Abstract
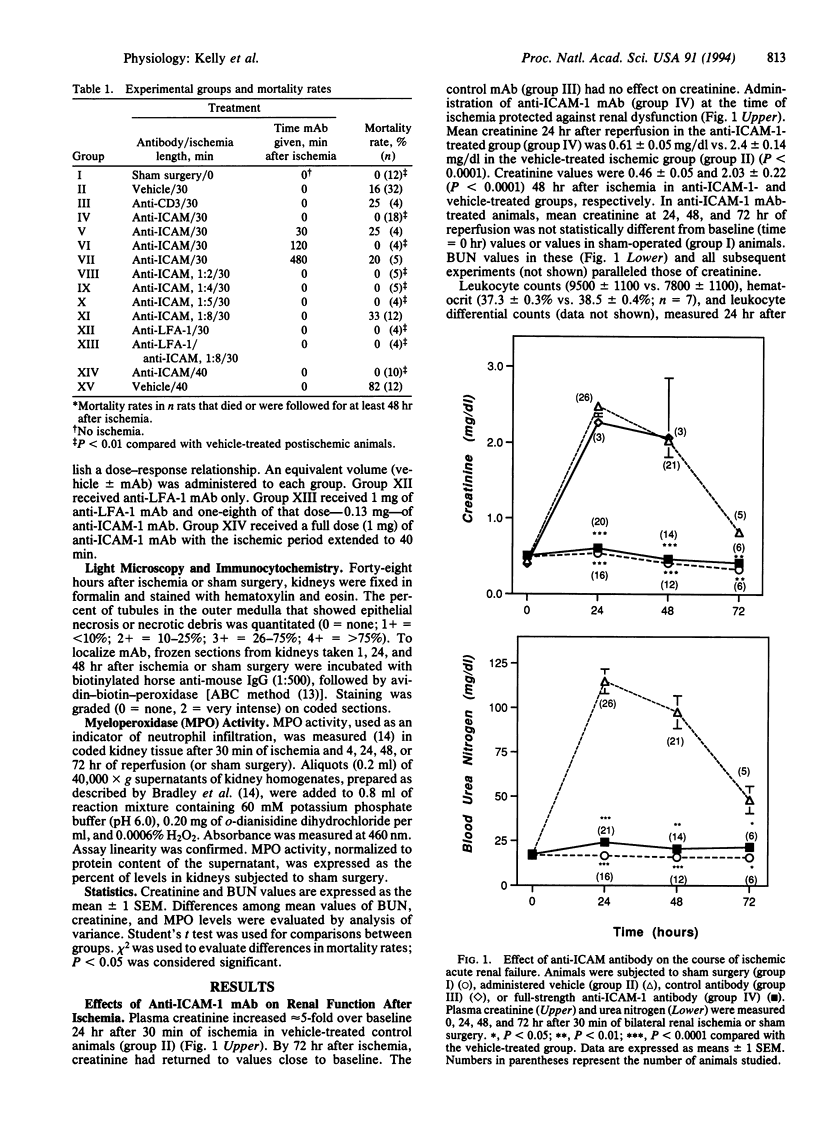
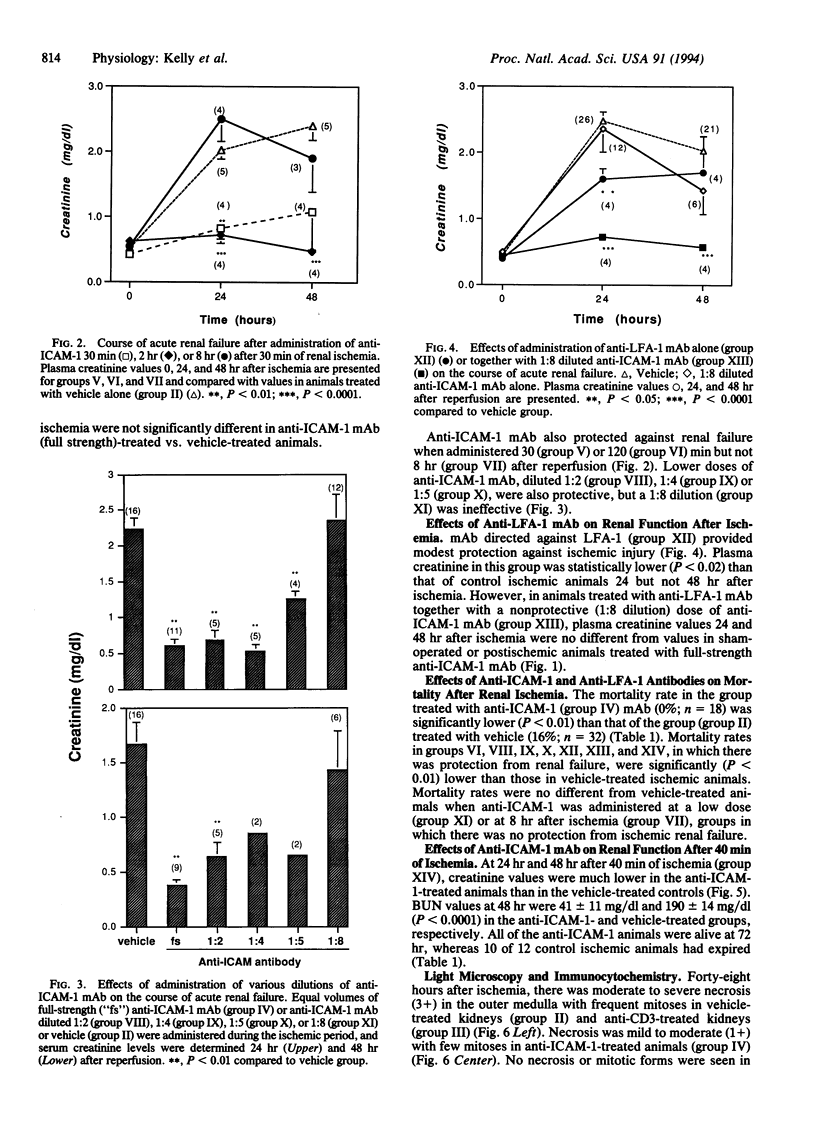
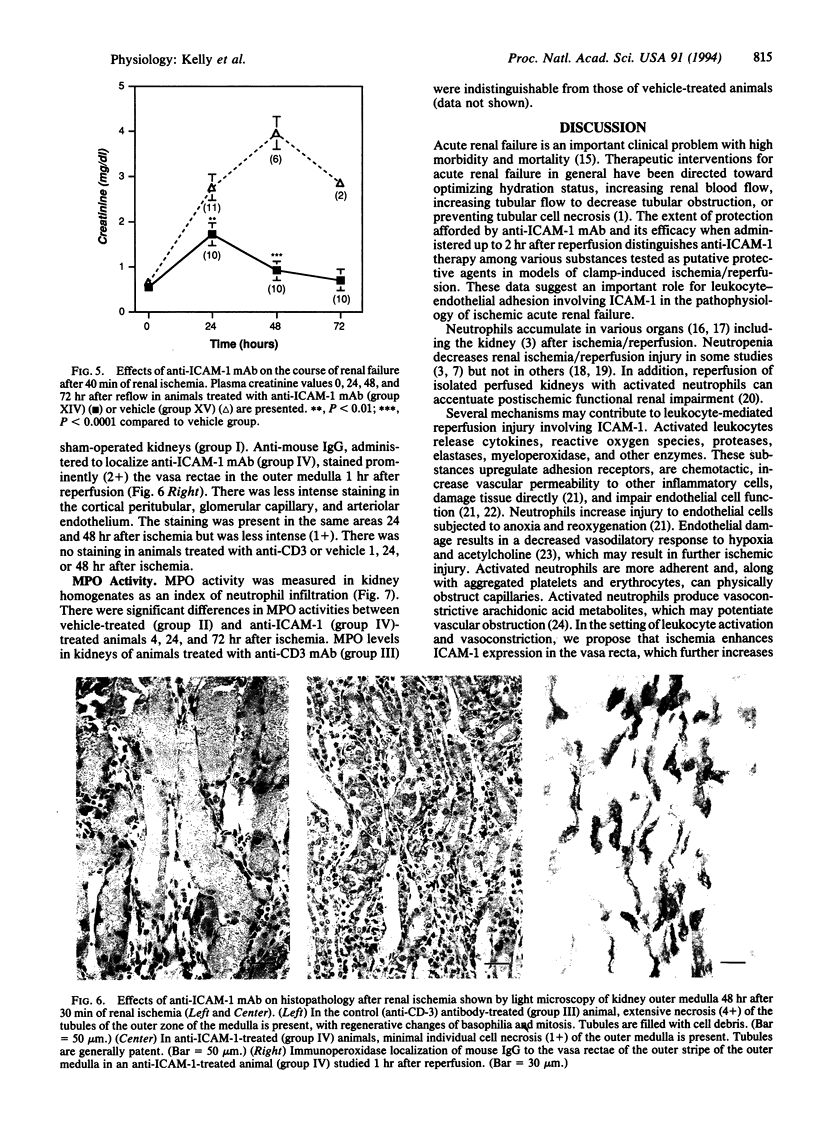
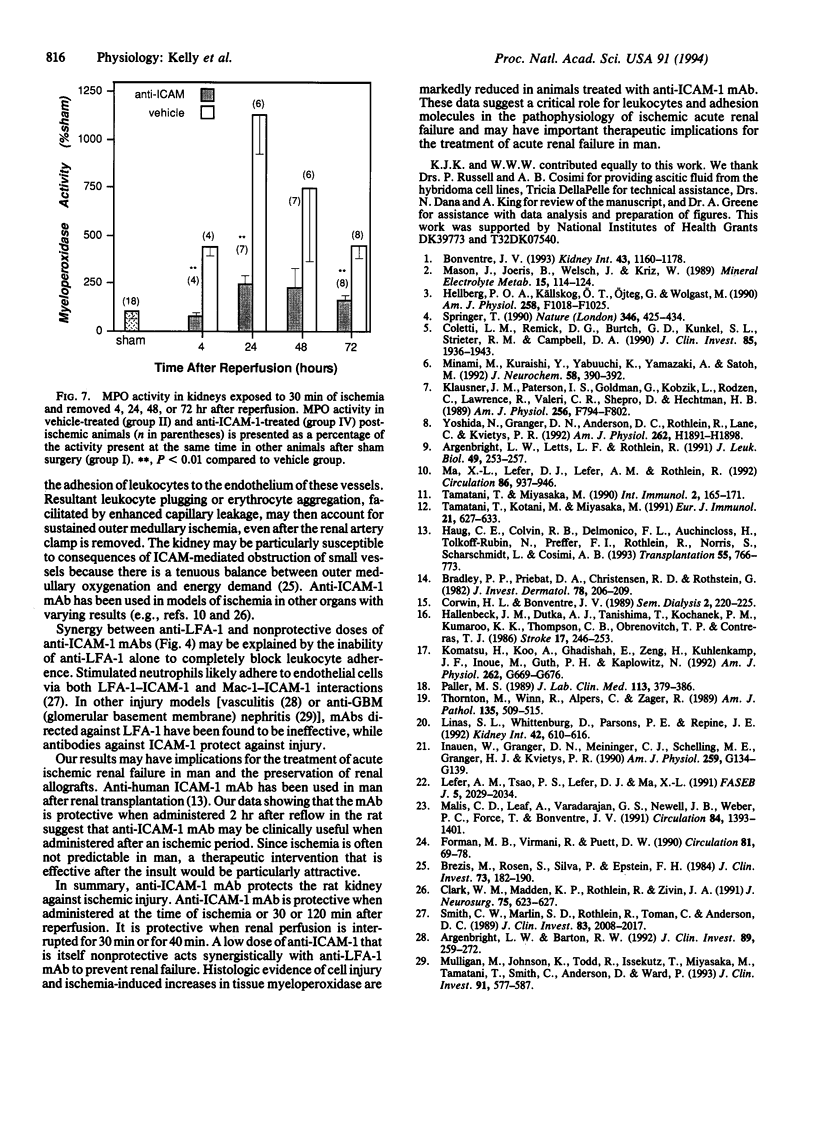
The pathophysiology of ischemic acute renal failure is complex, and the role of leukocyte adhesion in this process is not well defined. A monoclonal antibody (mAb) against intracellular adhesion molecule 1 (anti-ICAM-1), administered at the time of bilateral renal ischemia in the rat, prevented both functional impairment and histologic changes of acute renal failure. Plasma creatinine measured (mg/dl) 24 hr after 30 min of ischemia was 0.61 +/- 0.05 in the anti-ICAM-1-treated animals compared with 2.4 +/- 0.14 (P < 0.0001) in the vehicle-treated ischemic group. Forty-eight hours after ischemia, creatinine values were 0.46 +/- 0.05 and 2.03 +/- 0.22 (P < 0.0001) in anti-ICAM-1 and vehicle-treated groups, respectively. A low dose of anti-ICAM-1 that was itself nonprotective, when given with partially protective doses of a mAb against lymphocyte function-associated antigen-1 (anti-LFA-1), acted synergistically to prevent renal failure. Anti-ICAM-1 mAb also protected the kidney when administered 0.5 or 2 hr but not 8 hr after restoration of blood flow and when the ischemic period was extended to 40 min. Ischemia-induced increases in tissue myeloperoxidase, a marker of neutrophil infiltration, were mitigated with anti-ICAM-1 treatment. Thus, anti-ICAM-1 mAb protected the kidney against ischemic renal failure, even when the antibody was administered after the ischemic period. These results suggest a critical role for leukocytes and adhesion molecules in the pathophysiology of ischemic injury and may have important therapeutic implications.