Abstract
Potassium channels (KChs) are the most diverse ion channels, in part due to extensive combinatorial assembly of a large number of principal and auxiliary subunits into an assortment of KCh complexes. This structural and functional diversity allows KChs to play diverse roles in neuronal function. Localization of KChs within specialized neuronal compartments defines their physiological role, and also fundamentally impacts their activity, due to localized exposure to diverse cellular determinants of channel function. Recent studies in mammalian brain reveal an exquisite refinement of KCh subcellular localization. This includes axonal KChs at the initial segment, and near/within nodes of Ranvier and presynaptic terminals, dendritic KChs found at sites reflecting specific synaptic input, and KChs defining novel compartments. Painting the remarkable diversity of KChs onto the complex architecture of mammalian neurons creates an elegant picture of electrical signal processing underlying the sophisticated function of individual neuronal compartments, and ultimately neurotransmission and behavior.
Introduction
Mammalian brain neurons are distinguished from other cells by extreme molecular and structural complexity that is intimately linked to the array of intra- and inter-cellular signaling events that underlie brain function. Integral to the functional complexity of neurons is the array of proteins they express (estimated to encompass the products of two-thirds of the genome), a complexity markedly enhanced by compartmentalization of specific proteins and their functions at highly restricted sites within the neuron’s complex structure. Moreover, dynamic changes in the subcellular localization of these proteins, in the absence of any changes in overall expression level, can confer functional plasticity to neurons. While this structural and molecular complexity provides neurons with a deep diversity and flexibility of function, it also creates challenges for understanding mechanisms controlling neuronal function at the proteomic level. As a prominent example of this molecular diversity is the expression of a large superfamily of plasma membrane ion channels that carry out the bulk of intercellular (via ionotropic receptors) and intracellular (via non-receptor ion channels) neurotransmission. Among the large and diverse set of non-receptor ion channels, which together comprise the third largest set of mammalian signaling proteins [after GPCRs and protein kinases; (Yu and Catterall, 2004)], is the subset defined by their selective passage of K+ ions, referred to generically as K+ channels (KChs). KChs are by far the most diverse group of mammalian ion channels, starting with the 80 paralogous genes encoding the principal transmembrane ion conducting or α subunits (http://www.genenames.org/genefamilies/KCN) and the dozen or so additional genes that encode auxiliary subunits, defined as stably-associated non-conducting or modulatory components of KCh complexes. These genes are expressed in distinct cellular expression patterns throughout the brain, such that particular neurons express specific combinations of KCh α and auxiliary subunits. However, the proteomic complexity of KChs is much greater, as KChs exist as multisubunit complexes formed by coassembly of multiple (typically four, but in the case of two pore KChs only two) α subunits, plus a variable number of auxiliary subunits. Co-assembly of different α and auxiliary subunits in a wide variety of combinations yields a huge diversity in KChs with distinct subunit composition and functional characteristics (Jan and Jan, 2012).
In spite of the challenges presented by the combined molecular complexity of KChs, and structural complexity of the mammalian brain, tremendous progress has been made in our understanding of the roles of specific brain KChs The cloning of KChs has allowed for generation of a reliable set of subtype-specific antibodies against KCh α and auxiliary subunits, some specific for distinct functional states, that, when combined with advances in sample preparation, labeling and imaging techniques, have allowed for numerous insights into the diversity of KCh subcellular localization, as reviewed here, that rivals that of any other family of mammalian proteins. In contrast to excitatory ionotropic receptors located primarily near the site of their neurotransmitter stimuli at synapses, triggers for gated KCh activity are generated at many different sites in neurons, such that KChs can, and do, operate at many different sites. KCh subcellular localization delineates their role in neuronal physiology, through their impact on local electrical signaling events that play specific roles in intra- and inter-cellular neurotransmission. Moreover, at these sites, specific and functionally distinct KCh complexes sense and respond to local changes in levels of their activating stimuli, associate with other cellular proteins in macromolecular complexes, and are modulated by a wide array of local activity-dependent and modulatory signaling pathways. These patterns of KCh subcellular localization map at the first level to the basic plasma membrane compartments of neurons (dendrite, cell body, axon), and then to distinct subcompartments (e.g., dendritic spines, axon initial segment (AIS), nodes of Ranvier (NoRs), presynaptic terminals, etc.). While each of these sites contains a unique repertoire of ion channels associated with the primary events in neurotransmission (e.g., ionotropic glutamate receptors at the excitatory post-synapse; voltage-gated Na+ (Nav) channels at the AIS; voltage-gated Ca2+ (Cav) channels in the presynaptic terminal, etc.), we now appreciate that each also harbors a distinct population of KChs, playing crucial roles as both determinants and modulators of these events. Moreover, the subcellular localization of certain KChs has defined novel neuronal plasma membrane subcompartments, whose function in neurotransmission is less well established, but that likely impact significant aspects of neuronal function, as the observed patterns of subcellular distributions are highly conserved across diverse mammalian species.
Molecular relationships among KChs
KCh principal or α subunits are multipass transmembrane polypeptides that contain the K+ selective pore, as well as the domains that allow them to respond to diverse stimuli. The 80 genes encoding mammalian KCh α subunits are segregated into four main branches (Figure 1) and numerous sub-branches, based on sequence similarity and function. The four main branches correspond to: constitutively open or “leak” KChs of the tandem pore or K2P branch (15 members); constitutively active, and G protein and ATP regulated inwardly rectifying KChs of the Kir branch (15 members); Ca2+ and Na+ activated KChs of the KCa branch (8 members); and voltage-gated KChs of the Kv branch (41 members). It should be noted that some KChs respond to more than one of these stimuli (e.g., KCa1.1 channels are calcium- and voltage-dependent), acting as coincidence detectors for chemical and electrical signaling events. Two pore KCh α subunits assemble into dimers to function, and all of the others into tetramers, and branches and then sub-branches provide strong inferences as to which KCh α subunits can efficiently co-assemble with one another to form homo- or hetero-meric channels [e.g., Kv1 α subunits can co-assemble with one another, but not with Kv2s or Kv3s, etc., and vice-versa; (Jan and Jan, 2012; Papazian, 1999)]. Auxiliary subunits generally co-assemble with these tetrameric α subunit complexes in a sub-branch-specific manner (e.g., Kvβ subunits with Kv1s, AMIGO with Kv2s, KChIPs and DPPs with Kv4s, etc.). The subunit composition of KChs defines their intrinsic functional properties, as well as diverse aspects of expression, localization and modulation. As such, defining the subunit composition of KChs found at specific sites in mammalian brain neurons is crucial to understanding the basic tenants of their function. Given that subunit composition determines pharmacology, accurately defining the subunit composition of KChs and recapitulating them in a format that allows for high-throughput screening of libraries of compounds and biologicals is a requisite component of drug discovery research (Rhodes and Trimmer, 2008). This has and will continue to achieve increased importance as we enter a new era of enhanced access of therapeutic antibodies and other biologicals to the brain (Niewoehner et al., 2014; Watts and Dennis, 2013).
Figure 1. Simplified overview of subcellular localization of KChs.
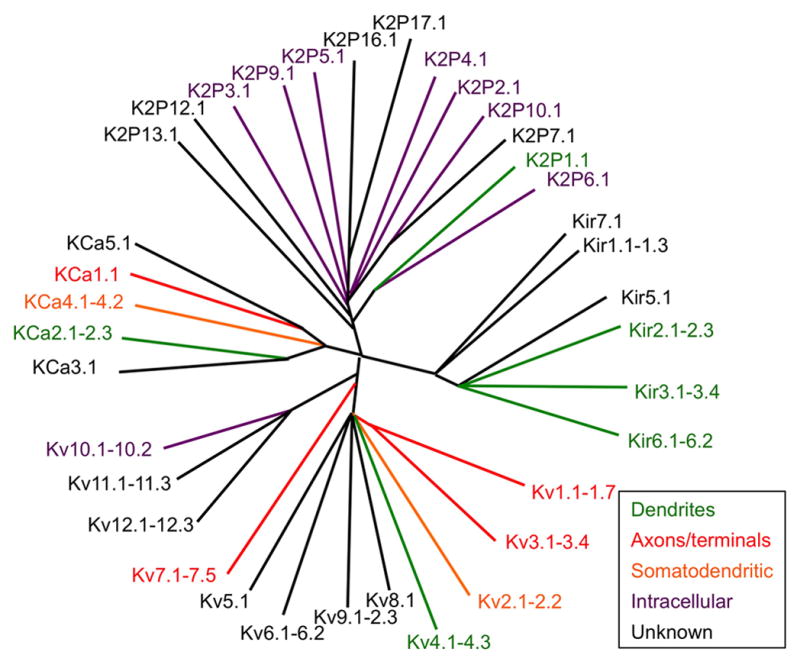
Dendrogram format derived from that available as an online resource courtesy of Fabrice Duprat (https://www.ipmc.cnrs.fr/~duprat/ipmc/nomenclature.htm), and is not meant to convey precise evolutionary relationships. Subcellular localization is based on certain brain neuron classes as detailed in text, and is meant as a general simplified overview and does not universally apply to all brain regions and neuron types.
Analyses of expression patterns of endogenous KCh transcripts in mammalian brain by in situ hybridization, including efforts focused on individual KChs [reviewed in (Vacher et al., 2008)] as well as large-scale (i.e., genome-wide) endeavors such as Genepaint (Geffers et al., 2012; Visel et al., 2004) and the Allen Brain Atlas (Lein et al., 2007; Sunkin et al., 2013), have provided a wealth of information as to the cellular expression patterns of individual KCh subunits. However, while the rules for KCh assembly are relatively simple (Papazian, 1999), the determinants defining which subunit combinations traffic through the endomembrane system and appear in the plasma membrane are more complex [e.g., (Manganas and Trimmer, 2000; Zerangue et al., 1999), etc.], such that subunit composition of native KChs is determined both by rules governing restrictive subunit assembly, and by the selective trafficking of KCh complexes containing specific subunit combinations. Thus, the expression levels of a particular KCh subunit in the plasma membrane do not necessarily reflect its mRNA levels. Furthermore, when considering subcellular localization of KChs, there exist considerations that do not hold for proteins such as transcription factors and chromatin components that, whether expressed in a cell that is simple and small, or (like many brain neurons) large and complex, these proteins are reliably found near the site of their mRNA within or near the nucleus. However, as KChs function in the plasma membrane, by definition they will be found at sites quite distinct from their encoding mRNAs. In many non-neuronal cell types, the distance between the nucleus and the cell boundary can be measured in microns or tens of microns, but in the case of vertebrate neurons, with their extensive dendritic and axonal processes, the boundary membrane can be millimeters, centimeters and even meters away. As such, understanding KCh protein expression, in terms of both the subunit composition of native KChs, and their subcellular localization cannot be resolved or accurately predicted from studies of mRNA expression, but need to be addressed at the proteomic level.
It remains a practical reality that how much we know of any particular protein is determined to a great extent by whether specific probes exist to study it (Edwards et al., 2011). Given the complexity of KCh subunit composition, and the complexity of mammalian brain neuron structure, it is clear that specific probes for efficiently and specifically labeling native, endogenously expressed KCh α and auxiliary subunits are especially crucial to understanding their diverse functional roles. Unlike the facile and straightforward design and synthesis of complementary nucleotide probes to label individual KCh mRNAs by in situ hybridization, current technology does not allow for the directed synthesis of probes for labeling KCh polypeptides. Given the diversity of KChs, it is not feasible to approach systematic analyses of subcellular localization in mammalian brain neurons using genetically encoded tagged KCh subunits, as one can do in yeast or C. elegans, even if one could be assured of accurate incorporation of the exogenous subunit into normal oligomeric complexes, and obviously this approach is impossible in human brain. As such we are left with the need for high quality probes that efficiently and specifically label endogenous KCh subunit proteins in situ.
The cloning of KCh subunits and determination of their deduced amino acid sequences, combined with advances in synthetic peptide synthesis and recombinant protein expression, has allowed for production of designer immunogens that can be used to generate antibodies against individual KCh subunits. Some of these anti-KCh antibodies were generated as singular efforts in individual research laboratories, others from commercial endeavors, and others from systematic NIH-funded efforts, for example at the UC Davis/NIH NeuroMab Facility (neuromab.ucdavis.edu). While much thought and effort has gone into generating and validating anti-KCh antibodies, monospecific antibodies for each KCh family member remains an unmet need. There is a documented propensity (the Harlow-Knapp effect) to focus research efforts on the same small subset of proteins about which we already know quite a bit (in many cases because reliable probes do exist), and to mostly ignore the rest (Fedorov et al., 2010; Grueneberg et al., 2008). Consistent with this, the availability of rigorously validated antibodies against certain KCh α and auxiliary subunits has led to an extensive knowledge base regarding their subunit composition and subcellular localization in mammalian brain, while hardly any or no validated probes exist for most others, about which we know little or nothing at all (Table 1, Figure 1). Recognizing the universal need for high quality protein-specific probes, government agencies have initiated large scale efforts to generate high affinity “binders”, in most cases antibodies, for (ultimately) every human protein (Taussig et al., 2013). However, we are still a long way from achieving this goal.
Table 1.
Overview of KCh Subcellular Localization in Mammalian Brain
| Subunit | Gene | AKA | Predominant localization in brain |
|---|---|---|---|
| KCa1.1 | KCNMA1 | BK, Slo1 | Axons, terminals |
| KCa2.1 | KCNN1 | SK1 | Dendrites |
| KCa2.2 | KCNN2 | SK2 | Dendrites/spines |
| KCa2.3 | KCNN3 | SK3 | Axons, terminals |
| KCa3.1 | KCNN4 | IKCA1, SK4 | Somata, dendrites |
| KCa4.1 | KCNT1 | Slack, Slo2.2 | Somatodendritic |
| KCa4.2 | KCNT2 | Slick, Slo2.1 | Somatodendritic |
| Kir2.2 | KCNJ12 | IRK2 | Dendritic |
| Kir2.3 | KCNJ4 | IRK3 | Axons/terminals |
| Kir3.1 | KCNJ3 | GIRK1 | Dendrites/spines |
| Kir3.2 | KCNJ6 | GIRK2 | Dendrites/spines |
| Kir3.3 | KCNJ9 | GIRK3 | Axons/terminals |
| Kir6.2 | KCNJ11 | KATP | Dendrites |
| Kv1.1 | KCNA1 | AIS, axon preterminals, JPN | |
| Kv1.2 | KCNA2 | AIS, axon preterminals, JPN | |
| Kv1.3 | KCNA3 | CoH terminals, microglia | |
| Kv1.4 | KCNA4 | Axon preterminals | |
| Kv1.6 | KCNA6 | Somatodendritic | |
| Kv2.1 | KCNB1 | Somata, proximal dendrites, AIS | |
| Kv2.2 | KCNB2 | Somata, proximal dendrites, AIS | |
| Kv3.1 | KCNC1 | Terminals, NoR | |
| Kv3.2 | KCNC2 | Terminals | |
| Kv3.3 | KCNC3 | Terminals | |
| Kv3.4 | KCNC4 | Terminals | |
| Kv4.2 | KCND2 | Dendrites/spines | |
| Kv4.3 | KCND3 | Dendrites/spines | |
| Kv7.2 | KCNQ2 | KCNQ2 | AIS, NoR, axons |
| Kv7.3 | KCNQ3 | KCNQ3 | AIS, NoR, axons |
| Kv7.5 | KCNQ5 | KCNQ5 | Terminals CoH |
| Non-neuronal cells | K2p3.1, Kir4.1, Kir6.1, Kv1.5 | ||
| Intracellular | K2p1.1, K2p2.1, K2p4.1, K2p5.1, K2p6.1, K2p9.1, K2p10.1, K2p18.1, Kir2.4, Kir3.4, Kv4.1, Kv10.1, Kv10.1, Kv11.1, Kv11.2 | ||
| No reports | K2p7.1, K2p12.1, K2p13.1, K2p15.1, K2p16.1, K2p17.1, KCa5.1, Kir1.1, Kir4.2, Kir5.1, Kir7.1, Kv1.7, Kv1.8, Kv5.1, Kv6.1, Kv6.2, Kv6.3, Kv6.4, Kv7.1, Kv7.4, Kv8.1, Kv8.2, Kv9.1, Kv9.2, Kv9.3, Kv12.1, Kv12.2, Kv12.3 | ||
While how much we know of the subcellular localization of KChs in mammalian brain is biased toward a select subset based on probe availability, it is also heavily weighted towards particular neurons and/or brain regions. We know a tremendous amount about the situation in hippocampus and cerebellum, in large part due to the relatively simple neuroanatomical organization of these regions, as well as a few other neurons with exceptional characteristics (e.g., globular bushy neurons in the anterior ventral cochlear nucleus or AVCN that give rise to the extraordinary calyx of Held nerve terminals), and much less about most other brain regions. We also know more about KChs localized within subcellular compartments that we have learned to visualize at the level of light microscopy (e.g., the AIS, NoRs, and surrounding regions), than in many others that remain to be characterized, due to their size, density and/or complexity (e.g., neocortical dendritic spines and presynaptic terminals). Lastly, among mammals we know the most about KCh subcellular localization in rat and mouse brain, and less so in human and non-human primates.
