Abstract
Regulatory T cells (Tregs) are generated to antigens (Ag) found in the retina. Some Tregs are the result of ectopic expression of the retinal Ags in the thymus, where developing T cells are committed to enter the regulatory lineage. However, the generation of retinal Ag-specific Tregs independent of the thymus was uncertain. Our studies show that Tregs can be generated from mature, peripheral T cells based on exposure to retinal Ags. These peripherally induced Tregs limited immune responses and experimental autoimmune disease induced by retinal Ags and thus constitute a crucial component of retinal immune privilege.
Keywords: Regulatory T cells, immune privilege, retina
Introduction
The proper function of the mammalian immune system requires a balance between effector responses that are sufficient to contain and clear pathogenic microorganisms and provide tumor immunity while avoiding excessive or aberrant immune responses that could result in unwanted tissue damage, allergic responses, or autoimmune disease. While the immune system employs a myriad of mechanisms to maintain this balance, it is now realized that regulatory T cells (Tregs) are critical in shaping and controlling antigen (Ag)-specific adaptive immune responses. In fact, it is speculated that any adaptive immune response generates not only effector T (Teffs) and/or B cells, but also a population of Tregs necessary to control that particular immune response and maintain immune homeostasis [1–3].
The idea that T cells could mediate immune regulation led to the search for cellular markers by which CD4+ T cells could be uniquely defined and characterized as Tregs. CD25 (IL-2 receptor, α chain) expression was the initial identifying marker of Treg function [4, 5] but it could not be considered a definitive Treg marker as it is also expressed on activated T cells. Genetic analysis showed that mutations in the gene for the transcription factor Foxp3 were associated with the autoimmunity seen in human IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) [6] and in the Scurfy mouse strain [7]. Subsequent analysis showed that Foxp3 was necessary for the development of CD4+ Tregs [8–10]. Foxp3+ Tregs can arise in either the thymus (natural Tregs, nTregs) or in the periphery from naïve T cells (induced Tregs, iTregs). While there are no markers that can distinguish nTregs from iTregs, the former are thought to control autoimmunity while the latter are important in modulating the immune response to microorganisms, allergens, and assist in controlling autoimmune inflammation [11, 12]. While Foxp3+ Tregs are considered the prototypical and dominant type of Treg, other lineages of T cells are known to have immunosuppressive functions (reviewed in [13, 14]) including the Ag-induced IL-10 producing Tr1 cells [15, 16], TGF-β producing Th3 cells [17, 18], several sublineages of CD8+ T cells [19], and even CD4−CD8− peripheral T cells [20]. The variety of Tregs speaks clearly for their importance in immune regulation and also suggests that local microenvironments can influence the development of Tregs from T cells present at the time of the immune response.
The eye is composed of a number of tissues, many of which are delicate and/or non-regenerative. Inflammatory or tissue destructive immune responses that are normally tolerated in other organs could have serious consequences on visual function. Therefore the eye has developed both unique anatomical features and a variety of physiological mechanisms that modulate or limit the immune responses in order to maintain the protective immune functions and eliminate the non-specific tissue damage associated with immune responses. Many of these features and mechanisms are discussed in other reviews of this volume or elsewhere [21, 22].
Tregs and Ocular Immune Regulation
This review will focus on recent studies of the generation of Tregs from mature, naïve T cells in the periphery to retinal specific Ags. Given the necessity for strict immune regulation in ocular tissue, it is not surprising that Tregs with a variety of origins and phenotypes have been identified and that this redundancy of Tregs and their cooperative effort is needed to maintain the overall immune homeostasis of the entire eye [23, 24]. Several of these Treg generating mechanisms are known or thought to contribute to immune privilege associated with the normal, quiescent retina.
It has been demonstrated that nTregs can be generated to retinal Ags when those Ags are expressed in the thymus. Resistance to the induction of experimental autoimmune uveoretinitis (EAU) in rodent strains correlated highly with thymic expression of retinal-specific Ags such as interphotoreceptor retinoid binding protein (IRBP) [25, 26]. Subsequent research showed that thymic expression of IRBP led to the negative selection of IRBP-specific Teffs, and the positive selection of IRBP-specific Tregs, since depletion of CD25+ T cells abrogated the resistance to EAU [27, 28]. However, negative selection is not perfect [29]. Other transgenic (Tg) mouse models expressing neo-self Ags under the control of retina-specific promoters have a significant, but incomplete, negative selection of Ag-specific autoreactive T cells [30, 31]. Therefore, the ability to generate Tregs in the periphery to retinal Ags would be advantageous, if not necessary, for maintaining retinal immune privilege.