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Argenbright L. W., Barton R. W. Interactions of leukocyte integrins with intercellular adhesion molecule 1 in the production of inflammatory vascular injury in vivo. The Shwartzman reaction revisited. J Clin Invest. 1992 Jan;89(1):259–272. doi: 10.1172/JCI115570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Argenbright L. W., Letts L. G., Rothlein R. Monoclonal antibodies to the leukocyte membrane CD18 glycoprotein complex and to intercellular adhesion molecule-1 inhibit leukocyte-endothelial adhesion in rabbits. J Leukoc Biol. 1991 Mar;49(3):253–257. doi: 10.1002/jlb.49.3.253. [DOI] [PubMed] [Google Scholar]
- Bonventre J. V. Mechanisms of ischemic acute renal failure. Kidney Int. 1993 May;43(5):1160–1178. doi: 10.1038/ki.1993.163. [DOI] [PubMed] [Google Scholar]
- Bradley P. P., Priebat D. A., Christensen R. D., Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J Invest Dermatol. 1982 Mar;78(3):206–209. doi: 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Brezis M., Rosen S., Silva P., Epstein F. H. Selective vulnerability of the medullary thick ascending limb to anoxia in the isolated perfused rat kidney. J Clin Invest. 1984 Jan;73(1):182–190. doi: 10.1172/JCI111189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark W. M., Madden K. P., Rothlein R., Zivin J. A. Reduction of central nervous system ischemic injury by monoclonal antibody to intercellular adhesion molecule. J Neurosurg. 1991 Oct;75(4):623–627. doi: 10.3171/jns.1991.75.4.0623. [DOI] [PubMed] [Google Scholar]
- Colletti L. M., Remick D. G., Burtch G. D., Kunkel S. L., Strieter R. M., Campbell D. A., Jr Role of tumor necrosis factor-alpha in the pathophysiologic alterations after hepatic ischemia/reperfusion injury in the rat. J Clin Invest. 