Abstract
There is anatomical and functional connectivity between the primary motor cortex (M1) and posterior parietal cortex (PPC) that plays a role in sensorimotor integration. In this study, we applied corticocortical paired-associative stimuli to ipsilateral PPC and M1 (parietal ccPAS) in healthy right-handed subjects to test if this procedure could modulate M1 excitability and PPC–M1 connectivity. One hundred and eighty paired transcranial magnetic stimuli to the PPC and M1 at an interstimulus interval (ISI) of 8 ms were delivered at 0.2 Hz. We found that parietal ccPAS in the left hemisphere increased the excitability of conditioned left M1 assessed by motor evoked potentials (MEPs) and the input–output curve. Motor behavior assessed by the Purdue pegboard task was unchanged compared with controls. At baseline, conditioning stimuli over the left PPC potentiated MEPs from left M1 when ISI was 8 ms. This interaction significantly attenuated at 60 min after left parietal ccPAS. Additional experiments showed that parietal ccPAS induced plasticity was timing-dependent, was absent if ISI was 100 ms, and could also be seen in the right hemisphere. Our results suggest that parietal ccPAS can modulate M1 excitability and PPC–M1 connectivity and is a new approach to modify motor excitability and sensorimotor interaction.
Keywords: associative plasticity, corticocortical paired-associative stimulation, motor cortex, parietal motor connectivity, posterior parietal cortex
Introduction
Associative plasticity is supposed to be a physiological mechanism of memory and learning (Hebb 1949; Antonov et al. 2003; Cassenaer and Laurent 2012). It could occur when an input to a postsynaptic neuron coincides with another input to the same neuron or postsynaptic depolarization with a specific temporal order. Paired-associative stimulation (PAS), as originally described, combines a peripheral somatosensory input with transcranial magnetic stimulation (TMS) over primary motor cortex (M1) at specific time intervals and can induce associative plasticity via mechanisms of spike-timing-dependent plasticity (Stefan et al. 2000, 2002; Wolters et al. 2003). Recently, several studies showed that similar associative plasticity can be induced by corticocortical PAS (ccPAS) in which the afferent stimulation of M1 generated by TMS at an interconnected area, such as contralateral M1, ventral premotor, or supplementary motor areas, is time-locked to TMS over the M1 (Koganemaru et al. 2009; Rizzo et al. 2009; Arai et al. 2011; Buch et al. 2011). The induced plasticity is specific to the interstimulus interval (ISI). Thus, ccPAS provides a method to modify the excitability and functional connectivity of M1.
The posterior parietal cortex (PPC) receives sensory input from sensory cortical regions and has strong reciprocal connections with frontal motor areas (Hyvarinen 1982; Babb et al. 1984; Prevosto et al. 2011). The PPC acts as the interface between sensory and motor cortical areas and is important for sensorimotor integration (Medendorp et al. 2005; Buneo and Andersen 2006; Bernier and Grafton 2010). The neurophysiological interaction between PPC and ipsilateral M1 was demonstrated by a paired-pulse TMS protocol. A conditioning TMS stimulus over PPC shortly prior to a test stimulus (TS) over the hand area of M1 can facilitate the motor evoked potentials (MEPs) when the interval between the PPC and M1 stimuli is 4–8ms (Koch et al. 2007; Karabanov et al. 2013); this indicates the alteration of M1 excitability by PPC activation and thus, implies functional PPC–M1 connectivity.
In this study, we introduce a novel parietal ccPAS protocol aimed to test the effects of repeated associative stimulation of PPC and M1 on M1 excitability, PPC–M1 connectivity, and a simple motor task (Purdue Pegboard task). The order and interval of the paired stimulations are important for effectively producing associative plasticity (Bi and Poo 1998; Wolters et al. 2003; Tzounopoulos et al. 2004), so we chose to stimulate left PPC 8 ms before left M1 since this interval showed the most consistent facilitation during previous studies from our group (Karabanov et al. 2013). We tested left M1 excitability, left PPC–M1 connectivity, and pegboard performance prior to and at several time points after parietal ccPAS. We hypothesized that parietal ccPAS at an ISI of 8 ms would enhance M1 excitability and modify the interaction strength between the PPC and M1. In an additional experiment, we tested the timing specificity of the observed parietal ccPAS effects by stimulating the left PPC 100 ms prior to left M1. Since hemispheric asymmetry in parietal–motor connectivity and parietal function has been reported by several TMS and imaging studies (Iacoboni 2006; Koch et al. 2011), we also tested whether similar effects could be produced by right parietal ccPAS.
Materials and Methods
Subjects
Thirty-five healthy volunteers (15 females), aged 20–46 years (29.7 ± 8.0 years), took part in the experiment. All subjects were right-handed, based on the Edinburgh Handedness Inventory (Oldfield 1971); none of the participants had a history of neurological or medical illness or was taking medication. All participants were naive to the design and hypotheses of the study. Three subjects were excluded from the study, because the spatial overlap of the parietal and motor TMS coils did not allow accurate positioning. Some subjects participated in more than one substudy, which were at least 3 weeks apart (average, 69.3 ± 33.3 days). The study was approved by the Institutional Review Board (IRB) of the National Institute of Neurological Disorders and Stroke (NINDS). Written informed consent was obtained from all subjects before the experiments. The experimental procedures were performed in accordance with the Declaration of Helsinki.
Recording Procedure
Electromyographic (EMG) activity was recorded from the first dorsal interosseous (FDI) muscle in a belly-tendon montage using a pair of Ag–AgCl surface electrodes. Impedance was kept <20 kΩ. The signal was amplified using an EMG system (Nicolet Viking) and band-pass filtered (20–2000 Hz). The signal was digitized at a frequency of 5 kHz using the Labview software (National Instruments, Austin, TX, USA) and stored on a computer for off-line analysis.
Participants were seated comfortably in an armchair with the chin placed in a chin rest. A pillow supported the participants' hands and arms and acoustic feedback from the EMG was used to ensure a relaxed state throughout the experiment. Magnetic stimulation was delivered to the M1 using a custom-made figure-of-eight coil (40 mm double coils with the handle pointing upward) connected to a Magstim 200 magnetic stimulator (Magstim Company Ltd, Whitland, Dyfed, UK). The coil was placed tangentially on the scalp such that the anteroposterior axis of the coil was perpendicular to the central sulcus, in an approximately 45° angle from the head's anteroposterior axis. The optimal scalp position of left M1 was defined as the point (left M1HAND) where stimulation evoked the largest MEPs recorded from the right FDI muscle. This position was marked for reference and the coil position was continually checked throughout the experiment. For each subject, MEPs were recorded and their amplitude was defined as the maximal peak-to-peak amplitude in each single trial. The rest motor threshold (rMT) was defined as the lowest stimulator intensity required to elicit MEPs of at least 50 μV in the relaxed FDI in at least 5 of 10 consecutive pulses (Rossini et al. 1994).
Parietal ccPAS: PPC–M1 Corticocortical Paired-Associative Stimulation
The parietal ccPAS intervention consisted of 180 paired TMS pulses over the left hemisphere. Each stimulus pair consisted of a TMS pulse given to the left PPC followed by a TMS pulse given to the left M1HAND. The PPC stimulus always preceded M1HAND stimulus by 8 ms. The 180 stimulus pairs were delivered every 5 s (0.2 Hz) over a period of 15 min and were given through 2 Magstim 200 magnetic stimulators connected to 2 custom-made 40-mm double coils. The coil position for the PPC was defined as the P3 position of the international 10–20 electrode (EEG) system. This point was chosen because its corresponding cortical location in different subjects could reliably be within the PPC near posterior intraparietal sulcus (Herwig et al. 2003; Okamoto et al. 2004). In our previous study mapping different intrahemispheric parietal–motor networks, we used a neuronavigation system, but there were considerable intersubject differences in parietal–motor connectivity and the most effective stimulation points within the parietal sulcus could not be determined by the anatomy (Karabanov et al. 2013). Here, we did not see any advantage in using that method in this study. The coil was placed tangentially to the skull and along the anterioposterior direction of head to induce a posterioanteriorly directed current in the underlying cortical tissue. The orientation was chosen according to Koch et al. (2007) and Karabanov et al. (2012). The intensity of TMS at the PPC was set at 90% of rMT and that at M1HAND was adjusted to evoke a MEP amplitude of approximately 1 mV in the contralateral relaxed FDI.