The down regulation of immune responses to Ags placed in the anterior chamber of the eye (anterior chamber associated immune deviation, ACAID) is mediated by both CD4+ and CD8+ Tregs [32, 33]. Other studies have shown that the ACAID-type of immune deviation extended to Ags placed in the vitreous cavity [34] and the sub-retinal space [35] suggesting that retinal immune privilege could be, in part, due to an ACAID-type regulatory response. However, as discussed in the following section, the role of ACAID-generated Tregs in maintaining the immune privilege to endogenous Ags of the normal retina is uncertain.
Retinal Ags Induce a Distinct Immunoregulation Mediated by CD3+4+25+ T Cells
To study unique immunoregulatory mechanisms associated with retinal Ags we employed the arrβgal Tg mice (B10.A background) that express E. coli beta-galactosidase (βgal) as a neo-self Ag under control of a truncated version of the rod photoreceptor arrestin (S-Ag) promoter [36–38]. Expression of βgal in these mice is tightly regulated. Greater than 99.9% of the βgal is expressed in the photoreceptor cells, with very low or trace levels seen in the pineal gland and in a very small number of brain cells. The arrβgal mice are advantageous in studying immunoregulation induced by retinal Ags for several reasons. First, since βgal has no eukaryotic homologs, the endogenous T cell repertoire has not been biased towards Ag-specific Teffs or Tregs by cross reaction with related self-proteins. Second, the βgal is endogenous and constitutively expressed, thus the immune regulation induced by the Ag is representative of that in the normal animal and not influenced by physical or physiological manipulations.
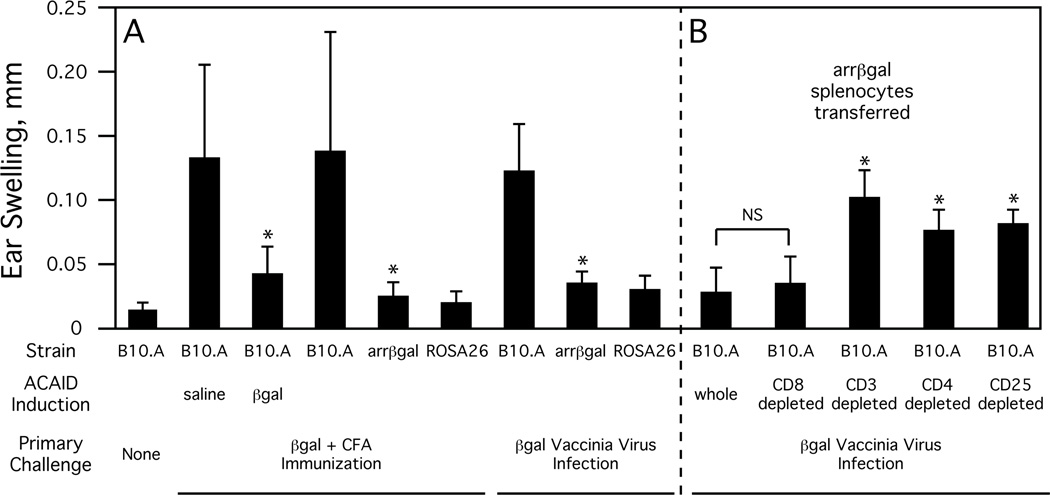
Just as ACAID induced to βgal can limit the delayed-type hypersensitivity (DTH) response to subsequent Ag challenge, we observed a similar, but spontaneous in nature, reduction in the DTH response in arrβgal mice (Fig. 1A). This reduction in the DTH response was observed both in arrβgal mice that were immunized with βgal mixed in complete Freund’s adjuvant (CFA) [39] or infected with a recombinant vaccinia virus expressing βgal [40]. By either method, the DTH response was limited in both the arrβgal mice and ROSA26 mice (Tg mice having a low level but widespread expression of βgal, including the thymus, but not photoreceptor cells) demonstrating that retinal expression of βgal by itself was sufficient to induce significant Ag-specific immunoregulation.
Fig. (1). Inhibition of the DTH (ear swelling) response to βgal in arrβal mice.
A. ACAID-induced and spontaneous downregulation of the DTH response to βgal. Indicated strain of mice were given a primary challenge either by immunization (βgal + CFA) or by infection (βgal vaccinia virus). Where indicated, some mice were given an injection of saline or βgal into the anterior chamber of the eyes to induce ACAID seven days prior to the primary challenge. All mice were given a second challenge of βgal into the ear pinna seven days after the primary challenge and DTH was assayed by ear swelling 48 hours later. All results given as mean ± SD, * = p < 0.05 (t test) compared to similarly treated B10.A mice. B. DTH inhibition is mediated by arrβgal CD3+4+25+ T cells. B10.A mice were transferred with the indicated fraction of arrβgal splenocytes and infected one day later with βgal vaccinia virus. DTH was assayed by ear swelling four weeks post-infection and measured at 48 hours. All results given as mean ± SD, * = p < 0.01 (t test) compared to mice transferred with whole splenocytes, NS = not significant (p > 0.05).