Having a collection of antibodies in hand is necessary but not sufficient; as to obtain reliable information on expression and subcellular localization of brain KChs these antibodies then need to be used in careful immunohistochemistry experiments. This involves using brain sections that have been prepared to preserve anatomical structure and immunoreactivity (Lorincz and Nusser, 2008b), labeled with optimal levels of high quality and rigorously validated primary (Rhodes and Trimmer, 2006) and secondary (Manning et al., 2012) antibodies. The latter issue deserves additional mention, due to the underutilization of high quality and widely available secondary antibodies specific for individual mouse IgG subclasses (IgG1, IgG2a, IgG2b being the most common) that allow for easy and reliable multiplex labeling by mixing and matching mouse monoclonal antibodies of different subclasses. This achieves increased importance given the availability of a large and growing number of high quality mouse monoclonal antibodies against KCh subunits and many other neuronal proteins, and that multiplex immunofluorescence labeling, especially when combined with optical sectioning, array tomography, or super-resolution imaging, allows for insights into subcellular colocalization well beyond what any single label experiment could provide. The labeling obtained then needs to be appropriately imaged, and the images correctly processed and analyzed to represent its true nature and extent.
Careful experimental design including attention to controls aimed at determining whether each of these goals was achieved underlies the validity of the results (Lorincz and Nusser, 2008b; Rhodes and Trimmer, 2006; Saper, 2005; Saper and Sawchenko, 2003). As pointed out astutely in a valuable toolbox article (Lorincz and Nusser, 2008b), the conditions used for sample preparation can fundamentally impact the results, such that an antibody validated for specificity under one set of experimental conditions can end up yielding incomplete or incorrect information under another set of conditions. While this is especially relevant when making big leaps, such as from immunoblots to immunohistochemistry, it also holds for immunohistochemistry performed under different conditions, for example “conventional conditions” used for preparing lightly fixed and detergent permeabilized brain sections for light microscope level analyses of immunoperoxidase/diaminobenzidine (LM-DAB) and immunofluorescence (LM-IF) labeling, versus non-permeabilized sections for pre-embedding immunoperoxidase/DAB (EM-DAB) or immunogold (EM-IG) labeling for electron microscopic analysis, versus the conditions used for more heavily fixed post-embedding immunogold labeling, or the exceptional requirements for SDS digested freeze fracture replica immunogold labeling (SDS-FRL) for electron microscopy (Masugi-Tokita and Shigemoto, 2007). However, as many questions regarding KCh expression and localization in brain neurons require using more than one of these approaches, it is important to have a reliable antibody, or set of antibodies, that together have been validated for efficacy and specificity under each condition used.
Two techniques deserve special mention. Recent advances in super-resolution imaging microscopy (Evanko, 2009), primarily applied to thin samples such as monolayer cell cultures, but more recently in relatively thick (i.e., 10–70 μm) brain sections [e.g., (Dani et al., 2010)], have allowed for insights into details of the subcellular localization of KChs. For example, structured illumination microscopy or SIM imaging of brain sections multiplex labeled with mouse monoclonal antibodies of different IgG subclasses allowed for the precise localization of clustered Kv2.1 channels at sites on the AIS deficient in expression of ankyrin-G (AnkG) defining these sites as unique ion channel clustering domains (King et al., 2014), and at sites over ryanodine receptors in striatal medium spiny neurons (Mandikian et al., 2014). The general application of superresolution imaging, using SIM and higher resolution methods (Evanko, 2009), to future studies of KCh subcellular localization will lead to additional insights into distinct neuronal domains and associated molecular components. The use of EM-IG labeling of SDS-FRL samples (Masugi-Tokita and Shigemoto, 2007) has recently emerged as a powerful approach for quantitative ultrastructural analyses of subcellular localization of K+ channels. This technique, while currently practiced in only a handful of laboratories, has yielded remarkable insights into KCh localization, and promises to play a major role in defining at exquisite detail quantitative aspects of subcellular localization with exquisite detail, as it has for ionotropic receptor ion channels at synapses [e.g., (Antal et al., 2008; Tarusawa et al., 2009)], and Nav channels on dendrites (Lorincz and Nusser, 2010).
Leitmotifs of KCh subcellular localization
Recent reviews have provided comprehensive treatment of cellular and subcellular expression of ion channels, including KChs in mammalian brain neurons (Lujan, 2010; Nusser, 2012; Vacher et al., 2008), and mechanisms underlying such localization (Gu and Barry, 2011; Jensen et al., 2011; Vacher and Trimmer, 2012). In addition, recent studies have revealed details of KCh compartmentalized subcellular localization, and striking similarities between KChs distantly related in molecular and functional characteristics (Table 1, Figure 1), and which has shaped our thinking of the role of specific subunits and combinations thereof in neuronal function. I emphasize that given the diversity of neuron classes in mammalian brain, it is not surprising that there will be certain neurons that have KChs localized in subcellular compartments distinct from those summarized in Table 1 and Figure 1. Prominent examples of neuron classes that seem to do things differently include local inhibitory interneurons, olfactory bulb neurons, and cerebellar Purkinje cells, among others, which appear to have distinct rules for subcellular localization (Vacher et al., 2008). However, these summaries, while an oversimplification of all mammalian species, all brain neuron classes, and even more strikingly, all developmental stages, are a useful summary of the most detailed studies in the literature. Here I provide some salient examples of these themes focusing on findings obtained from a set of specific neurons and/or specific neuronal compartments.
Dendritic KChs: input-specific localization in hippocampal CA1 pyramidal cell apical dendrites
Diverse populations of KChs and dynamic modulation of their activity are major determinants of the electrical compartmentalization of dendrites, from distinct proximal to distal gradients (Hoffman et al., 1997; Korngreen and Sakmann, 2000; Schaefer et al., 2007), to differences between oblique dendrites that yield branch-specific differences in dendritic excitability (Losonczy et al., 2008). Additionally, local activity of KChs at or near spines impacts maturation (Kim and Hoffman, 2012) and function (Bloodgood and Sabatini, 2007; Sala and Segal, 2014; Yuste, 2013) of individual synapses. Given these important roles, it is not surprising that disruption of the normal patterns of expression, localization and function of dendritic KChs in brain neurons has emerged as the likely pathophysiological basis for a number of neurological and psychiatric disorders (Poolos and Johnston, 2012). Much of what we know of dendritic KChs is from studies in the apical dendrites of hippocampal CA1 pyramidal neurons, in part due to the focus of many neurophysiological studies on the Schaffer collateral CA3:CA1 synapses located here, but also due to the large size of the primary apical dendrite that is amenable to direct patch clamp analyses (Chen and Johnston, 2006).
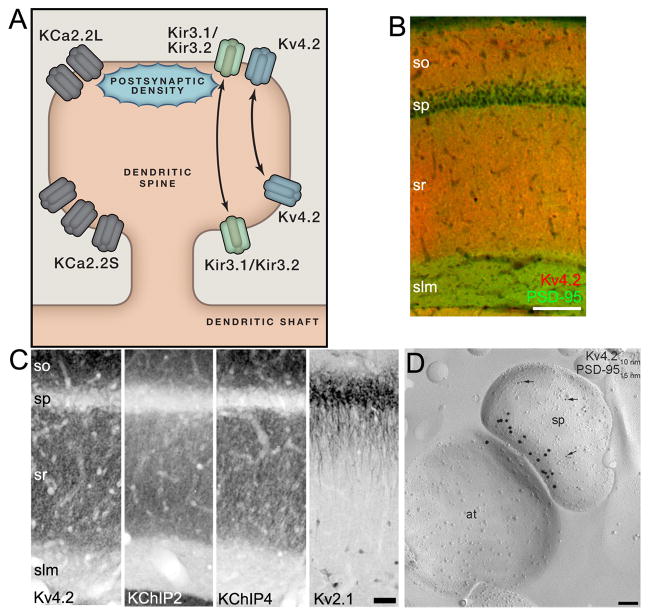
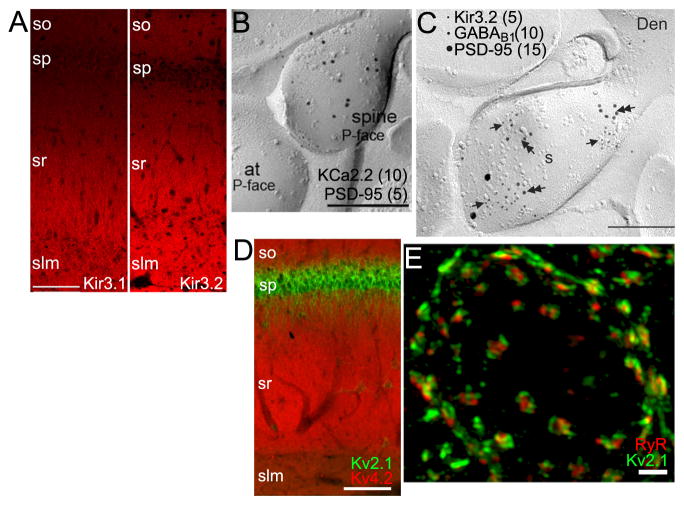
Transient A-type Kv4 channels, small conductance Ca2+-activated KCa2/SK and G protein-coupled inwardly rectifying Kir3/GIRK channels are consistently found in dendrites (Table 1, Figure 1). In many neurons, these KChs exhibit input-specific compartmentalization, being enriched at specific subcompartments of the dendritic membrane that correspond to sites of distinct synaptic input. Kv4.2 immunolabeling in the apical dendrites of CA1 pyramidal neurons (Figure 2B, 2C, 3D) has a non-uniform input-specific distribution (Rhodes et al., 2004; Sheng et al., 1992), being more robust in stratum radiatum (sr), the site of Schaffer collateral input from hippocampal CA3, than in stratum lacunosum moleculare (slm), the site of lateral perforant path input into CA1 from entorhinal cortex. Cytoplasmic KChIP2 and KChIP4 (Rhodes et al., 2004) auxiliary subunits exquisitely co-localize with Kv4.2 in sr of CA1 (Figure 2C), while DPP6 auxiliary subunits are expressed uniformly across sr and slm, perhaps suggesting a function in distal dendrites distinct from acting as a Kv4.2 auxiliary subunit (Clark et al., 2008). In contrast to labeling for Kv4.2, light microscope level immunolabeling for small conductance calcium activated KCa2.2 α subunits labeling is sparse in sr of CA1, and robust in slm (Sailer et al., 2002; Sailer et al., 2004). Labeling for Kir3.1 and Kir3.2 G protein coupled KCh α subunits is also prominent in slm, as well as distal sr (Figure 3A) (Drake et al., 1997; Kirizs et al., 2014; Liao et al., 1996; Miyashita and Kubo, 1997). This suggests input-specific requirements for specific KChs on CA1 apical dendrites.
Figure 2. K+ channel localization in hippocampal CA1 apical dendrites.
A. Cartoon showing localization of KChs in dendritic spines. See text for details. B. Double LM-IF labeling for Kv4.2 (red) and PSD-95 (green) in rat CA1 showing robust labeling for Kv4.2 in sr versus slm. Scale bar: 100 μm. C. LM-DAB labeling for Kv4.2 (far left), KChIP2 (middle left), KChIP4 (middle right) and Kv2.1 (far right) in rat CA1. Scale bar: 50 μm. Modified from (Rhodes et al., 2004). D. EM-IG labeling for Kv4.2 (small particles) and PSD-95 (large particles) in a SDS-FRL sample from rat CA1 showing a single excitatory synapse (at: axon terminal; sp: spine). Scale bar: 0.1 μm. From (Kerti et al., 2012). Anatomical labels for panels B, C: CA1: so: stratum oriens; sp: stratum pyramidale; sr: stratum radiatum; slm: stratum lacunosum moleculare; and for dentate gyrus: ml: molecular layer.
Figure 3. K+ channel localization in dendrites and somata.
A. LM-IF labeling for Kir3.1 (left) and Kir3.2 (right) in rat CA1. From (Kirizs et al., 2014). Scale bar: 100 μm. B. EM-IG labeling for KCa2.2 (large particles, 10 nm) and PSD-95 (small particles, 5 nm, at arrow) showing extrasynaptic KCa2.2 within a dendritic spine (sp) at an excitatory synapse (at: axon terminal) in a SDS-FRL sample from mouse CA1. Scale bar: 0.2 μm. From (Ballesteros-Merino et al., 2012). C. EM-IG labeling of Kir3.2, GABA-B1 receptors, and PSD-95 in a SDS-FRL sample from rat CA1 showing colocalization of Kir3.2, GABA-B1 receptors in the extrasynaptic membrane of a dendritic spine (s) emerging from a dendrite (Den). Scale bar: 0.2 μm. From (Kulik et al., 2006). D. Double LM-IF labeling for Kv4.2 (red) and Kv2.1 (green) in rat CA1 showing precise compartmentalization of these KChs across different hippocampal strata. Scale bar: 100 μm. E. Super-resolution (SIM) image of a double label LM-IF of Kv2.1 (green) and ryanodine receptor (red) in a mouse striatal medium spiny neuron. Scale bar: 2 μm. From (Mandikian et al., 2014). Anatomical labels for panels A, D as in Figure 2.
Recent studies employing quantitative EM-IG labeling of SDS digested freeze fracture replicas (SDS-FRL) of CA1 apical dendrite plasma membrane KChs consistent with these light microscopy analyses. The highest levels of Kv4.2 EM-IG labeling are observed in CA1 sr (Kerti et al., 2012), and Kir3.2 labeling in CA1 slm (Kirizs et al., 2014). Patch clamp mapping studies show that in CA1 sr, the density of Kv4.2 mediated A-type channels on CA1 apical dendrites is approximately five-fold higher in distal versus proximal regions (Hoffman et al., 1997). This gradient is crucial for distance-dependent scaling of Schaffer collateral inputs on CA1 dendrites, as the normal increases in mEPSC amplitude with increasing distance from the soma (Andrasfalvy and Magee, 2001) are absent in Kv4.2 knockout mice (Andrasfalvy et al., 2008). However, consistent with the light microscope level studies of expression of both Kv4.2 and KChIPs (Figure 2B, 2C, 3D) (Kerti et al., 2012; Rhodes et al., 2004), the quantitative mapping studies of plasma membrane Kv4.2 immunogold particles in SDS-FRL samples (Figure 2D) suggest only a small if any gradient of labeling along the CA1 apical dendritic arbor, and within radial oblique braches (Kerti et al., 2012). This suggests that either the subset of Kv4.2 α and auxiliary subunits with enhanced expression in distal dendrites are refractory to detection with antibodies, or that not all Kv4.2 channels in the plasma membrane are active, with an enhanced representation of “silent” channels in proximal versus distal dendrites. The former scenario seems unlikely, as similar results on the lack of a gradient have been obtained in multiple laboratories using a wide variety of labeling techniques and employing a large collection of independent antibodies. However, Kv4.2 channel activity is modulated by phosphorylation (Jerng et al., 2004) and there are suggestions of compartment specific labeling with certain anti-Kv4.2 phospho-specific antibodies has been observed (Varga et al., 2000).
