Abstract
Osteoarthritis (OA) is a painful and life-altering disease that severely limits the daily activities of millions of Americans, and it is one of the most common causes of disability in the world. With obesity on the rise and the world’s population living longer, the prevalence of OA is expected to increase dramatically in the coming decades, generating burdensome socioeconomic costs. This review summarizes current pharmaceutical, nonpharmaceutical, and prospective new treatments for OA, with primary focus on the dietary supplement avocado/soybean unsaponifiables (ASU). ASU modulates OA pathogenesis by inhibiting a number of molecules and pathways implicated in OA. Anticatabolic properties prevent cartilage degradation by inhibiting the release and activity of matrix metalloproteinases and increasing tissue inhibitors of these catabolic enzymes. ASU also inhibits fibrinolysis by stimulating the expression of plasminogen activator inhibitor. Anabolic properties promote cartilage repair by stimulating collagen and aggrecan synthesis via inhibition of inflammatory cytokines such as interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor, ERK, and prostaglandin E2. Chondroprotective effects are mediated by correcting growth factor abnormalities, increasing TGF-β, and decreasing vascular endothelial growth factor (VEGF) in synovial fluid. ASU also inhibits cholesterol absorption and endogenous cholesterol biosynthesis, which mediate reactive oxygen species pathology in chondrocytes. At the clinical level, ASU reduces pain and stiffness while improving joint function, resulting in decreased dependence on analgesics.
Keywords: osteoarthritis, cartilage, dietary supplements, avocado soybean unsaponifiables (ASU), Arthrocen
Introduction
Osteoarthritis (OA) is a chronic synovial joint disease, characterized by two main features: (1) progressive damage of articular cartilage, bone remodeling, and new bone formation (osteophytes and subchondral bone sclerosis) and (2) synovial inflammation and fibrosis of ligaments, tendons, menisci, and capsules. All joints may be affected, but the most commonly involved are knees, hands, and hips (Fig. 1). While chronic OA used to be regarded as a “wear and tear disease,” researchers now believe that low-grade inflammation and growth of blood vessels and nerves from the subchondral bone into articular cartilage, as well as metabolic disorders, play a major role in disease pathology.1-4 Patients with OA suffer from pain, inflammation, and limited joint function. Pharmacological interventions are mostly palliative, focusing on alleviation of symptoms or slowing disease progression until damaged hip or knee joints are eventually replaced.5-10 Women are more severely affected than men by knee OA.11 Differences in knee anatomy (narrower femurs, thinner patellae, larger quadriceps angles, and differences in tibial condylar size), previous knee trauma, and genetic and hormonal influences may play a role. Other factors such as age and obesity are also common factors. In general, women present for treatment in more advanced stages of OA and have more debilitating pain than men. Women also have less cartilage volume, greater cartilage wear, and overall differences in mechanical alignment.
Figure 1.
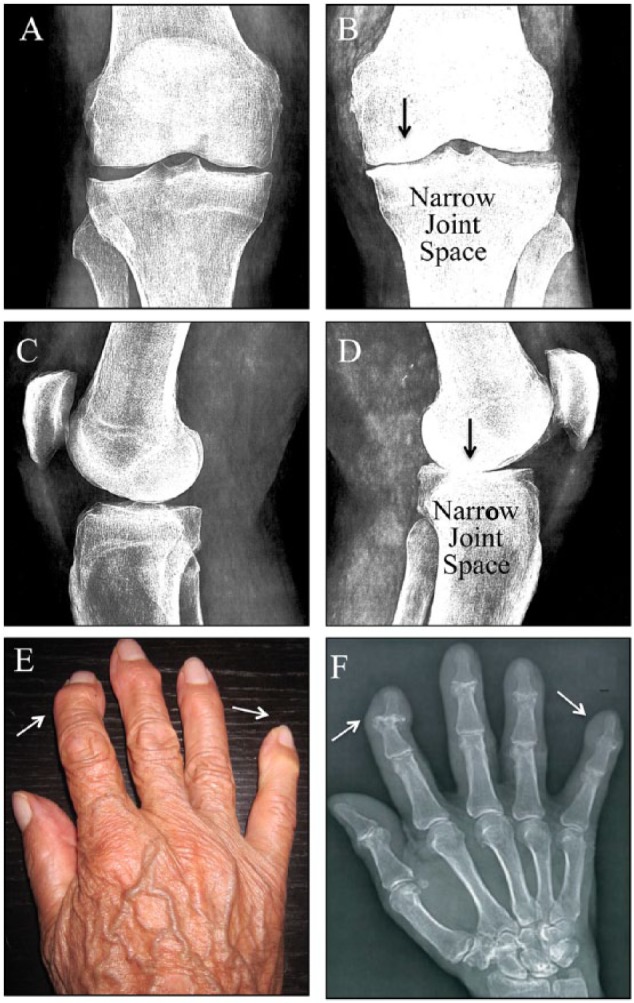
Anteroposterior and cross-table lateral of knee osteoarthritis (OA). Symptomatic knee OA typically presents with narrowing of the joint space and bone spurs (arrows). (A and B) During the development of OA, articular cartilage breaks down over time and becomes thin. As a result, the bone surfaces rub against each other, further damaging the cartilage and bone and causing pain. (C and D) Joints with late-stage OA are often painful, warm to the touch, possibly red, swollen, have subchondral cysts, and notable loss of function.
Current Pharmacologic Therapies
Pain medications currently used to treat the symptoms of OA include acetaminophen, topical capsaicin, topical and oral nonsteroidal anti-inflammatory drugs (NSAIDs; i.e., naproxen and ibuprofen), and the synthetic opioids tramadol and codeine. However, each of these therapies has potential drawbacks that may limit their widespread use. Analgesics can be addictive, whereas acetaminophen can have serious side effects, such as kidney and liver damage.12 Patients who do not respond to acetaminophen may be prescribed NSAIDs. Treatment with NSAIDs, which inhibit cyclooxygenases (COX1 and COX2), thereby blocking prostaglandin synthesis, improves quality of life and decreases pro-inflammatory cytokines including interleukin-6 (IL-6), vascular endothelial growth factor (VEGF), and tumor necrosis factor-α (TNF-α) in synovial fluid and mitogen-activated protein kinases (MAPKs) in knee OA.13 However, NSAIDs can also cause serious side effects, including upper gastrointestinal (GI) toxicity (dyspepsia, ulcers, perforation, obstructions, and bleeding) and liver dysfunction. As such, they are typically prescribed for the shortest possible duration at the lowest effective dose. To reduce the risk of these upper GI complications, the US FDA has approved the use of the NSAID HZT501 (Duexis), a drug containing 800 mg ibuprofen, in combination with 26.6 mg famotidine, a histamine H2-receptor antagonist.14 Alternatively the NSAID celecoxib has less risk of upper GI complications by selectively inhibiting the isoenzyme COX-2, which is specific to inflamed tissue, versus COX-1, which is constitutive in many tissues including the GI tract. It should be noted that daily treatment with celecoxib is more effective in patients with normal body mass index (BMI) than obese patients.15
Intra-articular injection of corticosteroids (GC) is recommended to relieve inflammation and pain in OA joints. However, GC injections are short acting, prone to adverse side effects, and have limited disease-modifying effects. For patients with knee OA, viscosupplementation with hyularonin may be used to replaces shock absorbing and lubricant material in the joint fluid, but the effects are similarly short-lived.
Current Nonpharmacologic Therapies
Currently, guidelines for OA management are available from numerous organizations, including the American Academy of Orthopedic Surgeons (AAOS), the American College of Rheumatology (ACR), the American Geriatrics Society (AGS), the American Pain Society (APS), and the Osteoarthritis Research Society International (OARSI) in the United States and the European League Against Rheumatism (EULAR) and the United Kingdom’s National Institute for Health and Clinical Excellence (NICE) in Europe. Collectively, these guidelines reflect the experience of physicians across a variety of medical disciplines. Whereas all generally use the same data sources (i.e., evidence-based research, expert opinion, patient experience, and cost-effectiveness analysis), they differ in focus. For instance, the AAOS and AGS guidelines reflect the perspective of specialists in orthopedic surgery, geriatrics, and pain management, whereas the EULAR and OARSI guidelines primarily emphasize the findings of experts in rheumatology. The NICE guidelines are developed jointly by physicians and other health care professionals working in conjunction with a range of clinical researchers. In addition, the scope varies, with some guidelines (e.g., AAOS, ACR, EULAR, and OARSI) addressing specific types of OA (i.e., knee, hip, or hand) and others (e.g., AGS, APS, and NICE) addressing OA more generally. As such, recommendations can vary widely, for instance, guidelines for use of NSAIDs.10,16-19 Recommended nonpharmacologic interventions range between therapeutic exercises, patient education, transcutaneous electrical nerve stimulation, acupuncture, orthotics and insoles, heat and cryotherapy, patellar tapping, and weight control. In an effort to evaluate these varying guidelines, the Appraisal of Guidelines Research and Evaluation (AGREE II) scored 17 clinical practice guidelines (CPGs) including EULAR, NICE, OARSI, AAOS, and ACR, on six different measures: D1, scope and purpose; D2, stakeholder involvement; D3, rigor of development; D4, clarity and presentation; D5, applicability; and D6, editorial independence.20 The general clinical management recommendations tended to be similar among high-quality CPGs, although interventions addressed varied. Nonpharmacological management interventions were superficially addressed in more than half of the selected CPGs.