However, a number of studies suggest that the immunoregulation associated with retinal Ag expression is fundamentally different than that of ACAID. First, antigenic challenge of splenocytes from βgal immunized arrβgal mice produced elevated levels of IL-4 but not IL-10 or TGF-β1 versus controls [39]. This differs from ACAID immunoregulation which is associated with increased levels of all three cytokines. Further, splenocytes from B10.A and arrβgal mice that were subjected to βgal-induced ACAID and then βgal immunized were blocked in their production of IL-2 and IFN-γ upon antigenic challenge, whereas βgal immunized arrβgal mice could only induce a partial reduction in IFN-γ levels compared to B10.A controls [41]. Second, the expression of βgal in photoreceptors is different than the placement of a bolus of Ag. Although highly expressed, the total amount of βgal in arrβgal mice (≈150 ng/retina) is small compared to the amount of Ag used in ACAID induction protocols, is intracellular, and is constitutively expressed. Third, recent research suggests that the generation of ACAID Tregs is not dependent on peripheral CD4+25+ T cells--which could be either nTregs or iTregs [42]. Further, while there is an expansion of a CD4+25+Foxp3+ subpopulation of ACAID Tregs during ACAID induction, they appear not to directly mediate immune suppression [43]. Together, these studies suggest that the Tregs generated by ACAID-type mechanisms are distinct from and secondary to the immunoregulatory mechanisms associated with endogenous Ags of the quiescent retina.
Evidence that retinal Ags can induce a population of prototypical CD4+25+Foxp3+ Tregs came first from experiments showing that B10.A mice, after receiving arrβgal mouse splenocytes, exhibited reduced DTH response after being infected and then challenged with βgal (Fig. 1B), and secondly, the transfer of fractionated arrβgal splenocytes showed that DTH inhibition was lost with removal of either the CD3+ cells, the CD4+ cells, or just the CD25+ cells, but not by removal of CD8+ cells (Fig. 1B) [41].
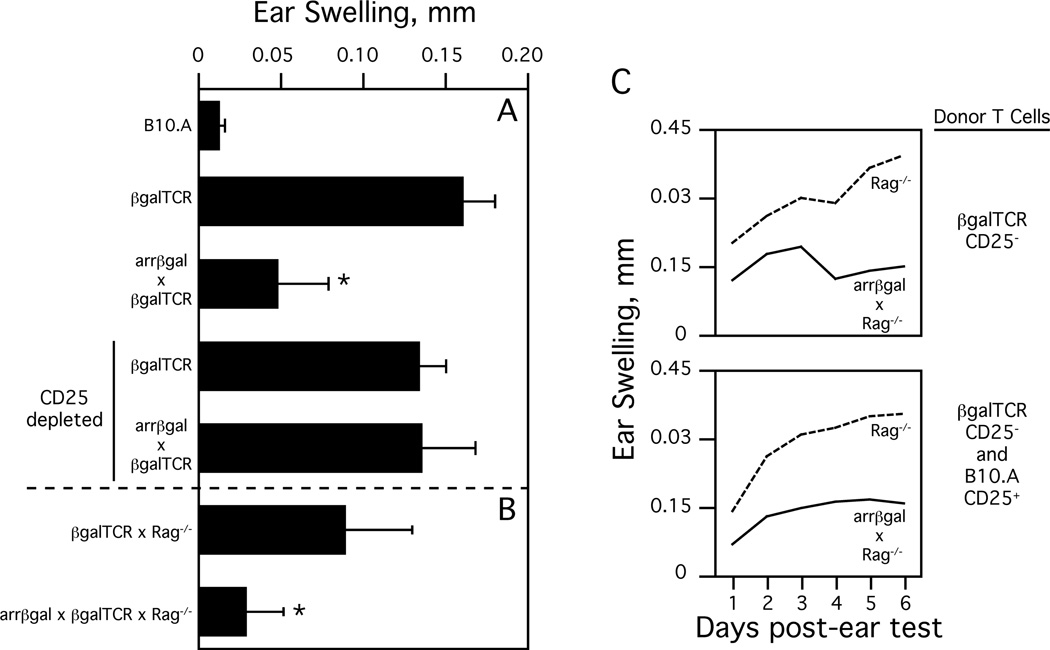
Although the observation that T cells from a normal, unmanipulated arrβgal mouse possessed βgal-specific immunoregulatory activity was strong evidence that Ags from normal, quiescent retina induce Tregs, further investigation required the use of a T cell receptor (TCR) Tg specific for βgal. To this end, we created the CD4+ T cell (class II MHC-restricted) βgalTCR mouse [44]. Injection of soluble βgal into the ear pinna without prior immunization was sufficient to induce a significant DTH response in βgalTCR mice (Fig 2A). However, this DTH response was inhibited in naïve arrβgal × βgalTCR double Tg mice, but the inhibition could be overcome in these mice by antibody depletion of CD25+ cells prior to ear testing (Fig. 2A) [41]. We have also obtained similar results with a CD4+ TCR Tg and a CD8+ TCR Tg (both specific for distinct βgal epitopes) crossed with arrβgal mice, all on the B6 background (unpublished observations). These results clearly indicate that retinal Ags can spontaneously induce CD25+ Tregs.