Experiment 1: Effects of Parietal ccPAS on M1 Excitability and Motor Behavior
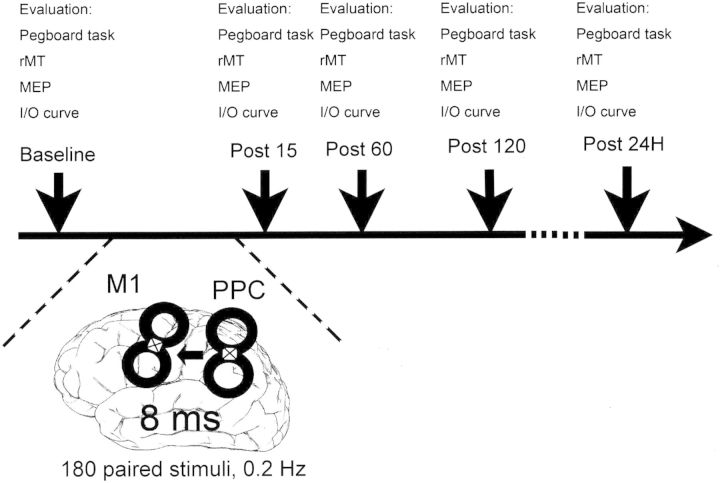
Twelve subjects were enrolled (5 women; 27 ± 7.4 years). This experiment was designed to investigate the effects of left parietal ccPAS on the excitability of the conditioned M1HAND. The induced changes of the conditioned M1HAND were assessed at 4 time points: Before (baseline) and at 15, 60, and 120 min (P15, P60, and P120) after ccPAS. A further time point at 24 h after ccPAS (P24H) was added after enrollment of the first 4 subjects, because a possible effect of ccPAS was still noted at P120. Each assessment consisted of the following measurements: (1) Behavioral measure of parietal–motor function; (2) the determination of the rMT; (3) short measure of cortical excitability by MEP amplitude; and (4) more detailed measure of cortical excitability by the input–output curve of the corticospinal tract (I/O curve; Fig. 1).
Figure 1.
Design of the parietal ccPAS study. The parietal ccPAS contained 180 pairs of TMS stimuli delivered at 0.2 Hz. In each pair of stimuli, the TMS stimulus at PPC was always 8 ms before the TMS stimulus at the ipsilateral M1. The coils we used for ccPAS are custom-made coils with the handle perpendicular to the plane of the coil. In this figure, the white square with a cross inside represents the handle. Pegboard task, rMT, MEP, and I/O curve were evaluated before (baseline), and 15, 60, 120 min and 24 h (P15, P60, P120, and P24H) after the parietal ccPAS.
The behavioral task was a modified version of the Purdue pegboard task, a widely used measure of fine finger control that was shown to be reliable and valid (Tiffin and Asher 1948). The Purdue pegboard consists of 2 rows of small holes surmounted by 4 cups containing small metal pins. Subjects were asked to pick up pins from the cups and place them into the holes on the board in order as quickly as possible. The number of correctly placed pins was recorded at the end of a 30-s trial. At the beginning of the experiment, subjects were instructed about the task and performed one practice trial. At each time point, participants performed 5 pegboard trials, which were averaged for statistical analysis.
For MEP recordings, the TMS stimulator was adjusted to an intensity that evoked an MEP amplitude of approximately 1 mV when applied to M1HAND before the ccPAS intervention. Ten consecutive MEPs were recorded at that intensity. This baseline stimulus intensity was also used to elicit MEPs at the time points following the cc-PAS intervention. To generate the I/O curve, intensities of the TMS stimuli were individually adapted according to the predefined rMT at each time point. Ten MEPs were recorded with each of the following TMS intensities in a pseudorandom order: 80%, 90%, 100%, 110%, 120%, 130%, 150%, 160%, and 170% of rMT. For each subject, the mean amplitude of 10 MEPs at each intensity was calculated for statistical analyses.
Experiment 2: Effects of Parietal ccPAS on PPC–M1 Connectivity
Twelve subjects were enrolled (5 women; 35.2 ± 8.7 years; 5 had participated in experiment 1). This experiment aimed to test the changes of PPC–M1 interaction after parietal cc-PAS. Since we assumed that the plastic changes induced by parietal ccPAS would occur only for a short time, the interaction was assessed before (baseline), and at 15 and 60 min (P15 and P60) after the end of ccPAS.
The left PPC–left M1 interaction was studied using dual-site paired-pulse TMS. The conditioning stimulus (CS) was applied to P3 first; the TS was applied to left M1HAND second with varying ISIs of 4, 6, and 8 ms. In all recordings, the CS was applied at 90% rMT and the TS was applied at an intensity set to evoke a MEP amplitude of 1 mV over the FDI at rest (Koch et al. 2007). Eighteen stimuli were applied for each of the 4 conditions (TS alone and CS + TS at 3 different ISIs). MEP amplitude was determined by averaging all the amplitudes under each condition.
Experiment 3: Interval Specificity of Parietal ccPAS
Eight subjects were enrolled (6 women; 28 ± 7.6 years; 7 had participated in experiment 1). This experiment was added to test whether the aftereffects of left parietal ccPAS were different for a specific ISI. An ISI of 100 ms was chosen in this experiment. With such a long interval between pulses, the conditioning volley should arrive at M1HAND too early to coincide with the second TMS pulse given directly to M1HAND and is unlikely to lead to associative plasticity.
We assessed the excitability of the conditioned left M1HAND before (baseline) and at 15, 60, and 120 min (P15, P60, and P120) after the end of cc-PAS. Excitability of the conditioned M1HAND was assessed by rMT, MEPs, and the I/O curve of the corticospinal tract in the relaxed right FDI.
Experiment 4: Learning Effects with the Pegboard
Twelve subjects were enrolled (5 women; 28.1 ± 7.3 years; none participated in experiment 1). In this experiment, we repeated the Purdue pegboard task in a different group of subjects to test whether the behavior differences before and after parietal ccPAS in experiment 1 were due to the effects of ccPAS or just simply a learning effect from repeated task performance. The contents of the Purdue pegboard test and the instructions for the subjects were all the same as in experiment 1. Each subject repetitively performed the Purdue pegboard task at 5 times points similar to the timing in experiment 1: the beginning, 75, 120, 180 min, and 24 h later (corresponding to baseline, P15, P60, P120, and P24H, respectively). No TMS stimulation was performed in this experiment.
Experiment 5: Parietal ccPAS in the Right Hemisphere
Seven subjects were enrolled (2 women; 34.6 ± 7.9 years; none had participated in experiment 1). In this experiment, we tested whether the right parietal ccPAS with an 8-ms interval would induce similar effects on the right M1HAND excitability. The coil position for the PPC was defined relative to the P4 position of the international 10–20 EEG system. We measured rMT, MEPs, and the I/O curve of the corticospinal tract from the left FDI to assess the cortical excitability of the conditioned right M1HAND before (baseline) and at 15, 60, and 120 min (P15, P60, and P120) after right parietal ccPAS. To test the site-specific effects of parietal ccPAS and to exclude any possible systemic confounding factors that might increase M1 excitability during the study, we also delivered TMS to left M1HAND and recorded the MEPs in the relaxed right FDI at the above time points.
Statistical Analysis
This study involved 5 quantitative outcome measures: Purdue pegboard test, rMT, MEPs, I/O curve, and PPC–M1 interaction by paired-pulse TMS. Since each measure was taken multiple times from every individual subject, repeated-measures analyses of variance (ANOVAs) were applied to assess main effects or interactions. Normality assumption was evaluated by the Shapiro–Wilk test. If the assumption was not met, natural logarithm transformation was applied.
