke C, Kocsis JD. Remyelination of the rat spinal cord by transplantation of identified bone marrow stromal cells. J Neurosci. 2002;22:6623–6630. doi: 10.1523/JNEUROSCI.22-15-06623.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen Q, Long Y, Yuan X, Zou L, Sun J, Chen S, Perez-Polo JR, Yang K. Protective effects of bone marrow stromal cell transplantation in injured rodent brain: synthesis of neurotrophic factors. J Neurosci Res. 2005;80:611–619. doi: 10.1002/jnr.20494. [DOI] [PubMed] [Google Scholar]
- 120.Kawada H, Takizawa S, Takanashi T, Morita Y, Fujita J, Fukuda K, Takagi S, Okano H, Ando K, Hotta T. Administration of hematopoietic cytokines in the subacute phase after cerebral infarction is effective for functional recovery facilitating proliferation of intrinsic neural stem/progenitor cells and transition of bone marrow-derived neuronal cells. Circulation. 2006;113:701–710. doi: 10.1161/CIRCULATIONAHA.105.563668. [DOI] [PubMed] [Google Scholar]
- 121.Caroni P, Schwab ME. Antibody against myelin-associated inhibitor of neurite growth neutralizes nonpermissive substrate properties of CNS white matter. Neuron. 1988;1:85–96. doi: 10.1016/0896-6273(88)90212-7. [DOI] [PubMed] [Google Scholar]
- 122.Schwartz M, Cohen A, Stein-Izsak C, Belkin M. Dichotomy of the glial cell response to axonal injury and regeneration. FASEB J. 1989;3:2371–2378. doi: 10.1096/fasebj.3.12.2676680. [DOI] [PubMed] [Google Scholar]
- 123.Heumann R, Lindholm D, Bandtlow C, Meyer M, Radeke MJ, Misko TP, Shooter E, Thoenen H. Differential regulation of mRNA encoding nerve growth factor and its receptor in rat sciatic nerve during development, degeneration, and regeneration: role of macrophages. Proc Natl Acad Sci USA. 1987;84:8735–8739. doi: 10.1073/pnas.84.23.8735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Barres BA, Hart IK, Coles HS, Burne JF, Voyvodic JT, Richardson WD, Raff MC. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- 125.Blight AR. Delayed demyelination and macrophage invasion: a candidate for secondary cell damage in spinal cord injury. Cent Nerv Syst Trauma. 1985;2:299–315. doi: 10.1089/cns.1985.2.299. [DOI] [PubMed] [Google Scholar]
- 126.Bunge RP, Puckett WR, Becerra JL, Marcillo A, Quencer RM. Observations on the pathology of human spinal cord injury. A review and classification of 22 new cases with details from a case of chronic cord compression with extensive focal demyelination. Adv Neurol. 1993;59:75–89. [PubMed] [Google Scholar]
- 127.Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- 128.Liu XZ, Xu XM, Hu R, Du C, Zhang SX, McDonald JW, Dong HX, Wu YJ, Fan GS, Jacquin MF, et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hawryluk GW, Rowland J, Kwon BK, Fehlings MG. Protection and repair of the injured spinal cord: a review of completed, ongoing, and planned clinical trials for acute spinal cord injury. Neurosurg Focus. 2008;25:E14. doi: 10.3171/FOC.2008.25.11.E14. [DOI] [PubMed] [Google Scholar]
- 130.Dittuno PL, Ditunno JF. Walking index for spinal cord injury (WISCI II): scale revision. Spinal Cord. 2001;39:654–656. doi: 10.1038/sj.sc.3101223. [DOI] [PubMed] [Google Scholar]
- 131.Kalsi-Ryan S, Curt A, Verrier MC, Fehlings MG. Development of the Graded Redefined Assessment of Strength, Sensibility and Prehension (GRASSP): reviewing measurement specific to the upper limb in tetraplegia. J Neurosurg Spine. 2012;17:65–76. doi: 10.3171/2012.6.AOSPINE1258. [DOI] [PubMed] [Google Scholar]


