Abstract
This study investigates involvement of β-catenin signalling in regulation of p-glycoprotein (p-gp) expression in endothelial cells derived from brain vasculature. Pharmacological interventions that enhance or that block β-catenin signalling were applied to primary rat brain endothelial cells and to immortalized human brain endothelial cells, hCMEC/D3, nuclear translocation of β-catenin being determined by immunocytochemistry and by western blot analysis to confirm effectiveness of the manipulations. Using the specific glycogen synthase kinase-3 (GSK-3) inhibitor 6-bromoindirubin-3′-oxime enhanced β-catenin and increased p-gp expression including activating the MDR1 promoter. These increases were accompanied by increases in p-gp-mediated efflux capability as observed from alterations in intracellular fluorescent calcein accumulation detected by flow cytometry. Similar increases in p-gp expression were noted with other GSK-3 inhibitors, i.e. 1-azakenpaullone or LiCl. Application of Wnt agonist [2-amino-4-(3,4-(methylenedioxy) benzylamino)-6-(3-methoxyphenyl)pyrimidine] also enhanced β-catenin and increased transcript and protein levels of p-gp. By contrast, down-regulating the pathway using Dickkopf-1 or quercetin decreased p-gp expression. Similar changes were observed with multidrug resistance protein 4 and breast cancer resistance protein, both known to be present at the blood–brain barrier. These results suggest that regulation of p-gp and other multidrug efflux transporters in brain vasculature can be influenced by β-catenin signalling.
Keywords: β-catenin, breast cancer resistant protein, glycogen synthase kinase-3, multidrug resistance protein 4, p-glycoprotein, brain endothelial cells
β-catenin is a protein that not only plays a role as an adhesion molecule within adherens junctions, but also functions as a key intermediate in the canonical Wnt signalling pathway (Hagen et al. 2004). In this signalling pathway, interactions of Wnt proteins with the cell surface Frizzled receptors and associated membrane proteins lead to inactivation of glycogen synthase kinase-3 (GSK-3), resulting in stabilization of β-catenin. As a result, free β-catenin is allowed to accumulate and be translocated to the nucleus, binding to the transcription factor Tcf/Lef to alter the expression of target genes (Logan and Nusse 2004). Wnt proteins can also activate non-canonical pathways that do not involve β-catenin.
There is evidence that Wnt signalling, particularly via the canonical pathway plays a role in vascular endothelial survival and proliferation (Wright et al. 1999; Masckauchan et al. 2005). Wnt ligands and Wnt ligand receptors have been identified in different types of vascular endothelial cells (Goodwin et al. 2006). Interactions between canonical and non-canonical pathways may be such that the one then modulates the effects of the other (Masckauchan and Kitajewski 2006). Certainly, Wnt signalling pathways are now of interest in providing possible new targets suitable for therapeutic modification of angiogenesis. The idea that Wnt signalling pathways may also influence the barrier properties of blood vessels has not yet been addressed. It is interesting to note however that Wnt signalling in blood vessels in the brain during development (Maretto et al. 2003) appears with the same time frame as appearance of the efflux transporter, p-glycoprotein (p-gp) (ABCB1) in the brain vasculature (Qin and Sato 1995). p-gp plays an important role at the blood–brain barrier in preventing access of unwanted substances to the brain (Schinkel 1999). Transcriptional regulation of p-gp is rather complex and is far from being completely understood. Amongst the many other transcription factor binding sites identified (Scotto 2003), the promoter of the MDR1 gene encoding p-gp is known to contain multiple T-cell factor 4(TCF4)-binding sequences and the gene was found to be transcriptionally down-regulated after inactivation of TCF4 in a human colorectal carcinoma cell line, suggesting that MDR1 is a direct target gene of the TCF4/β-catenin transcriptional complex (Yamada et al. 2000). More recently, it has been shown in another cell type i.e. in 3T3-L1 cells that ectopic expression of Wnt-1 induces up-regulation of MDR1 (Longo et al. 2002) as detected via microarray analysis.
This study takes the initial steps towards determining the influence of the Wnt/β-catenin canonical pathway on blood–brain barrier properties by analysing the effects of activation downstream i.e. β-catenin signalling on p-gp expression in rat primary and human immortalized brain endothelial cells. Various pharmacological interventions that inhibit GSK-3 activity and enhance β-catenin signalling reveal that p-gp can be positively regulated in these cells by such activation. Expression of two other ATP-binding cassette (ABC) transporters known to be present at the blood–brain barrier, i.e. multidrug resistance protein, MRP4 (ABCC4) and breast cancer resistance protein, BCRP (ABCG2), is similarly enhanced.
Materials and methods
Reagents were of analytical, tissue culture, or molecular biology grade, as appropriate and were obtained from Sigma Aldrich Co. Ltd. (Poole, Dorset, UK), except where stated.
Cell isolation and culture
Primary cultures of rat brain endothelial cells (RBECs) were grown from microvessels isolated from the brains of male Wistar rats weighing 200–250 g (Charles Rivers, Margate, UK) and seeded into collagen IV and fibronectin-coated flasks (Lim et al. 2007). Puromycin treatment as described by Perriere et al. (2005) was performed to ensure purity of the endothelial cells. The endothelial cells proliferated in endothelial cell basal medium 2 (Cambrex Bio Science, Wokingham, UK) containing 20% bovine plasma-derived serum with 80 μg/mL heparin, 5 μg/mL ascorbic acid, 100 U/mL penicillin, 100 μg/mL streptomycin, 2 mM l-glutamine and 75 μg/mL endothelial cell growth supplement (from First Link, Birmingham, UK). Experiments were performed on cells from the first passage only.