Fig. (2). Peripheral generation of Tregs to retinal Ags.
A. DTH (ear swelling) response in βgalTCR and arrβgal × βgalTCR mice 48 hours after injection of βgal into the ear pinna. Where indicated, the mice were depleted of CD25+ T cells prior to ear testing. All results given as mean ± SD, * = p < 0.01 (t test) compared to normal βgalTCR mice and CD25-depleted mice. B. DTH response in βgalTCR × Rag−/− and arrβgal × βgalTCR × Rag−/− mice at 48 hours after βgal injection. All results given as mean ± SD, * = p < 0.01 (t test) compared βgalTCR × Rag−/− mice. C. DTH response in Rag−/− and arrβgal × Rag−/− mice transferred with CD25− βgalTCR T cells with (bottom) or without (top) CD25+ B10.A T cells. Mice were ear tested ten weeks post-transfer. Results are given as mean, p < 0.02 (t test) between Rag−/− and arrβgal × Rag−/− recipients for both transfers at each day post-ear test.
Tregs to Retinal Ags Can Be Peripherally Induced
Another advantage of the arrβgal mouse in studying retinal immune privilege, and the one that is highly suggestive for retinal Ags being able to induce peripherally generated Tregs, is the apparent lack of thymic βgal expression in the arrβgal mouse. Interestingly, we [40] and others [25] have detected by RT-PCR a low level of arrestin transcripts in both the B10.A and arrβgal thymus, but we have not found thymic expression of βgal either by RT-PCR or immunohistochemical assays. Although we speculate that the truncated version of the arrestin promoter used to make the arrβgal mice probably does not allow thymic βgal expression or its genomic insertion site modifies expression, we cannot rule out a transient or temporally regulated expression, outside of the times of our analysis, resulting in the generation of nTregs to retinal Ags. However, there is compelling evidence suggesting that peripherally generated iTregs constitute a significant portion of retinal Ag-specific Tregs.
A comparison of the fate of βgalTCR T cells maturing in a mouse with thymic βgal expression (ROSA26), versus those thought to lack thymic βgal expression (arrβgal), or those known to lack any βgal (B10.A), showed clearly that thymic Ag expression, does and would impose a profoundly different immunoregulatory phenotype than retinal Ag expression [44]. βgalTCR bone marrow engrafted into B10.A and arrβgal mice developed similar, substantial populations of βgalTCR T cells, which failed to develop in ROSA26 mice. Further, splenocytes recovered from similarly engrafted ROSA26 mice respond poorly to antigenic challenge, but those from the engrafted B10.A and arrβgal mice respond in an equally robust manner and are phenotypically indistinguishable for markers associated with T cell activation and memory T cell development. While the above results are consistent with negative selection, thymic expression of βgal also affects Treg levels. Analysis of splenocytes for CD4+25+Foxp3+ cells showed similar levels (≈ 3–4%) in both the βgalTCR and arrβgal × βgalTCR mice but ROSA26 × βgalTCR mice had four times as many Tregs [40].
Given the drastic effects that even a modest level of thymic βgal expression has on βgalTCR T cells, it seems likely that any expression of βgal in the arrβgal thymus would result in phenotypic and/or functional changes in βgalTCR T cells. Although it appears that βgalTCR T cells lack evidence for any recognition of photoreceptor βgal, it is clear that retinal Ag does impose immune regulation upon them that is independent of the thymus, as evidenced by the altered DTH response discussed above. Further, we have noted that none of our βgal × βgals-specific TCR double Tg mice develop spontaneous EAU and immunization protocols that induce EAU in arrβgal mice failed to generate disease in arrβgal × βgalTCR double Tg mice [40, 44]. Given that 80–90% of the CD4+ T cells in these mice carry the TCR specific for βgal, these results suggest that disease protection resulted from βgal-specific Tregs generated from naïve, βgal-specific T cells in the periphery.