Prospective New Treatments
New noninvasive, disease-modifying therapies for OA are lacking and needed by millions of patients. A number of prospective new treatments targeting pro-inflammatory mediators, cytokines, bone turnover, and angiogenic and neurogenic factors are being investigated, with varying success in clinical trials and clinical use.21
Interleukin-1 (IL-1) may prove an effective target, as IL-1 induces matrix metalloproteinase (MMP) production, resulting in the degradation of aggrecan and other matrix constituents. IL-1 also induces high levels of COX2 and prostaglandin E2 (PGE2), which may explain the pain associated with OA degeneration.22 The drug diacerein, an inhibitor of IL-1, may modify both disease symptoms and disease structure in OA. Oral diacerein has proven effective in reducing pain, although evidence from clinical trials and scientific literature suggest that the effectiveness in OA is weak. It can be used in conjunction with NSAIDs or viscosupplementation therapies for additive effects due to its alternative mechanism of action. The most common side effects of diacerein are gastrointestinal, such as diarrhea, and changes in the color of urine. Meanwhile, the IL-1-receptor antagonists anakinra and orthokin are reported to improve Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores.23-25 In addition, the IL-1β antibody “gevokizumab” is in phase II clinical trials for safety and biological activity in the treatment of hand OA.26,27
Nerve growth factor (NGF) has also been recognized as an important mediator of chronic pain in OA. Tanezumab, a monoclonal antibody against β-NGF receptor tyrosine kinase (TrkA), inhibits NGF action and reduces pain in patients.28 Two randomized phase III clinical trials indicate that tanezumab provides superior pain relief while improving physical function and global disease assessment scores in patients with painful hip OA.27,29-33 Although in most cases tanezumab is well tolerated, the unexpected occurrence of rapid destructive arthropathies suggests there may be safety issues. Alternatively, using the drug adalimumab to inhibit TNF-α, which upregulates β-NGF, does not improve global disease assessment scores in OA of the hand.27,34
Several studies have explored therapies aimed at modifying bone turnover for treatment of OA. Strontium ranelate (SrRa), an element similar to calcium, is easily taken up by the body and incorporated into bones in place of calcium. SrRa is currently indicated for the prevention of fracture in severe osteoporosis. The SEKOIA (SrRa Efficacy in Knee OsteoarthrItis triAl) trial, a 3-year randomized, double-blind, placebo-controlled trial, evaluated the efficacy, safety, and disease-modifying effects of SrRa given at 1 to 2 g/day in patients with knee OA. Magnetic resonance imaging (MRI) data indicate that SrRa significantly reduced cartilage volume loss and bone marrow lesion progression. Symptoms also improved in terms of pain and physical function after 6 and 12 months, respectively,35 although treatment was deemed safe and well tolerated. These data indicate that SrRa could be a promising new symptom and disease-modifying treatment for OA. Additionally, there is a need for further investigations to establish the optimal dosage and to better clarify the mechanism of action of SrRa in OA.35-37 Several clinical studies have investigated the effects of anti-resorptive therapies such as bisphosphonates on OA symptoms. A study by Carbone et al. found that alendronate (ALN) use in OA patients decreased bone abnormalities and attenuated knee pain, yet cartilage degeneration was still present in the MRI scans of treated patients.38 Spector et al. determined that risedronate use led to significant improvements in WOMAC scores and preservation of knee joint space compared with placebo in a 1-year randomized control trial involving patients with moderate OA.39 However, a 2-year randomized control trial of risedronate treatment revealed contradictory results, with no significant improvement of WOMAC score or joint space retention in the knee.40 Similarly, Nishii et al. observed no inhibition of OA progression in treated hip OA patients after 2 years of ALN treatment.41 Therefore, in spite of the growing body of clinical work investigating the subject, no definitive conclusion can be reached on the practicality of using bisphosphonates to treat patients with OA.
Antidepressants have shown promising preliminary results for treatment of pain associated with OA by increasing serotonin levels in the brain. Serotonin-norepinephrine reuptake inhibitors duloxetine (Cymbalta) and milnacipran significantly improve pain in OA.27,42 An open-label trial also suggested analgesic effectiveness of methotrexate, an anti-inflammatory drug that acts by inhibiting the metabolism of folic acid, demonstrating that up to 20 mg/week for 6 months achieved OARSI responder criteria in knee OA and warranted a randomized controlled trial.43
Other treatments are aimed at improving disease pathology by building cartilage. The small molecule kartogenin was identified in an image-based high-throughput screen to promote chondrocyte differentiation. It shows chondroprotective effects in vitro and is efficacious in two animal models of OA. Kartogenin induces chondrogenesis by disrupting the interaction between filamin A and the transcription factor core-binding factor b subunit (CBFβ), thereby altering CBFβ-RUNX1 and possibly RUNX2 transcriptional programs.44 Autologous injection of platelet-rich plasma (PRP) has been used to stimulate cartilage repair and healing in OA patients,27,45,46 but the presence of other growth factors in PRP may be problematic. Furthermore, bone morphogenic protein 7 (BMP7), FGF-8, and botulium toxin A (BoNT-A) are used in the treatment of knee OA.47 BoNT-A has an analgesic effect by temporarily suppressing acetylcholine secretion at presynaptic nueuromuscular junctions and appears to be effective and safe for the management of advanced knee OA. However, these results cannot be generalized to patients with mild knee joint pain or nonspecific soft tissue pain in the knee joint region. Further research is necessary to investigate possible complications such as aggravation of infection, effect on muscle strength, and neuropathic joint degeneration.
Current nonsurgical and reconstructive surgical therapies are unsuccessful in reversing OA. Recently, a phase I trial was reported in which chondrocytes were modified via intra-articular DNA injection to produce TGF-β1 in patients with advanced knee OA.48 Intra-articular injection of adipose-derived stem cell (ADSC) therapy in a new European program is also under investigation.49 ADSC induced the release of trophic factors that exerted anti-inflammatory effects on both synoviocytes and chondrocytes, with no MMP1, MMP3, or MMP13 production, suggesting safe and effective use of ADSCs for clinical applications. However, both treatments need proof-of-concept studies in larger patient populations. Alternatively, intra-articular injection of human mesenchymal stem cells can lead to articular cartilage protection through the SDF-1/CXCR4 axis.50-54
Dietary Supplements
Natural products can be safer than prescription medications with less undesirable side effects. Dietary supplements including avocado soybean unsaponifiables (ASU), chondroitin sulfate, hyaluronan, and glucosamine sulfate have been reported to modify EULAR symptoms for the treatment of OA.55,56 They are used to treat mild to moderate pain and alleviate symptoms to reduce the consumption of NSAIDs.
Several trials for chondroitin sulfate, glucosamine sulfate, and hyaluronan (C14H21NO11)n are in process.56,57 Chondroitin sulfate, glucosamine sulfate, and hyaluronan are building blocks for proteoglycan synthesis, and major constituents of the extracellular matrix in cartilage and synovial fluid.58 They are produced by chondrocytes and syonivocytes or obtained through diet.59-65 Hyaluronan and hyaluronic acid (Hyalgan hylan-GF20/Synvisc) can be injected into the knee joint of patients with OA who cannot tolerate NSAIDs or are awaiting joint surgery.66 A recent report indicates that viscosupplementation with Hylan-GF20 slows type II collagen degradation and joint inflammation in patients with OA.67 However, Hylan-GF20 was not present in granulomas, an indicator of inflammation, raising the question of clinical significance in pain reduction.68 Also, viscosupplementation with hyaluronic acid itself does not significantly improve disease outcome, and little is known about long-term effects.
The efficacy of glucosamine and/or chondroitin in treating knee OA pain was evaluated in the Glucosamine/Chondroitin Arthritis Intervention Trial, funded by the National Center for Complementary and Alternative Medicine and the National Institute of Arthritis and Musculoskeletal and Skin Diseases. Patients were treated daily for 24 weeks with glucosamine alone (1,500 mg), chondroitin sulfate alone (1,200 mg), glucosamine and chondroitin sulfate combined (same doses), a placebo, or celecoxib (200 mg), which served as a positive control. Although there were no statistically significant differences between any of the experimental treatments and the placebo overall, patients with moderate-to-severe pain given both glucosamine and chondroitin sulfate did show improvement (79% experienced pain reduction vs. 54% for placebo). Because of the small size of this subgroup, these findings should be considered preliminary and need to be confirmed in further studies.61,69-71
Glucosamine does not appear to slow arthritis progression over the long term and has many potential complications. The most common adverse effects are epigastric pain or tenderness, heartburn, diarrhea, and nausea. Glucosamine also may cause allergic reactions in patients with seafood allergies, as a product of lobster, crab, and shrimp shells. Glucosamine may interact with various pharmaceuticals, such as warfarin (Coumadin) and diabetes medications, dangerously modifying their efficacy.
Similarly, chondroitin sulfate appears not to provide meaningful benefit for patients with OA, and their combination has not proven effective for either pain management or functional improvement. OARSI and NICE no longer recommend the use of glucosamine or chondroitin sulfate alone, or in combination, if no effects are observed after 6 months or radiographic changes are marginal.55,61,62,72
A network meta-analysis of 10 trials in 3,803 patients by Juni and collaborators in 2012 found no clinically significant improvements in OA pain alleviation or JSN parameters with glucosamine, chondroitin, or combined treatment compared with placebo. Despite these results, many patients believe otherwise, potentially due to the natural course of disease, regression to the mean, or the placebo effect. The authors conclude that such patients should be permitted to use these supplements if they cover the cost themselves, since neither of these preparations was found to be dangerous.73
SierraSil is a dietary supplement marketed for joint pain relief that is derived from the mineral-rich clay found in the high Sierra Mountains in the United States. Clinical trial testing short-term efficacy of SierraSil at doses of 2 and 3 g per day failed to show sustained benefits over placebo, and iron toxicity has been reported.74
Some OA patients experience pain relief from topical creams containing capsaicin, the active component of chili peppers. However, use of these creams may introduce side effects such as burning, stinging, and redness of the skin and eyes.10
Avocado and Soybean Unsaponifiables
Avocado/soybean unsaponifiables are natural vegetable extracts made from avocado and soybean oils, consisting of the leftover fraction (approximately 1%) that cannot be made into soap after saponification. ASU is composed of one third avocado and two thirds soybean unsaponifiables (A1S2U). The major components of ASU are phytosterols β-sitosterol, campesterol, and stigmasterol, which are rapidly incorporated into cells. ASU is a complex mixture of many compounds including fat-soluble vitamins, sterols, triterpene alcohols, and possibly furan fatty acids. The identity of the active component(s) remains unknown. The sterol contents of ASU preparations are the primary contributors to biological activity in articular chondrocytes.75 Preclinical in vitro and in vivo studies have demonstrated that ASUs have beneficial effects on OA.76-90
ASU possesses chondroprotective, anabolic, and anticatabolic properties. It inhibits the breakdown of cartilage and promotes cartilage repair by inhibiting a number of molecules and pathways implicated in OA (Tables 1 and 2). ASU stimulates the synthesis of collagen and aggrecan by inhibiting inflammatory cytokines such as IL-1, IL-6, IL-8, TNF, and PGE2 through modulation of NF-kappaB.91-94 The combination of ASU and epigallocatechin gallate (EGCG; a major component of green tea catechins) affects an array of inflammatory molecules including expression of COX-2 and production of PGE2 in chondrocytes.95 COX-2 regulates the production of PGE2; both are mediators involved in the process of cartilage breakdown. ASU also inhibits the release and activity of collagenase (MMP2) and stromelysin 1 (MMP3) in cultured chondrocytes,77,96 increases tissue inhibitors of metalloproteinases (TIMP-1),79,97 and inhibits IL1-induced ERK but not p38 or JNK in chondrocytes in vitro.86
Table 1.