Subpopulations of each of these dendritic KChs are found within spines, both within the PSD itself, and in extra-synaptic regions of the spine head membrane, as seen for Kv4.2 (Figure 2D) (Kerti et al., 2012), KCa2.2 (Figure 3B) (Allen et al., 2011; Ballesteros-Merino et al., 2012) and Kir3.2 (Figure 3C) (Drake et al., 1997; Fernandez-Alacid et al., 2011; Kirizs et al., 2014; Koyrakh et al., 2005). It should be noted that in general the PSD itself has been shown to be difficult to access with antibodies (Fukaya and Watanabe, 2000), such that the use of pre-embedding methods [LM-DAB, LM-IF and pre-embedding EM-DAB and EM-IG; (Masugi-Tokita and Shigemoto, 2007)], could yield an underestimate of true levels of expression within the PSD. In cultured neurons, whether Kv4.2 is in spines versus dendritic shafts is dynamically regulated through changes in phosphorylation state and protein-protein interactions (Hoffman, 2013). KCa2.2 mRNA is alternatively spliced to generate short and long forms that have similar functional characteristics when expressed in heterologous cells (Strassmaier et al., 2005), but that exhibit distinct compartmentalization within dendritic spines (Allen et al., 2011). Transgenic mice that express only the short form of KCa2.2 (i.e., long form-specific knockouts) have selective loss of KCa2.2 EM-IG labeling in the PSD, while labeling in the extrasynaptic spine is unaffected. These mice have aberrant synaptically evoked EPSPs and defects in learning (Allen et al., 2011), and the synaptic defects are rescued by reintroduction of exogenous KCa2.2 long form (Allen et al., 2011). This suggests a crucial and distinct role for precise targeting of KCa2.2 to the PSD in normal synaptic function. Finally, Kir3.2 colocalizes with components of G protein signaling pathways (GABA-B1 receptors, RGS7, Gβ5) in dendritic spines, both within and around the PSD (Figure 3C), and less so in dendritic shafts (Fajardo-Serrano et al., 2013; Kulik et al., 2006). In cultured neurons, whether Kir3.2 is in dendritic spines versus shafts is dynamic and is associated with changes in Kir3.2 phosphorylation state (Chung et al., 2009).
Input-specific compartmentalization of dendritic KChs in other neurons
Input-specific compartmentalization of Kv4 α and auxiliary subunits, KCa2.2 and Kir3.2 is also seen in other neurons. CA3 pyramidal neurons and dentate granule cells express high levels of both Kv4.2 and Kv4.3, as well as KChIP auxiliary subunits, in prominent and restricted sites in their apical dendrites. In CA3, pronounced labeling is seen in distal dendrites in sr, but little or no labeling for these subunits is observed in the more proximal stratum lucidum, the site of massive dentate granule cell mossy fiber input into CA3, or in cell bodies. In dentate granule cells, little immunolabeling for these Kv4 subunits is present in cell bodies, or in the inner third of the molecular layer, the site of commissural/associational input, while robust labeling is seen in the middle and outer thirds of the molecular layer, the sites of perforant path input to dentate gyrus (Menegola and Trimmer, 2006; Monaghan et al., 2008; Rhodes et al., 2004). This uneven input-specific compartmentalization of Kv4.2 localization is exacerbated after seizures, with an accumulation resembling a barrier at the boundary between the inner and middle third of the molecular layer (Monaghan et al., 2008), presumably in response to the recurrent input resulting from seizure-induced mossy fiber sprouting, and to prevent overstimulation of distal dendrites by this ectopic input, and/or homeostatically suppress input from the medial perforant path. Kir3.1 and Kir3.2 also exhibit enhanced expression in the outer two-thirds of the dentate molecular layer (Fernandez-Alacid et al., 2011), suggesting a requirement for a diverse collection of KChs at the specific sites of perforant path input on dentate granule cell dendrites.
Kv2 channels: unique localization in proximal dendrites
In contrast to the KCh described above that exhibit enhanced expression in distal dendrites, delayed rectifier Kv2 α subunits in neurons across the brain are distinguished restricted high level expression in proximal dendrites. This is consistent with prominent expression of sustained or delayed rectifier Kv channels in proximal but not distal regions of apical dendrites of neocortical layer 5 pyramidal neurons (Korngreen and Sakmann, 2000; Schaefer et al., 2007) that defines the distal dendrite as a low threshold region for synaptic signal amplification. This is very apparent for Kv2.1 expressed in hippocampal CA1 and CA3 pyramidal neurons (Figures 2C, 3D, 5A) (Maletic-Savatic et al., 1995; Mandikian et al., 2014; Rhodes et al., 2004; Rhodes et al., 1995; Sarmiere et al., 2008; Speca et al., 2014) as well as for Kv2.2 (Speca et al., 2014). Quantitative immunogold labeling of SDS-FRL samples from adult rat CA1 reveals substantial Kv2.1 labeling on the proximal dendrites, and no detectable labeling at any site distal to this (Kirizs et al., 2014). Live cell imaging of cultured neurons reveals that Kv2.1 is carried in transport vesicles distinct from those containing distally directed Kv4.2, and that selective interaction of Kv2.1-containing vesicles with myosin II is crucial to their proximal localization, perhaps through interaction with a distinct pool of randomly oriented actin filaments present in proximal but not distal dendrites (Jensen et al., 2014). Kv2.1 (Figure 3E) (Trimmer, 1991) and Kv2.2 (Kihira et al., 2010) are prominently expressed in large clusters on somata that define a novel neuronal plasma membrane domain located over subsurface cisternae (Du et al., 1998; Mandikian et al., 2014), sites of intracellular Ca2+ release, that in certain neurons contain high densities of ryanodine receptor release channels (Figure 3E) that are juxtaposed to plasma membrane Kv2.1 labeling (Antonucci et al., 2001; Mandikian et al., 2014; Misonou et al., 2005).
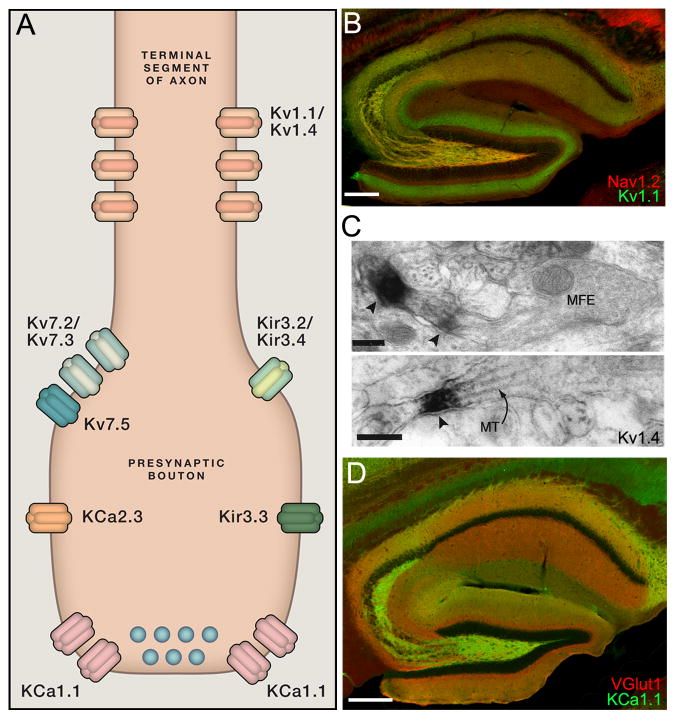
Figure 5. K+ channel localization in hippocampal mossy fibers.
A. Cartoon showing localization of KChs in presynaptic terminals. See text for details. B. Double label LM-IF labeling for Kv1.1 (green) and Nav1.2 (red) in mouse hippocampus, showing localization in MFs. Scale bar: 200 μm. C. EM-DAB labeling for Kv1.4 in stratum lucidum of CA3 in rat. From (Cooper et al., 1998). MFE = mossy fiber expansion. MT = microtubules. Scale bars: 0.2 μm. D. Double LM-IF labeling for KCa1.1 (green) and VGlut1 (red) in mouse hippocampus, showing localization in MFs. Scale bar: 200 μm.
KChs in Axons and Presynaptic Terminals
KChs play a prominent role in repolarizing axons after action potential initiation at the AIS, regulating propagation of action potentials in unmyelinated and myelinated axons, determining how action potentials invade nerve terminals to trigger neurotransmitter release, and in subsequent repolarization of the preterminal membrane after an excitatory event (Debanne et al., 2011). Accordingly, the molecular heterogeneity of axonal KChs, and the restricted subcompartmentalization of different isoforms within axons, is extensive (Table 1, Figure 1). Disruption of these defined patterns of subcellular localization is associated with a variety of neurological pathologies (Child and Benarroch, 2014), and axonal KChs have both established utility (e.g., retigabine, dalfampridine) and future potential (Judge et al., 2007) as drug targets.
KChs at the axon initial segment
KChs at the AIS play a prominent and diverse role in regulating initiation of action potentials, both in the anterograde direction down the axon, and retrograde direction back through the cell body and into dendrites (Bender and Trussell, 2012; Kole and Stuart, 2012). Precise compartmentalization of ion channels within distinct AIS subcompartments defines the properties of spike initiation (Brette, 2013). Moreover, the KChs that are highly concentrated at the AIS are dynamically regulated to yield distinct local regulation of ion channel function (Bender and Trussell, 2012; Kole and Stuart, 2012). While this unique neuronal subcompartment makes up only a tiny fraction of the neuronal plasma membrane surface area, it is emerging as a “hot spot” for disease, with a preponderance of mutations associated with inherited epilepsies in genes encoding proteins, including KChs, selectively expressed at the AIS (Child and Benarroch, 2014; Wimmer et al., 2010). The localization of KChs and other AIS proteins is also altered in response to injury and disease, and in numerous neurological and psychiatric disorders (Buffington and Rasband, 2011; Buttermore et al., 2013),
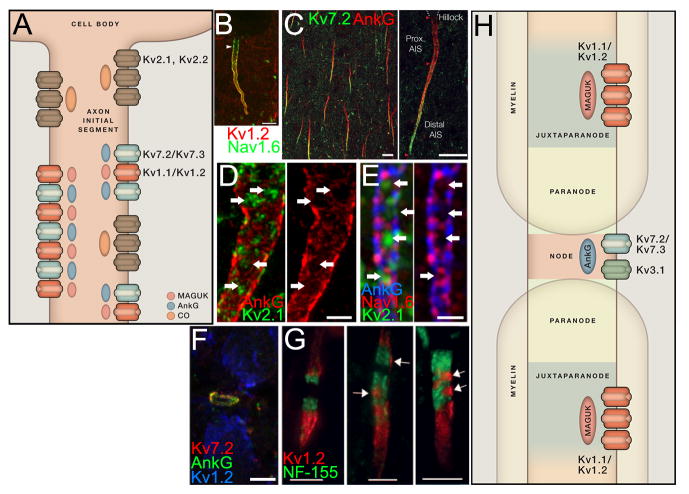
Within the AIS, low threshold delayed rectifier Kv1.1 and Kv1.2 α subunits are selectively expressed in the distal region (Figure 4B) (Goldberg et al., 2008; King et al., 2014; Lorincz and Nusser, 2008a; Ogawa et al., 2008). While in some neurons this localization can be observed in samples prepared using conventional fixation conditions (Dodson et al., 2002; Inda et al., 2006; Van Wart et al., 2007), in many brain neurons, antigen retrieval or altered fixation conditions are needed to see Kv1 labeling at the AIS (Lorincz and Nusser, 2008a, b). Kv1 channels at the distal AIS play prominent roles in determining the properties of action potentials, and in repolarizing the distal AIS, the site of spike initiation, after an action potential has been fired (Kole et al., 2007; Rowan et al., 2014). Kv7.2 and Kv7.3 are subunits of neuronal M-type KChs (Wang et al., 1998), and that are also found at the distal AIS (Figure 4C) of many mammalian brain neurons (Battefeld et al., 2014; Devaux et al., 2004; Klinger et al., 2011; Pan et al., 2006). Immunolabeling for Kv7 subunits at the AIS can be observed in frozen unfixed but not conventionally fixed samples. Kv7.2 and Kv7.3 contain a consensus AnkG binding motif that directs their localization to the AIS (Pan et al., 2006), while Kv1 α subunits lack such motifs, and may localize to distal regions of the AIS through a mechanism involving MAGUKs (Ogawa et al., 2008). That Kv7 channels are compartmentalized in more distal AIS (Battefeld et al., 2014; Pan et al., 2006), while AnkG is uniformly expressed in both proximal and distal AIS suggests that Kv7 localization within the AIS is regulated by factors other than the simple presence of the AnkG binding motif. This is also the case for Nav channels, which all contain virtually identical AnkG binding motifs (Lemaillet et al., 2003), yet different Nav channel α subunit isoforms exhibit compartmentalized localization within the AIS (Hu et al., 2009; Lorincz and Nusser, 2008a; Van Wart et al., 2007), contributing to their distinct roles in anterograde (e.g., distal Nav1.6; Figure 4B) and retrograde (e.g., proximal Nav1.1, Nav1.2) action potential initiation (Hu et al., 2009), and that is crucial to the sharpness of spike initiation (Brette, 2013). AnkG interaction and overall targeting of Nav channels to the AIS is influenced by phosphorylation (Brechet et al., 2008), raising the possibility that localization of Kv7 (and Nav) channels within specific AIS domains could be determined by phosphorylation or other post-translational modification or protein-protein interactions that are compartmentalized within the AIS. Perhaps consistent with this, the protein kinase casein kinase 2 is concentrated in the AIS, where it plays a role in stabilizing Nav channel: ankyrin G interaction (Brechet et al., 2008), and also interacts with and regulates Kv7.2/Kv7.3 channels (Kang et al., 2014), and both Kv7.2 and Kv7.3 are extensively phosphorylated but at sites in the primary sequence distinct from the AnkG binding motif [reviewed in (Trimmer and Misonou, 2014)]. Calmodulin binding to a region distinct from the AnkG binding motif promotes formation of Kv7.2/Kv7.3 heteromers (Liu and Devaux, 2014), and as heteromeric assembly of Kv7.2 with Kv7.3 promotes its targeting to the AIS, via the stronger AnkG binding motif in Kv7.3 (Chung et al., 2006; Rasmussen et al., 2007), calmodulin binding promotes AIS targeting (Liu and Devaux, 2014). The involvement of calmodulin may link Ca2+ signaling to dynamic regulation of Kv7 channel expression in the AIS. I note that in contrast to the discordant results discussed above that show gradients of dendritic Kv4.2 expression by electrophysiology but not by immunolabeling, combined electrophysiology and immunostaining results reveal similar patterns of Kv7 channel expression and function in proximal versus distal AIS (Battefeld et al., 2014). The genes encoding Kv7.2 (Biervert et al., 1998; Singh et al., 1998) and Kv7.3 (Charlier et al., 1998) were initially identified as harboring partial loss of function mutations in genetic analyses of benign familial neonatal convulsions, and many more mutations have ben identified in these patients [reviewed in (Wimmer et al., 2010)], as well as recently identified dominant-negative de novo mutations that yield a syndrome of epilepsy, global developmental delay, and autism (Orhan et al., 2014; Weckhuysen et al., 2012). Consistent with the crucial role of AIS KChs in neuronal excitability (Kole and Stuart, 2012). Kv7.2 and Kv7.3 are targets for the anti-epileptic drug retigabine (Main et al., 2000), a first in class positive allosteric modulator of KCh activity (Stafstrom et al., 2011) that yields reduced intrinsic excitability, presumably at least in part through modulation of AIS Kv7 channels (Brown and Passmore, 2009). A recent report suggests epistasis between Kv7 and AnkG in bipolar disorder (Judy et al., 2013), consistent with a key role for correct subcellular localization of AIS (or NoR, see below) Kv7 channels in normal neuronal function. Kv7 channels remain prime targets for development of therapeutics for a variety of neurological and psychiatric disorders (Grunnet et al., 2014).
Figure 4. K+ channel localization in the axon initial segment.
A. Cartoon showing localization of KChs in the AIS. See text for details. B. Double LM-IF labeling for Kv1.2 (red) and Nav1.6 (green) in a pyramidal neuron in rat neocortex layer 5 showing distal compartmentalization of Kv1.2. So: soma. Scale bar: 5 μm. From (Lorincz and Nusser, 2008a). C. Double LM-IF labeling for Kv7.2 (green) and AnkG (red) in a field of pyramidal neurons in rat neocortex layer 5 (right panel) and a single neuron (left panel) showing distal compartmentalization of Kv7.2. Scale bars: 10 μm. From (Battefeld et al., 2014). D. Super-resolution (SIM) image of double label LM-IF of Kv2.1 (green), and AnkG (red) showing the localization of Kv2.1 in “holes” in the AnkG matrix within the AIS of a rat neocortical pyramidal neuron. Right panel is same image without Kv2.1. Scale bar: 2 μm. From (King et al., 2014). E. Super-resolution (SIM) image of triple label LM-IF of Kv2.1 (green), Nav1.6 (red) and AnkG (blue), showing the mosaic of expression of these proteins within the AIS of a rat neocortical pyramidal neuron. Right panel is same image without Kv2.1, showing sites of Kv2.1 labeling are in holes of AnkG and Nav1.6 labeling. Scale bar: 1 μm. F. Triple label LM-IF of nodal Kv7.2 (red) and AnkG (green), and Kv1.2 (blue) at JPNs in rat brainstem, sample fixed at pH 6.0. Scale bar: 5 μm. G. Kv1.2 (red) at JPNs and neurofascin-155 (green, to mark paranodes) from multiple sclerosis patients. Images shows labeling at JPNs of normal appearing white matter (far left), and two examples of demyelinated white matter in which Kv1.2 labeling is disrupted. Scale bars: 5 μm. From (Howell et al., 2006). H. Cartoon showing overall expression of axonal KChs in domains at/near the node of Ranvier. See text for details.
