Abstract
Ocular dominance plasticity (ODP) in the cat primary visual cortex (V1) is induced during waking by monocular deprivation (MD) and consolidated during subsequent sleep. The mechanisms underlying this process are incompletely understood. Extracellular signal-regulated kinase (ERK) is activated in V1 during sleep after MD, but it is unknown whether ERK activation during sleep is necessary for ODP consolidation. We investigated the role of ERK in sleep-dependent ODP consolidation by inhibiting the ERK-activating enzyme MEK in V1 (via U0126) during post-MD sleep. ODP consolidation was then measured with extracellular microelectrode recordings. Western blot analysis was used to confirm the efficacy of U0126 and to examine proteins downstream of ERK. U0126 abolished ODP consolidation and reduced both phosphorylation of eukaryotic initiation factor 4E (eIF4E) and levels of the synaptic marker PSD-95. Furthermore, interfering with ERK-mediated translation by inhibiting MAP kinase-interacting kinase 1 (Mnk1) with CGP57380 mimicked the effects of U0126. These results demonstrate that ODP consolidation requires sleep-dependent activation of the ERK-Mnk1 pathway.
Keywords: consolidation, MAP kinase-interacting kinase, monocular deprivation, ocular dominance, protein synthesis
Introduction
Ocular dominance plasticity (ODP) is a canonical model of synaptic remodeling in vivo that has revealed many general principles underlying experience-dependent plasticity (reviewed in Berardi et al. 2003). In kittens, occluding vision in one eye (monocular deprivation, MD) during a critical period of development causes cells in the primary visual cortex (V1) to shift from being predominantly binocular to being primarily driven by nondeprived eye stimulation, as measured by single neuron recordings (Wiesel and Hubel 1963; Hubel and Wiesel 1970). ODP can be induced with as little as 6 h of MD during waking, and is consolidated during subsequent sleep (Frank et al. 2001). Although the mechanisms underlying sleep-dependent consolidation are largely unidentified, they include NMDA receptor and protein kinase A (PKA) activation (Aton et al. 2009), as well as translation via the mammalian target of rapamycin (mTOR) pathway (Seibt et al. 2012). The functional role of other kinases activated during post-MD sleep is unknown. For example, extracellular signal-regulated kinase (ERK) is phosphorylated downstream of NMDA receptor activation during post-MD sleep (Aton et al. 2009), but it is unclear whether ERK activity during sleep is required for ODP consolidation.
ERK has been implicated in many forms of neuronal plasticity, and is therefore an attractive candidate mechanism for regulating sleep-dependent plasticity. For example, ERK is necessary for visual cortical long-term potentiation (LTP) and long-term depression (LTD) in vitro (DiCristo et al. 2001; McCoy and McMahon 2007; McCoy et al. 2008). Furthermore, ERK and mTOR co-regulate translation (via eukaryotic initiation factor 4E (eIF4E) and eIF4E binding protein 1 (4E-BP1) phosphorylation) in multiple forms of hippocampal plasticity (Kelleher et al. 2004; Banko et al. 2006; Gelinas et al. 2007; Tsokas et al. 2007). ERK promotes eIF4E phosphorylation by activating an intermediate kinase, MAP kinase-interacting kinase 1 (Mnk1) (Waskiewicz et al. 1997; Ueda et al. 2004). Mnk1 phosphorylation is elevated during hippocampal LTP (Gelinas et al. 2007) and required for hippocampal LTP consolidation (Panja et al. 2009). However, the precise role of the ERK-Mnk1 pathway in sleep-dependent plasticity has not been previously explored. Continuous infusion of ERK inhibitors (during both sleep and wake) for 1 week blocks ODP in vivo (DiCristo et al. 2001), but this approach does not reveal the specific role of ERK activity in sleep, and the molecular mechanisms downstream of ERK were not investigated. There are also no previous studies of Mnk1 in the induction or consolidation of ODP.
We hypothesized that activation of the ERK-Mnk1 transduction pathway during sleep is required for ODP consolidation. To test this hypothesis, we inhibited ERK (with U0126) or Mnk1 (with CGP57380) activity in the sleeping, remodeling visual cortex. We found that neither compound altered sleep architecture, but both profoundly reduced the enhancement of plasticity normally observed after sleep. In addition, ERK and Mnk1 inhibition during sleep both reduced eIF4E phosphorylation and PSD-95 levels. These results demonstrate that the ERK-Mnk1 pathway plays an important role in sleep-dependent synaptic plasticity in vivo, and suggest it may do so by regulating synthesis of plasticity-related proteins.
Materials and Methods
All animal experiments were approved by the University of Pennsylvania IACUC and were performed as required by USDA regulations. Cats were bred in-house and housed with their queens on a 12:12 light:dark cycle prior to experimentation.
Surgical Procedures for Polysomnography and Intracortical Infusion
Eight female and 15 male cats were anesthetized and prepared for surgery as described previously (Frank et al. 2001; Jha et al. 2005). A craniotomy was performed bilaterally over V1 and the dura was partially reflected. Cannula guides with 2 attached EEG electrodes (Plastics One, Roanoke, VA, USA) were positioned over V1 (see Supplementary Fig. S1). An internal “dummy” cannula (solid stainless steel) was inserted into the cannula guide and remained in place until the start of the infusion to protect the cortex from infection. Five bilateral frontoparietal EEG and 3 nuchal EMG electrodes attached to an electrical socket were also implanted. Cannula assemblies and electrodes were affixed to the skull with bone screws and dental acrylic. Following a minimum of 4 days postoperative recovery and treatments, male and female animals were randomly assigned to the experiments described below.
Monocular Deprivation and Infusion Procedures
At the peak of the critical period for visual cortical plasticity (Postnatal day 28–35), cats were singly housed in a recording chamber. As previously described (Jha et al. 2005), EEG and EMG signals were routed from the animal to an amplifier system (Grass Technologies, Warwick, RI, USA) via an electrical cable tether/commutator attached to the implanted electrical sockets. The bipolar electrode pair attached to each cannula guide recorded EEG activity local to the infusion site. Polygraphic signals were high-pass filtered at 0.1 Hz (EEG) or 10 Hz (EMG), and low-pass filtered at 100 Hz. Signals were digitized at 200 Hz and recorded on a personal computer running commercial sleep-recording software (VitalRecorder, Kissei Comtec America, Inc., Irvine, CA, USA). After at least 6 h of baseline recording, animals were briefly anesthetized with isoflurane and the right eyelid was sutured shut. Animals were then kept awake for 6 h in lighted conditions, ensuring that all cats received 6 h of monocular vision to induce cortical remodeling (Frank et al. 2001). At the end of this period, the dummy cannula was removed from the cannula guide and replaced with a sterile stainless steel cannula (28G internal diameter with the tip extending 1 mm below the pial surface) attached to sterile tubing (see Supplementary Fig. S1). Tubing and cannulas were prefilled with drug or vehicle solution. Animals were then allowed sleep ad libitum in complete darkness for 1–6 h, during which time U0126 (5.26 mM; Promega, Madison, WI, USA) (Schafe et al. 2000), CGP57380 (2 mM; Tocris Bioscience, Ellisville, MO, USA) (Panja et al. 2009), or vehicle was infused bilaterally into V1 at a rate of 0.3 μL/min (Harvard Apparatus, Holliston, MA, USA). U0126 and CGP57380 were first dissolved in DMSO (Sigma-Aldrich, St. Louis, MO, USA), then diluted to their final concentrations 1:1 in ACSF (Harvard Apparatus) prior to infusion. An additional group of cats (MD-only) received infusions of vehicle in one hemisphere and U0126 in the other during the MD period; the drug-infused hemisphere was alternated between animals. These animals did not receive post-MD sleep.