For the MEPs and rMT, the effect of Time (within-subject variable) was assessed by one-factor repeated-measures ANOVA. For the I/O curve, first a natural logarithm transformation was applied, and then the effects of Time (within-subject variable), TMS intensity (within-subject variable), and their interaction were assessed by 2-factor repeated-measures ANOVA. For the Purdue pegboard task, the effects of Time (within-subject variable), Intervention (between-subject variable), and the interaction between Time and Intervention were assessed by 2-factor repeated-measures ANOVA with subjects treated as the random effect. For paired-pulse TMS, first, the effect of ISI (TS alone, 4, 6, and 8 ms; within-subject variable) was assessed using the data at each time point (baseline, P15, and P60) by one-factor repeated-measure ANOVA. Then, a 2-factor repeated-measures ANOVA using ISI (within-subject variable) and Time (within-subject variable) was performed to test the changes of PPC–M1 interaction before and after parietal ccPAS. Dunnett-Hsu was used for multiple comparisons with the control group. Numerical variables are expressed as the mean ± SD. A P-value of < 0.05 was considered significant. All statistical analyses were performed using SAS version 9.2.
Results
Experiment 1: Effects of Left Parietal ccPAS on Left M1 Excitability (n = 12)
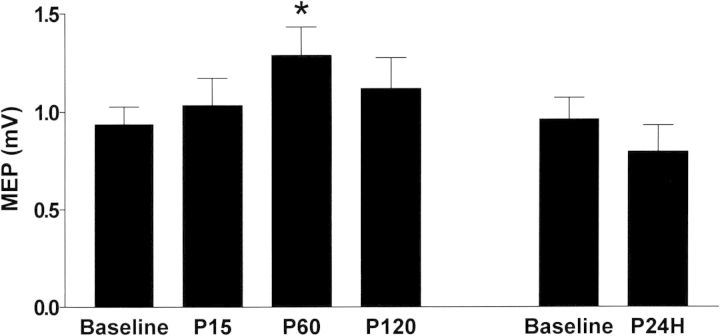
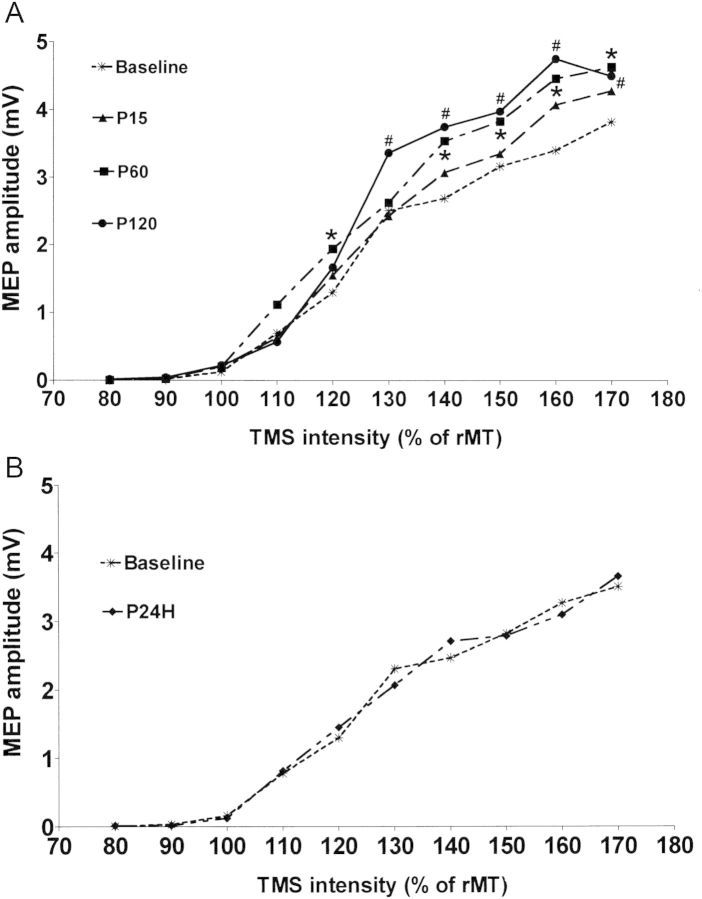
The mean rMTs for the left M1HAND at baseline and time points after left parietal ccPAS are presented in Table 1. There were no significant effects of ccPAS on the rMT (F = 1.63, P = 0.181). In MEPs evoked by a fixed TMS intensity, the mean intensity was 52.5 ± 9.2% of the maximum stimulator output. Figure 2 shows the average MEP amplitude from all subjects over time. Repeated-measures ANOVA showed a significant effect of Time on MEP amplitude (F = 4.38, P = 0.004), and post hoc analysis revealed a relative increase in MEP amplitude at 60 min after the end of ccPAS (P60) compared with the baseline (P = 0.031). There was no difference in MEP amplitude between baseline and 1 day after ccPAS (P24H) (P = 0.37). For the I/O curve, Figure 3A shows the mean MEP amplitudes calculated from all subjects with TMS intensities of 80–170% rMT at all points in time. Two-factor repeated-measures ANOVA revealed a significant effect of Time (F = 13.60, P < 0.001) and the Time × Intensity interaction (F = 1.8, P = 0.01). Post hoc testing for the Time × Intensity interaction showed significant increases of the MEP amplitude compared with the baseline at intensities of 120%, 140%, 150%, 160%, and 170% rMT at P60, and at intensities of 130%, 140%, 150%, 160%, and 170% rMT at P120. When comparing the I/O curve between baseline and P24H (Fig. 3B), there was no effect of Time (P = 0.849) or Time × Intensity interaction (P = 0.755).
Table 1.
Parameters of corticospinal excitability and motor behavior before and after left-to-right cc-PAS
| Measurement | Baseline | P15 | P60 | P120 | P24H |
|---|---|---|---|---|---|
| rMT, left ccPAS (ISI = 8 ms) (% of maximum stimulator output) | 43.6 ± 5.8 | 44.0 ± 6.1 | 43.3 ± 6.2 | 43.7 ± 6.0 | 44.5 ± 4.6 |
| MEP, left ccPAS (ISI = 8 ms) (mV) | 0.94 ± 0.31 | 1.04 ± 0.48 | 1.29 ± 0.50 | 1.12 ± 0.55 | 0.80 ± 0.39 |
| Pegboard task, left ccPAS (pin number) | 15.4 ± 1.4 | 15.9 ± 1.8 | 16.1 ± 1.7 | 16.1 ± 1.5 | 17.4 ± 1.3 |
| Pegboard task, controls (pin number) | 16.4 ± 2.0 | 16.4 ± 1.9 | 16.7 ± 1.8 | 17.1 ± 1.5 | 17.4 ± 1.5 |
| rMT, left ccPAS (ISI = 100 ms) (% of maximum stimulator output) | 41.3 ± 4.0 | 41.5 ± 4.0 | 41.8 ± 43.4 | 41 ± 3.7 | – |
| MEP, left ccPAS (ISI = 100 ms) (mV) | 1.13 ± 0.36 | 1.01 ± 0.31 | 1.10 ± 0.61 | 1.13 ± 0.621 | – |
| rMT, right ccPAS (ISI = 8 ms) (% of maximum stimulator output) | 45.7 ± 4.6 | 45.4 ± 6.1 | 46.1 ± 4.5 | 46 ± 4.7 | – |
| MEP of conditioned right M1, right ccPAS (ISI = 8 ms) (mV) | 1.11 ± 0.23 | 1.20 ± 0.56 | 1.96 ± 1.03 | 1.50 ± 0.70 | – |
| MEP of unconditioned left M1, right ccPAS (ISI = 8 ms) (mV) | 1.07 ± 0.38 | 0.74 ± 0.53 | 0.91 ± 0.47 | 1.03 ± 0.60 | – |
Note: Each value corresponds to the mean ± SD.
ISI: interstimulus interval;
rMT: rest motor threshold;
MEP: motor evoked potential.
Figure 2.
Effects of left parietal ccPAS on MEPs of left M1HAND. MEPs by left M1 TMS were recorded from right FDI muscle and were averaged from all subjects. There was a significant effect of Time in the corresponding repeated-measures ANOVA; the post hoc analysis showed a significant increase in MEP amplitude at P60 relative to the baseline, indicating left parietal ccPAS enhanced the cortical excitability of left M1. *P < 0.05.
Figure 3.