1990 Jun;85(6):1936–1943. doi: 10.1172/JCI114656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallenbeck J. M., Dutka A. J., Tanishima T., Kochanek P. M., Kumaroo K. K., Thompson C. B., Obrenovitch T. P., Contreras T. J. Polymorphonuclear leukocyte accumulation in brain regions with low blood flow during the early postischemic period. Stroke. 1986 Mar-Apr;17(2):246–253. doi: 10.1161/01.str.17.2.246. [DOI] [PubMed] [Google Scholar]
- Haug C. E., Colvin R. B., Delmonico F. L., Auchincloss H., Jr, Tolkoff-Rubin N., Preffer F. I., Rothlein R., Norris S., Scharschmidt L., Cosimi A. B. A phase I trial of immunosuppression with anti-ICAM-1 (CD54) mAb in renal allograft recipients. Transplantation. 1993 Apr;55(4):766–773. doi: 10.1097/00007890-199304000-00016. [DOI] [PubMed] [Google Scholar]
- Hellberg P. O., Källskog O. T., Ojteg G., Wolgast M. Peritubular capillary permeability and intravascular RBC aggregation after ischemia: effects of neutrophils. Am J Physiol. 1990 Apr;258(4 Pt 2):F1018–F1025. doi: 10.1152/ajprenal.1990.258.4.F1018. [DOI] [PubMed] [Google Scholar]
- Inauen W., Granger D. N., Meininger C. J., Schelling M. E., Granger H. J., Kvietys P. R. An in vitro model of ischemia/reperfusion-induced microvascular injury. Am J Physiol. 1990 Jul;259(1 Pt 1):G134–G139. doi: 10.1152/ajpgi.1990.259.1.G134. [DOI] [PubMed] [Google Scholar]
- Klausner J. M., Paterson I. S., Goldman G., Kobzik L., Rodzen C., Lawrence R., Valeri C. R., Shepro D., Hechtman H. B. Postischemic renal injury is mediated by neutrophils and leukotrienes. Am J Physiol. 1989 May;256(5 Pt 2):F794–F802. doi: 10.1152/ajprenal.1989.256.5.F794. [DOI] [PubMed] [Google Scholar]
- Komatsu H., Koo A., Ghadishah E., Zeng H., Kuhlenkamp J. F., Inoue M., Guth P. H., Kaplowitz N. Neutrophil accumulation in ischemic reperfused rat liver: evidence for a role for superoxide free radicals. Am J Physiol. 1992 Apr;262(4 Pt 1):G669–G676. doi: 10.1152/ajpgi.1992.262.4.G669. [DOI] [PubMed] [Google Scholar]