Stimulatory Effects of Avocado Soybean Unsaponifiable on Anti-Inflammatory, Anabolic Mediators That Protect Against Osteoarthritis.
| Molecular Mediator | Target Tissue/Organ | Organism | Assay | References |
|---|---|---|---|---|
| Collagen synthesis | Articular synoviocytes, chondrocytes, skin fibroblasts | Rabbit, bovine, human | In vitro | 81, 84, 93 |
| Collagen II mRNA | Chondrocytes + subchondral bone osteoblasts (SBO) | Human | In vitro | 84 |
| Agreecan proteoglycan | Chondrocytes, SBO | Equine, human | In vitro | 84, 94 |
| TGF-β1 | Knee joint fluid, osseointegration in tibiae | Rat, canine | In vivo, in vitro | 84, 98, 100, 101 |
| TGF-β2 | Knee joint fluid | Canine | In vitro, in vivo | 98, 100 |
| TGF-β3 | Chondrocytes + SBO | Human | In vitro | 84 |
| BMP-2 | Osseointegration in tibiae | Rat | In vivo | 101 |
| Osteocalcin | Chondrocytes + SBO | Human | In vitro | 84 |
| Chondroprotector | Bone implant | Rat | In vivo | 96 |
| Delayed destruction of the joints | Radiological evaluation | Human | In vivo | 81 |
| Plasminogen activator inhibitor 1 (PAI-1) | Chondrocytes, osteoblasts | Bovine | In vitro | 98 |
Table 2.
Inhibitory Effects of Avocado Soybean Unsaponifiable on Inflammatory and Catabolic Mediators of Osteoarthritis.
| Molecular Mediator | Target Tissue/Organ | Organism | Assay | References |
|---|---|---|---|---|
| Interleukin-1 beta (IL-1β) | Synoviocytes, chondrocytes | Mice, rabbit, human | In vitro | 77, 81, 116 |
| Interleukin-4 (IL-4) | Chondrocyte | In vitro | ||
| Interleukin-6 (IL-6) | Chondrocyte | Human | In vitro | 81, 92 |
| Interleukin-8 (IL-8) | Chondrocyte | Human | In vitro | 92 |
| Macrophage inhibitory protein-1beta (MIP-1β) | Chondrocyte | Human | In vitro | 87, 116 |
| MMP-2 (also known as collagenase, or gelatinase-2) | Fibroblasts, chondrocyte | Human | In vitro | 79, 81, 92 |
| MMP-3 (also known as stromelysin) | Fibroblasts chondrocyte + subchondral bone osteoblasts (SBO) | Mice, human | In vitro | 79, 81, 84, 86, 92, 101 |
| MMP-13, collagenase-3 | Chondrocytes, chondrocytes + SBO | Mice, human | In vivo | 84, 86 |
| Tissue inhibitors of MMP (TIMP-1) | Fibroblasts + SBO | Human | In vitro | 79, 84 |
| COX2 | Chondrocytes, monocyte/Macrophage-like cells, chondrocytes + SBO | Equine, human | In vitro | 84, 116, 115 |
| Prostagladine-2 (PGE2) | Hyalin chondrocytes, monocyte/macrophage-like cells | Mice, equine, human | In vitro | 86, 92, 116 |
| NF-κB | Hyalin chondrocytes, nuclear translocation of p65 | Mice, equine, human | In vitro | 86, 115 |
| ERK1/2 | Hyalin chondrocytes | Mice, human | In vitro | 86 |
| TNF-α | Chondrocytes | Human | In vitro | 84 |
| iNOS | Chondrocytes, monocyte/macrophage-like cells | Human | In vitro | 84, 116 |
| NO | Chondrocytes, monocyte/macrophage-like cells | Human | In vitro | 116 |
| oLDL | Osteoblasts | Human | Serum | 104 |
| Fibronectin | Chondrocytes | Human | In vitro | 81 |
| Alkaline phosphatase | Osteoblasts | Human | In vitro |
In vitro studies show that ASU inhibits fibrinolysis by stimulating the expression of plasminogen activator inhibitor (PAI-1).98 PAI-1 inhibits tissue plasminogen activator and urokinase (uPA), thereby blocking plasminogen activation and inhibiting fibrinolysis (the physiological breakdown of blood clots). This fibrinolytic and tissue destructive proteinase cascade may play a role in OA joint inflammation via altered expression of uPA receptors.99
ASUs alter growth factor levels implicated in OA pathogenesis, increasing TGF-β1 and TGF-β2 in the canine knee joint fluid,100 to repair cartilage and decreasing VEGF, which is markedly elevated in synovial fluid of patients.13,97 In a study of implant osseointegration in rat tibiae, ASU administration improved markers of bone growth, including bone morphogenic protein 2 (BMP-2) and transforming growth factor beta 1 (TGF-β1), though histomorphometric analysis of implant osseointegration was only slightly improved.101 ASU also inhibits cholesterol absorption and endogenous cholesterol biosynthesis.102 Sixty percent of patients with OA exhibit high levels of oxidized low-density lipoproteins (oLDL) in serum, which mediates reactive oxygen species (ROS) activity in chondrocytes and OA pathology.103,104 Treating patients with a daily dose of 300 mg ASU for 3 months decreased oLDL levels.105
At the clinical level, ASU reduces pain and stiffness while improving function in joints, resulting in decreased dependence on analgesics. ASU efficacy and safety during and after treatment have been assessed in various randomized, double-blind, multicenter trials in patients with symptomatic knee or hip OA. Two studies conducted over a 3-month period report that standard treatment with 300 mg/day of ASU improved indices of pain, stiffness, and physical function, as measured by WOMAC, and decreased analgesic drug demanded in patients with OA.106-109 A third trial conducted over 6 months reports similarly improved function compared with placebo, measured by the Lequense Functional Index, with persistent effects after termination of treatment.108 In a 6-month trial on patients with femorotibial gonarthrosis, ASU was as effective as 400 mg of chondroitin sulfate three times per day, as measured by WOMAC.110 Most recently, a 3-year randomized trial on patients with hip OA, performed under the ACR criteria (minimum of 1-4 mm hip JSW on the pelvic radiographs), reports excellent safety, but no significant reduction in the mean rate of JSN after 1 year. However, analyzing the results under different parameters reveals a significant 20% reduction in the rate of progression in patients with severe hip OA (P = 0.04), indicating a potential structure modifying effect of ASU,111 as confirmed in the ERADIAS study. In a clinical trial of patients with hip OA, the effects of ASU treatment over 3 years were evaluated by radiography to identify joint pathology and disease progression on the structural level. Although JSN was not statistically significant between ASU and placebo treatment, secondary analysis of disease progression, measured by JSN (0.5 mm) or total hip replacements, indicated 20% improvement with ASU (42.2% vs. 51.4% of placebo group, P = 0.054). Computerized image analysis also showed significant histological differences not detectable by traditional scoring methods.112 In sheep, ASU treatment following cartilage insult improved articular integrity, as measured by toluidine blue staining, after 6 months compared with untreated animals. These improvements were the result of decreased catabolism and increased anabolism of cartilage by ASU.78 Indeed, ASU reduces inflammation-mediated cartilage degradation by reducing IL-1, PGE2, and MMP-3 production, while also inducing proteoglycan, noncollagenous protein (NCP), and collagen synthesis within 72 hours of administration to bovine cells in culture.75 A recent study in patients with nonspecific dorsalgia demonstrated analgesic effect of Piascledine with positive outcome after 1 month.113 However, a randomized, double-blind, placebo-controlled clinical trial carried out in 14 obese adult volunteers over 3 months reports no significant effect on these parameters, as measured by hyperglycemic–hyperinsulinemic clamp technique.114
Four double-blind placebo-controlled randomized human clinical trials (RCTs) evaluate ASU’s impact on knee and hip OA.109 Two of these indicated that ASU treatment decreased NSAID intake over 3 months.106,107 Another found that ASU improves LFI compared with placebo over the course of 6 months, and also that improvements took 2 months to take effect, and subside after treatment ended.108 Alternatively, a long-term study indicated no significant difference in JSN, or other parameters of disease, after 2 years of ASU treatment,80 indicating that the beneficial impact of ASU on OA may be limited to short-term effects. However, this study also focused on identifying structure-modifying effects, versus symptom-modifying effects; although these two different measures of OA severity often correlate, ASU may affect each uniquely. Evidence for symptom-modifying effects of ASU is much stronger, and thus an alternative explanation for these contradictory findings is that while ASU does not improve structural damage of OA, as measured in this study, it does improve symptoms such as pain and mobility, as measured in previous studies.82 In a study of chronic nonspecific back pain, treatment with ASU (piascledine) combined with the NSAID artrosiline (320 mg/day) showed significant analgesic effect over NSAID treatment alone. The positive effect of ASU was demonstrated after 1 month of treatment. The authors suggest that further RCTs are needed to confirm results. To this end, the ERADIAS trial determined whether ASU Expanscience treatment slowed the radiological progression of hip OA.113 As for safety, none of the four RCTs reported significant differences in adverse effects between ASU and placebo.