Effects of left parietal ccPAS on the I/O curve of left M1HAND. (A) The MEPs were recorded from the right FDI with the intensities of TMS at 80%, 90%, 100%, 110%, 120%, 130%, 150%, 160%, and 170% of rMT. The plots represent the mean MEP amplitude averaged from all 12 subjects at different time points. The MEP amplitudes as a function of the TMS intensity at P60 and P120 were significantly different from those at baseline. The post hoc analysis showed an increased MEP amplitude compared with baseline at intensities of 120%, 140%, 150%, 160%, and 170% rMT at P60 and at intensities of 130%, 140%, 150%, 160%, and 170% rMT at P120. (B) There was no difference in the I/O curve between the baseline and 24 h after ccPAS. *P < 0.05; #P < 0.05.
Experiments 1 and 4: Effects of Left Parietal ccPAS on Motor Behavior (n = 24)
For the Purdue pegboard test, the mean number of pins from subjects (n = 12) with left parietal ccPAS and from control subjects without ccPAS intervention at different time points are summarized in Table 1. A 2-factor repeated-measures ANOVA was applied with Time (baseline, P15, P60, P120, and P24H), ccPAS intervention (with or without ccPAS), and the interaction between Time and ccPAS intervention as the fixed effects. There was a significant effect of Time on performance (F = 14.91, P < 0.001) but not the ccPAS intervention (F = 1.30, P = 0.266) or Time × ccPAS interaction (F = 1.09, P = 0.368), suggesting no significant effects of parietal ccPAS on the motor behavior assessed by the Purdue pegboard.
Experiment 2: Effects of Parietal ccPAS on PPC–M1 Connectivity (n = 12)
In the left PPC–M1 paired-pulse experiment, the mean intensities for the left M1HAND and PPC were 53.3 ± 8.0% and 40.7 ± 5.8%, respectively, of the maximum stimulator output at baseline, 52.6 ± 7.3% and 40.5 ± 5.5% of at P15, and 52.4 ± 7.4% and 41 ± 5.7% of at P60. Repeated-measures ANOVA showed no significant difference in the stimulus intensity of M1HAND and PPC at baseline and after PPC–M1 ccPAS. There was also no significant difference in the amplitude of MEPs evoked by TS alone among the baseline, P15, and P60 (F = 2.53, P = 0.103).
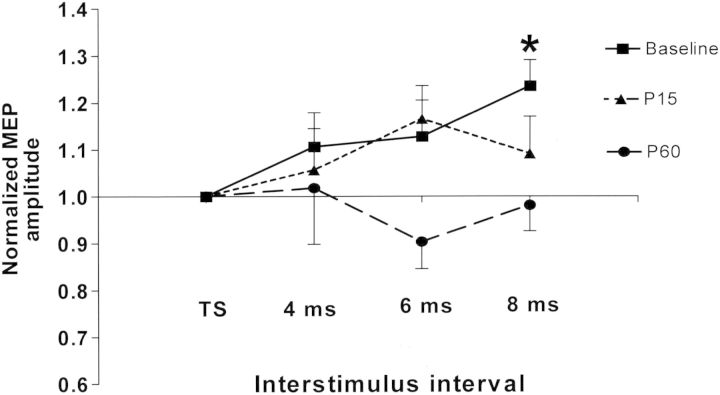
Before analyzing PPC–M1 connectivity, the PPC–M1 paired-pulse data were normalized to the amplitude of the TS alone. Figure 4 shows the normalized MEPs of each ISI at different time points. To investigate the PPC–M1 interaction, repeated-measures ANOVA to test the effects of ISI (TS alone, 4, 6, and 8 ms) was performed for baseline, P15, and P60, respectively. The results revealed a significant effect of ISI (F = 3.01; P = 0.044) at baseline, but there was no effect of ISI at P15 (F = 1.35, P = 0.276) and P60 (F = 0.66, P = 0.584). The post hoc analysis showed that a significant potentiation of MEPs was obtained at baseline when the CS was given 8 ms prior to the TS (P = 0.01). To explore the changes in the PPC–M1 interaction induced by parietal ccPAS, 2-factor repeated-measures ANOVA with Time (baseline, P15, and P60) and ISI (TS alone, 4, 6, and 8 ms) showed a significant effect of Time (F = 4.34; P = 0.016), but not the ISI (F = 1.68, P = 0.191) or Time × ISI interaction (F = 1.13, P = 0.349). The post hoc analysis revealed the normalized MEPs produced from left PPC–M1 paired-pulse TMS at P60 were significantly different from those at baseline (P = 0.011). We further focused on the effects of parietal ccPAS on the potentiation induced with ISIs of 8 ms. Another repeated-measures ANOVA with Time (baseline, P15, and P60) and ISI (TS alone or 8 ms) was performed. The results revealed a significant effect of Time × ISI interaction (F = 3.94; P = 0.027). Further analysis showed a significant effect of parietal ccPAS on the PPC–M1 interaction with ISI of 8 ms at P60 compared with baseline (P = 0.019).
Figure 4.
Effects of parietal ccPAS on the PPC–M1 interaction. The mean MEP amplitudes for all 3 ISI (4, 6, and 8 ms) are expressed as percentage of the unconditioned MEP amplitude (TS alone) at each time point. Repeated-measures ANOVA showed an effect of ISI only at baseline, and a significant potentiation of MEPs was noted at ISI of 8 ms. This facilitating the effect of PPC on the ipsilateral M1 faded gradually after PPC–M1 ccPAS. *P < 0.05. TS: test stimulus alone.
Experiment 3: Interval Specificity of Parietal ccPAS (n = 8)
Left parietal ccPAS was performed at a different ISI to test whether the conditioning effects depended on the interval between the 2 TMS stimuli of left PPC and M1. TMS of the left PPC was delivered 100 ms prior to stimulation of the left M1. The mean rMTs and MEP amplitudes for left M1HAND at baseline and time points after ccPAS are presented in Table 1. Repeated-measures ANOVA showed no differences in rMT or MEP amplitude at baseline, P15, P60, and P120 (F = 0.745, P = 0.538 for rMT; F = 0.17, P = 0.915 for MEP amplitude). For the I/O curve, in contrast to an ISI of 8 ms, 2-factor repeated-measures ANOVA revealed no effects of Time (F = 1.32, P = 0.27) and Time × Intensity interaction (F = 0.68, P = 0.887). These findings suggest no obvious changes in motor excitability before and after parietal ccPAS at an ISI of 100 ms.
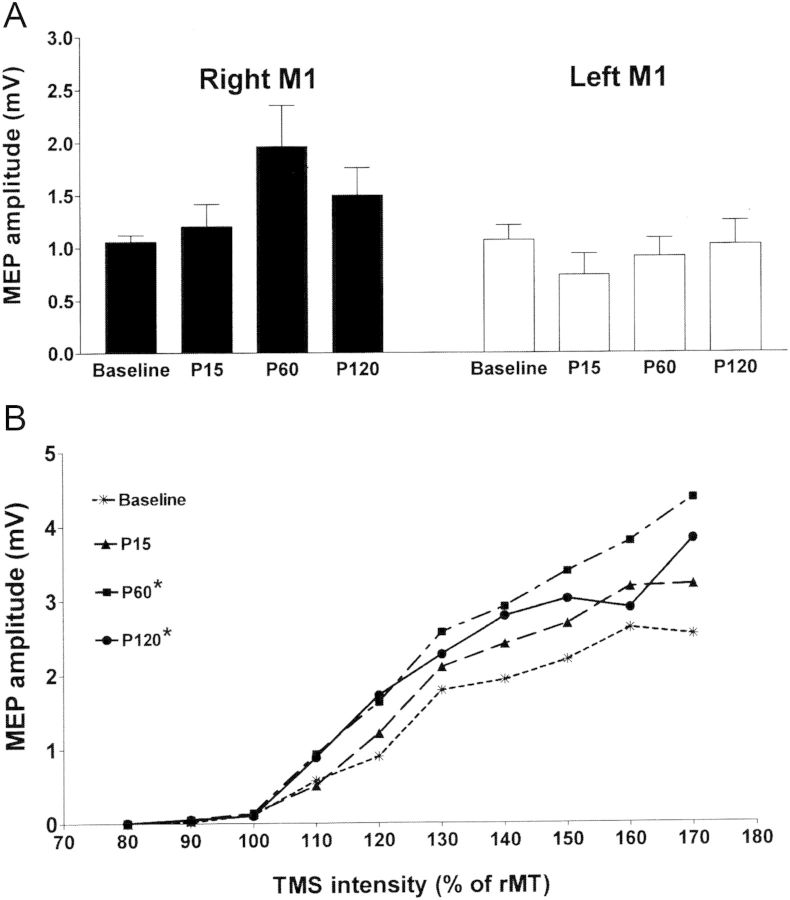
Experiment 5: Parietal ccPAS in the Right Hemisphere (n = 7)
Right parietal ccPAS with an ISI of 8 ms was performed to test if the same conditioning effects could be induced in the right hemisphere. There were no changes in the rMT of right M1 before and after right parietal ccPAS (Table 1) (F = 0.35, P = 0.79). Figure 5A compares MEP amplitude evoked from the conditioned right M1HAND and the unconditioned left M1HAND. The mean amplitude of MEPs showed an increment in the conditioned right M1, but a decrement in the unconditioned left M1 after right parietal ccPAS. Repeated-measures ANOVA showed a trend of effects of Time on the MEP amplitude evoked from the conditioned right M1 (F = 2.87, P = 0.065), but no effects of Time on the MEP amplitude from the unconditioned left M1 (F = 1.00, P = 0.414). Two-factor repeated-measures ANOVA analysis of the I/O curve revealed a significant effect of Time (F = 16.87, P < 0.001). Post hoc analysis showed that MEP amplitudes as a function of the TMS intensity were significantly different at 60 and 120 min after right parietal ccPAS compared with the baseline (both P < 0.001) (Fig. 5B).