- Lefer A. M., Tsao P. S., Lefer D. J., Ma X. L. Role of endothelial dysfunction in the pathogenesis of reperfusion injury after myocardial ischemia. FASEB J. 1991 Apr;5(7):2029–2034. doi: 10.1096/fasebj.5.7.2010056. [DOI] [PubMed] [Google Scholar]
- Linas S. L., Whittenburg D., Parsons P. E., Repine J. E. Mild renal ischemia activates primed neutrophils to cause acute renal failure. Kidney Int. 1992 Sep;42(3):610–616. doi: 10.1038/ki.1992.325. [DOI] [PubMed] [Google Scholar]
- Ma X. L., Lefer D. J., Lefer A. M., Rothlein R. Coronary endothelial and cardiac protective effects of a monoclonal antibody to intercellular adhesion molecule-1 in myocardial ischemia and reperfusion. Circulation. 1992 Sep;86(3):937–946. doi: 10.1161/01.cir.86.3.937. [DOI] [PubMed] [Google Scholar]
- Malis C. D., Leaf A., Varadarajan G. S., Newell J. B., Weber P. C., Force T., Bonventre J. V. Effects of dietary omega 3 fatty acids on vascular contractility in preanoxic and postanoxic aortic rings. Circulation. 1991 Sep;84(3):1393–1401. doi: 10.1161/01.cir.84.3.1393. [DOI] [PubMed] [Google Scholar]
- Mason J., Joeris B., Welsch J., Kriz W. Vascular congestion in ischemic renal failure: the role of cell swelling. Miner Electrolyte Metab. 1989;15(3):114–124. [PubMed] [Google Scholar]
- Minami M., Kuraishi Y., Yabuuchi K., Yamazaki A., Satoh M. Induction of interleukin-1 beta mRNA in rat brain after transient forebrain ischemia. J Neurochem. 1992 Jan;58(1):390–392. doi: 10.1111/j.1471-4159.1992.tb09324.x. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Johnson K. J., Todd R. F., 3rd, Issekutz T. B., Miyasaka M., Tamatani T., Smith C. W., Anderson D. C., Ward P. A. Requirements for leukocyte adhesion molecules in nephrotoxic nephritis. J Clin Invest. 1993 Feb;91(2):577–587. doi: 10.1172/JCI116237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller M. S. Effect of neutrophil depletion on ischemic renal injury in the rat. J Lab Clin Med. 1989 Mar;113(3):379–386. [PubMed] [Google Scholar]