Factors like BMI, severity of disease, and activity level may influence the effect of ASU, as these conditions exacerbate inflammatory conditions and mechanical stresses that contribute to OA. Adipose tissue plays an important role by producing metabolic factors with catabolic and pro-inflammatory properties, including cytokines, chemokines, and adipokines (IL-6 and TNF-α, IL-8, IFN-γ), which orchestrate pathophysiological processes in OA. Soluble mediators produced by adipocytes may also modulate chondrocyte metabolism and contribute to cartilage degradation. ASU may counteract these inflammatory processes by inhibiting the translocation of the transcription factor NF-κB from the cytoplasm to the nucleus, which controls transcription of many pro-inflammatory factors (Table 2).86,115 As such, ASU acts as an anabolic agent in vitro, reducing the production of pro-inflammatory mediators, including IL-1, IL-6, IL-8, macrophage inflammatory protein-1, NO, MMP-13, TNF-α, and COX2/PGE288,94,115,116 from various cell types (Table 1). In mice, ASU decreases pro-inflammatory interferon-γ (IFN-γ) and IL-4 production, in the context of parasitic diseases.117,118 Although more studies need to be conducted to show the effects of ASU in patients with varying BMI, the anti-inflammatory effects of ASU are likely to protect cartilage from obesity-associated inflammatory degradation and improve OA symptoms. Indeed, ASU significantly decreased the rate of OA progression to 40% compared with 50% in the placebo group in one study.111 However, this study showed that ASU did not influence the rate of OA progression in the obese subset of patients with mean symptom duration 4 and BMI of 27 kg/m2.
However, excessive inflammation associated with obesity may also impede efficacy, as it does with celecoxib (NSAID) treatment, which is not as effective in obese patients (BMI in excess of 30 kg/m2).119 The influence of obesity, and how it influences ASU efficacy, may also depend on the parameters used to measure and define disease. In a study examining the relationship between BMI and OA in patients scheduled to undergo hip replacement, increasing BMI was associated with increasing levels of pain and functional disability, but not radiographic joint damage. Thus, obesity might influence some aspects of disease and treatment but not others. This should be taken into account when designing and assessing studies intended to examine the impact of obesity on treatment efficacy.119
ASU has anti-inflammatory effects in mice when administered in conjunction with the anti-parasitic drug Praziquantel, reducing inflammatory cytokines IFN-γ and IL-4, as well as granuloma size, while increasing cidal activity.117,118 ASU also protects gingival elastic fibers from degradation by human leukocyte elastase,120 hypodermatitis,121 and ischemic damage.122
A recent electronic database analysis demonstrated the benefits and harms of oral medicinal plant products in treating OA. The authors used standard methods for trial selection and data extraction, and they assessed the quality of the body of evidence using the GRADE approach for major outcomes such as pain, function, radiographic joint changes, quality of life, withdrawals due to adverse events, total adverse events, and serious adverse events. The ASU product Piasclidine formed a small and clinically questionable improvement in symptoms, compared with placebo after 3 to 12 months treatment. Radiographic joint changes, as change in joint space width (JSW), did not differ between ASU 300 mg treatment and placebo. Moderate-quality evidence from a single study confirmed possible benefits of ASU 600 mg over placebo. There is no evidence that Piasclidine significantly improves joint structure, and limited evidence that it prevents joint space narrowing. The authors suggest further investigations are required to determine optimum daily doses producing clinical benefits without adverse events.123
ASU is considered as drug in most countries and is therefore prescribed by physicians. However, in the United States it is classified as dietary supplement and can be purchased as over-the-counter supplements, Avoca ASU (ASU-NMX1000, Nutramax Laboratories Inc., Edgewood, MD) and Maximize ASU 300/SierraSil (Maximum International Inc., Pompano Beach, FL). Avoca ASU, a combination of ASU and glucosamine sulfate, has been shown to suppress TNF-α, IL-1β, COX2, iNOS, PGE2, NF-κB activation and nitrite production in articular chondrocytes and monocytes/macrophages, reducing pain and inflammation in OA patients.115,116,124 However, conflicting reports indicate the complete absence of specific ASU molecules in Avoca ASU when compared with Piascledine.75,125-127
Questions remain about the efficacy and safety of ASUs for treatment of OA (Table 4). Macaigne and colleagues published a case report in 2004 describing a female with lymphatic colitis associated with Piascledine treatment.128 Further prospective multicenter studies are warranted to investigate whether other microscopic colitis cases129 are observed in patients treated with Piascledine. Avoca ASU that contains glucosamine can induce allergic reaction in people with shellfish allergy. Even in very small quantities, these people may experience mild symptoms, such as hives or nasal congestion, or more severe, even life-threatening, symptoms.
Table 4.
Adverse Effects of Avocado Soybean Unsaponifiable.
| Organ | Side Effects | Frequencies | Drug Withdrawal | References |
|---|---|---|---|---|
| Skin | Eczema | 32.5% | 130 | |
| Hives | ||||
| Photosensitivity | ||||
| Hypersensitivity syndrome | ||||
| Liver | Liver injury | 16.2% | Return to normal | 130 |
| Bilirubin | ||||
| ALKP | ||||
| GGTP | ||||
| Gastrointestinal | Regurgitation | 12% | Return to normal | 106, 129, 130 |
| Heartburn | ||||
| Nausea | ||||
| Epigastric pain | ||||
| Dyspepsia | ||||
| Diarrhea | ||||
| Constipation | ||||
| Microscopic colitis | ||||
| Coagulation | Platelets | 6.8% | 130 |
ALKP = alkaline phosphatase; GGTP = gamma-glutamyl transpeptidase.
An alternative ASU formulation is Arthrocen (Pharmin, USA, LLC, San Jose, CA). Arthrocen is an extract from avocado and soybean oils that does not contain any ingredients of animal origin, artificial flavor, sweetener, preservative, or color. Each capsule contains 100 mg unsaponifiable persea gratissima unsaponifiable (avocado) and 200 mg unsaponifiable glycine max (soybean) extracts, silica, magnesium stearate (E470b—manufactured from vegetable oil), and gelatin fines.
In general, the FDA does not hold dietary supplements to the stringent standards of pharmaceutical manufacture. If ASU is to be widely used for the treatment of OA, serious consideration should be given to their current regulatory status in order to ensure potency, purity, and as well as the excipients. Many studies have demonstrated substantial variation between the content listed on the labels of these products and the actual content. The sterols content of ASU have been demonstrated to have biological activities in culture and in animal models. This approach allowed us to compare the contents of three commercial supplements (Fig. 2). We found multiple peaks were present in the Piascledine-300 (Expanscience) mass spectrometry analysis (Agilent 7890 GC System, 7693 Auto Sampler, Agilent VL MSD with triple Axis Detector, Brea, CA), compared to the Arthrocen 300 mg (PharminUSA) or ASU300-Avocado Soy Unsaponifiable with Sierra 600 mg (Maximize, Maximum Int.) preparations (Fig. 2, Table 3). Similar results were found for Piascledine-300,75 with mass spectra sterol content of C20H30O2, C20H28O2, sitosterol, stigmasterol, campesterol, squalene, β-tocopherol, desmethyl tocopherol, oleic acid docosane, α-amyrin, and cholesterol. In two letters to the editor, Msika et al.126 and Henroitin125 claimed that the exact ingredients and preparation of ASU-Expanscience was an intellectual proprietary, protected by patent. Msika further emphasized that the tocopherols, sterols, and patented specific molecules from avocado contribute to the originality of the product, different from natural avocado unsaponifiables. In contrast, they analyzed Dasuquin with MSM, Dasuquin, and Avoca ASU (Nutramax), and compared them with ASU Expanscience. The analyses revealed content of products were significantly different from those indicated on the Nutramax labels—with no citrostadienol, and brassicasterol present in ASU Expanscience. Contrary to that they found contents included high level of rapeseed oil and unsaponifiables products with very low tocopherol, and without respected ratio of 1:2 for avocado to soybean unsaponifiables. The original ASU Piascledine 300 pills contains 100 mg of avocado unsaponifiables and 200 mg of soybean unsaponifiables. The difference in sterol content is based on the A/S ratio and avocado-specific modified unsaponifiables obtained by a patented process. Henrotin et al. have shown ASU effects are best when the ASU ratio is 2:1.92 In support, Henroitin et al. recommended additional studies to ensure efficacy of Nutramax products.125 These issues and reported adverse effects of ASU (Table 4)128,130 raises concerns about the content and purity of ASU supplements on the market, with implications for patient safety.
Figure 2.
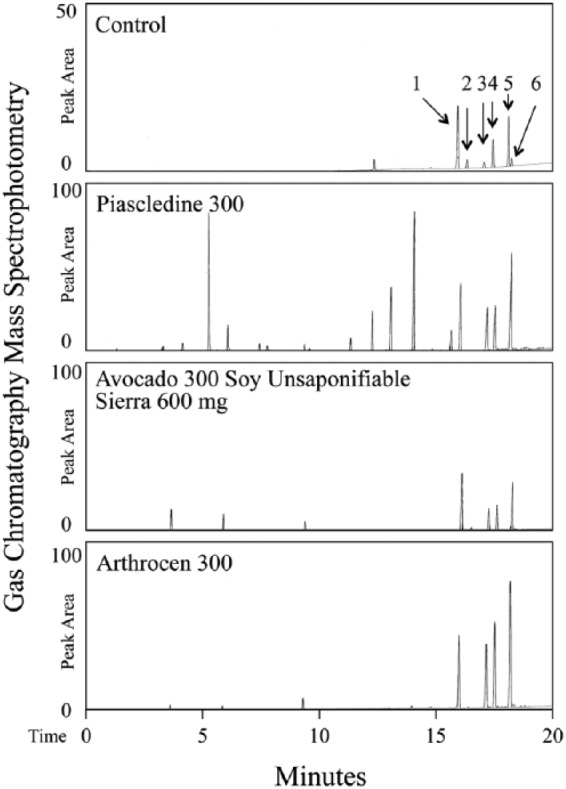
Gas chromatography–mass spectrometry analysis of major sterol components of Piascledine 300, Avocado 300 Soy Unsaponifiable with Sierra 600 mg, and Arthrocen 300. Control sample exhibited characteristic peaks corresponding to 1 = Dihydrocholesterol (5α-Cholestan-3β-ol; internal control; Sigma Aldrich), 2 = Brass (Brassicasterol), 3 = Camp (Campesterol), 4 = Stigmn (Stigmastanol), 5 = β-Sito (β-sitosterol), 6 = Stigma (Stigmasterol).