The use of recombination-activating gene knockout mice (Rag−/−), which lack endogenous Tregs, furthered the analysis of the ability of retinal Ags to generate iTregs. Although the issue is still unsettled, there is evidence that Foxp3+ Tregs can develop in the periphery of lymphopenic hosts from CD25− precursors [45, 46] and that there is positive selection of a significant number of CD4+25+Foxp3+ cells in thymus of TCR × cognate Ag double Tg mice on the Rag−/− background when cognate Ag is expressed in the thymus [47, 48]. Accordingly, we analyzed splenocytes and thymocytes from βgalTCR × Rag−/− and arrβgal × βgalTCR × Rag−/− mice [40]. Comparing these mice, there was no difference in the level of CD4+25+Foxp3+ cells from the spleen and no discrete population of CD25+Foxp3+ cells could be identified in either thymus. We then analyzed T cells from Rag−/− and arrβgal × Rag−/− mice transferred with naïve CD25− T cells from βgalTCR × Rag−/− mice. Although about twice as many T cells could be recovered from Rag−/− versus arrβgal × Rag−/− mice the percentage of T cells that were CD25+Foxp3+ was similar (≈ 4%) between the types of recipient mice. Thus, while cells with the Treg phenotype can develop in Rag−/− mice from either endogenous or transferred precursors, the similarity between Rag−/− and arrβgal × Rag−/− mice suggests that there is no expression of thymic βgal to skew the Treg population.
The phenomena in immunocompetent mice of βgalTCR T cells appearing ignorant of photoreceptor βgal, yet capable of developing a population of Tregs that can modulate DTH responses and disease induction, is repeated when the mice are placed on the Rag−/− background. The inhibition of the DTH response in arrβgal × βgalTCR versus βgalTCR mice is also seen in arrβgal × βgalTCR × Rag−/− versus βgalTCR × Rag−/− mice (Fig. 2B) [40]. Since these mice are on the Rag−/− background, the Tregs could only have been generated from the βgalTCR T cell population. Further evidence that retinal Ag can induce functional, Ag-specific Tregs in the periphery came from analysis of the DTH response in Rag−/− and arrβgal × Rag−/− mice transferred with naïve CD25− T cells from βgalTCR mice. Although both types of recipients developed a similar percentage of Tregs, the Rag−/− recipients had an unusually strong and progressive response when ear tested with βgal while, in contrast, arrβgal × Rag−/− recipients exhibited a smaller, self-limiting response (Fig. 2C) [40]. The addition of CD25+ T cells from B10.A mice to the βgalTCR CD25− T cells did not alter the DTH response in either Rag−/− or arrβgal × Rag−/− recipient mice (Fig. 2C) suggesting that it is the generation of βgal-specific Tregs that is necessary for the reduction in DTH. Together, these experiments provide strong evidence for the ability of retinal βgal to impose peripheral tolerance on Ag-specific T cells since the Rag−/− background requires that Teffs and Tregs be generated from the same mature, peripheral βgalTCR T cell precursors.
The Presence of the Retina is Critical for Treg Mediated Immunoregulation
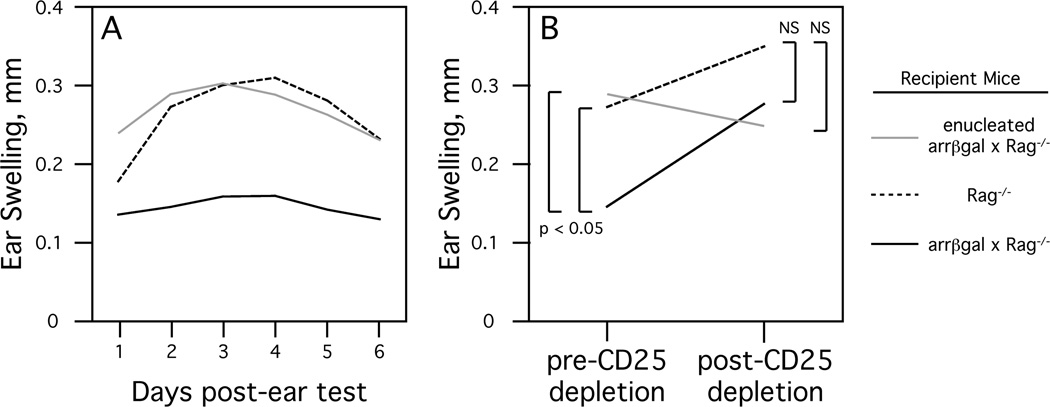
The idea that retinal Ags induce an iTreg mediated immunoregulation led to the prediction that manipulations to the retina should alter the tolerance towards retinal Ags. Since greater than 99.9% of the βgal in arrβgal mice is found in the retina, enucleation (removal of the eyes) provided an effective method for testing the role of the retina in the generation of retinal Ag-specific iTregs. Initial experiments involved enucleation of βgal Tg or control B10.A mice followed by irradiation to eliminate existing T cells, including Tregs. When tested five months later, enucleated arrβgal mice had lost the ability to limit DTH after immunization and subsequent challenge with βgal, whereas enucleated ROSA26 mice had retained their ability to limit DTH [40]. Conversely, in experiments that did not involve irradiation, arrβgal × βgalTCR × Rag−/− mice four months post-enucleation maintained the ability to limit βgal-induced DTH compared to both normal and enucleated βgalTCR × Rag−/− mice (unpublished observation). These results indicated