We chose U0126 because this compound potently inhibits the kinase that activates ERK (mitogen-activated protein kinase kinase, MEK) without nonspecific effects on other kinases (including the plasticity-related kinases PKC, CaMKII, and PKA) (Favata et al. 1998; Roberson et al. 1999). Similarly, CGP57380 inhibits Mnk1 without nonspecific effects on other kinases, including ERK and PKC (Knauf et al. 2001).
Tissue Collection and Western Blotting
Western blotting was used to determine the efficacy of kinase inhibitors on target proteins and to examine the downstream effects of kinase inhibition. Eight cats were used in these experiments (Vehicle: 3 cats; U0126: 3 cats; CGP57380: 2 cats). Immediately following 1 h of post-MD sleep with intracortical infusion, animals were anesthetized with isoflurane and sacrificed with an intracardiac injection of pentobarbital/phenytoin. V1 tissue near to (within 1 mm) and far from the cannula was rapidly collected and frozen on dry ice. Whole-tissue extracts were prepared and analyzed as previously described (Aton et al. 2009). Briefly, tissue was homogenized in lysis buffer containing 100 mM NaCl, 10 mM Na4P2O7·10H2O, 10 mM NaPO4, 50 mM NaF, 1 mM Na3VO4, 5 mM EDTA, 5 mM EGTA, 1% SDS, and phosphatase and protease inhibitor cocktails (1:100; Sigma). Lysates were then centrifuged to remove cellular debris and boiled in gel loading buffer (2.5% SDS) for 10 min. Protein concentration was quantified using a micro-BCA assay (Thermo Scientific, Rockford, IL, USA). Proteins (40 μg/well) were separated on precast polyacrylamide gels (Bio-Rad, Hercules, CA, USA), and transferred onto nitrocellulose membranes (0.45 μM pore size; Invitrogen, Carlsbad, CA, USA). Membranes were blocked at room temperature in Odyssey blocking buffer (Li-Cor, Lincoln, NE, USA) then incubated overnight with primary antibodies diluted in blocking buffer and 0.1% Tween-20 (Sigma-Aldrich) at 4 °C. Primary antibodies were raised in rabbit and obtained from Cell Signaling Technology (Danvers, MA, USA), unless otherwise noted. The following primary antibodies were used: anti-ERK1/2 (1:000), anti-phospho-ERK1/2 (Thr202/Tyr204 and Thr185/Tyr187, respectively) (1:500), anti-CaMKIIα (1:1500) (Abcam, Cambridge, MA, USA), anti-phospho-CaMKII (Thr286) (1:1000) (Abcam), anti-eIF4E (1:1000), anti-phospho-eIF4E (Ser209) (1:500) (Millipore, Billerica, MA), anti-CREB (1:500), anti-phospho-CREB (Ser133) (1:500) (Millipore), anti-eEF2 (1:1000), anti-phospho-eEF2 (Thr56) (1:500), and anti-PSD-95 (1:500) (Thermo Scientific). We attempted to measure phosphorylation of additional ERK substrates that regulate transcription and translation (Elk-1 and Mnk1, respectively), but these antibodies did not work in feline tissue. Mouse anti-β-actin (1:10 000) (Sigma-Aldrich) was included with each primary antibody as a loading control. Membranes were incubated with fluorescently conjugated goat anti-rabbit (800 nm) and anti-mouse (700 nm) antibodies simultaneously (Li-Cor) (1:20 000 in blocking buffer/0.1% Tween) for 1 h at room temperature in the dark. Each membrane was scanned at both 700 nm (β-actin loading control) and 800 nm (protein of interest) and quantified with the Odyssey infrared scanner and image quantification software (Li-Cor). Variation across gels was controlled for by normalizing to a common sample run on multiple gels. Quantified proteins from U0126- and CGP57380-infused groups were compared with the same vehicle control group.
Single-Unit Recording
Separate groups of cats (15 cats total) were used to determine the role of ERK and Mnk1 in sleep-dependent ODP consolidation. Immediately following six hours of intracortical infusion, cats were prepared for single unit recording (Jha et al. 2005). Animals were anesthetized with isoflurane and Nembutal and the skull above V1 was removed. Animals were paralyzed with a continuous intravenous infusion of Flaxedil and ventilated. The dura above V1 was removed and a 16-electrode array (FHC, Inc., Bowdoin, ME, USA) was lowered into V1 at sites near to (within 1 mm) and far from the cannula. Contact lenses were used to focus the eyes on a monitor positioned 40 cm away. Extracellular single-unit recordings were obtained while presenting one eye at a time with a blank screen or full-field reversing, drifting square gratings at 8 stimulus orientations. Each presentation was repeated 4 times per recording block. Between recording blocks, the electrode array was advanced deeper into the brain in 100 μm steps or moved to a new location. Two to three penetrations were made at each recording site.
Units were sorted off-line using principal component analysis (Offline Sorter; Plexon, Inc., Dallas, TX, USA) and analyzed as previously described (Jha et al. 2005; Aton et al. 2009). For comparison, unit data from 4 cats with binocular vision and unmanipulated sleep (referred to as “normal” animals) were reproduced with permission from earlier studies (Aton et al. 2009; Seibt et al. 2012). Briefly, each unit was assigned an ocular dominance (OD) category of 1–7 according to its left eye/right eye firing ratio. Visually unresponsive neurons (those that responded more strongly to blank screen than to gratings) were excluded from the analysis. OD scores were then used to calculate scalar measures of ODP (Issa et al. 1999). The contralateral eye bias index (CBI) is a weighted average of OD scores from the population of neurons sampled, where higher values indicate a stronger bias toward the contralateral eye. This measure was calculated separately for near and far recording sites in each hemisphere and converted to the nondeprived eye bias index (NBI) (where higher values indicate a stronger bias for the non-deprived eye) for clarity (Aton et al. 2009). The shift index (SI), a measure of overall changes in OD across both hemispheres of an animal, was calculated as CBIipsilateral to deprived eye − CBIcontralateral to deprived eye. The monocularity index (MI) was also calculated, where a value of 0 indicates that all recorded neurons respond equally to both eyes and 1 indicates a complete loss of binocular responses. Scalar ODP measures from U0126- and CGP57380-infused groups were compared with values from the same vehicle control group.
In addition to analyzing unit recordings on a population level, firing properties of individual units were examined. These properties include normalized peak firing rate, normalized spontaneous firing rate, evoked response index (ERI), and orientation selectivity index (OSI). For each unit, the peak and spontaneous firing rates were normalized to the average firing rate of all neurons that were recorded at the same location, as described previously (Aton et al. 2009). The OSI, a measure of orientation tuning, was calculated as the unit's response strength at its preferred orientation versus the orthogonal (90°) orientation. The ERI, a measure of how selectively a neuron fires in response to visual stimulation versus spontaneous activity, was calculated as 1−spontaneous firing rate /peak firing rate. Neuronal properties were averaged within each recording site (Aton et al. 2009; Seibt et al. 2012).
Sleep/Wake Analysis
Vigilance states were manually scored as rapid eye movement (REM) sleep, non-REM (NREM) sleep, or wake in 8-s epochs by a trained experimenter (M.C.D.). States were scored based on frontoparietal EEG and EMG signals (SleepSign for Animal; Kissei Comtec America, Inc., Irvine, CA, USA) according to previously described criteria (Frank et al. 2001). Percentage of total recording time and average bout duration for each vigilance state was calculated for baseline, MD, and post-MD periods. The number of sleep–wake transitions and the total number of state transitions were also calculated for the post-MD period. Fast Fourier transforms were performed on post-MD frontoparietal and V1 EEG recordings and averaged in 2-h bins to quantify changes in EEG activity during the infusion. For each hemisphere, power was averaged within the delta (0.5–4 Hz), sigma (10–15 Hz), theta (5–8 Hz), and high-frequency (HF) (15–40 Hz) bands and normalized to mean baseline power (Jha et al. 2005). Two vehicle-infused hemispheres were excluded from the power analysis because the EEG signals at the cannula sites were unusable.