Figure 5.
Effects of parietal ccPAS in the right hemisphere. (A) Parietal ccPAS was performed in the right hemisphere and the MEPs evoked from the right or left M1HAND were recorded from the contralateral FDI muscle. Repeated-measures ANOVA showed that Time had a trend of facilitatory effects on the MEPs from the conditioned right M1 (P = 0.065), but not the unconditioned left M1 (P = 0.414). (B) Effects of right parietal ccPAS on the I/O curve of the right M1HAND. The MEP amplitudes as a function of the TMS intensity at P60 and P120 were significantly higher than those at baseline. *P < 0.05.
Discussion
The present study demonstrated that associative transcranial stimulation of the parietal and motor cortex can induce changes in motor excitability and parietal–motor connectivity. One hundred and eighty PAS delivered at 0.2 Hz were sufficient to produce such plasticity. This associative plasticity is timing-dependent and is observed in both hemispheres. This agrees with a series of recent studies, demonstrating that cortical associative plasticity can be induced by time-locked stimuli over 2 connected cortical areas, such as M1 and supplementary motor area, M1 and ventral premotor cortex, and bihemispheric M1 (Rizzo et al. 2009; Arai et al. 2011; Buch et al. 2011). Results of the present study showed that associative plasticity can also be induced through the PPC–M1 network.
In the present study, the coil for the PPC was positioned relative to P3 or P4 of the 10–20 EEG system. According to previous investigations, the underlying cortex projected by these locations was within the PPC close to the posterior part of intraparietal sulcus (Homan et al. 1987; Herwig et al. 2003; Okamoto et al. 2004). The posterior intraparietal sulcus plays an important role in multisensory integration (Galati et al. 2001; Gentile et al. 2011), online motor control of manual actions, and the cognitive aspect of motor action (Rizzolatti et al. 1997; Graziano and Gross 1998; Haaland et al. 2000; Andersen and Buneo 2002; Gold and Shadlen 2007). Anatomical studies in primates and cats showed strong corticocortical interconnections between PPC and motor areas in the frontal cortex (Matelli and Luppino 2001; Makris et al. 2005). The functional interaction between PPC and ipsilateral M1 was first demonstrated by Koch et al. (2007) using the dual-site paired-pulse TMS paradigm. Subsequent studies revealed that this functional interaction could be modified during planning of reaching movements and was impaired in pathological conditions such as Parkinson's disease (Koch et al. 2008; Palomar et al. 2013). In the present study, we introduce ccPAS over the PPC–M1 network based on their anatomic-functional interplay. Our results showed that associative stimulations of the PPC–M1 network can induce transient and reversible plasticity in the motor cortex and the PPC–M1 connectivity.
In our study, facilitation of motor excitability is consistently seen for MEPs at a fixed intensity and for the whole I/O curve. Facilitation evolved gradually, reached maximum 1 h after ccPAS, lasted at least for 2 h, and then returned to baseline on the next day. These aftereffects resembled the associative long-term potentiations that are induced by coincident presynaptic activity and postsynaptic depolarization in a variety of models, such as cortical slice preparation and intact anesthetized experimental animals (Baranyi and Feher 1981; Magee and Johnston 1997; Jacob et al. 2007). The same principle has was applied in humans to induce plasticity at the M1 or primary somatosensory cortex by pairing a peripheral nerve stimulation with TMS of contralateral motor or somatosensory cortex (Stefan et al. 2000; Wolters et al. 2005) and by pairing 2 cortical stimuli (Rizzo et al. 2009; Buch et al. 2011). In our ccPAS, the conditioning TMS pulse at PPC probably stimulates the corticocortical connection between the PPC and M1 and activates the presynaptic afferents of M1. The TMS at M1 probably activates intracortical fibers traveling horizontally and eventually leads to activation of postsynaptic pyramidal output cells (Tong et al. 1996; Hamada et al. 2007). At an ISI of 8ms, we believe that the corticocortical volley elicited by the parietal TMS would arrive at the M1 slightly before or at the same time as the TMS given to M1, resulting in convergent activation at M1. In contrast, when ISI was 100ms, the parietal and M1 inputs would not be coincident and there would be no plastic changes at M1HAND. Our results indicate that the temporal relationship between the PPC and M1 TMS is crucial for developing the aftereffects. A recently published study also showed changes of motor cortex excitability after a similar ccPAS protocol between ipsilateral PPC and M1 (Koch et al. 2013). In that study, motor excitability increased after ccPAS when ISI was 5 ms and the M1 coil was held to induce an anterior–posterior directed current at the underlying M1 cortex. In contrast to the anterior–posterior directed current which preferentially induces I3 waves, in our parietal ccPAS protocol, the M1 coil was held to induce posterior–anterior directed current which preferentially activates I1 waves. The I1 wave precedes the I3 wave by ∼3 ms. Therefore, the convergent activations at M1 from presynaptic input and postsynaptic activation by the parietal ccPAS protocol with ISI of 5 ms and anterior–posterior directed current at M1 might be temporally similar to our parietal ccPAS protocol with ISI of 8 ms and posterior–anterior directed current at M1.
In the present study, we cannot exclude a contribution from polysynaptic circuits on the effects of the parietal ccPAS. In the parietal ccPAS paradigm, stimulating PPC might induce remote effects over the contralateral parietal cortex via transcallosal connections. These remote effects from the opposite parietal cortex might contribute to the ipsilateral effects on M1 (Koch et al. 2009). There are also major corticocortical projections from PPC to premotor cortex (Battaglia-Mayer et al. 2003; Rushworth et al. 2006), and this polysynaptic pathway linking PPC–premotor–M1 might also involve in the effects of parietal ccPAS. Further work is needed to explore whether the effects of PPC stimulation are mediated by direct pathways to M1 or indirect pathways via an interconnected cortical region.
Our results showed that the excitability of M1HAND expressed by MEPs or I/O curve were grossly increased after ccPAS. The facilitation only became significant at 60 and 120 min, but not at 15 min after ccPAS, a timing pattern often seen for associative plasticity. The classical PAS reached its maximal efficiency 15–30 min after intervention (Stefan et al. 2000). The left-to-right M1 cc-PAS increased corticospinal excitability at 30 and 60 min, but not immediately after cc-PAS (Rizzo et al. 2009). In animal studies, potentiation or depression of synapse strength develops gradually for tens of minutes after repetitive correlated spiking of pre- and postsynaptic neurons (Bi and Poo 1998; Fino et al. 2005). The cellular mechanism for the development of increased M1 excitability in our parietal ccPAS protocol is not established. NMDA receptor-mediated modulation of cortical excitability was a necessary step in the plasticity induced by classic PAS (Stefan et al. 2002). Other cellular changes involving post-translational modification of ion channels, transmitter receptor, or cellular trafficking might also contribute (Dan and Poo 2004; Sunyer et al. 2008). Further studies are needed to explore such issues.