Table 3.
Content Analysis of Supplements Containing Avocado Soybean Unsaponifiable.
| Company/Manufacturer | Brand Name | Dosage Form | Other Ingredients | Excipients on the Label |
|---|---|---|---|---|
| Nuramax Laboratories, Inc. | Avoca ASU | Avocado/Soybean Unsaponifiables, non-shellfish glucosamine, NMX1000 | OptiMSM | |
| Methylsulfonylmeth | ||||
| Green tea extract | ||||
| Helseudsalg Faaborg Denmark | AvoSol | Avocado 100 mg/Soy 200 mg Unsaponifiables | Vitamin C 30 mg | Glucose syrup, ox gelatin, soy protein isolate, extract rich in tocopherol, silicon dioxide, magnesium salts from fatty acids |
| Dr. Theos Official, USA | Avosoy | Avocado-Soybean Unsaponifiables | Vitamin C 60 mg | Cellulose, dicalcium phosphate, sodium croscarmellose, silicon dioxide, gum acacia, vegetable stearic acid, film coating, magnesium stearate |
| Vitamin E 30 U | ||||
| Manganese 2 mg | ||||
| Dr. Theos Official, USA | AvosoyComplete | Avocado-Soybean Unsaponifiables 300 mg | Vitamin C 60 mg | |
| Glucosamine 1,500 mg | Vitamin E 30 U | |||
| Porcine chondroitin 800 mg | Manganese 2 mg | |||
| Swanson Health Products, Fargo, ND, USA | AvoVida | 100 mg Unsaponifiables Persea gratissima | 30% β-sitosterol, campesterol, stigmasterol | Soy protein isolate, mixed tocopherols, silica |
| Glycine max | Microcrystalline cellulose (plant fiber), gelatin, magnesium stearate | |||
| Pharmin, USA, LLC, Formulation Technology, USA | Arthrocen 300 mg | Avocado 100 mg/Soy 200 mg Unsaponifiables | Persea gratissima | Silica, magnesium stearate (E470b manufactured from vegetable oil), and gelatin fines |
| Glycine max | ||||
| Nuramax Laboratories, Inc. | Cosamin ASU | Avocado/Soybean Unsaponifiables | Glucosamine Sulfate | |
| Chondoitine Sulfate | ||||
| Maximum International, USA | Maximize | ASU300-Avocado Soy Unsaponifiables | Iron: 1.1 mg (from SierraSil) | Microcrystalline cellulose, maltodextrin, croscarmellose sodium, silicon dioxide, stearic acid, hydroxypropylm ethylcellulose, hydroxypropylcellulos, magnesium stearate, polyethylene glycol |
| SierraSil: 600 mg | Iron toxicity recommendation on the container | |||
| Expanscience Lab, Courbevoie, France; Pharmascience, Montreal, Canada; Pharma Inv., Chile SA, Santiago; Solvay Ins.; Biol. Chenioterapicosells; Microsules y Bernabo Siegfried, Rhein | Piascledine 300 | Avocado/Soybean Unsaponifiable | Not described | Butylated hydroxytoluene (BHT) 0.05 mg/capsule |
| Colloidal anhydrous silica |
Conclusion
Osteoarthritis inflicts pain and physical limitation on millions of people. Improving joint function and patient activity is a central public health concern to improve quality and length of life. The aim is not only to treat pain but also to prevent the onset of disease. There is no cure for OA, and even symptomatic treatment options are scarce, dominated by pain management and surgical intervention. ASUs may prove to be an effective treatment option for symptomatic OA, as they have been shown to possess chondroprotective, anabolic, and anticatabolic properties, as well as anti-inflammatory properties. At the clinical level, ASUs reduce pain and stiffness while improving joint function. Importantly, ASUs are a natural, slow-acting agent that do not merely address acute pain but actively prevent progression of OA symptoms. Further studies are required to determine the specific mechanisms and target molecules of ASU function on OA at the cellular and metabolic level.
Footnotes
Acknowledgement and Funding: We are grateful to Susan Eastman, health librarian at Stanford Hospital Health Library. We are grateful to many authors for their generous contributions while writing this review. We also apologize to authors of many relevant articles whose works are not cited due to space constraints. Weacknowledge PL for providing us photos and X-ray images of his hands and knees. Dr. Christiansen is funded by the National Institute of Arthritis and Musculoskeletal and Skin Diseases, part of the National Institutes of Health, under Award Number AR062603, and by the Department of Defense–Congressionally Directed Medical Research Programs, under Award Number PR110178.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SimritBhatti and Dr. Ramin Goudarzi are employees of Formulation Technology Inc. and Pharmin USA, LLC, respectively. However, neither Formulation Technology nor Pharmin USA contributed funds or resources to this study or to the coauthors.
Ethical Approval: This review does not include previously unpublished research involving human subjects, and therefore no institutional approval was necessary. Images in Figure 1 were provided directly by PL, and are used with his full knowledge and approval.
References
- 1. Li Y, Wei X, Zhou J, Wei L. The age-related changes in cartilage and osteoarthritis. Biomed Res Int. 2013;2013:916530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115-26. [DOI] [PubMed] [Google Scholar]
- 3. Loeser RF. Aging processes and the development of osteoarthritis. Curr Opin Rheumatol. 2013;25(1):108-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Houard X, Goldring MB, Berenbaum F. Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr Rheumatol Rep. 2013;15(11):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lequesne M, Brandt K, Bellamy N, Moskowitz R, Menkes CJ, Pelletier JP, et al. Guidelines for testing slow acting drugs in osteoarthritis. J Rheumatol Suppl. 1994;41:65-71. [PubMed] [Google Scholar]
- 6. Jordan KM, Arden NK, Doherty M, Bannwarth B, Bijlsma JW, Dieppe P, et al. EULAR Recommendations 2003: an evidence based approach to the management of knee osteoarthritis: report of a Task Force of the Standing Committee for International Clinical Studies Including Therapeutic Trials (ESCISIT). Ann Rheum Dis. 2003;62(12):1145-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhang W, Doherty M, Arden N, Bannwarth B, Bijlsma J, Gunther KP, et al. EULAR evidence based recommendations for the management of hip osteoarthritis: report of a task force of the EULAR Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2005;64(5):669-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, part II: OARSI evidence-based, expert consensus guidelines. Osteoarthritis Cartilage. 2008;16(2):137-62. [DOI] [PubMed] [Google Scholar]
- 9. Richette P. Pharmacological therapies for osteoarthritis. Therapie. 2011;66(5):383-90. [DOI] [PubMed] [Google Scholar]
- 10. Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken). 2012;64(4):465-74. [DOI] [PubMed] [Google Scholar]
- 11. Hame SL, Alexander RA. Knee osteoarthritis in women. Curr Rev Musculoskelet Med. 2013;6(2):182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Curatolo M, Bogduk N. Pharmacologic pain treatment of musculoskeletal disorders: current perspectives and future prospects. Clin J Pain. 2001;17(1):25-32. [DOI] [PubMed] [Google Scholar]
- 13. Gallelli L, Galasso O, Falcone D, Southworth S, Greco M, Ventura V, et al. The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial. Osteoarthritis Cartilage. 2013;21(9):1400-8. [DOI] [PubMed] [Google Scholar]
- 14. Bello AE. DUEXIS® (ibuprofen 800 mg, famotidine 26.6 mg): a new approach to gastroprotection for patients with chronic pain and inflammation who require treatment with a nonsteroidal anti-inflammatory drug. Ther Adv Musculoskelet Dis. 2012;4(5):327-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sands GH, Brown PB, Essex MN. The efficacy of continuous versus intermittent celecoxib treatment in osteoarthritis patients with body mass index ≥30 and <30 kg/m2. Open Rheumatol J. 2013;7:32-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hauk L. Treatment of knee osteoarthritis: a clinical practice guideline from the AAOS. Am Fam Physician. 2014;89(11):918-20. [PubMed] [Google Scholar]
- 17. Wise J. NICE keeps paracetamol in UK guidelines on osteoarthritis. BMJ. 2014;348:g1545. [DOI] [PubMed] [Google Scholar]
- 18. McAlindon TE, Bannuru RR, Sullivan MC, Arden NK, Berenbaum F, Bierma-Zeinstra SM, et al. OARSI guidelines for the non-surgical management of knee osteoarthritis. Osteoarthritis Cartilage. 2014;22(3):363-88. [DOI] [PubMed] [Google Scholar]
- 19. Balmaceda CM. Evolving guidelines in the use of topical nonsteroidal anti-inflammatory drugs in the treatment of osteoarthritis. BMC Musculoskelet Disord. 2014;15:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brosseau L, Rahman P, Toupin-April K, Poitras S, King J, De Angelis G, et al. A systematic critical appraisal for non-pharmacological management of osteoarthritis using the appraisal of guidelines research and evaluation II instrument. PLoS One. 2014;9(1):e82986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mobasheri A. The future of osteoarthritis therapeutics: emerging biological therapy. Curr Rheumatol Rep. 2013;15(12):385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Abraham CL, Maas SA, Weiss JA, Ellis BJ, Peters CL, Anderson AE. A new discrete element analysis method for predicting hip joint contact stresses. J Biomech. 2013;46(6):1121-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dougados M, Nguyen M, Berdah L, Mazieres B, Vignon E, Lequesne M. Evaluation of the structure-modifying effects of diacerein in hip osteoarthritis: ECHODIAH, a three-year, placebo-controlled trial. Evaluation of the chondromodulating effect of diacerein in OA of the hip. Arthritis Rheum. 2001;44(11):2539-47. [DOI] [PubMed] [Google Scholar]