Statistical Analysis
Statistical tests were performed using SigmaPlot 11.0 (Systat Software, Inc., San Jose, CA, USA). Groups were compared using 1-tailed Student's t-tests, 1-way ANOVAs, or 2-way ANOVAs, as indicated. The use of planned, 1-tailed t-tests (α = 0.05, 95% confidence level) was specified prior to analysis, as we were interested in whether the drugs specifically impaired ODP and its underlying biochemical changes (Zar 1999). Nonparametric tests (Mann–Whitney U-tests, ANOVAs on ranks) were used in cases where data were not normally distributed.
Results
U0126 Inhibits ERK Phosphorylation
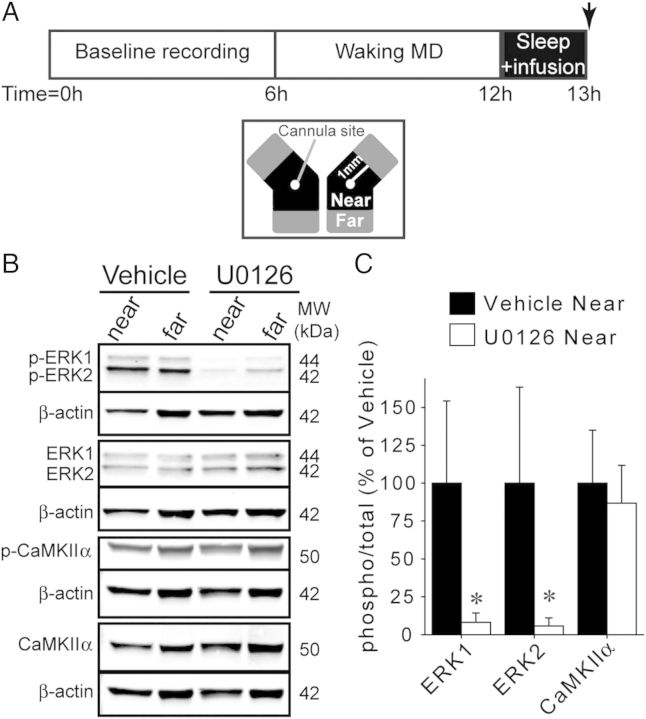
To confirm the efficacy of U0126, we measured ERK phosphorylation in V1 infused with U0126 or vehicle for 1 h of post-MD sleep, at a time when ERK phosphorylation is normally high (Aton et al. 2009) (Fig. 1A). Western blot analysis revealed that near the cannula, U0126 significantly decreased ERK1 and ERK2 phosphorylation to 8.13 ± 2.53 and 5.71 ± 2.18% of vehicle levels, respectively (Fig. 1B,C). Furthermore, within U0126-infused hemispheres, ERK1/2 phosphorylation was lower near to the cannula than far from the cannula (P = 0.009, Mann–Whitney U-test; data not shown). There was no difference between near and far sites in vehicle-infused hemispheres (P > 0.05, Mann–Whitney U-test; data not shown).
Figure 1.
U0126 infusion inhibits ERK, but not CaMKIIα, phosphorylation. (A) Top: Experimental design. Arrow indicates time at which V1 tissue was collected. Bottom: Schematic of bilaterally infused V1 tissue. Tissue was collected near (within 1 mm) and far from the cannula site. (B) Representative western blots showing total and phosphorylated ERK and CaMKIIα in V1 after 1 h of sleep with vehicle or U0126 infusion. Each protein of interest is shown above β-actin bands from the same blot. (C) Average (±SD) ERK and CaMKIIα phosphorylation near the infusion sites. Phospho-ERK levels were decreased by U0126, while CaMKIIα phosphorylation was unaffected. *P = 0.002, Mann–Whitney U-test; n = 6 hemispheres/group.
Similar to ERK, Ca2+/calmodulin-dependent protein kinase II (CaMKII) phosphorylation is elevated in the sleeping visual cortex following MD (Aton et al. 2009). To verify that the effects of U0126 could not be explained by nonspecific inhibition of CaMKII, western blots for total and phosphorylated CaMKIIα in vehicle and U0126-infused V1 tissue were performed. U0126 did not alter CaMKIIα phosphorylation (Fig. 1B,C), consistent with the previous observation that U0126 does not affect CaMKII phosphorylation in the hippocampus (Roberson et al. 1999).
ERK Inhibition During Sleep Impairs ODP Consolidation
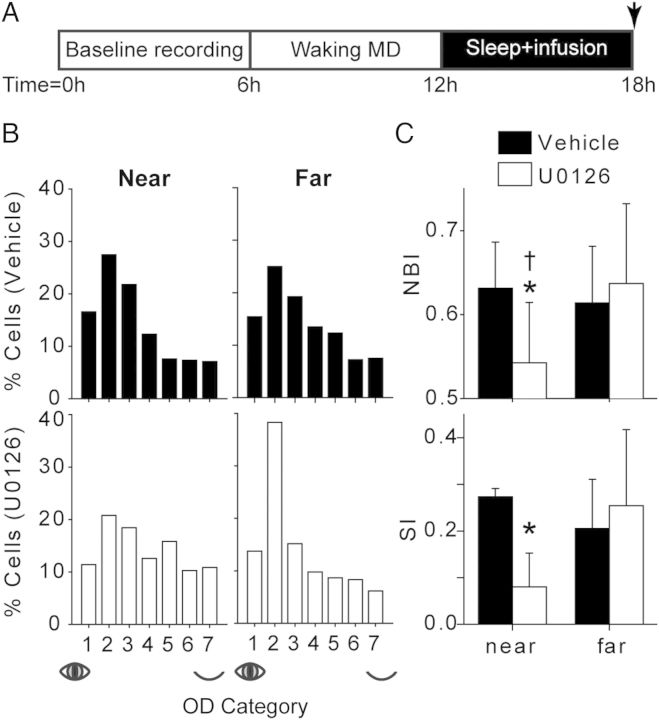
Inhibition of ERK in the sleeping, remodeling visual cortex abolished the normal effects of sleep on ODP. OD histograms revealed that the distribution of recorded cells was biased toward the nondeprived eye in vehicle-treated hemispheres and far from the cannula in U0126-infused hemispheres. In contrast, U0126 blocked this shift near the site of the infusion (Fig. 2B).
Figure 2.
ERK inhibition abolishes sleep-dependent plasticity. (A) Experimental design. Arrow indicates the time point at which the animal was prepared for single unit recording. (B) OD histograms showing the distribution of single-unit eye preference near and far from vehicle and U0126 infusion sites. Vehicle near n = 423 cells; U0126 near n = 342; vehicle far n = 416; U0126 far n = 277. (C) Average (±SD) NBI and SI (n = 7 hemispheres/group). U0126 infusion significantly attenuated the NBI and SI near the cannula compared with vehicle. The NBI was also significantly lower near versus far from the cannula. *P < 0.02, U0126 near versus vehicle near; †P < 0.03, U0126 near versus U0126 far; t-test.