that retinal Ags induce a T cell mediated immunoregulation and the presence of the retina is required to generate, but not maintain, retinal Ag-specific Tregs. In subsequent experiments designed to determine whether the quiescent, unirradiated retina can generate Tregs de novo from mature T cells, arrβgal × Rag−/− mice that were transferred with CD25− βgalTCR T cells were significantly limited in their DTH response compared to similarly transferred enucleated arrβgal × Rag−/− mice (Fig 3A). Further, treatment of the transferred arrβgal × Rag−/− mice with anti-CD25 antibody after the initial DTH assay resulted in a loss of DTH inhibition to subsequent challenge with βgal (Fig. 3B) [40]. Since none of these described experiments involve any manipulation of the thymus it seems clear that retinal Ags can elicit immune regulation from mature, peripheral T cells.
Fig. (3). Presence of the retina drives the formation of retinal Ag-specific Tregs.
A. DTH (ear swelling) response in recipient mice transferred with CD25− βgalTCR T cells. Enucleation was done seven days prior to transfer and ear testing was done eight weeks post-transfer. Results are given as mean, p < 0.01 (t test) for arrβgal × Rag−/− versus enucleated arrβgal × Rag−/− mice at each day post-ear test, p < 0.01 for arrβgal × Rag−/− versus Rag−/− for days 2–6 post-ear test. B. CD25+ T cells inhibit DTH in non-enucleated arrβgal × Rag−/− mice. Mice from A were rested for nine days after ear testing, treated with anti-CD25 antibody, and then ear tested again. Results of ear tests, 48 hours post-βgal injection in mice pre or post CD25+ T cell depletion, are given as mean. NS = not significant (p > 0.05).
Summary
Retinal immune privilege is the sum of a variety of immunoregulatory mechanisms. Our studies show that Tregs generated in the periphery to retinal Ags are an important component of retinal immune privilege as evidenced by our findings that they can limit DTH responses and autoimmune pathology associated with retinal Ags. While fundamental questions about the origin and function of the antigen presenting cells that gather retinal Ag and the actual site of Treg generation remain, our work demonstrates that the retinal origin of the Ag can induce Tregs independent of the thymus.
Acknowledgements
We thank Chunzhi Dou, Thien Sam, Kathleen Lew, Katie Pierson, and Heidi Roehrich for technical assistance. Our work was supported by National Institutes of Health Grants P30-EY011374, RO1-EY011542, RO1-EY016376; Research to Prevent Blindness Inc.; and the Minnesota Lions and Lionesses Clubs.
References
- 1.Hill JA, Benoist C, Mathis D. Treg cells: guardians for life. Nat Immunol. 2007;8(2):124–125. doi: 10.1038/ni0207-124. [DOI] [PubMed] [Google Scholar]
- 2.Feuerer M, Hill JA, Mathis D, Benoist C. Foxp3+ regulatory T cells: differentiation, specification, subphenotypes. Nat Immunol. 2009;10(7):689–695. doi: 10.1038/ni.1760. [DOI] [PubMed] [Google Scholar]
- 3.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133(5):775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25) J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 5.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188(2):287–296. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, et al. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106(12):R75–R81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, et al. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;1:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 8.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 9.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 10.Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat Immunol. 2003;4(4):337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 11.Curotto de Lafaille MA, Lafaille JJ. Natural and adaptive foxp3+ regulatory T cells: more of the same or a division of labor? Immunity. 2009;30(5):626–635. doi: 10.1016/j.immuni.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Josefowicz SZ, Rudensky A. Control of regulatory T cell lineage commitment and maintenance. Immunity. 2009;5:616–625. doi: 10.1016/j.immuni.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shevach EM. From vanilla to 28 flavors: multiple varieties of T regulatory cells. Immunity. 2006;2:195–201. doi: 10.1016/j.immuni.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Jutel M, Akdis CA. T-cell regulatory mechanisms in specific immunotherapy. Chem Immunol Allergy. 2008;94:158–177. doi: 10.1159/000155000. [DOI] [PubMed] [Google Scholar]