- 24. Henrotin Y, Sanchez C, Balligand M. Pharmaceutical and nutraceutical management of canine osteoarthritis: present and future perspectives. Vet J. 2005;170(1):113-23. [DOI] [PubMed] [Google Scholar]
- 25. Yang KG, Raijmakers NJ, van Arkel ER, Caron JJ, Rijk PC, Willems WJ, et al. Autologous interleukin-1 receptor antagonist improves function and symptoms in osteoarthritis when compared to placebo in a prospective randomized controlled trial. Osteoarthritis Cartilage. 2008;16(4):498-505. [DOI] [PubMed] [Google Scholar]
- 26. Baltzer AW, Ostapczuk MS, Stosch D, Seidel F, Granrath M. A new treatment for hip osteoarthritis: clinical evidence for the efficacy of autologous conditioned serum. Orthop Rev (Pavia). 2013;5(2):59-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Smelter E, Hochberg MC. New treatments for osteoarthritis. Curr Opin Rheumatol. 2013;25(3):310-6. [DOI] [PubMed] [Google Scholar]
- 28. Lane NE, Schnitzer TJ, Birbara CA, Mokhtarani M, Shelton DL, Smith MD, et al. Tanezumab for the treatment of pain from osteoarthritis of the knee. N Engl J Med. 2010;363(16):1521-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chevalier X, Eymard F, Richette P. Biologic agents in osteoarthritis: hopes and disappointments. Nat Rev Rheumatol. 2013;9(7):400-10. [DOI] [PubMed] [Google Scholar]
- 30. Brown MT, Murphy FT, Radin DM, Davignon I, Smith MD, West CR. Tanezumab reduces osteoarthritic hip pain: results of a randomized, double-blind, placebo-controlled phase III trial. Arthritis Rheum. 2013;65(7):1795-803. [DOI] [PubMed] [Google Scholar]
- 31. Spierings EL, Fidelholtz J, Wolfram G, Smith MD, Brown MT, West CR. A phase III placebo- and oxycodone-controlled study of tanezumab in adults with osteoarthritis pain of the hip or knee. Pain. 2013;154(9):1603-12. [DOI] [PubMed] [Google Scholar]
- 32. Seidel MF, Wise BL, Lane NE. Nerve growth factor: an update on the science and therapy. Osteoarthritis Cartilage. 2013;21(9):1223-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sanga P, Katz N, Polverejan E, Wang S, Kelly KM, Haeussler J, et al. Efficacy, safety, and tolerability of fulranumab, an anti-nerve growth factor antibody, in the treatment of patients with moderate to severe osteoarthritis pain. Pain. 2013;154(10):1910-9. [DOI] [PubMed] [Google Scholar]
- 34. Teich N. Topical application of TNF-blockers. Dtsch Med Wochenschr. 2013;138(8):381-5. [DOI] [PubMed] [Google Scholar]
- 35. Pelletier JP, Roubille C, Raynauld JP, Abram F, Dorais M, Delorme P, et al. Disease-modifying effect of strontium ranelate in a subset of patients from the phase III knee osteoarthritis study SEKOIA using quantitative MRI: reduction in bone marrow lesions protects against cartilage loss. Ann Rheum Dis. Epub 2013. December 2. [DOI] [PubMed] [Google Scholar]
- 36. Bruyere O, Reginster JY, Bellamy N, Chapurlat R, Richette P, Cooper C. Clinically meaningful effect of strontium ranelate on symptoms in knee osteoarthritis: a responder analysis. Rheumatology (Oxford). 2014;53(8):1457-64. [DOI] [PubMed] [Google Scholar]
- 37. Tenti S, Cheleschi S, Guidelli GM, Galeazzi M, Fioravanti A. What about strontium ranelate in osteoarthritis? Doubts and securities. Mod Rheumatol. Epub 2014. March 19. [DOI] [PubMed] [Google Scholar]
- 38. Carbone LD, Nevitt MC, Wildy K, Barrow KD, Harris F, Felson D, et al. The relationship of antiresorptive drug use to structural findings and symptoms of knee osteoarthritis. Arthritis Rheum. 2004;50(11):3516-25. [DOI] [PubMed] [Google Scholar]
- 39. Spector TD, Conaghan PG, Buckland-Wright JC, Garnero P, Cline GA, Beary JF, et al. Effect of risedronate on joint structure and symptoms of knee osteoarthritis: results of the BRISK randomized, controlled trial [ISRCTN01928173]. Arthritis Res Ther. 2005;7(3):R625-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Buckland-Wright JC, Messent EA, Bingham CO, 3rd, Ward RJ, Tonkin C. A 2 yr longitudinal radiographic study examining the effect of a bisphosphonate (risedronate) upon subchondral bone loss in osteoarthritic knee patients. Rheumatology. 2007;46(2):257-64. [DOI] [PubMed] [Google Scholar]
- 41. Nishii T, Tamura S, Shiomi T, Yoshikawa H, Sugano N. Alendronate treatment for hip osteoarthritis: prospective randomized 2-year trial. Clin Rheumatol. 2013;32(12):1759-66. [DOI] [PubMed] [Google Scholar]
- 42. Chappell AS, Desaiah D, Liu-Seifert H, Zhang S, Skljarevski V, Belenkov Y, et al. A double-blind, randomized, placebo-controlled study of the efficacy and safety of duloxetine for the treatment of chronic pain due to osteoarthritis of the knee. Pain Pract. 2011;11(1):33-41. [DOI] [PubMed] [Google Scholar]
- 43. Wenham CY, Grainger AJ, Hensor EM, Caperon AR, Ash ZR, Conaghan PG. Methotrexate for pain relief in knee osteoarthritis: an open-label study. Rheumatology (Oxford). 2013;52(5):888-92. [DOI] [PubMed] [Google Scholar]
- 44. Johnson K, Zhu S, Tremblay MS, Payette JN, Wang J, Bouchez LC, et al. A stem cell-based approach to cartilage repair. Science. 2012;336(6082):717-21. [DOI] [PubMed] [Google Scholar]
- 45. Zhu Y, Yuan M, Meng HY, Wang AY, Guo QY, Wang Y, et al. Basic science and clinical application of platelet-rich plasma for cartilage defects and osteoarthritis: a review. Osteoarthritis Cartilage. 2013;21(11):1627-37. [DOI] [PubMed] [Google Scholar]
- 46. Andia I, Maffulli N. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nat Rev Rheumatol. 2013;9(12):721-30. [DOI] [PubMed] [Google Scholar]
- 47. Chou CL, Lee SH, Lu SY, Tsai KL, Ho CY, Lai HC. Therapeutic effects of intra-articular botulinum neurotoxin in advanced knee osteoarthritis. J Chin Med Assoc. 2010;73(11):573-80. [DOI] [PubMed] [Google Scholar]
- 48. Madry H, Cucchiarini M. Advances and challenges in gene-based approaches for osteoarthritis. J Gene Med. 2013;15(10):343-55. [DOI] [PubMed] [Google Scholar]
- 49. Jorgensen C. ADIPOA: cell therapy with stromal adipocytes cells. Rev Med Interne. 2011;32(Suppl 2):S203. [DOI] [PubMed] [Google Scholar]
- 50. Shen W, Chen J, Zhu T, Chen L, Zhang W, Fang Z, et al. Intra-articular injection of human meniscus stem/progenitor cells promotes meniscus regeneration and ameliorates osteoarthritis through stromal cell-derived factor-1/CXCR4-mediated homing. Stem Cells Transl Med. 2014;3(3):387-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Emadedin M, Aghdami N, Taghiyar L, Fazeli R, Moghadasali R, Jahangir S, et al. Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med. 2012;15(7):422-8. [PubMed] [Google Scholar]
- 52. Sato M, Uchida K, Nakajima H, Miyazaki T, Guerrero AR, Watanabe S, et al. Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 2012;14(1):R31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Koh YG, Choi YJ. Infrapatellar fat pad-derived mesenchymal stem cell therapy for knee osteoarthritis. Knee. 2012;19(6):902-7. [DOI] [PubMed] [Google Scholar]
- 54. Shen W, Chen J, Zhu T, Yin Z, Chen X, Chen L, et al. Osteoarthritis prevention through meniscal regeneration induced by intra-articular injection of meniscus stem cells. Stem Cells Dev. 2013;22(14):2071-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Richette P. Management of osteoarthritis: oral therapies. Rev Prat. 2012;62(5):654-60. [PubMed] [Google Scholar]
- 56. Reginster JY, Gillot V, Bruyere O, Henrotin Y. Evidence of nutriceutical effectiveness in the treatment of osteoarthritis. Curr Rheumatol Rep. 2000;2(6):472-7. [DOI] [PubMed] [Google Scholar]
- 57. Hochberg M, Chevalier X, Henrotin Y, Hunter DJ, Uebelhart D. Symptom and structure modification in osteoarthritis with pharmaceutical-grade chondroitin sulfate: what’s the evidence? Curr Med Res Opin. 2013;29(3):259-67. [DOI] [PubMed] [Google Scholar]
- 58. Hui AY, McCarty WJ, Masuda K, Firestein GS, Sah RL. A systems biology approach to synovial joint lubrication in health, injury, and disease. Wiley Interdiscip Rev Syst Biol Med. 2012;4(1):15-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357(9252):251-6. [DOI] [PubMed] [Google Scholar]
- 60. Pavelka K, Gatterova J, Olejarova M, Machacek S, Giacovelli G, Rovati LC. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2002;162(18):2113-23. [DOI] [PubMed] [Google Scholar]
- 61. Clegg DO, Reda DJ, Harris CL, Klein MA, O’Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795-808. [DOI] [PubMed] [Google Scholar]