The extent of the OD shift was quantified using the NBI and the SI (see Materials and Methods section) (Fig. 2C). The NBI ranges from 0 (only responsive to the deprived eye) to 1 (only responsive to the nondeprived eye), while the SI ranges from −1 (only responsive to the deprived eye) to 1 (only responsive to the nondeprived eye). Both the NBI and SI were significantly lower in U0126- than vehicle-infused V1 when measured near the cannula. Furthermore, within U0126-infused hemispheres, the NBI was significantly lower near the cannula than far from the cannula. These results confirm that ERK activity in V1 during sleep is required for ODP consolidation.
We have previously shown that mTOR activity is required for the sleep-dependent consolidation of ODP, but not its induction during wakefulness (Seibt et al. 2012). We therefore examined whether ERK was necessary for ODP induction during waking MD. We infused vehicle or U0126 into V1 during 6 h MD in awake animals, then obtained single-unit recordings near to the cannula and compared the resulting MI with normal animals (see Supplementary Fig. S2). We examined the MI in this case because it is a more sensitive measure of this early stage of ODP than the NBI (Aton et al. 2009). Consistent with previous results (Frank et al. 2001; Aton et al. 2009), 6 h of MD with vehicle infusion produced a small but significant increase in the MI relative to cats with normal binocular vision. MD-only hemispheres infused with U0126 also had a significantly higher MI than normal controls, indicating that ERK activity specifically during wake is not necessary for ODP induction.
U0126 Infusion During Sleep Impairs eIF4E Phosphorylation
ERK can promote plasticity by phosphorylating many substrates, including those involved in transcriptional and translational control (reviewed in Adams and Sweatt 2002). However, it is unknown which of these pathways are activated by ERK during sleep-dependent consolidation. Therefore, we used western blot analysis to measure proteins phosphorylated downstream of ERK activation (eIF4E; eukaryotic elongation factor 2, eEF2; cAMP response element binding protein, CREB) in U0126- and vehicle-infused V1. These proteins regulate mRNA translation initiation (eIF4E), translation elongation (eEF2), and transcription (CREB). We also measured levels of postsynaptic density protein 95 (PSD-95), a postsynaptic scaffolding protein whose expression increases AMPA receptor currents during experience-dependent plasticity in vivo (Ehrlich and Malinow 2004). Sleep promotes the synthesis of PSD-95 in V1 (Seibt et al. 2012), but it is unknown whether this requires ERK activity.
U0126 significantly decreased eIF4E phosphorylation and levels of PSD-95 (Fig. 3). In contrast, we did not find a significant effect of U0126 on eEF2 or CREB phosphorylation compared with vehicle. Together, these results suggest that ERK activity in the sleeping, remodeling cortex promotes the synthesis of proteins such as PSD-95, which may contribute to consolidation.
Figure 3.
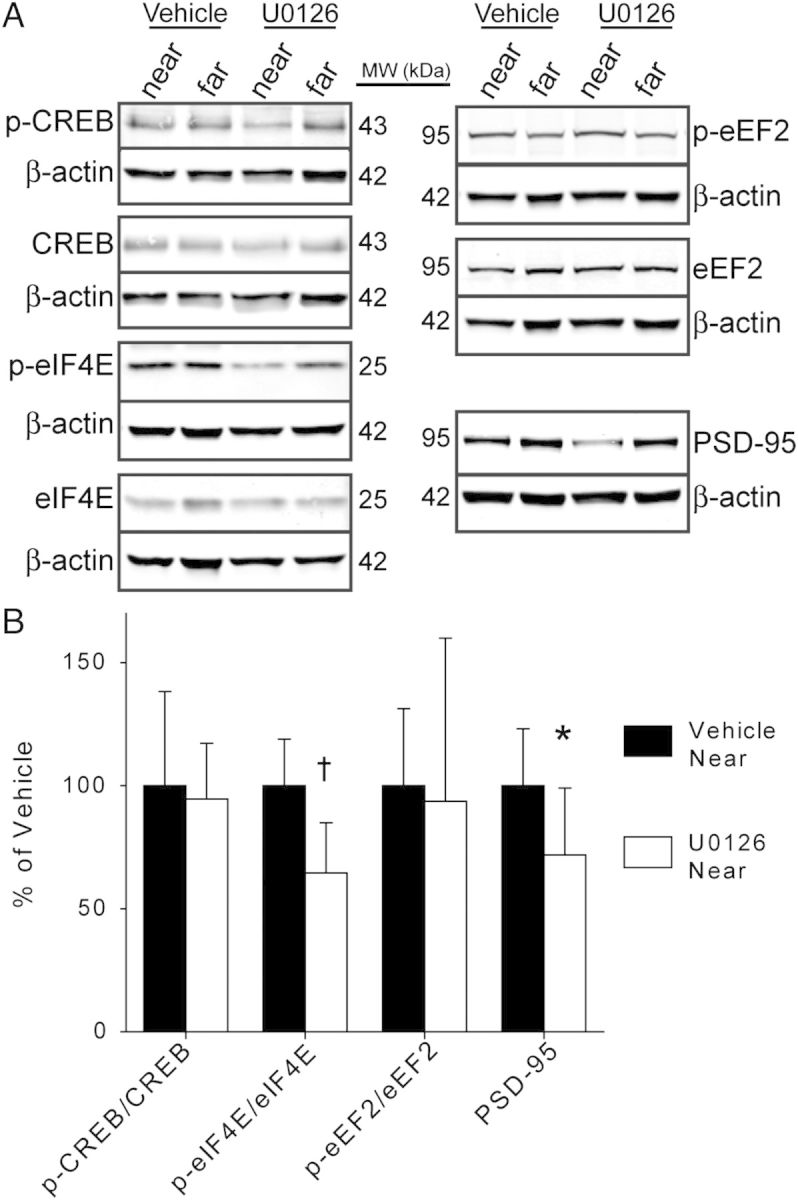
U0126 inhibits downstream molecular effectors of ERK. (A) Representative western blots from vehicle and U0126-infused V1. Bands for each protein of interest are shown above β-actin bands from the same blot. (B) Average (±SD) levels of p-CREB, p-eIF4E, p-eEF2, and PSD-95. U0126 significantly decreased eIF4E phosphorylation and PSD-95 levels versus vehicle. *P = 0.041, †P = 0.0054, n = 6 hemispheres/group, t-test.
Mnk1 Inhibition During Sleep Mimics ERK Inhibition
Mnk1 is a direct target of ERK that phosphorylates eIF4E (Waskiewicz et al. 1997). As U0126 infusion attenuated eIF4E phosphorylation, we investigated whether directly inhibiting Mnk1 activity could mimic the effects of U0126. To do this, we infused the Mnk1 inhibitor CGP57380 into V1 during post-MD sleep. We first collected V1 tissue after 1 h of post-MD sleep to confirm the efficacy and specificity of the drug. CGP57380 infusion significantly decreased eIF4E phosphorylation and PSD-95 levels near to the cannula site without affecting ERK phosphorylation (Fig. 4A,B). CGP57380 did not affect phosphorylation of any other proteins examined (p-CaMKIIα, p-CREB, p-eEF2: P > 0.1, CGP57380 n = 4 hemispheres, vehicle n = 6; t-test; data not shown).
Figure 4.