- 15.Roncarolo MG, Gregori S, Battaglia M, Bacchetta R, Fleischhauer K, Levings MK. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol Rev. 2006;212:28–50. doi: 10.1111/j.0105-2896.2006.00420.x. [DOI] [PubMed] [Google Scholar]
- 16.Wu K, Bi Y, Sun K, Wang C. IL-10-producing type 1 regulatory T cells and allergy. Cell Mol Immunol. 2007;4(4):269–275. [PubMed] [Google Scholar]
- 17.Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. I. Induction of Foxp3-positive regulatory T cells by Th3 cells derived from TGF-beta T cell-transgenic mice. J Immunol. 2007;178(1):179–185. doi: 10.4049/jimmunol.178.1.179. [DOI] [PubMed] [Google Scholar]
- 18.Carrier Y, Yuan J, Kuchroo VK, Weiner HL. Th3 cells in peripheral tolerance. II. TGF-beta-transgenic Th3 cells rescue IL-2-deficient mice from autoimmunity. J Immunol. 2007;178(1):172–178. doi: 10.4049/jimmunol.178.1.172. [DOI] [PubMed] [Google Scholar]
- 19.Lu L, Cantor H. Generation and regulation of CD8(+) regulatory T cells. Cell Mol Immunol. 2008;5(6):401–406. doi: 10.1038/cmi.2008.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen W, Zhou D, Torrealba JR, Waddell TK, Grant D, Zhang L. Donor lymphocyte infusion induces long-term donor-specific cardiac xenograft survival through activation of recipient double-negative regulatory T cells. J Immunol. 2005;175(5):3409–3416. doi: 10.4049/jimmunol.175.5.3409. [DOI] [PubMed] [Google Scholar]
- 21.Streilein JW. Ocular immune privilege: the eye takes a dim but practical view of immunity and inflammation. J Leukoc Biol. 2003;74(2):179–185. doi: 10.1189/jlb.1102574. [DOI] [PubMed] [Google Scholar]
- 22.Taylor AW. Ocular immune privilege. Eye (London, England) 2009;23(10):1885–1889. doi: 10.1038/eye.2008.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stein-Streilein J, Taylor AW. An eye's view of T regulatory cells. J Leukoc Biol. 2007;81(3):593–598. doi: 10.1189/jlb.0606383. [DOI] [PubMed] [Google Scholar]
- 24.Niederkorn JY. Regulatory T cells and the eye. Chem Immunol Allergy. 2007;92:131–139. doi: 10.1159/000099263. [DOI] [PubMed] [Google Scholar]
- 25.Egwuagu CE, Charukamnoetkanok P, Gery I. Thymic expression of autoantigens correlates with resistance to autoimmune disease. J Immunol. 1997;159:3109–3112. [PubMed] [Google Scholar]
- 26.Ham DI, Fujimoto C, Gentleman S, Chan CC, Yu CR, Yu S, et al. The level of thymic expression of RPE65 inversely correlates with its capacity to induce experimental autoimmune uveitis (EAU) in different rodent strains. Exp Eye Res. 2006;83(4):897–902. doi: 10.1016/j.exer.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 27.Avichezer D, Grajewski RS, Chan CC, Mattapallil MJ, Silver PB, Raber JA, et al. An immunologically privileged retinal antigen elicits tolerance: major role for central selection mechanisms. J Exp Med. 2003;198(11):1665–1676. doi: 10.1084/jem.20030413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grajewski RS, Silver PB, Agarwal RK, Su SB, Chan CC, Liou GI, et al. Endogenous IRBP can be dispensable for generation of natural CD4+CD25+ regulatory T cells that protect from IRBP-induced retinal autoimmunity. J Exp Med. 2006;203(4):851–856. doi: 10.1084/jem.20050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gallegos AM, Bevan MJ. Central tolerance: good but imperfect. Immunol Rev. 2006;209:290–296. doi: 10.1111/j.0105-2896.2006.00348.x. [DOI] [PubMed] [Google Scholar]
- 30.Ham DI, Kim SJ, Chen J, Vistica BP, Fariss RN, Lee RS, et al. Central immunotolerance in transgenic mice expressing a foreign antigen under control of the rhodopsin promoter. Invest Ophthalmol Vis Sci. 2004;45(3):857–862. doi: 10.1167/iovs.03-1028. [DOI] [PubMed] [Google Scholar]