When labeling for primarily somatodendritic Kv2.1 is combined with that for AnkG to mark the AIS, a very minor population of Kv2.1 at the AIS can be detected (King et al., 2014; Sarmiere et al., 2008). Unlike the other AIS KChs discussed above, Kv2.1 is distinct in both being present along the entire length (i.e., in both proximal and distal regions) of the AIS (King et al., 2014; Sarmiere et al., 2008), and by not being expressed elsewhere on axons (Du et al., 1998; Kirizs et al., 2014; Rhodes et al., 2004; Rhodes et al., 1995; Trimmer, 1991). Multiplex LM-IF labeling and super-resolution imaging reveal Kv2.1 has a clustered subcellular localization in a non-canonical AIS membrane compartment devoid of underlying AnkG, and distinct from Kv1 and Nav channels (Figures 4D, 4E), but adjacent to axo-axonal GABAergic synapses, and overlying intracellular cisternal organelles (King et al., 2014). Together these studies show that the AIS, while representing a miniscule fraction of the total neuronal plasma membrane area, contains high densities of a specific set of KChs (and other ion channels) precisely segregated into distinct membrane subcompartments, forming a mosaic of ion channel expression and function that is a dynamic determinant of neuronal excitability.
KChs at the nodes of Ranvier of myelinated axons
A number of KChs exhibit highly enriched and specific subcellular localization at nodes of Ranvier (NoRs) of myelinated axons, including within the node itself, and also at the associated juxtaparanode (JPN) domains [recently reviewed in (Buttermore et al., 2013; Chang and Rasband, 2013)]. Kv1.1 and Kv1.2 (Figure 4F, 4G) are found at high levels at JPNs, and their discovery here (Wang et al., 1993; Wang et al., 1994) provided the first evidence for a molecular distinction between this compartment and the nearby node, and flanking paranode and internode domains. Kv1.1 and Kv1.2, together with the auxiliary Kvβ2 subunit (Rhodes et al., 1997; Shi et al., 1996) are seen consistently at JPNs of myelinated axons throughout the vertebrate nervous system (Rasband, 2004), including in humans (Figure 4G) (Howell et al., 2006; Howell et al., 2010), although there are less frequent examples of transient or A-type Kv1.4 subunits at JPNs (Rasband et al., 2001). Kv1 channels, which at JPNs are located underneath the myelin, are thought to act to prevent repetitive firing and contribute to intermodal resting potential (Faivre-Sarrailh and Devaux, 2013), and their exposure during development, or due to demyelination in disease or injury, leads to conduction failure (Susuki, 2013). The Kv1 channels at JPNs are targets for therapeutics (e.g., dalfampridine) that improve function in patients with demyelinating disorders such as multiple sclerosis (Goodman and Stone, 2013). Clustering of Kv1 channels at JPNs is one of the latest events in the formation of NoRs (Vabnick et al., 1999), and their presence is dynamic and dependent on formation and maintenance of intact myelin (Dupree et al., 1999; Rasband et al., 1999; Rasband et al., 1998), such that Kv1 localization at JPNs is a sensitive diagnostic readout that has been used to define myelination state in animal studies of developmental NoR formation (Susuki et al., 2013), neuropathic disease (Golan et al., 2013), aging (Hinman et al., 2006), injury (Choo et al., 2009), and in vivo models of remyelination by stem cells (Eftekharpour et al., 2007; Ruff et al., 2013; Uchida et al., 2012), and in post mortem analyses of donors with human demyelinating diseases such as multiple sclerosis (Figure 4G) (Howell et al., 2006). The molecular basis of the labile nature of Kv1 channel localization at JPNs, including mechanisms to sense and respond to changes in myelination state has not been established. Kv1 α subunits contain C-terminal PDZ binding motifs and interact directly with MAGUKs such as PSD-95 in vitro (Kim et al., 1995), and are clustered by MAGUKs in heterologous cells (Kim et al., 1995; Tiffany et al., 2000). Kv1 channels and MAGUKs copurify from brain (Schulte et al., 2011) and Kv1 subunits colocalize with PSD-95 at JPNs (Baba et al., 1999; Rasband and Trimmer, 2001). However, Kv1 channel localization at JPNs remains intact in the absence of PSD-95 (Rasband et al., 2002), although other MAGUKs, such as PSD-93, are also expressed at JPNs (Ogawa et al., 2010) and could potentially compensate. Kv1.2 is phosphorylated at numerous C-terminal sites, and while all known sites are in regions distinct in primary structure from the PDZ binding motif (Trimmer and Misonou, 2014; Yang et al., 2007), it is possible that altered phosphorylation underlies the sensitivity of Kv1 localization at JPNs to myelination state. The role of MAGUKs in localizing Kv1 channels, and other JPN proteins with PDZ binding motifs such as Caspr2 (Poliak et al., 1999) and ADAM22 (Ogawa et al., 2010) remains elusive (Chang and Rasband, 2013).
Immunolabeling for the high threshold Kv3.1b α subunit is found within the NoR of a subset of myelinated axons in unfixed but not conventionally fixed brain sections (Devaux et al., 2003). Kv3.1b and AnkG coimmunoprecipitate from brain (Devaux et al., 2003), and interact directly through an AnkG binding motif present on Kv3.1b that is distinct from that found on Nav and Kv7 channels (Xu et al., 2007). Kv3.1b is also distinct from other AnkG binding ion channels (e.g., Kv7.2, Kv7.3 and Nav channels) in not being present at the AIS (Devaux et al., 2003), suggesting unimpeded transport of NoR-destined Kv3.1b containing vesicles through the AnkG-rich AIS without binding, a process that may involve direct Kv3.1b binding to kinesin I (Xu et al., 2010). Kv7.2 and Kv7.3 are also found at the NoR in frozen samples (Devaux et al., 2004), or in samples prepared with low pH fixation (Figure 4F) [see (Lorincz and Nusser, 2008b) for method], and consistent with the canonical AnkG binding motif on these subunits (Pan et al., 2006). These heteromeric Kv7.2/Kv7.3 channels at the NoR stabilize the membrane potential and increase Nav channel availability and action potential amplitude (Battefeld et al., 2014). Much remains to be learned of the mechanisms involved in generating precise localization of KChs at specific sites in myelinated axons, the sensing mechanisms for maintaining the appropriate levels of particular KChs at these sites in healthy adult axons, and the pathways that lead to their disruption in response to injury and disease.
KChs within/near presynaptic terminals
A diverse set of KChs also plays a key role in regulating neurotransmitter release from nerve terminals. The subunits that comprise these KChs are important targets for physiological neuromodulation to confer plasticity to transmitter release, and for its therapeutic modulation. In this category are KChs at the preterminal segment of the axon, acting as filters and/or gatekeepers regulating access of axonal electrical signals to the presynaptic terminal, and those within the nerve terminal itself, acting to regulate the presynaptic membrane potential and activity of the Cav channels that underlie release (Debanne, 2004).
Mossy fiber (MFs) axons of dentate granule cells make a diverse set of glutamatergic excitatory synapse types onto multiple classes of target neurons in dentate gyrus hilus and CA3 stratum lucidum, and MF synapses exhibit target-specific differences in structure (Rollenhagen and Lubke, 2010), function (Toth and McBain, 2000) and plasticity (Scott et al., 2008). MF axons and/or terminals exhibit prominent immunolabeling for Kv1.1 (Figure 5B) (Wang et al., 1993) and Kv1.4 (Rettig et al., 1992; Sheng et al., 1992), as well Kvβ1 auxiliary subunits (Rhodes et al., 1997). Electron microcopy studies show immunolabeling for Kv1.1 (Wang et al., 1994) and Kv1.4 (Cooper et al., 1998) in preterminal segments of MF axons (Figure 5C), similar to the metabotropic glutamate receptor mGluR2 (Shigemoto et al., 1997; Yokoi et al., 1996), raising the possibility of efficient coupling of metabotropic signaling to modulation of Kv1 channel function. The localization is distinct from that proposed for the Nav1.2 channel, which is highly expressed in MFs (Figure 5B) (Gong et al., 1999; Westenbroek et al., 1989), but which electrophysiology experiments suggest is present within the terminal itself and where it confers somewhat unique excitability to MF terminals (Engel and Jonas, 2005). Fast inactivating DTX-sensitive KChs, presumably Kv1.1/Kv1.4/Kvβ1 complexes, are modulated by CamKII (Roeper et al., 1997) and opioid signaling (Simmons and Chavkin, 1996), and play an important role in dynamic control of Ca2+ influx into MF terminals (Geiger and Jonas, 2000). MFs undergo a dramatic reorganization upon MF sprouting that occurs in animal models of mesial temporal lobe epilepsy (MTLE) (Jones et al., 1992) and MTLE patients (Spencer and Spencer, 1994), leading to the formation of ectopic synapses, most prominent in the inner molecular layer of the dentate gyrus, and that contain immunolabeling for Kv1.4 (Monaghan et al., 2008). This suggests that unlike other MF KChs that are not found to these ectopic sites (see below), Kv1.4-containing channels have potential as therapeutic targets for regulating activity of this aberrant recurrent circuit in MTLE patients.
Kv1 channels are also present in axon preterminal segments of other neurons, most prominently studied in the exceptionally large GABAergic terminals of cerebellar basket cells (BCTs), and of AVCN neurons in auditory brainstem that form calyx of Held terminals. Ultrastructural studies of Kv1.1 and Kv1.2 in BCTs show compartmentalization within the preterminal axon plexus and at septate junctions that form between adjacent basket cell axon branches, but not in the terminals themselves (Laube et al., 1996; McNamara et al., 1996; Wang et al., 1994). Immunolabeling for Kv1 α subunits at these septate junctions colocalizes with labeling for PSD-95 (Laube et al., 1996), as predicted from LM-IF colocalization (Kim et al., 1995). However, PSD-95 is not required for localization of Kv1 channels in BCTs, as immunolabeling for these K+ channels remains intact in mice lacking PSD-95 (Rasband et al., 2002). Kv1.1 knockout mice have an increase in spontaneous GABA release from BCTs and increased GABA inhibition of Purkinje neuron output (Zhang et al., 1999) that presumably underlies the ataxic phenotype seen in these mice. Loss-of-function mutations in Kv1.1 underlie episodic ataxia type 1, and disruption of the normal function of BCTs is presumed to contribute to the ataxia seen in these patients (Kullmann and Waxman, 2010), as supported by results from transgenic mice expressing a human episodic ataxia type 1 Kv1.1 mutation, which exhibit both enhanced GABA release onto cerebellar Purkinje neurons, and ataxia (Herson et al., 2003).
Kv1.1 and Kv1.2 α subunits underlie the low threshold currents in the preterminal segment or transition zone between the axon and calyx of Held terminal, and are not found within the terminals themselves (Dodson et al., 2003). This preterminal zone of the axon, also called the heminode (as AVCN axons are myelinated), also contains high levels of immunolabeling for Nav1.6 (Leao et al., 2005), suggesting some similarities between this final segment of the axon and the AIS. Pharmacological dissection of Kv1 currents in the preterminal segment reveals coexistence of homomeric Kv1.2 channels and Kv1.1/Kv1.2 heteromers that is not apparent in light microscope level immunolabeling (Dodson et al., 2003), showing that two different Kv1 channels of distinct but related subunit composition are targeted to the same small subcompartment of these axons. Presumably, the homomeric Kv1.3 channels recently found in calyx of Held terminals (Gazula et al., 2010) bypass this zone to be specifically retained in the terminal. That a distinct Kv1 channel (a Kv1.1/Kv1.2/Kv1.6 heteromer) is present on somata of AVCN globular bushy cells (Dodson et al., 2003) that give rise to calyx of Held terminals implies that these coexpressed Kv1 α subunits can selectively partition into tetrameric channels with distinct subunit compositions, or that these distinct KChs arise from non-overlapping pulses of Kv1 α subunit gene expression. The distinct polarized expression (i.e., somatodendritic versus axonal), and compartmentalized subcellular localization (pre-terminal segment versus terminal) of these highly similar Kv1 channels at the calyx of Held is a remarkable example of precision in subcellular localization of highly related KChs in distinct neuronal compartments.
Kv7.2 and Kv7.3 subunits are also present in mossy fibers (Cooper et al., 2001; Devaux et al., 2004; Klinger et al., 2011), although their localization in specific subcompartments, such as terminals or preterminal segments of axons, has not been determined, nor has their specific role in mossy fiber function. Kv7.5 immunolabeling is present in calyx of Held nerve terminals (Caminos et al., 2007). Kv7.5 expressed in calyx of Held nerve terminals is an important determinant of the resting membrane potential, and as such plays a key role in determining glutamate release probability (Huang and Trussell, 2011).
High threshold Kv3 channels play a critical role in repolarization of MF axons and terminals (Alle et al., 2011; Riazanski et al., 2001), and immunolabeling for Kv3.4 (Rettig et al., 1992; Veh et al., 1995) and Kv3.3 (Chang et al., 2007) is prominent in hippocampal MFs, although ultrastructural studies to determine whether the Kv3 channels are in the MF boutons themselves, or in axons and preterminal segments, have not been performed. Kv3.4 immunolabeling in MFs is reduced in the pilocarpine model of MTLE (Pacheco Otalora et al., 2011), perhaps contributing to network hyperexcitability associated with this model. In BCTs, Kv3.4 is found uniformly distributed over the pinceau (Laube et al., 1996), demonstrating distinct local compartmentalization of Kv1 and Kv3 channels within BCTs following delivery from their site of synthesis in the basket cell body. EM-IG labeling reveals Kv3.2 is also present in pinceau in a pattern similar to that observed for Kv3.4 (Bobik et al., 2004). The high threshold current in the calyx of Held is from Kv3.1-containing channels (Dodson et al., 2003), with potential contributions from Kv3.3 (Korber et al., 2014; Weiser et al., 1994), and/or Kv3.4 α subunits (Ishikawa et al., 2003). Distinct from preterminal segment Kv1 KChs, immunolabeling for Kv3.1 is found within the calyx of Held nerve terminal itself (Dodson et al., 2003), albeit on the non-synaptic (i.e., back) side of the terminal (Elezgarai et al., 2003), presumably to reduce the potential for transynaptic effects on membrane potential (Schneggenburger and Forsythe, 2006). Based on a combination of their distinct functional properties and subcellular localizations, the Kv1 and Kv3 channels at the calyx of Held, like those in MFs and BCTs, have distinct roles, with preterminal Kv1.1/Kv1.2 channels reducing nerve terminal excitability and preventing aberrant neurotransmitter release, and Kv3 channels within the terminal shortening the presynaptic action potential to regulate neurotransmitter release (Ishikawa et al., 2003). A recent study suggests Kv1 and Kv3 channels near/at MF terminals are substrates for a novel form of plasticity, whereby arachidonic acid released as a retrograde messenger from CA3 dendrites/spines inhibits these KChs, resulting in a broadening of action potentials and an increase in glutamate release (Carta et al., 2014). In heterologous cells, arachidonic acid enhances inactivation to suppress activity of Kv1 and Kv3 channels, while PIP2 removes inactivation and enhances their activity (Oliver et al., 2004). Future studies will determine whether this form of plasticity occurs in other central synapses that have Kv1 and Kv3 channels near/at presynaptic terminals.