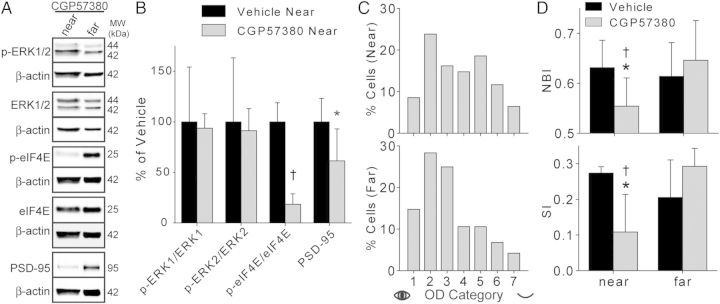
Mnk1 inhibition abolishes sleep-dependent plasticity. (A) Representative western blots. Each protein of interest is paired with β-actin bands from the same blot. (B) CGP57380 significantly decreased mean (±SD) eIF4E phosphorylation and PSD-95 levels without affecting ERK phosphorylation. Vehicle values are reproduced from Figures 1 and 3. *P = 0.0275; †P ≤ 0.001; CGP57380 n = 4 hemispheres; t-test. (C) OD histograms revealed an attenuated shift near to, but not far from, the site of CGP57380 infusion. Near n = 420 cells; far n = 502. (D) Average (±SD) NBI and SI (CGP57380 n = 6 hemispheres). CGP57380 significantly impaired the OD shift near to, but not far from, the infusion site. Vehicle data are reproduced from Figure 2. *P < 0.03, CGP57380 near vs. vehicle near; †P < 0.03, CGP57380 near vs. CGP57380 far; t-test.
We then measured the effects of infusing CGP57380 during 6 h of post-MD sleep on ODP. Single-unit recordings revealed that CGP57380 attenuated sleep-dependent plasticity, mimicking the effects of U0126. As shown in Figure 4C, CGP57380 prevented the normal sleep-dependent shift in the OD histogram toward the nondeprived eye near the cannula site. CGP57380 also significantly reduced scalar measures of ODP (the NBI and SI) compared with vehicle and compared with sites far from the cannula (Fig. 4D). These results confirm that Mnk1 activation is required during sleep for ODP consolidation.
Single-Unit Firing Properties Following Drug Infusion
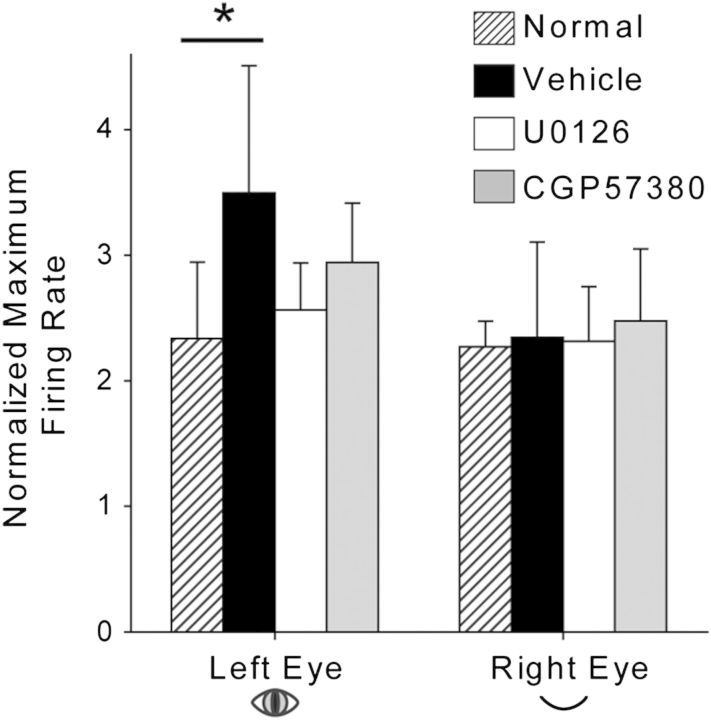
We have previously shown that post-MD sleep enhances V1 neuronal responses to nondeprived eye stimulation (Aton et al. 2009; Seibt et al. 2012). As ERK activity is required for LTP in rodent V1 (DiCristo et al. 2001), we hypothesized that the ERK-Mnk1 pathway is also required for sleep-dependent enhancement of nondeprived eye responses. To test this, we compared the normalized peak firing rate in response to stimulation of each eye between normal control, vehicle-infused, U0126-infused, and CGP57380-infused hemispheres. As expected, relative to normal controls, responses to the nondeprived eye were significantly enhanced in vehicle-infused V1; however, U0126 and CGP57380 infusion prevented this enhancement (Fig. 5). No differences across groups were observed in response to deprived eye stimulation. These results confirm that ERK and Mnk1 promote sleep-dependent ODP consolidation by enhancing nondeprived eye responses, rather than attenuating deprived eye responses.
Figure 5.
ERK and Mnk1 inhibition prevent enhancement of neuronal responses to nondeprived eye stimulation. Average (±SD) normalized peak firing rates in response to left- and right-eye visual stimulation are shown. Left (nondeprived) eye responses were enhanced in vehicle-treated V1 compared with normal animals that did not undergo manipulations of vision or sleep. This increase was blocked near to the site of U0126 and CGP57380 infusion. *P < 0.05, ANOVA on ranks followed by Dunn's post hoc test. Normal n = 8 hemispheres; vehicle n = 7; U0126 n = 7; CGP57380 n = 6.
The effects of U0126 and CGP57380 on plasticity could not be ascribed to nonspecific effects of these drugs on visual cortical neurons. As shown in Table 1, there were no significant differences between groups on any measure of single unit firing characteristics (percent of visually responsive units, ERI, OSI, and normalized spontaneous firing rate).
Table 1.
Firing properties of V1 neurons
| % Visually responsive neurons | Evoked response index |
Orientation selectivity index |
Normalized spontaneous firing rate |
||||
|---|---|---|---|---|---|---|---|
| Left eye | Right eye | Left eye | Right eye | Left eye | Right eye | ||
| Vehicle | 99.05±1.9 | 0.73±0.046 | 0.62±0.18 | 0.75±0.10 | 0.69±0.12 | 0.65±0.17 | 0.59±0.30 |
| U0126 | 99.80±0.52 | 0.71±0.12 | 0.67±0.06 | 0.72±0.05 | 0.67±0.06 | 0.57±0.17 | 0.61±0.17 |
| CGP57380 | 99.26±1.2 | 0.78±0.09 | 0.71±0.14 | 0.72±0.07 | 0.75±0.05 | 0.51±0.24 | 0.52±0.27 |
Firing properties of V1 neurons were unaffected by U0126 and CGP57380. Average (±SD) firing properties of units recorded near to the site of drug infusion are shown. No significant differences were observed across drug treatments for any property. ERI, OSI, spontaneous firing rate: 1-way ANOVA P > 0.25; % visually responsive neurons: ANOVA on ranks P > 0.6; vehicle n = 7 hemispheres, U0126 n = 7, CGP57380 n = 6.