- 62. Henrotin Y, Mobasheri A, Marty M. Is there any scientific evidence for the use of glucosamine in the management of human osteoarthritis? Arthritis Res Ther. 2012;14(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Altman RD, Abramson S, Bruyere O, Clegg D, Herrero-Beaumont G, Maheu E, et al. Commentary: osteoarthritis of the knee and glucosamine. Osteoarthritis Cartilage. 2006;14(10):963-6. [DOI] [PubMed] [Google Scholar]
- 64. Henrotin Y, Chevalier X, Herrero-Beaumont G, McAlindon T, Mobasheri A, Pavelka K, et al. Physiological effects of oral glucosamine on joint health: current status and consensus on future research priorities. BMC Res Notes. 2013;6:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Henrotin Y, Lambert C. Chondroitin and glucosamine in the management of osteoarthritis: an update. Curr Rheumatol Rep. 2013;15(10):361. [DOI] [PubMed] [Google Scholar]
- 66. Rutjes AW, Juni P, da Costa BR, Trelle S, Nuesch E, Reichenbach S. Viscosupplementation for osteoarthritis of the knee: a systematic review and meta-analysis. Ann Intern Med. 2012;157(3):180-91. [DOI] [PubMed] [Google Scholar]
- 67. Henrotin Y, Chevalier X, Deberg M, Balblanc JC, Richette P, Mulleman D, et al. Early decrease of serum biomarkers of type II collagen degradation (Coll2-1) and joint inflammation (Coll2-1 NO(2)) by hyaluronic acid intra-articular injections in patients with knee osteoarthritis: a research study part of the Biovisco study. J Orthop Res. 2013;31(6):901-7. [DOI] [PubMed] [Google Scholar]
- 68. Waddell DD, Beyer A, Thompson TL, Morawiak J, Elkins C, Rosenberg A, et al. No conclusive evidence that histologically found granulomas and acute local reactions following hylan G-F 20 injections are related or have clinical significance. J Knee Surg. 2014;27(2):99-104. [DOI] [PubMed] [Google Scholar]
- 69. Sawitzke AD, Shi H, Finco MF, Dunlop DD, Harris CL, Singer NG, et al. Clinical efficacy and safety of glucosamine, chondroitin sulphate, their combination, celecoxib or placebo taken to treat osteoarthritis of the knee: 2-year results from GAIT. Ann Rheum Dis. 2010;69(8):1459-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Sawitzke AD, Shi H, Finco MF, Dunlop DD, Bingham CO, 3rd, Harris CL, et al. The effect of glucosamine and/or chondroitin sulfate on the progression of knee osteoarthritis: a report from the glucosamine/chondroitin arthritis intervention trial. Arthritis Rheum. 2008;58(10):3183-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. The NIH Glucosamine/Chondroitin Arthritis Intervention Trial (GAIT). J Pain Palliat Care Pharmacother. 2008;22(1):39-43. [DOI] [PubMed] [Google Scholar]
- 72. Miller KL, Clegg DO. Glucosamine and chondroitin sulfate. Rheum Dis Clin North Am. 2011;37(1):103-18. [DOI] [PubMed] [Google Scholar]
- 73. Wandel S, Juni P, Tendal B, Nuesch E, Villiger PM, Welton NJ, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Miller MJ, Mehta K, Kunte S, Raut V, Gala J, Dhumale R, et al. Early relief of osteoarthritis symptoms with a natural mineral supplement and a herbomineral combination: a randomized controlled trial [ISRCTN38432711]. J Inflamm (Lond). 2005;2:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lippiello L, Nardo JV, Harlan R, Chiou T. Metabolic effects of avocado/soy unsaponifiables on articular chondrocytes. Evid Based Complement Alternat Med. 2008;5(2):191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Thiers MH. Unsaponifiable constituents of avocado and soya oils. Treatment of certain forms of arthralgia. J Med Lyon. 1972;53(222):195-8. [PubMed] [Google Scholar]
- 77. Mauviel A, Loyau G, Pujol JP. Effect of unsaponifiable extracts of avocado and soybean (Piascledine) on the collagenolytic action of cultures of human rheumatoid synoviocytes and rabbit articular chondrocytes treated with interleukin-1. Rev Rhum Mal Osteoartic. 1991;58(4):241-5. [PubMed] [Google Scholar]
- 78. Cake MA, Read RA, Guillou B, Ghosh P. Modification of articular cartilage and subchondral bone pathology in an ovine meniscectomy model of osteoarthritis by avocado and soya unsaponifiables (ASU). Osteoarthritis Cartilage. 2000;8(6):404-11. [DOI] [PubMed] [Google Scholar]
- 79. Kut-Lasserre C, Miller CC, Ejeil AL, Gogly B, Dridi M, Piccardi N, et al. Effect of avocado and soybean unsaponifiables on gelatinase A (MMP-2), stromelysin 1 (MMP-3), and tissue inhibitors of matrix metalloproteinase (TIMP- 1 and TIMP-2) secretion by human fibroblasts in culture. J Periodontol. 2001;72(12):1685-94. [DOI] [PubMed] [Google Scholar]
- 80. Lequesne M, Maheu E, Cadet C, Dreiser RL. Structural effect of avocado/soybean unsaponifiables on joint space loss in osteoarthritis of the hip. Arthritis Rheum. 2002;47(1):50-8. [DOI] [PubMed] [Google Scholar]
- 81. Kucharz EJ. Application of avocado/soybean unsaponifiable mixtures (piascledine) in treatment of patients with osteoarthritis. Ortop Traumatol Rehabil. 2003;5(2):248-51. [PubMed] [Google Scholar]
- 82. Ernst E. Avocado-soybean unsaponifiables (ASU) for osteoarthritis—a systematic review. Clin Rheumatol. 2003;22(4-5):285-8. [DOI] [PubMed] [Google Scholar]
- 83. Angermann P. Avocado/soybean unsaponifiables in the treatment of knee and hip osteoarthritis. Ugeskr Laeger. 2005;167(33):3023-5. [PubMed] [Google Scholar]
- 84. Henrotin YE, Deberg MA, Crielaard JM, Piccardi N, Msika P, Sanchez C. Avocado/soybean unsaponifiables prevent the inhibitory effect of osteoarthritic subchondral osteoblasts on aggrecan and type II collagen synthesis by chondrocytes. J Rheumatol. 2006;33(8):1668-78. [PubMed] [Google Scholar]
- 85. Kawcak CE, Frisbie DD, McIlwraith CW, Werpy NM, Park RD. Evaluation of avocado and soybean unsaponifiable extracts for treatment of horses with experimentally induced osteoarthritis. Am J Vet Res. 2007;68(6):598-604. [DOI] [PubMed] [Google Scholar]
- 86. Gabay O, Gosset M, Levy A, Salvat C, Sanchez C, Pigenet A, et al. Stress-induced signaling pathways in hyalin chondrocytes: inhibition by avocado-soybean unsaponifiables (ASU). Osteoarthritis Cartilage. 2008;16(3):373-84. [DOI] [PubMed] [Google Scholar]
- 87. Christensen R, Bartels EM, Astrup A, Bliddal H. Symptomatic efficacy of avocado-soybean unsaponifiables (ASU) in osteoarthritis (OA) patients: a meta-analysis of randomized controlled trials. Osteoarthritis Cartilage. 2008;16(4):399-408. [DOI] [PubMed] [Google Scholar]
- 88. Boileau C, Martel-Pelletier J, Caron J, Msika P, Guillou GB, Baudouin C, et al. Protective effects of total fraction of avocado/soybean unsaponifiables on the structural changes in experimental dog osteoarthritis: inhibition of nitric oxide synthase and matrix metalloproteinase-13. Arthritis Res Ther. 2009;11(2):R41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Dinubile NA. A potential role for avocado- and soybean-based nutritional supplements in the management of osteoarthritis: a review. Phys Sportsmed. 2010;38(2):71-81. [DOI] [PubMed] [Google Scholar]
- 90. Altinel L, Sahin O, Kose KC, Bas O, Ozen OA, Saritas ZK, et al. Healing of osteochondral defects in canine knee with avocado/soybean unsaponifiables: a morphometric comparative analysis. Eklem Hastalik Cerrahisi. 2011;22(1):48-53. [PubMed] [Google Scholar]
- 91. Lamaud E, Robert AM, Wepierre J. Biochemical effects of unsaponifiable lipidic components of avocado and soya bean administered percutaneously on the connective tissue components of hairless rat skin. Int J Cosmet Sci. 1979;1(4):213-9. [DOI] [PubMed] [Google Scholar]
- 92. Henrotin YE, Labasse AH, Jaspar JM, De Groote DD, Zheng SX, Guillou GB, et al. Effects of three avocado/soybean unsaponifiable mixtures on metalloproteinases, cytokines and prostaglandin E2 production by human articular chondrocytes. Clin Rheumatol. 1998;17(1):31-9. [DOI] [PubMed] [Google Scholar]
- 93. Mauviel A, Daireaux M, Hartmann DJ, Galera P, Loyau G, Pujol JP. Effects of unsaponifiable extracts of avocado/soy beans (PIAS) on the production of collagen by cultures of synoviocytes, articular chondrocytes and skin fibroblasts. Rev Rhum Mal Osteoartic. 1989;56(2):207-11. [PubMed] [Google Scholar]
- 94. Henrotin YE, Sanchez C, Deberg MA, Piccardi N, Guillou GB, Msika P, et al. Avocado/soybean unsaponifiables increase aggrecan synthesis and reduce catabolic and proinflammatory mediator production by human osteoarthritic chondrocytes. J Rheumatol. 2003;30(8):1825-34. [PubMed] [Google Scholar]
- 95. Ownby SL, Fortuno LV, Au AY, Grzanna MW, Rashmir-Raven AM, Frondoza CG. Expression of pro-inflammatory mediators is inhibited by an avocado/soybean unsaponifiables and epigallocatechin gallate combination. J Inflamm (Lond). 2014;11(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Khayyal MT, el-Ghazaly MA. The possible “chondroprotective” effect of the unsaponifiable constituents of avocado and soya in vivo. Drugs Exp Clin Res. 1998;24(1):41-50. [PubMed] [Google Scholar]