Immunolabeling for the KCa1.1 α subunit of BK channels is also present at high levels in MFs (Figure 5D) (Knaus et al., 1996; Misonou et al., 2006a; Sailer et al., 2006). No EM level studies have been published to define this labeling as being in axons, preterminal segments or terminals, although KCa1.1 immunolabeling is present in the active zone of glutamatergic Schaffer collateral nerve terminals located in CA1 sr (Hu et al., 2001; Sailer et al., 2006). KCa1.1 in MF axons/terminals is downregulated in the pilocarpine model of MTLE (Pacheco Otalora et al., 2008). Unlike Kv1.4 which is present in the ectopic MFs in the inner molecular layer of the dentate gyrus (Monaghan et al., 2008), the residual KCa1.1 remains in MFs within hilar and CA3 regions, and is not observed in the ectopic MFs (Pacheco Otalora et al., 2008). This suggests selective targeting to/retention in normal regions of MFs, and/or exclusion from ectopic regions, and presumably exacerbating the impact of MF sprouting on network hyperexcitability.
Coupling function of diverse ion channels through subcellular colocalization
Given the functional interplay between different classes of ion channels in the electrical events that define neurotransmission, it is not surprising that the subcellular localization of KChs appears to be coordinated with that of other ion channels. KChs play diverse roles in determining the membrane environment that controls Nav channel function, influencing the amplitude and duration of the Nav channel based action potential, and the Nav channel refractory period that impacts the interspike interval. There exist numerous prominent examples where KChs are precisely localized at/near sites of clustered Nav channels, including at the distal AIS, where microclusters of Kv1 and Kv7 channels are interspersed with Nav channels in a fine mosaic of coexpression, which also includes segregated clusters of Kv2.1. At the NoR, Kv3 and Kv7 channels co-cluster with Nav channels at high densities within the node itself, while Kv1 channels are segregated into the neighboring JPN. Kv7 and Kv3 α subunits contain AnkG binding motifs, as do Nav channels, and presumably directing their colocalization. In certain axons (e.g., at the calyx of Held), the high concentration of Kv1 channels in preterminal segments of axons is paired with a high density of Nav channels, and is separated from the Kv3 and Kv7 channels in the terminals, which lack Nav channels. In other axons and terminals (e.g., MFs) Kv1 channels are at preterminal sites distinct from the nerve terminal themselves, which contain Nav channels. The mechanisms for generating and maintaining this diverse array of associated yet distinct patterns of subcellular localization of KChs and Nav channels on axons/terminals in a neuron-specific manner is not completely understood, and remains a major focus of much research activity.
There also exist numerous examples of KChs that exhibit precise subcellular colocalization, and in some cases physical association, with components of diverse neuronal Ca2+ signaling microdomains. These include KChs that are intrinsically Ca2+-sensitive, for which changes in intracellular Ca2+ levels are the primary determinant of their activity, whether it be through direct binding, as for KCa1.1 α subunits of BK channels, or through constitutively associated calmodulin, as for KCa2 α subunits of SK channels. It is not surprising to see the precise colocalization (and in certain cases direct intermolecular association) of such KChs with Ca2+ channels, both plasma membrane Cav channels and/or intracellular Ca2+ release channels, as close association underlies effective coupling of Ca2+ channel-mediated Ca2+ flux to activation of these Ca2+-sensitive KChs, including intimate coupling of Ca2+ flux through individual Cav channels to individual molecules of physically associated KCa1.1 channels (Fakler and Adelman, 2008). An emerging theme is the colocalization, association and functional coupling of Ca2+ channels with KChs whose α subunits are not themselves intrinsically Ca2+-sensitive (i.e., those outside the KCa family). These includes Kv4 channels that acquire Ca2+-sensitivity and associate with Cav channels in macromolecular complexes through their EF hand containing/Ca2+-binding KChIP auxiliary subunits (Turner and Zamponi, 2014), and Kv2 channels, found at/near sites of intracellular Ca2+ release (Antonucci et al., 2001; King et al., 2014; Mandikian et al., 2014), which may influence their modulation by Ca2+-regulated protein kinases and phosphatases (Misonou et al., 2006b). For Kv2.1 channels it is clear that their colocalization with intracellular Ca2+ release machinery is dynamic and reversible, and regulated by activity-dependent changes in Kv2.1 phosphorylation state (Misonou et al., 2005). It is likely, given the large number of KChs expressed in neurons, that there will be additional examples that colocalize and perhaps even directly associate with ion channels and other signaling proteins in specific compartments.
Overall considerations and future directions
The overall picture that has emerged from the studies detailed above, and many others not covered here, is that individual KCh types are present at precise and diverse locations on neuronal somata, axons and dendrites. As the neuronal plasma membrane surface area can be up to 10,000 times larger than that of a typical mammalian cell (Gonzalez and Couve, 2014), it is striking that the area in which any particular KCh is found can be so tiny. For example, in axons of human neocortical Betz cells, a KCh localized at the thousand or so NoRs in the meter long axon is concentrated in an area representing ≈0.1% of the axonal membrane area (and one within the AIS only 0.005%!), percentages that become even smaller when the extensive dendritic arbor area is included. To so strategically traffic KChs to such exact locations, and then faithfully maintain them there at precise levels, represents a remarkable feat of cell biology whose underlying mechanisms are for the most part not yet clear. Given what we know about the complexity of the molecular machinery underlying the organization of dendritic spines (Sala and Segal, 2014), it is somewhat intimidating to contemplate defining the molecular mechanisms that generate, maintain, and dynamically regulate the diverse array of restricted plasma membrane domains populated, and in some cases defined, by specific KChs. Such efforts remain important, as in many regards, subcellular localization defines native KCh function, in terms of its impact on neuronal physiology, but also how the function and regulation of the KCh is impacted by local environment.
As is clear from the details above, there is much known of the subcellular localization of many different KChs, and this has provided important insights into the specific roles of these important determinants of neurotransmission. However, there remain many KChs for which little information exists, including many members of entire KCh classes, such as K2P leak KChs, electrically silent/modulatory Kv5, Kv6, Kv8 and Kv9 α subunits, and Kv10, Kv11 and Kv12 EAG and EAG-related KChs, whose subcellular localization in mammalian brain has not been described (Table 1, Figure 1). Whether the lack of such information is sufficient justification for a “KCh-ome-wide” initiative to generate validated “binders” for each of the 90 or so KCh α and auxiliary subunits is not clear. Such a prospect is confounded by the fact that it is oftentimes not enough to generate a single probe and “check off the box”. To start, it is important to obtain similar results with at least two independent antibodies with distinct binding sites (Rhodes and Trimmer, 2006). Moreover, certain KCh mRNAs are subjected to alternative splicing, which in some cases leads to changes in subcellular localization in neurons [e.g., Kv3.1 (Ozaita et al., 2002), KCa2.2 (Allen et al., 2011), etc.], and in other cases [e.g., KCa1.1 (Fodor and Aldrich, 2009)] generates a diversity of subunits that compromises the practical reality or utility of obtaining probes for each variant. Clearly, some combination of concerted and individual efforts will continue to provide the probes that will allow for insights into those KChs who are poorly understood, not out of lack of importance, but from a simple lack of tools.
State-specific probes for KChs, both intentional and unintentional
While it remains an unmet challenge to obtain general (i.e., state-independent) probes for each KCh subunit, it is important to recognize the utility of applying state-dependent or -specific antibodies in the study of KChs. As a prominent example, antibodies that recognize specific phosphorylation states of a given KCh are useful reagents for defining both the regulation of that KCh, but also the activity/signaling state of the neuron and/or specific neuronal subcellular compartment in which it resides, as exemplified by studies of activity-dependent regulation of Kv4.2 (Varga et al., 2000), Kv3.1 (Song et al., 2005) and Kv2.1 (Misonou et al., 2006b). Rigorously defined state-specific antibodies, whether specific for phosphosites, other post-translational modifications, or for KChs of specific folding state or subunit composition, have substantial value. However, that it is possible to generate state-specific antibodies intentionally raises the specter that they could be also generated inadvertently. As virtually all “general” anti-KCh antibodies are made against “naked” (i.e., unmodified) fragments of KCh subunits, one can imagine antibodies that selectively recognize only a subset of specific states in which a KCh can exist, each of which is arguably distinct from the naked fragment used for immunization. These include antibodies whose epitope is occluded in states intrinsic to the target KCh subunit itself (e.g., specific conformational states assumed by the full length subunit, or the presence/absence of post-translational modifications), and by states conferred by environment (co-assembled subunits, interacting proteins, overall protein environment/density). Using such antibodies in studies aimed at subcellular localization of KChs would presumably have the potential to label specific but undefined subsets of target KCh subunits. Examples were detailed above of antibodies that label KChs at certain subcellular locations only when specific sample preparation conditions are used (e.g., Kv7 channels at the AIS and NoR; Kv3 at the NoR; Kv1 at the AIS), but label other compartments (e.g., Kv7 and Kv1 unmyelinated axons and terminals, Kv1 at JPNs, etc.) under most if not all conditions. Presumably, the difficulty in labeling proteins at the AIS and NoR is a reflection of local environment, distinguished by an extremely dense meshwork of interacting proteins (Rasband, 2011), similar to the difficulty of labeling proteins within the PSD (Fukaya and Watanabe, 2000), although other targets at the AIS and NoR (e.g., AnkG and Nav channels) can be effectively labeled mostly regardless of conditions. These considerations and examples underscore the need to carefully validate the specificity of antibodies under the conditions under which they will be used (Lorincz and Nusser, 2008b).
Distinguishing distinct compartments
Higher resolution analyses for KChs, including multiplex LM-IF combined with superresolution microscopy and/or array tomography, and EM-IG labeling on conventional thin sections and SDS-FRL samples, are needed to better define the specific subcompartment harboring KChs. Such analyses to date have provided crucial details on the distribution of certain KCh subunits in specific neuronal subcompartments, but additional analyses using these approaches are needed to fully appreciate the repertoire of KChs in the different functional compartments of neurons, especially in compartments too small to distinguish at the level of the light microscope such as presynaptic terminals and dendritic spines. It remains a challenge that many antibodies that work well for conventional labeling by LM-DAB or LM-IF do not work for labeling samples prepared for array tomography (Micheva et al., 2010) or for SDS-FRL (Masugi-Tokita and Shigemoto, 2007). In general, EM-based analyses, while providing exquisite ultrastructural detail and the opportunity for molecular quantitation, are difficult to perform on large populations of neurons, such as a systematic analysis of KCh across populations of neocortical synapses, a subject that could be addressed with array tomography (O’Rourke et al., 2012) Application of these techniques to the study of the subcellular localization of KChs in mammalian brain will add to the existing knowledge base and provide new insights into these important regulators of neuronal activity.
Acknowledgments
Work by J.S.T. was supported by NIH grants R01NS034383, R01NS042225, and U24NS050606. I thank current and former members of the Trimmer laboratory for their contributions, and especially Colleen Manning for providing numerous unpublished images used here, as well as JoAnne Engebrecht, Colleen Manning and Hannah Bishop for careful readings of the manuscript. I acknowledge authors cited and uncited who contributed to the work reviewed here, and apologize for any omissions or errors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alle H, Kubota H, Geiger JR. Sparse but highly efficient Kv3 outpace BKCa channels in action potential repolarization at hippocampal mossy fiber boutons. J Neurosci. 2011;31:8001–8012. doi: 10.1523/JNEUROSCI.0972-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen D, Bond CT, Lujan R, Ballesteros-Merino C, Lin MT, Wang K, Klett N, Watanabe M, Shigemoto R, Stackman RW, Jr, et al. The SK2-long isoform directs synaptic localization and function of SK2-containing channels. Nat Neurosci. 2011;14:744–749. doi: 10.1038/nn.2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrasfalvy BK, Magee JC. Distance-dependent increase in AMPA receptor number in the dendrites of adult hippocampal CA1 pyramidal neurons. J Neurosci. 2001;21:9151–9159. doi: 10.1523/JNEUROSCI.21-23-09151.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrasfalvy BK, Makara JK, Johnston D, Magee JC. Altered synaptic and non-synaptic properties of CA1 pyramidal neurons in Kv4.2 knockout mice. J Physiol. 2008;586:3881–3892. doi: 10.1113/jphysiol.2008.154336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal M, Fukazawa Y, Eordogh M, Muszil D, Molnar E, Itakura M, Takahashi M, Shigemoto R. Numbers, densities, and colocalization of AMPA- and NMDA-type glutamate receptors at individual synapses in the superficial spinal dorsal horn of rats. J Neurosci. 2008;28:9692–9701. doi: 10.1523/JNEUROSCI.1551-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antonucci DE, Lim ST, Vassanelli S, Trimmer JS. Dynamic localization and clustering of dendritic Kv2.1 voltage-dependent potassium channels in developing hippocampal neurons. Neuroscience. 2001;108:69–81. doi: 10.1016/s0306-4522(01)00476-6. [DOI] [PubMed] [Google Scholar]
- Baba H, Akita H, Ishibashi T, Inoue Y, Nakahira K, Ikenaka K. Completion of myelin compaction, but not the attachment of oligodendroglial processes triggers K(+) channel clustering. J Neurosci Res. 1999;58:752–764. [PubMed] [Google Scholar]
- Ballesteros-Merino C, Lin M, Wu WW, Ferrandiz-Huertas C, Cabanero MJ, Watanabe M, Fukazawa Y, Shigemoto R, Maylie J, Adelman JP, Lujan R. Developmental profile of SK2 channel expression and function in CA1 neurons. Hippocampus. 2012;22:1467–1480. doi: 10.1002/hipo.20986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battefeld A, Tran BT, Gavrilis J, Cooper EC, Kole MH. Heteromeric Kv7.2/7.3 channels differentially regulate action potential initiation and conduction in neocortical myelinated axons. J Neurosci. 2014;34:3719–3732. doi: 10.1523/JNEUROSCI.4206-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender KJ, Trussell LO. The physiology of the axon initial segment. Annu Rev Neurosci. 2012;35:249–265. doi: 10.1146/annurev-neuro-062111-150339. [DOI] [PubMed] [Google Scholar]
- Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, Steinlein OK. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–406. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- Bloodgood BL, Sabatini BL. Nonlinear regulation of unitary synaptic signals by CaV(2.3) voltage-sensitive calcium channels located in dendritic spines. Neuron. 2007;53:249–260. doi: 10.1016/j.neuron.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Bobik M, Ellisman MH, Rudy B, Martone ME. Potassium channel subunit Kv3.2 and the water channel aquaporin-4 are selectively localized to cerebellar pinceau. Brain Res. 2004;1026:168–178. doi: 10.1016/j.brainres.2004.07.088. [DOI] [PubMed] [Google Scholar]
- Brechet A, Fache MP, Brachet A, Ferracci G, Baude A, Irondelle M, Pereira S, Leterrier C, Dargent B. Protein kinase CK2 contributes to the organization of sodium channels in axonal membranes by regulating their interactions with ankyrin G. J Cell Biol. 2008;183:1101–1114. doi: 10.1083/jcb.200805169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brette R. Sharpness of spike initiation in neurons explained by compartmentalization. PLoS Comput Biol. 2013;9:e1003338. doi: 10.1371/journal.pcbi.1003338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. Br J Pharmacol. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington SA, Rasband MN. The axon initial segment in nervous system disease and injury. Eur J Neurosci. 2011;34:1609–1619. doi: 10.1111/j.1460-9568.2011.07875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttermore ED, Thaxton CL, Bhat MA. Organization and maintenance of molecular domains in myelinated axons. J Neurosci Res. 2013;91:603–622. doi: 10.1002/jnr.23197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caminos E, Garcia-Pino E, Martinez-Galan JR, Juiz JM. The potassium channel KCNQ5/Kv7.5 is localized in synaptic endings of auditory brainstem nuclei of the rat. J Comp Neurol. 2007;505:363–378. doi: 10.1002/cne.21497. [DOI] [PubMed] [Google Scholar]
- Carta M, Lanore F, Rebola N, Szabo Z, Da Silva SV, Lourenco J, Verraes A, Nadler A, Schultz C, Blanchet C, Mulle C. Membrane lipids tune synaptic transmission by direct modulation of presynaptic potassium channels. Neuron. 2014;81:787–799. doi: 10.1016/j.neuron.2013.12.028. [DOI] [PubMed] [Google Scholar]
- Chang KJ, Rasband MN. Excitable domains of myelinated nerves: axon initial segments and nodes of Ranvier. Curr Top Membr. 2013;72:159–192. doi: 10.1016/B978-0-12-417027-8.00005-2. [DOI] [PubMed] [Google Scholar]
- Chang SY, Zagha E, Kwon ES, Ozaita A, Bobik M, Martone ME, Ellisman MH, Heintz N, Rudy B. Distribution of Kv3.3 potassium channel subunits in distinct neuronal populations of mouse brain. J Comp Neurol. 2007;502:953–972. doi: 10.1002/cne.21353. [DOI] [PubMed] [Google Scholar]
- Charlier C, Singh NA, Ryan SG, Lewis TB, Reus BE, Leach RJ, Leppert M. A pore mutation in a novel KQT-like potassium channel gene in an idiopathic epilepsy family. Nat Genet. 1998;18:53–55. doi: 10.1038/ng0198-53. [DOI] [PubMed] [Google Scholar]
- Chen X, Johnston D. Voltage-gated ion channels in dendrites of hippocampal pyramidal neurons. Pflugers Arch. 2006;453:397–401. doi: 10.1007/s00424-006-0097-y. [DOI] [PubMed] [Google Scholar]
- Child ND, Benarroch EE. Differential distribution of voltage-gated ion channels in cortical neurons: implications for epilepsy. Neurology. 2014;82:989–999. doi: 10.1212/WNL.0000000000000228. [DOI] [PubMed] [Google Scholar]
- Choo AM, Liu J, Liu Z, Dvorak M, Tetzlaff W, Oxland TR. Modeling spinal cord contusion, dislocation, and distraction: characterization of vertebral clamps, injury severities, and node of Ranvier deformations. J Neurosci Methods. 2009;181:6–17. doi: 10.1016/j.jneumeth.2009.04.007. [DOI] [PubMed] [Google Scholar]
- Chung HJ, Jan YN, Jan LY. Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc Natl Acad Sci U S A. 2006;103:8870–8875. doi: 10.1073/pnas.0603376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Qian X, Ehlers M, Jan YN, Jan LY. Neuronal activity regulates phosphorylation-dependent surface delivery of G protein-activated inwardly rectifying potassium channels. Proc Natl Acad Sci U S A. 2009;106:629–634. doi: 10.1073/pnas.0811615106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark BD, Kwon E, Maffie J, Jeong HY, Nadal M, Strop P, Rudy B. DPP6 localization in brain supports function as a Kv4 channel associated protein. Front Mol Neurosci. 2008;1:8. doi: 10.3389/neuro.02.008.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EC, Harrington E, Jan YN, Jan LY. M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. J Neurosci. 2001;21:9529–9540. doi: 10.1523/JNEUROSCI.21-24-09529.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper EC, Milroy A, Jan YN, Jan LY, Lowenstein DH. Presynaptic localization of Kv1.4-containing A-type potassium channels near excitatory synapses in the hippocampus. J Neurosci. 1998;18:965–974. doi: 10.1523/JNEUROSCI.18-03-00965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani A, Huang B, Bergan J, Dulac C, Zhuang X. Superresolution imaging of chemical synapses in the brain. Neuron. 2010;68:843–856. doi: 10.1016/j.neuron.2010.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debanne D. Information processing in the axon. Nat Rev Neurosci. 2004;5:304–316. doi: 10.1038/nrn1397. [DOI] [PubMed] [Google Scholar]
- Debanne D, Campanac E, Bialowas A, Carlier E, Alcaraz G. Axon physiology. Physiol Rev. 2011;91:555–602. doi: 10.1152/physrev.00048.2009. [DOI] [PubMed] [Google Scholar]
- Devaux J, Alcaraz G, Grinspan J, Bennett V, Joho R, Crest M, Scherer SS. Kv3.1b is a novel component of CNS nodes. J Neurosci. 2003;23:4509–4518. doi: 10.1523/JNEUROSCI.23-11-04509.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. J Neurosci. 2004;24:1236–1244. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson PD, Barker MC, Forsythe ID. Two heteromeric Kv1 potassium channels differentially regulate action potential firing. J Neurosci. 2002;22:6953–6961. doi: 10.1523/JNEUROSCI.22-16-06953.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodson PD, Billups B, Rusznak Z, Szucs G, Barker MC, Forsythe ID. Presynaptic rat Kv1.2 channels suppress synaptic terminal hyperexcitability following action potential invasion. J Physiol. 2003;550:27–33. doi: 10.1113/jphysiol.2003.046250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake CT, Bausch SB, Milner TA, Chavkin C. GIRK1 immunoreactivity is present predominantly in dendrites, dendritic spines, and somata in the CA1 region of the hippocampus. Proc Natl Acad Sci U S A. 1997;94:1007–1012. doi: 10.1073/pnas.94.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Tao-Cheng JH, Zerfas P, McBain CJ. The K+ channel, Kv2.1, is apposed to astrocytic processes and is associated with inhibitory postsynaptic membranes in hippocampal and cortical principal neurons and inhibitory interneurons. Neuroscience. 1998;84:37–48. doi: 10.1016/s0306-4522(97)00519-8. [DOI] [PubMed] [Google Scholar]
- Dupree JL, Girault JA, Popko B. Axo-glial interactions regulate the localization of axonal paranodal proteins. J Cell Biol. 1999;147:1145–1152. doi: 10.1083/jcb.147.6.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AM, Isserlin R, Bader GD, Frye SV, Willson TM, Yu FH. Too many roads not taken. Nature. 2011;470:163–165. doi: 10.1038/470163a. [DOI] [PubMed] [Google Scholar]
- Eftekharpour E, Karimi-Abdolrezaee S, Wang J, El Beheiry H, Morshead C, Fehlings MG. Myelination of congenitally dysmyelinated spinal cord axons by adult neural precursor cells results in formation of nodes of Ranvier and improved axonal conduction. J Neurosci. 2007;27:3416–3428. doi: 10.1523/JNEUROSCI.0273-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elezgarai I, Diez J, Puente N, Azkue JJ, Benitez R, Bilbao A, Knopfel T, Donate-Oliver F, Grandes P. Subcellular localization of the voltage-dependent potassium channel Kv3.1b in postnatal and adult rat medial nucleus of the trapezoid body. Neuroscience. 2003;118:889–898. doi: 10.1016/s0306-4522(03)00068-x. [DOI] [PubMed] [Google Scholar]
- Engel D, Jonas P. Presynaptic action potential amplification by voltage-gated Na+ channels in hippocampal mossy fiber boutons. Neuron. 2005;45:405–417. doi: 10.1016/j.neuron.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Evanko D. Primer: fluorescence imaging under the diffraction limit. Nat Methods. 2009;6:19–20. [Google Scholar]
- Faivre-Sarrailh C, Devaux JJ. Neuro-glial interactions at the nodes of Ranvier: implication in health and diseases. Front Cell Neurosci. 2013;7:196. doi: 10.3389/fncel.2013.00196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo-Serrano A, Wydeven N, Young D, Watanabe M, Shigemoto R, Martemyanov KA, Wickman K, Lujan R. Association of Rgs7/Gbeta5 complexes with Girk channels and GABAB receptors in hippocampal CA1 pyramidal neurons. Hippocampus. 2013;23:1231–1245. doi: 10.1002/hipo.22161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron. 2008;59:873–881. doi: 10.1016/j.neuron.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Fedorov O, Muller S, Knapp S. The (un)targeted cancer kinome. Nat Chem Biol. 2010;6:166–169. doi: 10.1038/nchembio.297. [DOI] [PubMed] [Google Scholar]
- Fernandez-Alacid L, Watanabe M, Molnar E, Wickman K, Lujan R. Developmental regulation of G protein-gated inwardly-rectifying K+ (GIRK/Kir3) channel subunits in the brain. Eur J Neurosci. 2011;34:1724–1736. doi: 10.1111/j.1460-9568.2011.07886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodor AA, Aldrich RW. Convergent evolution of alternative splices at domain boundaries of the BK channel. Annu Rev Physiol. 2009;71:19–36. doi: 10.1146/annurev.physiol.010908.163124. [DOI] [PubMed] [Google Scholar]
- Fukaya M, Watanabe M. Improved immunohistochemical detection of postsynaptically located PSD-95/SAP90 protein family by protease section pretreatment: a study in the adult mouse brain. J Comp Neurol. 2000;426:572–586. [PubMed] [Google Scholar]
- Gazula VR, Strumbos JG, Mei X, Chen H, Rahner C, Kaczmarek LK. Localization of Kv1.3 channels in presynaptic terminals of brainstem auditory neurons. J Comp Neurol. 2010;518:3205–3220. doi: 10.1002/cne.22393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geffers L, Herrmann B, Eichele G. Web-based digital gene expression atlases for the mouse. Mamm Genome. 2012;23:525–538. doi: 10.1007/s00335-012-9413-3. [DOI] [PubMed] [Google Scholar]
- Geiger JR, Jonas P. Dynamic control of presynaptic Ca(2+) inflow by fast-inactivating K(+) channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- Golan N, Kartvelishvily E, Spiegel I, Salomon D, Sabanay H, Rechav K, Vainshtein A, Frechter S, Maik-Rachline G, Eshed-Eisenbach Y, et al. Genetic deletion of Cadm4 results in myelin abnormalities resembling Charcot-Marie-Tooth neuropathy. J Neurosci. 2013;33:10950–10961. doi: 10.1523/JNEUROSCI.0571-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg EM, Clark BD, Zagha E, Nahmani M, Erisir A, Rudy B. K+ channels at the axon initial segment dampen near-threshold excitability of neocortical fast-spiking GABAergic interneurons. Neuron. 2008;58:387–400. doi: 10.1016/j.neuron.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong B, Rhodes KJ, Bekele-Arcuri Z, Trimmer JS. Type I and type II Na(+) channel alpha-subunit polypeptides exhibit distinct spatial and temporal patterning, and association with auxiliary subunits in rat brain. J Comp Neurol. 1999;412:342–352. [PubMed] [Google Scholar]
- Gonzalez C, Couve A. The axonal endoplasmic reticulum and protein trafficking: cellular bootlegging south of the soma. Semin Cell Dev Biol. 2014;27:23–31. doi: 10.1016/j.semcdb.2013.12.004. [DOI] [PubMed] [Google Scholar]
- Goodman AD, Stone RT. Enhancing neural transmission in multiple sclerosis (4-aminopyridine therapy) Neurotherapeutics. 2013;10:106–110. doi: 10.1007/s13311-012-0156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grueneberg DA, Degot S, Pearlberg J, Li W, Davies JE, Baldwin A, Endege W, Doench J, Sawyer J, Hu Y, et al. Kinase requirements in human cells: I. Comparing kinase requirements across various cell types. Proc Natl Acad Sci U S A. 2008;105:16472–16477. doi: 10.1073/pnas.0808019105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunnet M, Strobaek D, Hougaard C, Christophersen P. Kv7 channels as targets for anti-epileptic and psychiatric drug-development. Eur J Pharmacol. 2014;726:133–137. doi: 10.1016/j.ejphar.2014.01.017. [DOI] [PubMed] [Google Scholar]
- Gu C, Barry J. Function and mechanism of axonal targeting of voltage-sensitive potassium channels. Prog Neurobiol. 2011;94:115–132. doi: 10.1016/j.pneurobio.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herson PS, Virk M, Rustay NR, Bond CT, Crabbe JC, Adelman JP, Maylie J. A mouse model of episodic ataxia type-1. Nat Neurosci. 2003;6:378–383. doi: 10.1038/nn1025. [DOI] [PubMed] [Google Scholar]
- Hinman JD, Peters A, Cabral H, Rosene DL, Hollander W, Rasband MN, Abraham CR. Age-related molecular reorganization at the node of Ranvier. J Comp Neurol. 2006;495:351–362. doi: 10.1002/cne.20886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA. K+ channel regulation of multicompartmental signal integration. Neuron. 2013;79:403–405. doi: 10.1016/j.neuron.2013.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Howell OW, Palser A, Polito A, Melrose S, Zonta B, Scheiermann C, Vora AJ, Brophy PJ, Reynolds R. Disruption of neurofascin localization reveals early changes preceding demyelination and remyelination in multiple sclerosis. Brain. 2006;129:3173–3185. doi: 10.1093/brain/awl290. [DOI] [PubMed] [Google Scholar]
- Howell OW, Rundle JL, Garg A, Komada M, Brophy PJ, Reynolds R. Activated microglia mediate axoglial disruption that contributes to axonal injury in multiple sclerosis. J Neuropathol Exp Neurol. 2010;69:1017–1033. doi: 10.1097/NEN.0b013e3181f3a5b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Shao LR, Chavoshy S, Gu N, Trieb M, Behrens R, Laake P, Pongs O, Knaus HG, Ottersen OP, Storm JF. Presynaptic Ca2+-activated K+ channels in glutamatergic hippocampal terminals and their role in spike repolarization and regulation of transmitter release. J Neurosci. 2001;21:9585–9597. doi: 10.1523/JNEUROSCI.21-24-09585.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W, Tian C, Li T, Yang M, Hou H, Shu Y. Distinct contributions of Na(v)1.6 and Na(v)1.2 in action potential initiation and backpropagation. Nat Neurosci. 2009;12:996–1002. doi: 10.1038/nn.2359. [DOI] [PubMed] [Google Scholar]
- Huang H, Trussell LO. KCNQ5 channels control resting properties and release probability of a synapse. Nat Neurosci. 2011;14:840–847. doi: 10.1038/nn.2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inda MC, DeFelipe J, Munoz A. Voltage-gated ion channels in the axon initial segment of human cortical pyramidal cells and their relationship with chandelier cells. Proc Natl Acad Sci U S A. 2006;103:2920–2925. doi: 10.1073/pnas.0511197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T, Nakamura Y, Saitoh N, Li WB, Iwasaki S, Takahashi T. Distinct roles of Kv1 and Kv3 potassium channels at the calyx of Held presynaptic terminal. J Neurosci. 2003;23:10445–10453. doi: 10.1523/JNEUROSCI.23-32-10445.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan LY, Jan YN. Voltage-gated potassium channels and the diversity of electrical signalling. J Physiol. 2012;590:2591–2599. doi: 10.1113/jphysiol.2011.224212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen CS, Rasmussen HB, Misonou H. Neuronal trafficking of voltage-gated potassium channels. Mol Cell Neurosci. 2011;48:288–297. doi: 10.1016/j.mcn.2011.05.007. [DOI] [PubMed] [Google Scholar]
- Jensen CS, Watanabe S, Rasmussen HB, Schmitt N, Olesen SP, Frost NA, Blanpied TA, Misonou H. Specific sorting and post-Golgi trafficking of dendritic potassium channels in living neurons. J Biol Chem. 2014;289:10566–10581. doi: 10.1074/jbc.M113.534495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerng HH, Pfaffinger PJ, Covarrubias M. Molecular physiology and modulation of somatodendritic A-type potassium channels. Mol Cell Neurosci. 2004;27:343–369. doi: 10.1016/j.mcn.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Jones RS, Heinemann UF, Lambert JD. The entorhinal cortex and generation of seizure activity: studies of normal synaptic transmission and epileptogenesis in vitro. Epilepsy Res Suppl. 1992;8:173–180. doi: 10.1016/b978-0-444-89710-7.50027-6. [DOI] [PubMed] [Google Scholar]