- 31.Lambe T, Leung JC, Ferry H, Bouriez-Jones T, Makinen K, Crockford TL, et al. Limited peripheral T cell anergy predisposes to retinal autoimmunity. J Immunol. 2007;178(7):4276–4283. doi: 10.4049/jimmunol.178.7.4276. [DOI] [PubMed] [Google Scholar]
- 32.Stein-Streilein J, Streilein JW. Anterior chamber associated immune deviation (ACAID): Regulation, biological relevance, and implications for therapy. Int Rev Immunol. 2002;21:123–152. doi: 10.1080/08830180212066. [DOI] [PubMed] [Google Scholar]
- 33.Niederkorn JY. The induction of anterior chamber-associated immune deviation. Chem Immunol Allergy. 2007;92:27–35. doi: 10.1159/000099251. [DOI] [PubMed] [Google Scholar]
- 34.Jiang LQ, Streilein JW. Immune privilege extended to allogeneic tumor cells in the vitreous cavity. Invest Ophthalmol Vis Sci. 1991;32(1):224–228. [PubMed] [Google Scholar]
- 35.Wenkel H, Streilein JW. Analysis of immune deviation elicited by antigens injected into the subretinal space. Invest Ophthalmol Vis Sci. 1998;39(10):1823–1834. [PubMed] [Google Scholar]
- 36.Kikuchi T, Raju K, Breitman ML, Shinohara T. The proximal promoter of the mouse arrestin gene directs gene expression in photoreceptor cells and contains an evolutionarily conserved retinal factor-binding site. Mol Cell Biol. 1993;13(7):4400–4408. doi: 10.1128/mcb.13.7.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gregerson DS, Torseth JW, McPherson SW, Roberts JP, Shinohara T, Zack DJ. Retinal expression of a neo-self antigen, beta-galactosidase, is not tolerogenic, and creates a target for autoimmune uveoretinitis. J Immunol. 1999;63(2):1073–1080. [PubMed] [Google Scholar]
- 38.Kimura A, Singh D, Wawrousek EF, Kikuchi M, Nakamura M, Shinohara T. Both PCE-1/RX and OTX/CRX interactions are necessary for photoreceptor- specific gene expression. J Biol Chem. 2000;275(2):1152–1160. doi: 10.1074/jbc.275.2.1152. [DOI] [PubMed] [Google Scholar]
- 39.Gregerson DS, Dou C. Spontaneous induction of immunoregulation by an endogenous retinal protein. Invest Ophthalmol Vis Sci. 2002;43(9):2984–2991. [PubMed] [Google Scholar]
- 40.Gregerson DS, Heuss ND, Lehmann U, McPherson SW. Peripheral induction of tolerance by retinal antigen expression. J Immunol. 2009;183(2):814–822. doi: 10.4049/jimmunol.0803748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gregerson DS, Heuss ND, Lehmann U, McPherson SW. Evidence for extrathymic generation of regulatory T cells specific for a retinal antigen. Ophthalmic Res. 2008;40(3-4):154–159. doi: 10.1159/000119868. [DOI] [PubMed] [Google Scholar]
- 42.Keino H, Takeuchi M, Kezuka T, Hattori T, Usui M, Taguchi O, et al. Induction of eye-derived tolerance does not depend on naturally occurring CD4+CD25+ T regulatory cells. Invest Ophthalmol Vis Sci. 2006;47(3):1047–1055. doi: 10.1167/iovs.05-0110. [DOI] [PubMed] [Google Scholar]
- 43.Saban DR, Cornelius J, Masli S, Schwartzkopff J, Doyle M, Chauhan SK, et al. The role of ACAID and CD4+CD25+FOXP3+ regulatory T cells on CTL function against MHC alloantigens. Mol Vis. 2008;14:2435–2442. [PMC free article] [PubMed] [Google Scholar]
- 44.McPherson SW, Heuss ND, Gregerson DS. Lymphopenia-induced proliferation is a potent activator for CD4+ T cell-mediated autoimmune disease in the retina. J Immunol. 2009;182(2):969–979. doi: 10.4049/jimmunol.182.2.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Curotto de Lafaille MA, Lino AC, Kutchukhidze N, Lafaille JJ. CD25- T cells generate CD25+Foxp3+ regulatory T cells by peripheral expansion. J Immunol. 2004;173(12):7259–7268. doi: 10.4049/jimmunol.173.12.7259. [DOI] [PubMed] [Google Scholar]
- 46.Wan YY, Flavell RA. Identifying Foxp3-expressing suppressor T cells with a bicistronic reporter. Proc Nat Acad Sci USA. 2005;102(14):5126–5131. doi: 10.1073/pnas.0501701102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cabarrocas J, Cassan C, Magnusson F, Piaggio E, Mars L, Derbinski J, et al. Foxp3+ CD25+ regulatory T cells specific for a neo-self-antigen develop at the double-positive thymic stage. Proc Nat Acad Sci USA. 2006;103(22):8453–8458. doi: 10.1073/pnas.0603086103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aschenbrenner K, D'Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8(4):351–358. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]