Sleep Architecture is Unaffected by Drug Infusion
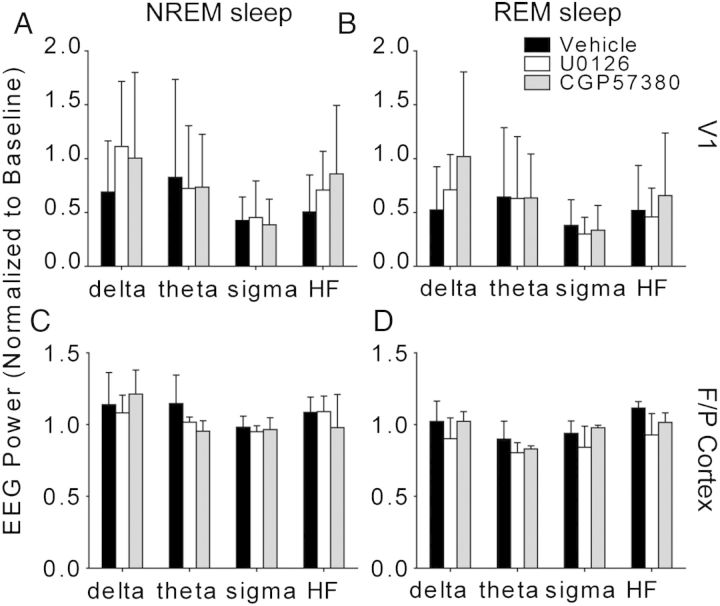
U0126 and CGP57380 also had no significant effects on global sleep architecture. Two-way ANOVAs revealed no significant main effect of drug (P ≥ 0.320) or drug × time interaction (P ≥ 0.423) for the state amount, state duration, or number of state transitions during post-MD sleep (main effects: drug treatment, post-MD time; vehicle N = 4 animals, U0126 N = 4, CGP57380 N = 3) (Fig. 6). We observed significant main effects of time over the course of the post-MD period: NREM amount (P < 0.001) and bout duration (P < 0.001) decreased, REM amount (P = 0.02) increased, wake amount (P < 0.001) and bout duration (P = 0.013) increased, sleep-to-wake transition number increased (P = 0.002), and total number of transitions between arousal states increased (P = 0.004). Two-way ANOVAs also showed no main effect of drug (P ≥ 0.205) or drug × time interaction (P ≥ 0.292) for frontoparietal EEG power in delta, theta, sigma, and HF bands in these animals (Fig. 7). We observed significant main effects of post-MD time on frontoparietal EEG power: NREM power in the theta (P < 0.001), sigma (P = 0.024), and HF bands (P = 0.019) decreased, REM theta power decreased (P = 0.012), and REM HF power increased (P = 0.011). However, these changes to sleep architecture and EEG power occurred in all treatment groups and reflect changes similar to those previously observed during post-MD sleep (Frank et al. 2001; Jha et al. 2005; Seibt et al. 2008). There were also no significant differences between groups in EEG activity at the infusion sites. Two-way ANOVAs (Vehicle n = 5; U0126 n = 7; CGP57380 n = 6 hemispheres) revealed no effect of drug (P ≥ 0.098), time (P ≥ 0.630), or drug × time interaction (P ≥ 0.334) on delta, theta, sigma, or HF power within V1 (Fig. 7A,B).
Figure 6.
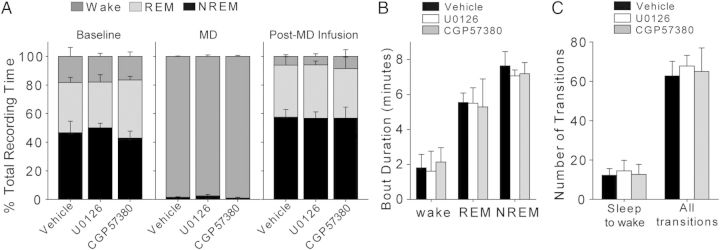
Sleep architecture is unaffected by drug infusion. Post-MD sleep characteristics are displayed as 6-hour averages for simplicity, but were divided into 2-hour bins for statistical analysis (see Results; no significant effects of drug or drug × time interaction were observed). Sleep architecture was determined using frontoparietal EEG and EMG recordings. Vehicle N = 4 cats; U0126 N = 4; CGP57380 N = 3. (A) Mean (±SD) amounts of wake, REM sleep, and NREM sleep during baseline, MD, and post-MD recording periods. (B) Mean (±SD) duration of wake, REM sleep, and NREM sleep bouts during the post-MD infusion period. (C) Average (±SD) number of transitions from sleep to wake and total number of transitions between all arousal states during the post-MD period.
Figure 7.
Drug infusion does not alter sleep EEG power. Data represent Fourier transformed EEG data, normalized to baseline values and averaged into standard frequency bands (delta, theta, sigma, and high frequency (HF)). Each frequency band is displayed as mean±SD and averaged across the entire 6 h recording period for simplicity, but was divided into 2-hour bins for statistical analysis (see Results; no significant drug or drug × time interaction effects were observed for any frequency band). (A,B) EEG power in V1, recorded from bipolar leads at the cannula site, did not differ between drug- and vehicle-infused cortex. (C,D) Drug infusion did not alter EEG activity in the frontoparietal (F/P) cortex.
Discussion
We show that activation of the ERK-Mnk1 pathway during sleep is necessary for ODP consolidation. More specifically, inhibition of ERK or Mnk1 during sleep in the visual cortex abolishes the normal potentiation in non-deprived visual pathways observed after sleep. The effects of ERK inhibition during sleep cannot be explained by indirect effects of drug infusion, since sleep architecture and neuronal firing properties in V1 were unaffected. This loss of plasticity is accompanied by reductions in phosphorylated eIF4E and levels of PSD-95. Phosphorylation of eIF4E correlates with increased 5′-cap-dependent mRNA translation rates. Although the underlying mechanism is unclear, phosphorylation of eIF4E promotes its dissociation from the 5′-cap, which may speed translation by facilitating polysome formation or increasing the pool of available cap-binding factors (Scheper and Proud 2002). In addition to regulating translation via eIF4E phosphorylation, ERK-Mnk signaling is required for translation initiation complex formation during plasticity in vivo (Panja et al. 2009). PSD-95 is a synaptic protein whose expression controls the localization of AMPA receptors to the postsynaptic membrane during plasticity in vivo (Ehrlich and Malinow 2004). Therefore, the current study suggests that the ERK-Mnk1 pathway is activated in the sleeping, remodeling cortex and promotes consolidation by upregulating the synthesis of plasticity-related proteins such as PSD-95.
Mechanisms of ERK-Dependent Consolidation
ERK regulates multiple pathways that can ultimately lead to the stabilization of plasticity, including transcription and translation (reviewed in Adams and Sweatt 2002). In hippocampal LTP, translation is controlled by concomitant activation of the ERK and mTOR pathways via eIF4E and 4E-BP1 phosphorylation (Kelleher et al. 2004; Gelinas et al. 2007; Tsokas et al. 2007; Connor et al. 2011). Seibt et al. (2012) showed that protein synthesis via mTOR is required during sleep for ODP consolidation; however, the role of ERK in sleep-dependent translation was not explored. Although we did not directly measure initiation complex formation or translation, our finding that U0126 and CGP57380 inhibit eIF4E phosphorylation and decrease PSD-95 levels is consistent with a dual role for ERK and mTOR in the synthesis of plasticity-related proteins during sleep-dependent consolidation.
Translation initiation, combined with decreased elongation via eEF2 phosphorylation, can promote the synthesis of a specific pool of proteins including CaMKIIα and Arc (Scheetz et al. 2000; Belelovsky et al. 2005; Park et al. 2008). Phosphorylation of eEF2 is elevated in V1 during post-MD sleep but is not blocked by mTOR inhibition (Seibt et al. 2012). Here, we found that eEF2 phosphorylation is also unaffected by U0126, suggesting that ERK and mTOR promote a general increase in translation during sleep, while an additional factor restricts the pool of translated mRNAs by increasing eEF2 phosphorylation. One candidate for this factor is PKA, which is required for sleep-dependent ODP consolidation (Aton et al. 2009) and can promote eEF2 phosphorylation (Redpath and Proud 1993; Diggle et al. 2001).