Associative plasticity is usually thought to be bidirectional, and both long-term potentiation and long-term depression can be induced in slice preparations and also with classical PAS (Bell et al. 1997; Stefan et al. 2000). In classical PAS, an ISI of 10 and 25 ms between the peripheral and the cortical stimulus results in lasting suppression and facilitation of M1 excitability, respectively. In our study, we can only report facilitation. In pilot studies, we tried parietal ccPAS at different ISIs (e.g. −4 and −6 ms), but could not find consistent depression effects. Due to the small number of participants in the groups, these results were not included here. In a recent study by Koch et al. (2013) using a similar ccPAS protocol between PPC and M1, they showed both long-term potentiation and depression at M1 by changing the ISI and the direction of induced current at M1. Bidirectional plasticity has not been often reported for ccPAS, and only the study by Arai et al. (2011) looking at SMA–M1 plasticity could show depression effect, whereas other studies only report facilitating effects (Koganemaru et al. 2009; Rizzo et al. 2009; Buch et al. 2011). Whether ccPAS protocols can also reliably induce inhibition of the motor cortex has to be investigated by further studies.
We investigated how local connectivity between PPC and M1 was affected by parietal ccPAS. At baseline, the conditioning stimuli over the left PPC potentiated MEPs evoked from ipsilateral M1 when the ISI was 8 ms. These results are compatible with a previous study from our group in which the different intrahemispheric parietal–motor networks were mapped using the neuronavigation system and paired-pulse TMS. The CS at the central and posterior intraparietal sulcus resulted in a facilitatory effect on ipsilateral M1; this facilitation was highest when the CS was given 8 ms prior to the testing stimulus at ipsilateral M1 (Karabanov et al. 2013). Similar findings in the present study suggested that the coil placement of PPC is a suitable estimate of posterior intraparietal sulcus. Our result was different from the study by Koch et al. (2007), which showed MEP facilitation at an ISI of 4 ms. However, there was variability in the ISI of PPC–M1 interaction ranging from 2 to 6 ms in previous studies (Remple et al. 2007; Koch et al. 2011; Karabanov et al. 2012), and there tended to be facilitation of MEPs at an ISI of 8 ms, although the trend was not statistically significant. The cause of this variability is still unknown. One possible explanation might be the polysynaptic activation of conditioning TMS at the PPC, for example, the PPC projections to ipsilateral M1 via the prefrontal areas (Petrides and Pandya 1984; Remple et al. 2007). Because of this variability, in future studies, to optimize parietal ccPAS, it might be reasonable to optimize the parietal stimulus empirically for best location and ISI. As more experiments are done, if the variability can be explained and reduced, we might also gain further insights into the more precise nature of the plastic effects.
The facilitating effects of the PPC–M1 interaction was not significantly altered at 15 min, but was down-regulated 1 h after ccPAS when the potentiation of MEPs by parietal ccPAS was significant. Due to the lasting facilitation of the MEPs, it might be that this increase in corticospinal excitability contributes to the reduced PPC–M1 interaction. However, we adjusted stimulus intensity after ccPAS and there was no difference in MEP amplitude of TS alone before and after ccPAS. These results suggest that parietal ccPAS modulated the strength of the PPC–M1 interaction. We had anticipated that the order of stimuli in the present parietal PAS protocol would enhance the synapse in the PPC–M1 connectivity based on the mechanism of spike-timing-dependent plasticity. However, parietal ccPAS resulted in decreased PPC facilitatory input to M1. Similar attenuation of corticocortical interaction was also shown in a recent study examining the effects of bihemispheric M1 ccPAS at an ISI of 8 ms. The interhemispheric inhibition attenuated after bihemispheric M1 ccPAS, suggesting the enhancement of transcallosal facilitatory rather than inhibitory pathways (Rizzo et al. 2009). The existence of an inhibitory interaction between PPC and M1 was demonstrated in human studies involving response inhibition (Watanabe et al. 2002; Hu and Li 2012), and the current parietal ccPAS might lead to an increase in the inhibitory influence on the ipsilateral M1. The attenuation of the PPC–M1 interaction in the present study was also compatible with a previous study from our group that revealed modulation of the PPC–M1 interaction following sensorimotor training. In that study, after 10 min of sensorimotor training involving tapping the index finger in synchrony to a rhythmic visual or auditory sequence, the PPC–M1 facilitation attenuated immediately. These results suggest that integrating sensory information into a coherent motor plan during sensorimotor training modified the PPC–M1 interaction (Karabanov et al. 2012). In parietal ccPAS, the associative stimulation of the PPC and M1 incorporated the input from PPC with activation of the motor cortex. This might simulate the cortical pathway during sensorimotor training and, thus, could induce attenuation of the PPC–M1 interaction.
Increased M1 excitability either by direct M1 stimulation (Kim et al. 2004; Teo et al. 2011) or by ccPAS of bihemispheric M1 (Koganemaru et al. 2009; Rizzo et al. 2009) was associated with increased motor performance. Since the parietal–motor connection is especially important for sensory-motor integration, in our study, we measured performance on the Purdue pegboard task that heavily relies on hand–eye coordination to assess the effects of parietal ccPAS. However, the difference in performance was not statistically significant between groups. While we could not demonstrate a change in motor performance, other types of motor behaviors involving sensorimotor integration might be assessed in future studies. For example, tapping the index finger in synchrony to a rhythmic sequence was shown to modulate the parietal–motor interaction (Karabanov et al. 2012).
The PPC–frontal motor network is important for sensorimotor integration (Andersen and Cui 2009), including praxis movements (Bohlhalter et al. 2009; Wheaton et al. 2009). Abnormalities of sensorimotor integration were described in a variety of neurological and psychiatric disorders (Abbruzzese and Berardelli 2003; Sailer et al. 2003; Kent et al. 2012). Recent studies showed impaired parietal–motor interaction in Parkinson's disease, dystonia, stroke with hemineglect, Alzheimer's disease, and schizophrenia (Oliveri et al. 2000; Quartarone et al. 2008; Bonni et al. 2013; Palomar et al. 2013). Improving sensorimotor disorganization by proprioceptive training in patients with musician's dystonia led to objective behavioral improvement (Rosenkranz et al. 2009). Modulation of parietofrontal connectivity of the intact hemisphere by repetitive TMS in stroke patients with hemineglect improved the neglect symptoms (Koch et al. 2012). In the present study, we showed that parietal ccPAS could induce transient associative plasticity at M1 and modulate the PPC–M1 interaction. Therefore, parietal ccPAS might be clinically applicable for the treatment or rehabilitation of neurological and psychiatric disorders associated with abnormal parietal–motor interactions.
Funding
This work was supported by (in part) by the Intramural Research Program of the National Institute of Neurological Disorder and Stroke, National Institutes of Health.
Notes
Conflict of Interest: None declared.