- 97. Cinelli M, Guiducci S, Del Rosso A, Pignone A, Del Rosso M, Fibbi G, et al. Piascledine modulates the production of VEGF and TIMP-1 and reduces the invasiveness of rheumatoid arthritis synoviocytes. Scand J Rheumatol. 2006;35(5):346-50. [DOI] [PubMed] [Google Scholar]
- 98. Boumediene K, Felisaz N, Bogdanowicz P, Galera P, Guillou GB, Pujol JP. Avocado/soya unsaponifiables enhance the expression of transforming growth factor beta1 and beta2 in cultured articular chondrocytes. Arthritis Rheum. 1999;42(1):148-56. [DOI] [PubMed] [Google Scholar]
- 99. Belcher C, Fawthrop F, Bunning R, Doherty M. Plasminogen activators and their inhibitors in synovial fluids from normal, osteoarthritis, and rheumatoid arthritis knees. Ann Rheum Dis. 1996;55(4):230-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Altinel L, Saritas ZK, Kose KC, Pamuk K, Aksoy Y, Serteser M. Treatment with unsaponifiable extracts of avocado and soybean increases TGF-beta1 and TGF-beta2 levels in canine joint fluid. Tohoku J Exp Med. 2007;211(2):181-6. [DOI] [PubMed] [Google Scholar]
- 101. de Oliveira GJ, de Paula LG, Spin-Neto R, Stavropoulos A, Spolidorio LC, Marcantonio E, Jr, et al. Effect of avocado/soybean unsaponifiables on osseointegration: a proof-of-principle preclinical in vivo study. Int J Oral Maxillofac Implants. 2014;29(4):949-57. [DOI] [PubMed] [Google Scholar]
- 102. Chevallier F, Lutton C, Sulpice JC, D’Hollander F. Influence of the daily ingestion of a total unsaponifiable extract from avocado and soy bean oils on cholesterol metabolism in the rat. Pathol Biol (Paris). 1975;23(3):225-30. [PubMed] [Google Scholar]
- 103. Zushi S, Akagi M, Kishimoto H, Teramura T, Sawamura T, Hamanishi C. Induction of bovine articular chondrocyte senescence with oxidized low-density lipoprotein through lectin-like oxidized low-density lipoprotein receptor 1. Arthritis Rheum. 2009;60(10):3007-16. [DOI] [PubMed] [Google Scholar]
- 104. Kishimoto H, Akagi M, Zushi S, Teramura T, Onodera Y, Sawamura T, et al. Induction of hypertrophic chondrocyte-like phenotypes by oxidized LDL in cultured bovine articular chondrocytes through increase in oxidative stress. Osteoarthritis Cartilage. 2010;18(10):1284-90. [DOI] [PubMed] [Google Scholar]
- 105. Zborovskii AB, Akhverdian Iu R, Sivordova LE, Simakova ES, Zavodovskii BV. Efficiency of unsaponifiable compounds of soya beans and avocado in health care personnel with osteoarthritis in Volgograd. Med Tr Prom Ekol. 2013;(2):41-4. [PubMed] [Google Scholar]
- 106. Blotman F, Maheu E, Wulwik A, Caspard H, Lopez A. Efficacy and safety of avocado/soybean unsaponifiables in the treatment of symptomatic osteoarthritis of the knee and hip. A prospective, multicenter, three-month, randomized, double-blind, placebo-controlled trial. Rev Rhum Engl Ed. 1997;64(12):825-34. [PubMed] [Google Scholar]
- 107. Appelboom T, Schuermans J, Verbruggen G, Henrotin Y, Reginster JY. Symptoms modifying effect of avocado/soybean unsaponifiables (ASU) in knee osteoarthritis. A double blind, prospective, placebo-controlled study. Scand J Rheumatol. 2001;30(4):242-7. [DOI] [PubMed] [Google Scholar]
- 108. Maheu E, Mazieres B, Valat JP, Loyau G, Le Loet X, Bourgeois P, et al. Symptomatic efficacy of avocado/soybean unsaponifiables in the treatment of osteoarthritis of the knee and hip: a prospective, randomized, double-blind, placebo-controlled, multicenter clinical trial with a six-month treatment period and a two-month follow-up demonstrating a persistent effect. Arthritis Rheum. 1998;41(1):81-91. [DOI] [PubMed] [Google Scholar]
- 109. Ameye LG, Chee WS. Osteoarthritis and nutrition. From nutraceuticals to functional foods: a systematic review of the scientific evidence. Arthritis Res Ther. 2006;8(4):R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pavelka K, Coste P, Geher P, Krejci G. Efficacy and safety of piascledine 300 versus chondroitin sulfate in a 6 months treatment plus 2 months observation in patients with osteoarthritis of the knee. Clin Rheumatol. 2010;29(6):659-70. [DOI] [PubMed] [Google Scholar]
- 111. Maheu E, Cadet C, Marty M, Moyse D, Kerloch I, Coste P, et al. Randomised, controlled trial of avocado-soybean unsaponifiable (Piascledine) effect on structure modification in hip osteoarthritis: the ERADIAS study. Ann Rheum Dis. 2014;73(2):376-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Shimizu C, Coutts RD, Healey RM, Kubo T, Hirasawa Y, Amiel D. Method of histomorphometric assessment of glycosaminoglycans in articular cartilage. J Orthop Res. 1997;15(5):670-4. [DOI] [PubMed] [Google Scholar]
- 113. Merkulova DM, Onsin AA, Merkulov YA. Piascledine in the treatment of chronic dorsalgia. Zh Nevrol Psikhiatr Im S S Korsakova. 2013;113:18-22. [PubMed] [Google Scholar]
- 114. Martinez-Abundis E, Gonzalez-Ortiz M, Mercado-Sesma AR, Reynoso-von-Drateln C, Moreno-Andrade A. Effect of avocado soybean unsaponifiables on insulin secretion and insulin sensitivity in patients with obesity. Obes Facts. 2013;6(5):443-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Heinecke LF, Grzanna MW, Au AY, Mochal CA, Rashmir-Raven A, Frondoza CG. Inhibition of cyclooxygenase-2 expression and prostaglandin E2 production in chondrocytes by avocado soybean unsaponifiables and epigallocatechin gallate. Osteoarthritis Cartilage. 2010;18(2):220-7. [DOI] [PubMed] [Google Scholar]
- 116. Au RY, Al-Talib TK, Au AY, Phan PV, Frondoza CG. Avocado soybean unsaponifiables (ASU) suppress TNF-alpha, IL-1beta, COX-2, iNOS gene expression, and prostaglandin E2 and nitric oxide production in articular chondrocytes and monocyte/macrophages. Osteoarthritis Cartilage. 2007;15(11):1249-55. [DOI] [PubMed] [Google Scholar]
- 117. Soliman MF. Evaluation of avocado/soybean unsaponifiable alone or concurrently with praziquantel in murine schistosomiasis. Acta Trop. 2012;122(3):261-6. [DOI] [PubMed] [Google Scholar]
- 118. Ndjonka D, Rapado LN, Silber AM, Liebau E, Wrenger C. Natural products as a source for treating neglected parasitic diseases. Int J Mol Sci. 2013;14(2):3395-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lubbeke A, Duc S, Garavaglia G, Finckh A, Hoffmeyer P. BMI and severity of clinical and radiographic signs of hip osteoarthritis. Obesity (Silver Spring). 2009;17(7):1414-9. [DOI] [PubMed] [Google Scholar]
- 120. Kut C, Assoumou A, Dridi M, Bonnefoix M, Gogly B, Pellat B, et al. Morphometric analysis of human gingival elastic fibres degradation by human leukocyte elastase protective effect of avocado and soybean unsaponifiables (ASU). Pathol Biol (Paris). 1998;46(7):571-6. [PubMed] [Google Scholar]
- 121. Chaze J. Treatment of hypodermatitis of the leg with unsaponifiable extracts of avocado and soya. Phlebologie. 1972;25(3):315-8. [PubMed] [Google Scholar]
- 122. Yaman M, Eser O, Cosar M, Bas O, Sahin O, Mollaoglu H, et al. Oral administration of avocado soybean unsaponifiables (ASU) reduces ischemic damage in the rat hippocampus. Arch Med Res. 2007;38(5):489-94. [DOI] [PubMed] [Google Scholar]
- 123. Cameron M, Chrubasik S. Oral herbal therapies for treating osteoarthritis. Cochrane Database Syst Rev. 2014;(5):CD002947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Frondoza CG, Heinecke LF, Grzanna MW, Au AY, Ownby SL. Modulation of cytokine-induced prostaglandin E(2) production in cultures of articular chondrocytes obtained from carpal joints of camels (Camelus dromedarius). Am J Vet Res. 2011;72(1):51-8. [DOI] [PubMed] [Google Scholar]
- 125. Henrotin Y. Avocado/soybean unsaponifiable (ASU) to treat osteoarthritis: a clarification. Osteoarthritis Cartilage. 2008;16(9):1118-9. [DOI] [PubMed] [Google Scholar]
- 126. Msika P, Baudouin C, Saunois A, Bauer T. Avocado/soybean unsaponifiables, ASU EXPANSCIENCE, are strictly different from the nutraceutical products claiming ASU appellation. Osteoarthritis Cartilage. 2008;16(10):1275-6. [DOI] [PubMed] [Google Scholar]
- 127. Frondoza CG. Response to letter to editor entitled: “Avocado/soybean unsaponifiables, ASU Expanscience, are strictly different from the nutraceutical products claiming ASU appellation” (4365). Osteoarthritis Cartilage. 2008;16:1590-1. [DOI] [PubMed] [Google Scholar]
- 128. Macaigne G, Ozon N, Dikov D, Auriault ML, Deplus R. Piascledine-associated lymphocytic colitis. Gastroenterol Clin Biol. 2004;28(4):412-3. [DOI] [PubMed] [Google Scholar]
- 129. Macaigne G, Lahmek P, Locher C, Lesgourgues B, Costes L, Nicolas MP, et al. Microscopic colitis or functional bowel disease with diarrhea: a French prospective multicenter study. Am J Gastroenterol. 2014;109(9):1461-70. [DOI] [PubMed] [Google Scholar]
- 130. Olivier P, Montastruc JL. Post-marketing safety profile of avocado-soybean unsaponifiables. Presse Med. 2010;39(10):e211-6. [DOI] [PubMed] [Google Scholar]