- Smith C. W., Marlin S. D., Rothlein R., Toman C., Anderson D. C. Cooperative interactions of LFA-1 and Mac-1 with intercellular adhesion molecule-1 in facilitating adherence and transendothelial migration of human neutrophils in vitro. J Clin Invest. 1989 Jun;83(6):2008–2017. doi: 10.1172/JCI114111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T. A. Adhesion receptors of the immune system. Nature. 1990 Aug 2;346(6283):425–434. doi: 10.1038/346425a0. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Kotani M., Miyasaka M. Characterization of the rat leukocyte integrin, CD11/CD18, by the use of LFA-1 subunit-specific monoclonal antibodies. Eur J Immunol. 1991 Mar;21(3):627–633. doi: 10.1002/eji.1830210314. [DOI] [PubMed] [Google Scholar]
- Tamatani T., Miyasaka M. Identification of monoclonal antibodies reactive with the rat homolog of ICAM-1, and evidence for a differential involvement of ICAM-1 in the adherence of resting versus activated lymphocytes to high endothelial cells. Int Immunol. 1990;2(2):165–171. doi: 10.1093/intimm/2.2.165. [DOI] [PubMed] [Google Scholar]
- Thornton M. A., Winn R., Alpers C. E., Zager R. A. An evaluation of the neutrophil as a mediator of in vivo renal ischemic-reperfusion injury. Am J Pathol. 1989 Sep;135(3):509–515. [PMC free article] [PubMed] [Google Scholar]
- Yoshida N., Granger D. N., Anderson D. C., Rothlein R., Lane C., Kvietys P. R. Anoxia/reoxygenation-induced neutrophil adherence to cultured endothelial cells. Am J Physiol. 1992 Jun;262(6 Pt 2):H1891–H1898. doi: 10.1152/ajpheart.1992.262.6.H1891. [DOI] [PubMed] [Google Scholar]