References
- Abbruzzese G, Berardelli A. Sensorimotor integration in movement disorders. Mov Disord. 2003;18:231–240. doi: 10.1002/mds.10327. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Buneo CA. Intentional maps in posterior parietal cortex. Annu Rev Neurosci. 2002;25:189–220. doi: 10.1146/annurev.neuro.25.112701.142922. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Cui H. Intention, action planning, and decision making in parietal-frontal circuits. Neuron. 2009;63:568–583. doi: 10.1016/j.neuron.2009.08.028. [DOI] [PubMed] [Google Scholar]
- Antonov I, Antonova I, Kandel ER, Hawkins RD. Activity-dependent presynaptic facilitation and Hebbian LTP are both required and interact during classical conditioning in Aplysia. Neuron. 2003;37:135–147. doi: 10.1016/s0896-6273(02)01129-7. [DOI] [PubMed] [Google Scholar]
- Arai N, Muller-Dahlhaus F, Murakami T, Bliem B, Lu MK, Ugawa Y, Ziemann U. State-dependent and timing-dependent bidirectional associative plasticity in the human SMA-M1 network. J Neurosci. 2011;31:15376–15383. doi: 10.1523/JNEUROSCI.2271-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb RS, Waters RS, Asanuma H. Corticocortical connections to the motor cortex from the posterior parietal lobe (areas 5a, 5b, 7) in the cat demonstrated by the retrograde axonal transport of horseradish peroxidase. Exp Brain Res. 1984;54:476–484. doi: 10.1007/BF00235473. [DOI] [PubMed] [Google Scholar]
- Baranyi A, Feher O. Synaptic facilitation requires paired activation of convergent pathways in the neocortex. Nature. 1981;290:413–415. doi: 10.1038/290413a0. [DOI] [PubMed] [Google Scholar]
- Battaglia-Mayer A, Caminiti R, Lacquaniti F, Zago M. Multiple levels of representation of reaching in the parieto-frontal network. Cereb Cortex. 2003;13:1009–1022. doi: 10.1093/cercor/13.10.1009. [DOI] [PubMed] [Google Scholar]
- Bell CC, Han VZ, Sugawara Y, Grant K. Synaptic plasticity in a cerebellum-like structure depends on temporal order. Nature. 1997;387:278–281. doi: 10.1038/387278a0. [DOI] [PubMed] [Google Scholar]
- Bernier PM, Grafton ST. Human posterior parietal cortex flexibly determines reference frames for reaching based on sensory context. Neuron. 2010;68:776–788. doi: 10.1016/j.neuron.2010.11.002. [DOI] [PubMed] [Google Scholar]
- Bi GQ, Poo MM. Synaptic modifications in cultured hippocampal neurons: dependence on spike timing, synaptic strength, and postsynaptic cell type. J Neurosci. 1998;18:10464–10472. doi: 10.1523/JNEUROSCI.18-24-10464.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohlhalter S, Hattori N, Wheaton L, Fridman E, Shamim EA, Garraux G, Hallett M. Gesture subtype-dependent left lateralization of praxis planning: an event-related fMRI study. Cereb Cortex. 2009;19:1256–1262. doi: 10.1093/cercor/bhn168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonni S, Lupo F, Lo Gerfo E, Martorana A, Perri R, Caltagirone C, Koch G. Altered parietal-motor connections in Alzheimer's disease patients. J Alzheimers Dis. 2013;33:525–533. doi: 10.3233/JAD-2012-121144. [DOI] [PubMed] [Google Scholar]
- Buch ER, Johnen VM, Nelissen N, O'Shea J, Rushworth MF. Noninvasive associative plasticity induction in a corticocortical pathway of the human brain. J Neurosci. 2011;31:17669–17679. doi: 10.1523/JNEUROSCI.1513-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buneo CA, Andersen RA. The posterior parietal cortex: sensorimotor interface for the planning and online control of visually guided movements. Neuropsychologia. 2006;44:2594–2606. doi: 10.1016/j.neuropsychologia.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Cassenaer S, Laurent G. Conditional modulation of spike-timing-dependent plasticity for olfactory learning. Nature. 2012;482:47–52. doi: 10.1038/nature10776. [DOI] [PubMed] [Google Scholar]
- Dan Y, Poo MM. Spike timing-dependent plasticity of neural circuits. Neuron. 2004;44:23–30. doi: 10.1016/j.neuron.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Fino E, Glowinski J, Venance L. Bidirectional activity-dependent plasticity at corticostriatal synapses. J Neurosci. 2005;25:11279–11287. doi: 10.1523/JNEUROSCI.4476-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galati G, Committeri G, Sanes JN, Pizzamiglio L. Spatial coding of visual and somatic sensory information in body-centred coordinates. Eur J Neurosci. 2001;14:737–746. doi: 10.1046/j.0953-816x.2001.01674.x. [DOI] [PubMed] [Google Scholar]
- Gentile G, Petkova VI, Ehrsson HH. Integration of visual and tactile signals from the hand in the human brain: an fMRI study. J Neurophysiol. 2011;105:910–922. doi: 10.1152/jn.00840.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold JI, Shadlen MN. The neural basis of decision making. Annu Rev Neurosci. 2007;30:535–574. doi: 10.1146/annurev.neuro.29.051605.113038. [DOI] [PubMed] [Google Scholar]
- Graziano MS, Gross CG. Spatial maps for the control of movement. Curr Opin Neurobiol. 1998;8:195–201. doi: 10.1016/s0959-4388(98)80140-2. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Knight RT. Neural representations of skilled movement. Brain. 2000;123:2306–2313. doi: 10.1093/brain/123.11.2306. [DOI] [PubMed] [Google Scholar]
- Hamada M, Hanajima R, Terao Y, Arai N, Furubayashi T, Inomata-Terada S, Yugeta A, Matsumoto H, Shirota Y, Ugawa Y. Quadro-pulse stimulation is more effective than paired-pulse stimulation for plasticity induction of the human motor cortex. Clin Neurophysiol. 2007;118:2672–2682. doi: 10.1016/j.clinph.2007.09.062. [DOI] [PubMed] [Google Scholar]
- Hebb D. The organization of behavior: a neuropsychological theory. New York: Wiley; 1949. [Google Scholar]
- Herwig U, Satrapi P, Schonfeldt-Lecuona C. Using the international 10–20 EEG system for positioning of transcranial magnetic stimulation. Brain Topogr. 2003;16:95–99. doi: 10.1023/b:brat.0000006333.93597.9d. [DOI] [PubMed] [Google Scholar]
- Homan RW, Herman J, Purdy P. Cerebral location of international 10–20 system electrode placement. Electroencephalogr Clin Neurophysiol. 1987;66:376–382. doi: 10.1016/0013-4694(87)90206-9. [DOI] [PubMed] [Google Scholar]
- Hu S, Li CS. Neural processes of preparatory control for stop signal inhibition. Hum Brain Mapp. 2012;33:2785–2796. doi: 10.1002/hbm.21399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyvarinen J. Posterior parietal lobe of the primate brain. Physiol Rev. 1982;62:1060–1129. doi: 10.1152/physrev.1982.62.3.1060. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Visuo-motor integration and control in the human posterior parietal cortex: evidence from TMS and fMRI. Neuropsychologia. 2006;44:2691–2699. doi: 10.1016/j.neuropsychologia.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Jacob V, Brasier DJ, Erchova I, Feldman D, Shulz DE. Spike timing-dependent synaptic depression in the in vivo barrel cortex of the rat. J Neurosci. 2007;27:1271–1284. doi: 10.1523/JNEUROSCI.4264-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabanov A, Jin SH, Joutsen A, Poston B, Aizen J, Ellenstein A, Hallett M. Timing-dependent modulation of the posterior parietal cortex-primary motor cortex pathway by sensorimotor training. J Neurophysiol. 2012;107:3190–3199. doi: 10.1152/jn.01049.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabanov AN, Chao CC, Paine R, Hallett M. Mapping different intra-hemispheric parietal-motor networks using twin coil TMS. Brain Stimul. 2013;6:384–389. doi: 10.1016/j.brs.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent JS, Hong SL, Bolbecker AR, Klaunig MJ, Forsyth JK, O'Donnell BF, Hetrick WP. Motor deficits in schizophrenia quantified by nonlinear analysis of postural sway. PLoS One. 2012;7:e41808. doi: 10.1371/journal.pone.0041808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YH, Park JW, Ko MH, Jang SH, Lee PK. Facilitative effect of high frequency subthreshold repetitive transcranial magnetic stimulation on complex sequential motor learning in humans. Neurosci Lett. 2004;367:181–185. doi: 10.1016/j.neulet.2004.05.113. [DOI] [PubMed] [Google Scholar]
- Koch G, Bonni S, Giacobbe V, Bucchi G, Basile B, Lupo F, Versace V, Bozzali M, Caltagirone C. Theta-burst stimulation of the left hemisphere accelerates recovery of hemispatial neglect. Neurology. 2012;78:24–30. doi: 10.1212/WNL.0b013e31823ed08f. [DOI] [PubMed] [Google Scholar]