In addition to regulating translation, ERK can also translocate to the nucleus and activate downstream transcription factors such as CREB (reviewed in Adams and Sweatt, 2002). Phospho-ERK nuclear translocation occurs in rat V1 after 24 h of MD, implicating ERK as a regulator of transcription during ODP (Takamura et al. 2007). However, we found that CREB phosphorylation is not blocked by U0126 during the first hour of post-MD sleep, suggesting that ERK does not exert transcriptional control during sleep. This finding agrees with previous observations that the time course of the effects of U0126 on LTP in V1 is consistent with a role for ERK in translation rather than transcription (DiCristo et al. 2001). This result is also consistent with our previous findings that transcription of the genes c-fos, arc, and bdnf in V1 is reduced during sleep (Seibt et al. 2012). Therefore, while it is possible that ERK may lead to CREB phosphorylation on a longer timescale (between 1 and 6 h of sleep), or that ERK may activate other transcription factors (such as Elk-1), our results suggest that ERK does not promote transcription during sleep.
ERK and Synaptic Potentiation
ODP occurs through decreased V1 responsiveness to deprived eye inputs, which is initiated during waking and maintained during sleep, as well as enhanced responsiveness to nondeprived eye inputs, which occurs during post-MD sleep (Aton et al. 2009; Seibt et al. 2012). ERK activation is required for LTP in V1 (DiCristo et al. 2001), but is also necessary for various forms of LTD, including NMDA receptor- and metabotropic glutamate receptor-dependent hippocampal LTD (Thiels et al. 2002; Gallagher et al. 2004), cerebellar LTD (Kawasaki et al. 1999; Endo and Launey 2003), and muscarinic acetylcholine receptor-dependent visual cortical LTD (McCoy and McMahon 2007; McCoy et al. 2008). Therefore, we considered the possibility that the ERK-Mnk1 pathway may promote ODP either by enhancing nondeprived eye responses or depressing deprived eye responses during sleep. Analysis of neuronal firing properties revealed that U0126 and CGP57380 selectively inhibited the strengthening of nondeprived eye responses in V1. Therefore, we conclude that ERK and Mnk1 activation during sleep promote ODP by potentiating synaptic responses in favor of the nondeprived eye.
Potential Mechanisms of ERK Activation
Although we have demonstrated a requirement for ERK in sleep-dependent plasticity, the cellular events that initiate ERK phosphorylation during sleep remain unclear. ERK can be activated by numerous mechanisms, including neuronal activity and activation of neuromodulatory inputs (reviewed in Adams and Sweatt, 2002). Aton et al. (2009) demonstrated that ERK activation during post-MD sleep requires NMDA receptor activity. Therefore, our results support a model in which cortical activity patterns unique to sleep activate NMDA receptors, thereby promoting ERK activation. However, cortical activity patterns and neuromodulatory tone vary greatly across sleep states, raising the question of whether ERK activation may occur preferentially during NREM or REM sleep. One possibility is that the combination of neuronal activity and high cholinergic tone during REM sleep could activate ERK via NMDA and muscarinic acetylcholine receptors. In support of this hypothesis, REM sleep deprivation has been shown to decrease ERK phosphorylation in the rat dorsal hippocampus (Ravassard et al. 2009), and spontaneous REM sleep elevates hippocampal ERK phosphorylation (Luo et al. 2013). Furthermore, Lopez et al. (2008) observed a trend for decreased hippocampal PSD-95 in young rats that had been deprived of REM sleep. However, it remains to be seen whether these effects of REM sleep deprivation hold true in the cortex, and whether REM sleep deprivation impairs ODP consolidation. Ultimately, more detailed investigations of kinase function during wake, REM sleep, and NREM sleep in the remodeling cortex are required to fully elucidate the mechanisms underlying cortical consolidation.
Conclusions
Although a number of mechanisms underlying neuronal plasticity in vitro are known, it is unclear how many of these mechanisms function in vivo. The present study furthers our understanding of how the ERK-Mnk1 pathway functions in the intact brain, and reveals a novel mechanism underlying sleep-dependent cortical consolidation.
Supplementary Material
Supplementary material can be found at: http://www.cercor.oxfordjournals.org/
Funding
This work was supported by departmental funds from the University of Pennsylvania and National Institutes of Health (EY019022 and HL114161 to M.G.F. and F31NS067935 to M.C.D.).
Supplementary Material
Notes
Conflict of Interest: None declared.
References
- Adams JP, Sweatt JD. Molecular psychology: roles for the ERK MAP kinase cascade in memory. Annu Rev Pharmacol Toxicol. 2002;42:135–163. doi: 10.1146/annurev.pharmtox.42.082701.145401. [DOI] [PubMed] [Google Scholar]
- Aton SJ, Seibt J, Dumoulin M, Jha SK, Steinmetz N, Coleman T, Naidoo N, Frank MG. Mechanisms of sleep-dependent consolidation of cortical plasticity. Neuron. 2009;61:454–466. doi: 10.1016/j.neuron.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banko JL, Hou L, Poulin F, Sonenberg N, Klann E. Regulation of eukaryotic initiation factor 4E by converging signaling pathways during metabotropic glutamate receptor-dependent long-term depression. J Neurosci. 2006;26:2167–2173. doi: 10.1523/JNEUROSCI.5196-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelovsky K, Elkobi A, Kaphzan H, Nairn A, Rosenblum K. A molecular switch for translational control in taste memory consolidation. Eur J Neurosci. 2005;22:2560–2568. doi: 10.1111/j.1460-9568.2005.04428.x. [DOI] [PubMed] [Google Scholar]
- Berardi N, Pizzorusso T, Ratto GM, Maffei L. Molecular basis of plasticity in the visual cortex. Trends Neurosci. 2003;26:369–378. doi: 10.1016/S0166-2236(03)00168-1. [DOI] [PubMed] [Google Scholar]
- Connor SA, Wang YT, Nguyen PV. Activation of beta-adrenergic receptors facilitates heterosynaptic translation-dependent long-term potentiation. J Physiol. 2011;589:4321–4340. doi: 10.1113/jphysiol.2011.209379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiCristo G, Berardi N, Cancedda L, Pizzorusso T, Putignano E, Ratto GM, Maffei L. Requirement of ERK activation for visual cortical plasticity. Science. 2001;292:2337–2340. doi: 10.1126/science.1059075. [DOI] [PubMed] [Google Scholar]
- Diggle TA, Subkhankulova T, Lilley KS, Shikotra N, Willis AE, Redpath NT. Phosphorylation of elongation factor-2 kinase on serine 499 by cAMP-dependent protein kinase induces Ca2+/calmodulin-independent activity. Biochem J. 2001;353:621–626. doi: 10.1042/0264-6021:3530621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich I, Malinow R. Postsynaptic density 95 controls AMPA receptor incorporation during long-term potentiation and experience-driven synaptic plasticity. J Neurosci. 2004;24:916–927. doi: 10.1523/JNEUROSCI.4733-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo S, Launey T. ERKs regulate PKC-dependent synaptic depression and declustering of glutamate receptors in cerebellar Purkinje cells. Neuropharmacology. 2003;45:863–872. doi: 10.1016/s0028-3908(03)00210-7. [DOI] [PubMed] [Google Scholar]
- Favata MF, Horiuchi KY, Manos EJ, Daulerio AJ, Stradley DA, Feeser WS, Van Dyk DE, Pitts WJ, Earl RA, Hobbs F, et al. Identification of a novel inhibitor of mitogen-activated protein kinase kinase. J Biol Chem. 1998;273:18623–18632. doi: 10.1074/jbc.273.29.18623. [DOI] [PubMed] [Google Scholar]