- Judge SI, Smith PJ, Stewart PE, Bever CT., Jr Potassium channel blockers and openers as CNS neurologic therapeutic agents. Recent Pat CNS Drug Discov. 2007;2:200–228. doi: 10.2174/157488907782411765. [DOI] [PubMed] [Google Scholar]
- Judy JT, Seifuddin F, Pirooznia M, Mahon PB, Jancic D, Goes FS, Schulze T, Cichon S, Noethen M, et al. Bipolar Genome Study C. Converging Evidence for Epistasis between ANK3 and Potassium Channel Gene KCNQ2 in Bipolar Disorder. Front Genet. 2013;4:87. doi: 10.3389/fgene.2013.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Xu M, Cooper EC, Hoshi N. Channel-anchored protein kinase CK2 and protein phosphatase 1 reciprocally regulate KCNQ2-containing M-channels via phosphorylation of calmodulin. J Biol Chem. 2014;289:11536–11544. doi: 10.1074/jbc.M113.528497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerti K, Lorincz A, Nusser Z. Unique somato-dendritic distribution pattern of Kv4.2 channels on hippocampal CA1 pyramidal cells. Eur J Neurosci. 2012;35:66–75. doi: 10.1111/j.1460-9568.2011.07907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kihira Y, Hermanstyne TO, Misonou H. Formation of heteromeric Kv2 channels in mammalian brain neurons. J Biol Chem. 2010;285:15048–15055. doi: 10.1074/jbc.M109.074260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Hoffman DA. Dynamic regulation of synaptic maturation state by voltage-gated A-type K+ channels in CA1 hippocampal pyramidal neurons. J Neurosci. 2012;32:14427–14432. doi: 10.1523/JNEUROSCI.2373-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- King AN, Manning CF, Trimmer JS. A unique ion channel clustering domain on the axon initial segment of mammalian neurons. J Comp Neurol. 2014;522:2594–2608. doi: 10.1002/cne.23551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirizs T, Kerti-Szigeti K, Lorincz A, Nusser Z. Distinct axo-somato-dendritic distributions of three potassium channels in CA1 hippocampal pyramidal cells. Eur J Neurosci. 2014;39:1771–1783. doi: 10.1111/ejn.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger F, Gould G, Boehm S, Shapiro MS. Distribution of M-channel subunits KCNQ2 and KCNQ3 in rat hippocampus. NeuroImage. 2011;58:761–769. doi: 10.1016/j.neuroimage.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus HG, Schwarzer C, Koch RO, Eberhart A, Kaczorowski GJ, Glossmann H, Wunder F, Pongs O, Garcia ML, Sperk G. Distribution of high-conductance Ca(2+)-activated K+ channels in rat brain: targeting to axons and nerve terminals. J Neurosci. 1996;16:955–963. doi: 10.1523/JNEUROSCI.16-03-00955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kole MH, Letzkus JJ, Stuart GJ. Axon initial segment Kv1 channels control axonal action potential waveform and synaptic efficacy. Neuron. 2007;55:633–647. doi: 10.1016/j.neuron.2007.07.031. [DOI] [PubMed] [Google Scholar]
- Kole MH, Stuart GJ. Signal processing in the axon initial segment. Neuron. 2012;73:235–247. doi: 10.1016/j.neuron.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Korber C, Dondzillo A, Eisenhardt G, Herrmannsdorfer F, Wafzig O, Kuner T. Gene expression profile during functional maturation of a central mammalian synapse. Eur J Neurosci. 2014 doi: 10.1111/ejn.12661. [DOI] [PubMed] [Google Scholar]
- Korngreen A, Sakmann B. Voltage-gated K+ channels in layer 5 neocortical pyramidal neurones from young rats: subtypes and gradients. J Physiol. 2000;525(Pt 3):621–639. doi: 10.1111/j.1469-7793.2000.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyrakh L, Lujan R, Colon J, Karschin C, Kurachi Y, Karschin A, Wickman K. Molecular and cellular diversity of neuronal G-protein-gated potassium channels. J Neurosci. 2005;25:11468–11478. doi: 10.1523/JNEUROSCI.3484-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulik A, Vida I, Fukazawa Y, Guetg N, Kasugai Y, Marker CL, Rigato F, Bettler B, Wickman K, Frotscher M, Shigemoto R. Compartment-dependent colocalization of Kir3.2-containing K+ channels and GABAB receptors in hippocampal pyramidal cells. J Neurosci. 2006;26:4289–4297. doi: 10.1523/JNEUROSCI.4178-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann DM, Waxman SG. Neurological channelopathies: new insights into disease mechanisms and ion channel function. J Physiol. 2010;588:1823–1827. doi: 10.1113/jphysiol.2010.190652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laube G, Roper J, Pitt JC, Sewing S, Kistner U, Garner CC, Pongs O, Veh RW. Ultrastructural localization of Shaker-related potassium channel subunits and synapse-associated protein 90 to septate-like junctions in rat cerebellar Pinceaux. Brain Res Mol Brain Res. 1996;42:51–61. doi: 10.1016/s0169-328x(96)00120-9. [DOI] [PubMed] [Google Scholar]
- Leao RM, Kushmerick C, Pinaud R, Renden R, Li GL, Taschenberger H, Spirou G, Levinson SR, von Gersdorff H. Presynaptic Na+ channels: locus, development, and recovery from inactivation at a high-fidelity synapse. J Neurosci. 2005;25:3724–3738. doi: 10.1523/JNEUROSCI.3983-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lein ES, Hawrylycz MJ, Ao N, Ayres M, Bensinger A, Bernard A, Boe AF, Boguski MS, Brockway KS, Byrnes EJ, et al. Genome-wide atlas of gene expression in the adult mouse brain. Nature. 2007;445:168–176. doi: 10.1038/nature05453. [DOI] [PubMed] [Google Scholar]
- Lemaillet G, Walker B, Lambert S. Identification of a conserved ankyrin-binding motif in the family of sodium channel {alpha} subunits. J Biol Chem. 2003;278:27333–27339. doi: 10.1074/jbc.M303327200. [DOI] [PubMed] [Google Scholar]
- Liao YJ, Jan YN, Jan LY. Heteromultimerization of G-protein-gated inwardly rectifying K+ channel proteins GIRK1 and GIRK2 and their altered expression in weaver brain. J Neurosci. 1996;16:7137–7150. doi: 10.1523/JNEUROSCI.16-22-07137.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Devaux JJ. Calmodulin orchestrates the heteromeric assembly and the trafficking of KCNQ2/3 (Kv7.2/3) channels in neurons. Mol Cell Neurosci. 2014;58:40–52. doi: 10.1016/j.mcn.2013.12.005. [DOI] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Cell-type-dependent molecular composition of the axon initial segment. J Neurosci. 2008a;28:14329–14340. doi: 10.1523/JNEUROSCI.4833-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Specificity of immunoreactions: the importance of testing specificity in each method. J Neurosci. 2008b;28:9083–9086. doi: 10.1523/JNEUROSCI.2494-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorincz A, Nusser Z. Molecular identity of dendritic voltage-gated sodium channels. Science. 2010;328:906–909. doi: 10.1126/science.1187958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losonczy A, Makara JK, Magee JC. Compartmentalized dendritic plasticity and input feature storage in neurons. Nature. 2008;452:436–441. doi: 10.1038/nature06725. [DOI] [PubMed] [Google Scholar]
- Lujan R. Organisation of potassium channels on the neuronal surface. J Chem Neuroanat. 2010;40:1–20. doi: 10.1016/j.jchemneu.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Main MJ, Cryan JE, Dupere JR, Cox B, Clare JJ, Burbidge SA. Modulation of KCNQ2/3 potassium channels by the novel anticonvulsant retigabine. Mol Pharmacol. 2000;58:253–262. doi: 10.1124/mol.58.2.253. [DOI] [PubMed] [Google Scholar]
- Maletic-Savatic M, Lenn NJ, Trimmer JS. Differential spatiotemporal expression of K+ channel polypeptides in rat hippocampal neurons developing in situ and in vitro. J Neurosci. 1995;15:3840–3851. doi: 10.1523/JNEUROSCI.15-05-03840.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandikian D, Bocksteins E, Parajuli LK, Bishop HI, Cerda O, Shigemoto R, Trimmer JS. Cell type-specific spatial and functional coupling between mammalian brain Kv2.1 K(+) channels and ryanodine receptors. J Comp Neurol. 2014;522:3555–3574. doi: 10.1002/cne.23641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manganas LN, Trimmer JS. Subunit composition determines Kv1 potassium channel surface expression. J Biol Chem. 2000;275:29685–29693. doi: 10.1074/jbc.M005010200. [DOI] [PubMed] [Google Scholar]
- Manning CF, Bundros AM, Trimmer JS. Benefits and pitfalls of secondary antibodies: why choosing the right secondary is of primary importance. PLoS One. 2012;7:e38313. doi: 10.1371/journal.pone.0038313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masugi-Tokita M, Shigemoto R. High-resolution quantitative visualization of glutamate and GABA receptors at central synapses. Curr Opin Neurobiol. 2007;17:387–393. doi: 10.1016/j.conb.2007.04.012. [DOI] [PubMed] [Google Scholar]
- McNamara NM, Averill S, Wilkin GP, Dolly JO, Priestley JV. Ultrastructural localization of a voltage-gated K+ channel alpha subunit (Kv1.2) in the rat cerebellum. Eur J Neurosci. 1996;8:688–699. doi: 10.1111/j.1460-9568.1996.tb01254.x. [DOI] [PubMed] [Google Scholar]
- Menegola M, Trimmer JS. Unanticipated region- and cell-specific downregulation of individual KChIP auxiliary subunit isotypes in Kv4.2 knock-out mouse brain. J Neurosci. 2006;26:12137–12142. doi: 10.1523/JNEUROSCI.2783-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micheva KD, Busse B, Weiler NC, O’Rourke N, Smith SJ. Single-synapse analysis of a diverse synapse population: proteomic imaging methods and markers. Neuron. 2010;68:639–653. doi: 10.1016/j.neuron.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Menegola M, Buchwalder L, Park EW, Meredith A, Rhodes KJ, Aldrich RW, Trimmer JS. Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol. 2006a;496:289–302. doi: 10.1002/cne.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Menegola M, Mohapatra DP, Guy LK, Park KS, Trimmer JS. Bidirectional activity-dependent regulation of neuronal ion channel phosphorylation. J Neurosci. 2006b;26:13505–13514. doi: 10.1523/JNEUROSCI.3970-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Mohapatra DP, Trimmer JS. Kv2.1: a voltage-gated k+ channel critical to dynamic control of neuronal excitability. Neurotoxicology. 2005;26:743–752. doi: 10.1016/j.neuro.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Kubo Y. Localization and developmental changes of the expression of two inward rectifying K(+)-channel proteins in the rat brain. Brain Res. 1997;750:251–263. doi: 10.1016/s0006-8993(96)01365-0. [DOI] [PubMed] [Google Scholar]
- Monaghan MM, Menegola M, Vacher H, Rhodes KJ, Trimmer JS. Altered expression and localization of hippocampal A-type potassium channel subunits in the pilocarpine-induced model of temporal lobe epilepsy. Neuroscience. 2008;156:550–562. doi: 10.1016/j.neuroscience.2008.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niewoehner J, Bohrmann B, Collin L, Urich E, Sade H, Maier P, Rueger P, Stracke JO, Lau W, Tissot AC, et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron. 2014;81:49–60. doi: 10.1016/j.neuron.2013.10.061. [DOI] [PubMed] [Google Scholar]
- Nusser Z. Differential subcellular distribution of ion channels and the diversity of neuronal function. Curr Opin Neurobiol. 2012;22:366–371. doi: 10.1016/j.conb.2011.10.006. [DOI] [PubMed] [Google Scholar]
- O’Rourke NA, Weiler NC, Micheva KD, Smith SJ. Deep molecular diversity of mammalian synapses: why it matters and how to measure it. Nat Rev Neurosci. 2012;13:365–379. doi: 10.1038/nrn3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Horresh I, Trimmer JS, Bredt DS, Peles E, Rasband MN. Postsynaptic density-93 clusters Kv1 channels at axon initial segments independently of Caspr2. J Neurosci. 2008;28:5731–5739. doi: 10.1523/JNEUROSCI.4431-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Oses-Prieto J, Kim MY, Horresh I, Peles E, Burlingame AL, Trimmer JS, Meijer D, Rasband MN. ADAM22, a Kv1 channel-interacting protein, recruits membrane-associated guanylate kinases to juxtaparanodes of myelinated axons. J Neurosci. 2010;30:1038–1048. doi: 10.1523/JNEUROSCI.4661-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver D, Lien CC, Soom M, Baukrowitz T, Jonas P, Fakler B. Functional conversion between A-type and delayed rectifier K+ channels by membrane lipids. Science. 2004;304:265–270. doi: 10.1126/science.1094113. [DOI] [PubMed] [Google Scholar]
- Orhan G, Bock M, Schepers D, Ilina EI, Reichel SN, Loffler H, Jezutkovic N, Weckhuysen S, Mandelstam S, Suls A, et al. Dominant-negative effects of KCNQ2 mutations are associated with epileptic encephalopathy. Ann Neurol. 2014;75:382–394. doi: 10.1002/ana.24080. [DOI] [PubMed] [Google Scholar]
- Ozaita A, Martone ME, Ellisman MH, Rudy B. Differential subcellular localization of the two alternatively spliced isoforms of the Kv3.1 potassium channel subunit in brain. J Neurophysiol. 2002;88:394–408. doi: 10.1152/jn.2002.88.1.394. [DOI] [PubMed] [Google Scholar]
- Pacheco Otalora LF, Hernandez EF, Arshadmansab MF, Francisco S, Willis M, Ermolinsky B, Zarei M, Knaus HG, Garrido-Sanabria ER. Down-regulation of BK channel expression in the pilocarpine model of temporal lobe epilepsy. Brain Res. 2008;1200C:116–131. doi: 10.1016/j.brainres.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco Otalora LF, Skinner F, Oliveira MS, Farrell B, Arshadmansab MF, Pandari T, Garcia I, Robles L, Rosas G, Mello CF, et al. Chronic deficit in the expression of voltage-gated potassium channel Kv3.4 subunit in the hippocampus of pilocarpine-treated epileptic rats. Brain Res. 2011;1368:308–316. doi: 10.1016/j.brainres.2010.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Z, Kao T, Horvath Z, Lemos J, Sul JY, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci. 2006;26:2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian DM. Potassium channels: some assembly required. Neuron. 1999;23:7–10. doi: 10.1016/s0896-6273(00)80746-1. [DOI] [PubMed] [Google Scholar]
- Poliak S, Gollan L, Martinez R, Custer A, Einheber S, Salzer JL, Trimmer JS, Shrager P, Peles E. Caspr2, a new member of the neurexin superfamily, is localized at the juxtaparanodes of myelinated axons and associates with K+ channels. Neuron. 1999;24:1037–1047. doi: 10.1016/s0896-6273(00)81049-1. [DOI] [PubMed] [Google Scholar]
- Poolos NP, Johnston D. Dendritic ion channelopathy in acquired epilepsy. Epilepsia. 2012;53(Suppl 9):32–40. doi: 10.1111/epi.12033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN. It’s “juxta” potassium channel! J Neurosci Res. 2004;76:749–757. doi: 10.1002/jnr.20073. [DOI] [PubMed] [Google Scholar]
- Rasband MN. Composition, assembly, and maintenance of excitable membrane domains in myelinated axons. Semin Cell Dev Biol. 2011;22:178–184. doi: 10.1016/j.semcdb.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Vanderah TW, Lai J, Porreca F, Trimmer JS. Distinct potassium channels on pain-sensing neurons. Proc Natl Acad Sci U S A. 2001;98:13373–13378. doi: 10.1073/pnas.231376298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Park EW, Zhen D, Arbuckle MI, Poliak S, Peles E, Grant SG, Trimmer JS. Clustering of neuronal potassium channels is independent of their interaction with PSD-95. J Cell Biol. 2002;159:663–672. doi: 10.1083/jcb.200206024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Trimmer JS. Developmental clustering of ion channels at and near the node of Ranvier. Dev Biol. 2001;236:5–16. doi: 10.1006/dbio.2001.0326. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Trimmer JS, Peles E, Levinson SR, Shrager P. K+ channel distribution and clustering in developing and hypomyelinated axons of the optic nerve. J Neurocytol. 1999;28:319–331. doi: 10.1023/a:1007057512576. [DOI] [PubMed] [Google Scholar]
- Rasband MN, Trimmer JS, Schwarz TL, Levinson SR, Ellisman MH, Schachner M, Shrager P. Potassium channel distribution, clustering, and function in remyelinating rat axons. J Neurosci. 1998;18:36–47. doi: 10.1523/JNEUROSCI.18-01-00036.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen HB, Frokjaer-Jensen C, Jensen CS, Jensen HS, Jorgensen NK, Misonou H, Trimmer JS, Olesen SP, Schmitt N. Requirement of subunit co-assembly and ankyrin-G for M-channel localization at the axon initial segment. J Cell Sci. 2007;120:953–963. doi: 10.1242/jcs.03396. [DOI] [PubMed] [Google Scholar]