- Koch G, Cercignani M, Bonni S, Giacobbe V, Bucchi G, Versace V, Caltagirone C, Bozzali M. Asymmetry of parietal interhemispheric connections in humans. J Neurosci. 2011;31:8967–8975. doi: 10.1523/JNEUROSCI.6567-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Ruge D, Schippling S, Caltagirone C, Rothwell JC. Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J Neurosci. 2007;27:6815–6822. doi: 10.1523/JNEUROSCI.0598-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Fernandez Del Olmo M, Cheeran B, Schippling S, Caltagirone C, Driver J, Rothwell JC. Functional interplay between posterior parietal and ipsilateral motor cortex revealed by twin-coil transcranial magnetic stimulation during reach planning toward contralateral space. J Neurosci. 2008;28:5944–5953. doi: 10.1523/JNEUROSCI.0957-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Ponzo V, Di Lorenzo F, Caltagirone C, Veniero D. Hebbian and anti-Hebbian spike-timing-dependent plasticity of human cortico-cortical connections. J Neurosci. 2013;33:9725–9733. doi: 10.1523/JNEUROSCI.4988-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch G, Ruge D, Cheeran B, Fernandez Del Olmo M, Pecchioli C, Marconi B, Versace V, Lo Gerfo E, Torriero S, Oliveri M, et al. TMS activation of interhemispheric pathways between the posterior parietal cortex and the contralateral motor cortex. J Physiol. 2009;587:4281–4292. doi: 10.1113/jphysiol.2009.174086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koganemaru S, Mima T, Nakatsuka M, Ueki Y, Fukuyama H, Domen K. Human motor associative plasticity induced by paired bihemispheric stimulation. J Physiol. 2009;587:4629–4644. doi: 10.1113/jphysiol.2009.174342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee JC, Johnston D. A synaptically controlled, associative signal for Hebbian plasticity in hippocampal neurons. Science. 1997;275:209–213. doi: 10.1126/science.275.5297.209. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, Pandya DN. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Matelli M, Luppino G. Parietofrontal circuits for action and space perception in the macaque monkey. Neuroimage. 2001;14:S27–S32. doi: 10.1006/nimg.2001.0835. [DOI] [PubMed] [Google Scholar]
- Medendorp WP, Goltz HC, Crawford JD, Vilis T. Integration of target and effector information in human posterior parietal cortex for the planning of action. J Neurophysiol. 2005;93:954–962. doi: 10.1152/jn.00725.2004. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Dan H, Sakamoto K, Takeo K, Shimizu K, Kohno S, Oda I, Isobe S, Suzuki T, Kohyama K, et al. Three-dimensional probabilistic anatomical cranio-cerebral correlation via the international 10–20 system oriented for transcranial functional brain mapping. Neuroimage. 2004;21:99–111. doi: 10.1016/j.neuroimage.2003.08.026. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oliveri M, Rossini PM, Filippi MM, Traversa R, Cicinelli P, Palmieri MG, Pasqualetti P, Caltagirone C. Time-dependent activation of parieto-frontal networks for directing attention to tactile space. A study with paired transcranial magnetic stimulation pulses in right-brain-damaged patients with extinction. Brain. 2000;123:1939–1947. doi: 10.1093/brain/123.9.1939. [DOI] [PubMed] [Google Scholar]
- Palomar FJ, Conde V, Carrillo F, Fernandez-del-Olmo M, Koch G, Mir P. Parieto-motor functional connectivity is impaired in Parkinson's disease. Brain Stimul. 2013;6:147–154. doi: 10.1016/j.brs.2012.03.017. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rhesus monkey. J Comp Neurol. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Prevosto V, Graf W, Ugolini G. Proprioceptive pathways to posterior parietal areas MIP and LIPv from the dorsal column nuclei and the postcentral somatosensory cortex. Eur J Neurosci. 2011;33:444–460. doi: 10.1111/j.1460-9568.2010.07541.x. [DOI] [PubMed] [Google Scholar]
- Quartarone A, Morgante F, Sant'angelo A, Rizzo V, Bagnato S, Terranova C, Siebner HR, Berardelli A, Girlanda P. Abnormal plasticity of sensorimotor circuits extends beyond the affected body part in focal dystonia. J Neurol Neurosurg Psychiatry. 2008;79:985–990. doi: 10.1136/jnnp.2007.121632. [DOI] [PubMed] [Google Scholar]
- Remple MS, Reed JL, Stepniewska I, Lyon DC, Kaas JH. The organization of frontoparietal cortex in the tree shrew (Tupaia belangeri): II. Connectional evidence for a frontal-posterior parietal network. J Comp Neurol. 2007;501:121–149. doi: 10.1002/cne.21226. [DOI] [PubMed] [Google Scholar]
- Rizzo V, Siebner HS, Morgante F, Mastroeni C, Girlanda P, Quartarone A. Paired associative stimulation of left and right human motor cortex shapes interhemispheric motor inhibition based on a Hebbian mechanism. Cereb Cortex. 2009;19:907–915. doi: 10.1093/cercor/bhn144. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Parietal cortex: from sight to action. Curr Opin Neurobiol. 1997;7:562–567. doi: 10.1016/s0959-4388(97)80037-2. [DOI] [PubMed] [Google Scholar]
- Rosenkranz K, Butler K, Williamon A, Rothwell JC. Regaining motor control in musician's dystonia by restoring sensorimotor organization. J Neurosci. 2009;29:14627–14636. doi: 10.1523/JNEUROSCI.2094-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Rushworth MF, Behrens TE, Johansen-Berg H. Connection patterns distinguish 3 regions of human parietal cortex. Cereb Cortex. 2006;16:1418–1430. doi: 10.1093/cercor/bhj079. [DOI] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Paradiso G, Gunraj CA, Lang AE, Chen R. Short and long latency afferent inhibition in Parkinson's disease. Brain. 2003;126:1883–1894. doi: 10.1093/brain/awg183. [DOI] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Benecke R, Cohen LG, Classen J. Mechanisms of enhancement of human motor cortex excitability induced by interventional paired associative stimulation. J Physiol. 2002;543:699–708. doi: 10.1113/jphysiol.2002.023317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Kunesch E, Cohen LG, Benecke R, Classen J. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- Sunyer B, Diao W, Lubec G. The role of post-translational modifications for learning and memory formation. Electrophoresis. 2008;29:2593–2602. doi: 10.1002/elps.200700791. [DOI] [PubMed] [Google Scholar]
- Teo JT, Swayne OB, Cheeran B, Greenwood RJ, Rothwell JC. Human theta burst stimulation enhances subsequent motor learning and increases performance variability. Cereb Cortex. 2011;21:1627–1638. doi: 10.1093/cercor/bhq231. [DOI] [PubMed] [Google Scholar]
- Tiffin J, Asher EJ. The Purdue pegboard; norms and studies of reliability and validity. J Appl Psychol. 1948;32:234–247. doi: 10.1037/h0061266. [DOI] [PubMed] [Google Scholar]
- Tong G, Malenka RC, Nicoll RA. Long-term potentiation in cultures of single hippocampal granule cells: a presynaptic form of plasticity. Neuron. 1996;16:1147–1157. doi: 10.1016/s0896-6273(00)80141-5. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7:719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Watanabe J, Sugiura M, Sato K, Sato Y, Maeda Y, Matsue Y, Fukuda H, Kawashima R. The human prefrontal and parietal association cortices are involved in NO-GO performances: an event-related fMRI study. Neuroimage. 2002;17:1207–1216. doi: 10.1006/nimg.2002.1198. [DOI] [PubMed] [Google Scholar]
- Wheaton L, Fridman E, Bohlhalter S, Vorbach S, Hallett M. Left parietal activation related to planning, executing and suppressing praxis hand movements. Clin Neurophysiol. 2009;120:980–986. doi: 10.1016/j.clinph.2009.02.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolters A, Sandbrink F, Schlottmann A, Kunesch E, Stefan K, Cohen LG, Benecke R, Classen J. A temporally asymmetric Hebbian rule governing plasticity in the human motor cortex. J Neurophysiol. 2003;89:2339–2345. doi: 10.1152/jn.00900.2002. [DOI] [PubMed] [Google Scholar]
- Wolters A, Schmidt A, Schramm A, Zeller D, Naumann M, Kunesch E, Benecke R, Reiners K, Classen J. Timing-dependent plasticity in human primary somatosensory cortex. J Physiol. 2005;565:1039–1052. doi: 10.1113/jphysiol.2005.084954. [DOI] [PMC free article] [PubMed] [Google Scholar]