- Frank MG, Issa NP, Stryker MP. Sleep enhances plasticity in the developing visual cortex. Neuron. 2001;30:275–287. doi: 10.1016/s0896-6273(01)00279-3. [DOI] [PubMed] [Google Scholar]
- Gallagher SM, Daly CA, Bear MF, Huber KM. Extracellular signal-regulated protein kinase activation is required for metabotropic glutamate receptor-dependent long-term depression in hippocampal area CA1. J Neurosci. 2004;24:4859–4864. doi: 10.1523/JNEUROSCI.5407-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelinas JN, Banko JL, Hou L, Sonenberg N, Weeber EJ, Klann E, Nguyen PV. ERK and mTOR signaling couple beta-adrenergic receptors to translation initiation machinery to gate induction of protein synthesis-dependent long-term potentiation. J Biol Chem. 2007;282:27527–27535. doi: 10.1074/jbc.M701077200. [DOI] [PubMed] [Google Scholar]
- Hubel D, Wiesel TN. The period of susceptibility to the physiological effects of unilateral eye closure in kittens. J Physiol. 1970;206:419–436. doi: 10.1113/jphysiol.1970.sp009022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issa NP, Trachtenberg JT, Chapman B, Zahs KR, Stryker MP. The critical period for ocular dominance plasticity in the ferret's visual cortex. J Neurosci. 1999;19:6965–6978. doi: 10.1523/JNEUROSCI.19-16-06965.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha SK, Jones BE, Coleman T, Steinmetz N, Law C-T, Griffin G, Hawk J, Dabbish N, Kalatsky VA, Frank MG. Sleep-dependent plasticity requires cortical activity. J Neurosci. 2005;25:9266–9274. doi: 10.1523/JNEUROSCI.2722-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki H, Fujii H, Gotoh Y, Morooka T, Shimohama S, Nishida E, Hirano T. Requirement for mitogen-activated protein kinase in cerebellar long term depression. J Biol Chem. 1999;274:13498–13502. doi: 10.1074/jbc.274.19.13498. [DOI] [PubMed] [Google Scholar]
- Kelleher RJ, Govindarajan A, Jung H-Y, Kang H, Tonegawa S. Translational control by MAPK signaling in long-term synaptic plasticity and memory. Cell. 2004;116:467–479. doi: 10.1016/s0092-8674(04)00115-1. [DOI] [PubMed] [Google Scholar]
- Knauf U, Tschopp C, Gram H. Negative regulation of protein translation by mitogen-activated protein kinase-interacting kinases 1 and 2. Mol Cell Biol. 2001;21:5500–5511. doi: 10.1128/MCB.21.16.5500-5511.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez J, Roffwarg HP, Dreher A, Bissette G, Karolewicz B, Shaffery JP. Rapid eye movement sleep deprivation decreases long-term potentiation stability and affects some glutamatergic signaling proteins during hippocampal development. Neuroscience. 2008;153:44–53. doi: 10.1016/j.neuroscience.2008.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Phan TX, Yang Y, Garelick MG, Storm DR. Increases in cAMP, MAPK activity, and CREB phosphorylation during REM sleep: implications for REM sleep and memory consolidation. J Neurosci. 2013;33:6460–6468. doi: 10.1523/JNEUROSCI.5018-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy P, Norton TT, McMahon LL. Layer 2/3 synapses in monocular and binocular regions of tree shrew visual cortex express mAChR-dependent long-term depression and long-term potentiation. J Neurophysiol. 2008;100:336–345. doi: 10.1152/jn.01134.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy PA, McMahon LL. Muscarinic receptor–dependent long-term depression in rat visual cortex is PKC independent but requires ERK1/2 activation and protein synthesis. J Neurophysiol. 2007;98:1862–1870. doi: 10.1152/jn.00510.2007. [DOI] [PubMed] [Google Scholar]
- Panja D, Dagyte G, Bidinosti M, Wibrand K, Kristiansen A-M, Sonenberg N, Bramham CR. Novel translational control in Arc-dependent long term potentiation consolidation in vivo. J Biol Chem. 2009;284:31498–31511. doi: 10.1074/jbc.M109.056077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim J-A, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, et al. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravassard P, Pachoud B, Comte J-C, Mejia-Perez C, Scoté-Blachon C, Gay N, Claustrat B, Touret M, Luppi P-H, Salin PA. Paradoxical (REM) sleep deprivation causes a large and rapidly reversible decrease in long-term potentiation, synaptic transmission, glutamate receptor protein levels, and ERK/MAPK activation in the dorsal hippocampus. Sleep. 2009;32:227–240. doi: 10.1093/sleep/32.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redpath NT, Proud CG. Cyclic AMP-dependent protein kinase phosphorylates rabbit reticulocyte elongation factor-2 kinase and induces calcium-independent activity. Biochem J. 1993;293:31–34. doi: 10.1042/bj2930031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberson ED, English JD, Adams JP, Selcher JC, Kondratick C, Sweatt JD. The mitogen-activated protein kinase cascade couples PKA and PKC to cAMP response element binding protein phosphorylation in area CA1 of hippocampus. J Neurosci. 1999;19:4337–4348. doi: 10.1523/JNEUROSCI.19-11-04337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafe GE, Atkins CM, Swank MW, Bauer EP, Sweatt JD, LeDoux JE. Activation of ERK/MAP kinase in the amygdala is required for memory consolidation of Pavlovian fear conditioning. J Neurosci. 2000;20:8177–8187. doi: 10.1523/JNEUROSCI.20-21-08177.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheetz AJ, Nairn AC, Constantine-Paton M. NMDA receptor-mediated control of protein synthesis at developing synapses. Nat Neurosci. 2000;3:211–216. doi: 10.1038/72915. [DOI] [PubMed] [Google Scholar]
- Scheper GC, Proud CG. Does phosphorylation of the cap-binding protein eIF4E play a role in translation initiation? Eur J Biochem. 2002;269:5350–5359. doi: 10.1046/j.1432-1033.2002.03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seibt J, Aton SJ, Jha SK, Coleman T, Dumoulin MC, Frank MG. The non-benzodiazepine hypnotic zolpidem impairs sleep-dependent cortical plasticity. Sleep. 2008;31:1381–1391. [PMC free article] [PubMed] [Google Scholar]
- Seibt J, Dumoulin MC, Aton SJ, Coleman T, Watson A, Naidoo N, Frank MG. Protein synthesis during sleep consolidates cortical plasticity in vivo. Curr Biol. 2012;22:676–682. doi: 10.1016/j.cub.2012.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamura H, Ichisaka S, Hayashi C, Maki H, Hata Y. Monocular deprivation enhances the nuclear signalling of extracellular signal-regulated kinase in the developing visual cortex. Eur J Neurosci. 2007;26:2884–2898. doi: 10.1111/j.1460-9568.2007.05908.x. [DOI] [PubMed] [Google Scholar]
- Thiels E, Kanterewicz BI, Norman ED, Trzaskos JM, Klann E. Long-term depression in the adult hippocampus in vivo involves activation of extracellular signal-regulated kinase and phosphorylation of Elk-1. J Neurosci. 2002;22:2054–2062. doi: 10.1523/JNEUROSCI.22-06-02054.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsokas P, Ma T, Iyengar R, Landau EM, Blitzer RD. Mitogen-activated protein kinase upregulates the dendritic translation machinery in long-term potentiation by controlling the mammalian target of rapamycin pathway. J Neurosci. 2007;27:5885–5894. doi: 10.1523/JNEUROSCI.4548-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol. 2004;24:6539–6549. doi: 10.1128/MCB.24.15.6539-6549.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waskiewicz AJ, Flynn A, Proud CG, Cooper JA. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 1997;16:1909–1920. doi: 10.1093/emboj/16.8.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesel TN, Hubel DH. Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol. 1963;26:978–993. doi: 10.1152/jn.1963.26.6.1003. [DOI] [PubMed] [Google Scholar]
- Zar JH. Biostatistical analysis. 4th ed. Upper Saddle River, NJ: Prentice Hall; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.