- Rettig J, Wunder F, Stocker M, Lichtinghagen R, Mastiaux F, Beckh S, Kues W, Pedarzani P, Schroter KH, Ruppersberg JP, et al. Characterization of a Shaw-related potassium channel family in rat brain. EMBO J. 1992;11:2473–2486. doi: 10.1002/j.1460-2075.1992.tb05312.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Carroll KI, Sung MA, Doliveira LC, Monaghan MM, Burke SL, Strassle BW, Buchwalder L, Menegola M, Cao J, et al. KChIPs and Kv4 alpha subunits as integral components of A-type potassium channels in mammalian brain. J Neurosci. 2004;24:7903–7915. doi: 10.1523/JNEUROSCI.0776-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Keilbaugh SA, Barrezueta NX, Lopez KL, Trimmer JS. Association and colocalization of K+ channel alpha- and beta-subunit polypeptides in rat brain. J Neurosci. 1995;15:5360–5371. doi: 10.1523/JNEUROSCI.15-07-05360.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Strassle BW, Monaghan MM, Bekele-Arcuri Z, Matos MF, Trimmer JS. Association and colocalization of the Kvbeta1 and Kvbeta2 beta-subunits with Kv1 alpha-subunits in mammalian brain K+ channel complexes. J Neurosci. 1997;17:8246–8258. doi: 10.1523/JNEUROSCI.17-21-08246.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Trimmer JS. Antibodies as valuable neuroscience research tools versus reagents of mass distraction. J Neurosci. 2006;26:8017–8020. doi: 10.1523/JNEUROSCI.2728-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Trimmer JS. Antibody-based validation of CNS ion channel drug targets. J Gen Physiol. 2008;131:407–413. doi: 10.1085/jgp.200709926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazanski V, Becker A, Chen J, Sochivko D, Lie A, Wiestler OD, Elger CE, Beck H. Functional and molecular analysis of transient voltage-dependent K+ currents in rat hippocampal granule cells. J Physiol. 2001;537:391–406. doi: 10.1111/j.1469-7793.2001.00391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeper J, Lorra C, Pongs O. Frequency-dependent inactivation of mammalian A-type K+ channel KV1.4 regulated by Ca2+/calmodulin-dependent protein kinase. J Neurosci. 1997;17:3379–3391. doi: 10.1523/JNEUROSCI.17-10-03379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollenhagen A, Lubke JH. The mossy fiber bouton: the “common” or the “unique” synapse? Front Synaptic Neurosci. 2010;2:2. doi: 10.3389/fnsyn.2010.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowan MJ, Tranquil E, Christie JM. Distinct Kv channel subtypes contribute to differences in spike signaling properties in the axon initial segment and presynaptic boutons of cerebellar interneurons. J Neurosci. 2014;34:6611–6623. doi: 10.1523/JNEUROSCI.4208-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruff CA, Ye H, Legasto JM, Stribbell NA, Wang J, Zhang L, Fehlings MG. Effects of adult neural precursor-derived myelination on axonal function in the perinatal congenitally dysmyelinated brain: optimizing time of intervention, developing accurate prediction models, and enhancing performance. J Neurosci. 2013;33:11899–11915. doi: 10.1523/JNEUROSCI.1131-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer CA, Hu H, Kaufmann WA, Trieb M, Schwarzer C, Storm JF, Knaus HG. Regional differences in distribution and functional expression of small-conductance Ca2+-activated K+ channels in rat brain. J Neurosci. 2002;22:9698–9707. doi: 10.1523/JNEUROSCI.22-22-09698.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sailer CA, Kaufmann WA, Kogler M, Chen L, Sausbier U, Ottersen OP, Ruth P, Shipston MJ, Knaus HG. Immunolocalization of BK channels in hippocampal pyramidal neurons. Eur J Neurosci. 2006;24:442–454. doi: 10.1111/j.1460-9568.2006.04936.x. [DOI] [PubMed] [Google Scholar]
- Sailer CA, Kaufmann WA, Marksteiner J, Knaus HG. Comparative immunohistochemical distribution of three small-conductance Ca2+-activated potassium channel subunits, SK1, SK2, and SK3 in mouse brain. Mol Cell Neurosci. 2004;26:458–469. doi: 10.1016/j.mcn.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Sala C, Segal M. Dendritic spines: the locus of structural and functional plasticity. Physiol Rev. 2014;94:141–188. doi: 10.1152/physrev.00012.2013. [DOI] [PubMed] [Google Scholar]
- Saper CB. An open letter to our readers on the use of antibodies. J Comp Neurol. 2005;493:477–478. doi: 10.1002/cne.20839. [DOI] [PubMed] [Google Scholar]
- Saper CB, Sawchenko PE. Magic peptides, magic antibodies: guidelines for appropriate controls for immunohistochemistry. J Comp Neurol. 2003;465:161–163. doi: 10.1002/cne.10858. [DOI] [PubMed] [Google Scholar]
- Sarmiere PD, Weigle CM, Tamkun MM. The Kv2.1 K+ channel targets to the axon initial segment of hippocampal and cortical neurons in culture and in situ. BMC Neurosci. 2008;9:112. doi: 10.1186/1471-2202-9-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer AT, Helmstaedter M, Schmitt AC, Bar-Yehuda D, Almog M, Ben-Porat H, Sakmann B, Korngreen A. Dendritic voltage-gated K+ conductance gradient in pyramidal neurones of neocortical layer 5B from rats. J Physiol. 2007;579:737–752. doi: 10.1113/jphysiol.2006.122564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Forsythe ID. The calyx of Held. Cell Tissue Res. 2006;326:311–337. doi: 10.1007/s00441-006-0272-7. [DOI] [PubMed] [Google Scholar]
- Schulte U, Muller CS, Fakler B. Ion channels and their molecular environments--glimpses and insights from functional proteomics. Semin Cell Dev Biol. 2011;22:132–144. doi: 10.1016/j.semcdb.2010.09.015. [DOI] [PubMed] [Google Scholar]
- Scott R, Lalic T, Kullmann DM, Capogna M, Rusakov DA. Target-cell specificity of kainate autoreceptor and Ca2+-store-dependent short-term plasticity at hippocampal mossy fiber synapses. J Neurosci. 2008;28:13139–13149. doi: 10.1523/JNEUROSCI.2932-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Tsaur ML, Jan YN, Jan LY. Subcellular segregation of two A-type K+ channel proteins in rat central neurons. Neuron. 1992;9:271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- Shi G, Nakahira K, Hammond S, Rhodes KJ, Schechter LE, Trimmer JS. Beta subunits promote K+ channel surface expression through effects early in biosynthesis. Neuron. 1996;16:843–852. doi: 10.1016/s0896-6273(00)80104-x. [DOI] [PubMed] [Google Scholar]
- Shigemoto R, Kinoshita A, Wada E, Nomura S, Ohishi H, Takada M, Flor PJ, Neki A, Abe T, Nakanishi S, Mizuno N. Differential presynaptic localization of metabotropic glutamate receptor subtypes in the rat hippocampus. J Neurosci. 1997;17:7503–7522. doi: 10.1523/JNEUROSCI.17-19-07503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons ML, Chavkin C. k-Opioid receptor activation of a dendrotoxin-sensitive potassium channel mediates presynaptic inhibition of mossy fiber neurotransmitter release. Mol Pharmacol. 1996;50:80–85. [PubMed] [Google Scholar]
- Singh NA, Charlier C, Stauffer D, DuPont BR, Leach RJ, Melis R, Ronen GM, Bjerre I, Quattlebaum T, Murphy JV, et al. A novel potassium channel gene, KCNQ2, is mutated in an inherited epilepsy of newborns. Nat Genet. 1998;18:25–29. doi: 10.1038/ng0198-25. [DOI] [PubMed] [Google Scholar]
- Song P, Yang Y, Barnes-Davies M, Bhattacharjee A, Hamann M, Forsythe ID, Oliver DL, Kaczmarek LK. Acoustic environment determines phosphorylation state of the Kv3.1 potassium channel in auditory neurons. Nat Neurosci. 2005;8:1335–1342. doi: 10.1038/nn1533. [DOI] [PubMed] [Google Scholar]
- Speca DJ, Ogata G, Mandikian D, Bishop HI, Wiler SW, Eum K, Wenzel HJ, Doisy ET, Matt L, Campi KL, et al. Deletion of the Kv2.1 delayed rectifier potassium channel leads to neuronal and behavioral hyperexcitability. Genes Brain Behav. 2014;13:394–408. doi: 10.1111/gbb.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SS, Spencer DD. Entorhinal-hippocampal interactions in medial temporal lobe epilepsy. Epilepsia. 1994;35:721–727. doi: 10.1111/j.1528-1157.1994.tb02502.x. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Grippon S, Kirkpatrick P. Ezogabine (retigabine) Nat Rev Drug Discov. 2011;10:729–730. doi: 10.1038/nrd3561. [DOI] [PubMed] [Google Scholar]
- Strassmaier T, Bond CT, Sailer CA, Knaus HG, Maylie J, Adelman JP. A novel isoform of SK2 assembles with other SK subunits in mouse brain. J Biol Chem. 2005;280:21231–21236. doi: 10.1074/jbc.M413125200. [DOI] [PubMed] [Google Scholar]
- Sunkin SM, Ng L, Lau C, Dolbeare T, Gilbert TL, Thompson CL, Hawrylycz M, Dang C. Allen Brain Atlas: an integrated spatio-temporal portal for exploring the central nervous system. Nucleic Acids Res. 2013;41:D996–D1008. doi: 10.1093/nar/gks1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susuki K. Node of Ranvier disruption as a cause of neurological diseases. ASN Neuro. 2013;5:209–219. doi: 10.1042/AN20130025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susuki K, Chang KJ, Zollinger DR, Liu Y, Ogawa Y, Eshed-Eisenbach Y, Dours-Zimmermann MT, Oses-Prieto JA, Burlingame AL, Seidenbecher CI, et al. Three mechanisms assemble central nervous system nodes of Ranvier. Neuron. 2013;78:469–482. doi: 10.1016/j.neuron.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarusawa E, Matsui K, Budisantoso T, Molnar E, Watanabe M, Matsui M, Fukazawa Y, Shigemoto R. Input-specific intrasynaptic arrangements of ionotropic glutamate receptors and their impact on postsynaptic responses. J Neurosci. 2009;29:12896–12908. doi: 10.1523/JNEUROSCI.6160-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taussig MJ, Schmidt R, Cook EA, Stoevesandt O. Development of proteome-wide binding reagents for research and diagnostics. Proteomics Clin Appl. 2013;7:756–766. doi: 10.1002/prca.201300060. [DOI] [PubMed] [Google Scholar]
- Tiffany AM, Manganas LN, Kim E, Hsueh YP, Sheng M, Trimmer JS. PSD-95 and SAP97 exhibit distinct mechanisms for regulating K(+) channel surface expression and clustering. J Cell Biol. 2000;148:147–158. doi: 10.1083/jcb.148.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, McBain CJ. Target-specific expression of pre- and postsynaptic mechanisms. J Physiol. 2000;525(Pt 1):41–51. doi: 10.1111/j.1469-7793.2000.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer JS. Immunological identification and characterization of a delayed rectifier K+ channel polypeptide in rat brain. Proc Natl Acad Sci U S A. 1991;88:10764–10768. doi: 10.1073/pnas.88.23.10764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer JS, Misonou H. Phosphorylation of voltage-gated ion channels. In: Zheng J, Trudeau MC, editors. Handbook of Ion Channels. Boca Raton, FL: CRC Press; 2014. [Google Scholar]
- Turner RW, Zamponi GW. T-type channels buddy up. Pflugers Arch. 2014;466:661–675. doi: 10.1007/s00424-013-1434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida N, Chen K, Dohse M, Hansen KD, Dean J, Buser JR, Riddle A, Beardsley DJ, Wan Y, Gong X, et al. Human neural stem cells induce functional myelination in mice with severe dysmyelination. Sci Transl Med. 2012;4:155ra136. doi: 10.1126/scitranslmed.3004371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabnick I, Trimmer JS, Schwarz TL, Levinson SR, Risal D, Shrager P. Dynamic potassium channel distributions during axonal development prevent aberrant firing patterns. J Neurosci. 1999;19:747–758. doi: 10.1523/JNEUROSCI.19-02-00747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher H, Mohapatra DP, Trimmer JS. Localization and targeting of voltage-dependent ion channels in mammalian central neurons. Physiol Rev. 2008;88:1407–1447. doi: 10.1152/physrev.00002.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacher H, Trimmer JS. Trafficking mechanisms underlying neuronal voltage-gated ion channel localization at the axon initial segment. Epilepsia. 2012;53(Suppl 9):21–31. doi: 10.1111/epi.12032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wart A, Trimmer JS, Matthews G. Polarized distribution of ion channels within microdomains of the axon initial segment. J Comp Neurol. 2007;500:339–352. doi: 10.1002/cne.21173. [DOI] [PubMed] [Google Scholar]
- Varga AW, Anderson AE, Adams JP, Vogel H, Sweatt JD. Input-specific immunolocalization of differentially phosphorylated Kv4.2 in the mouse brain. Learn Mem. 2000;7:321–332. doi: 10.1101/lm.35300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veh RW, Lichtinghagen R, Sewing S, Wunder F, Grumbach IM, Pongs O. Immunohistochemical localization of five members of the Kv1 channel subunits: contrasting subcellular locations and neuron-specific co-localizations in rat brain. Eur J Neurosci. 1995;7:2189–2205. doi: 10.1111/j.1460-9568.1995.tb00641.x. [DOI] [PubMed] [Google Scholar]
- Visel A, Thaller C, Eichele G. GenePaint.org: an atlas of gene expression patterns in the mouse embryo. Nucleic Acids Res. 2004;32:D552–556. doi: 10.1093/nar/gkh029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Schwartzkroin PA, Tempel BL. Localization of Kv1.1 and Kv1.2, two K channel proteins, to synaptic terminals, somata, and dendrites in the mouse brain. J Neurosci. 1994;14:4588–4599. doi: 10.1523/JNEUROSCI.14-08-04588.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- Watts RJ, Dennis MS. Bispecific antibodies for delivery into the brain. Curr Opin Chem Biol. 2013;17:393–399. doi: 10.1016/j.cbpa.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Weckhuysen S, Mandelstam S, Suls A, Audenaert D, Deconinck T, Claes LR, Deprez L, Smets K, Hristova D, Yordanova I, et al. KCNQ2 encephalopathy: emerging phenotype of a neonatal epileptic encephalopathy. Ann Neurol. 2012;71:15–25. doi: 10.1002/ana.22644. [DOI] [PubMed] [Google Scholar]
- Weiser M, Vega-Saenz de Miera E, Kentros C, Moreno H, Franzen L, Hillman D, Baker H, Rudy B. Differential expression of Shaw-related K+ channels in the rat central nervous system. J Neurosci. 1994;14:949–972. doi: 10.1523/JNEUROSCI.14-03-00949.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenbroek RE, Merrick DK, Catterall WA. Differential subcellular localization of the RI and RII Na+ channel subtypes in central neurons. Neuron. 1989;3:695–704. doi: 10.1016/0896-6273(89)90238-9. [DOI] [PubMed] [Google Scholar]
- Wimmer VC, Reid CA, So EY, Berkovic SF, Petrou S. Axon initial segment dysfunction in epilepsy. J Physiol. 2010;588:1829–1840. doi: 10.1113/jphysiol.2010.188417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Cao R, Xiao R, Zhu MX, Gu C. The axon-dendrite targeting of Kv3 (Shaw) channels is determined by a targeting motif that associates with the T1 domain and ankyrin G. J Neurosci. 2007;27:14158–14170. doi: 10.1523/JNEUROSCI.3675-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M, Gu Y, Barry J, Gu C. Kinesin I transports tetramerized Kv3 channels through the axon initial segment via direct binding. J Neurosci. 2010;30:15987–16001. doi: 10.1523/JNEUROSCI.3565-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JW, Vacher H, Park KS, Clark E, Trimmer JS. Trafficking-dependent phosphorylation of Kv1.2 regulates voltage-gated potassium channel cell surface expression. Proc Natl Acad Sci U S A. 2007;104:20055–20060. doi: 10.1073/pnas.0708574104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokoi M, Kobayashi K, Manabe T, Takahashi T, Sakaguchi I, Katsuura G, Shigemoto R, Ohishi H, Nomura S, Nakamura K, et al. Impairment of hippocampal mossy fiber LTD in mice lacking mGluR2. Science. 1996;273:645–647. doi: 10.1126/science.273.5275.645. [DOI] [PubMed] [Google Scholar]
- Yu FH, Catterall WA. The VGL-chanome: a protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE. 2004;2004:re15. doi: 10.1126/stke.2532004re15. [DOI] [PubMed] [Google Scholar]
- Yuste R. Electrical compartmentalization in dendritic spines. Annu Rev Neurosci. 2013;36:429–449. doi: 10.1146/annurev-neuro-062111-150455. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Schwappach B, Jan YN, Jan LY. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane K(ATP) channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Messing A, Chiu SY. Specific alteration of spontaneous GABAergic inhibition in cerebellar purkinje cells in mice lacking the potassium channel Kv1.1. J Neurosci. 1999;19:2852–2864. doi: 10.1523/JNEUROSCI.19-08-02852.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]