Immortalized human brain endothelial cells of the hCMEC/D3 line (Weksler et al. 2005) were seeded in collagen IV and fibronectin-coated flasks and maintained in endothelial cell basal medium 2 containing 10% foetal calf serum along with heparin, ascorbic acid, penicillin, streptomycin, l-glutamine and endothelial cell growth supplement at concentrations described above. This cell line has been used successfully as a model of human brain endothelial cells in several recent studies (Afonso et al. 2007; Cucullo et al. 2007; Schreibelt et al. 2007).
Pharmacological interventions
Brain endothelial cells cultured as described above were grown to confluence in 6-well plate and then exposed to various agents that activate or inhibit β-catenin signalling for the periods of time stated. Agents designed to activate β-catenin signalling included pan Wnt agonist [2-amino-4-(3,4-(methylenedioxy) benzylamino)-6-(3-methoxyphenyl)pyrimidine] (Merck Chemical Ltd., Nottingham, UK) and the GSK-3β inhibitors, 6-bromoindirubin-3′-oxime (BIO) (Calbiochem), 1-azakenpaullone (Calbiochem) and LiCl (Sigma Aldrich Co. Ltd.). Agents providing inhibition included Wnt antagonist, Dkk-1 (R&D Systems Europe Ltd., Abingdon, UK) that blocks upstream Wnt receptor/ligand interactions and quercetin (Sigma Aldrich Co. Ltd.) that interferes with downstream transcriptional events. Cells were subsequently harvested for qRT-PCR and/or for western blot analysis as outlined below.
Reporter assays
To detect activation of the MDR1 promoter or of TCF target genes, hCMEC/D3 cells following trypsinization were transfected in suspension (6 × 105 cells per cuvette) by electroporation using a basic nucleofector kit for primary endothelial cells (Amaxa AG, Koln, Germany); 2 μg of the plasmid, pMDR-Luc (gift from Dr S. Hirohashi, National Cancer Centre Research Institute, Tokyo, Japan) (Yamada et al. 2000) containing the MDR1 promoter or 2.5 μg of the Topflash TCF reporter construct (Upstate, Charlottesville, VA, USA) were used per transfection, each together with co-transfected pCMV-SEAP, a reporter vector encoding secreted alkaline phosphatase under the control of a constitutive cytomegalovirus-derived promoter (gift from Dr M. Fussenegger, Zurich, Switzerland). In some experiments, 0.25–0.5 μg dominant negative mutant TCF4 (dnTCF4) (Hagen et al. 2004) or empty vector was included in the transfection. Following electroporation, cells were divided between six and nine wells of a 24-well plate and maintained in medium with or without BIO or lithium added 16–24 h later. Two to three days post-transfection, the medium was harvested for alkaline phosphatase activity and the cells analysed for luciferase activity as described previously (Pulaski et al. 2005). Alkaline phosphatase activity was determined in 100 μL aliquots of medium transferred to a new plate and incubated in 70 °C for 1 h. After adjusting to 21 °C, 100 μL of reaction mixture (1 mg/mL p-nitrophenyl phosphate in 2 M diethanolamine and 1 mM MgCl2, pH 9.8) was added to each well. Alkaline phosphatase activity was assessed from the changes in absorbance at 405 nm detected using a Multi Skan Ascent spectrophotometric plate reader (ThermoLabsystems, Basingstoke, UK). For luciferase activity, cells were washed in phosphate-buffered saline, scraped from the plates, resuspended in a lysis buffer (25 mM Tris-phosphate, 4 mM EGTA, 1% Triton X-100, 15 mM MgSO4 and 2 mM dithiothreitol, pH 7.8) and activity then determined in a Turner TD-20e luminometer (Turner Designs Inc., Mt View, CA, USA) using a commercial luciferase substrate (Becton Dickinson UK Ltd., Oxford, UK). Values were normalized to the alkaline phosphatase activity of co-transfected pCMV-SEAP.
RNA isolation, cDNA synthesis and real-time PCR
One microgram of RNA was used for cDNA synthesis using random hexamers (Promega UK Ltd., Southampton, UK) and BioScript MMLV reverse transcriptase according to the manufacturer’s instructions (Bioline, London, UK). PCR amplification mixtures (20 μL) contained 8 μL template cDNA, 2× SensiMix (Quantace Ltd, Watford, UK) containing SYBR Green (12 μL) and 500 nM forward and reverse primers. Sequences of these primers (shown as supplementary Table S1) were obtained from RTPrimerDB (http://medgen.ugent.be/rtprimerdb), a public database for primer and probe sequences used in real-time PCR assays (Pattyn et al. 2006). Amplification was allowed to proceed in a Rotor-Gene 3000™ (Corbett Research, Sydney, NSW, Australia) using the following cycle conditions: an initial denaturation step for 10 min, followed by 40 cycles of 94 °C for 30 s, 55 °C for 30 s and 72 °C for 30 s, with fluorescent detection at 72 °C and a final step of 72 °C for 2 min. Melt curve analysis was performed from 72 °C to 99 °C in 1 °C steps, 15 s for the first step and 5 s for each step thereafter. The cycle number at threshold, values were determined using the ‘comparative quantitation’ feature of the Rotor-Gene 6000 software (version 1.7) (Corbett Research).
Selection of reference genes using geNorm analysis
To determine the most stable reference genes in our study, the data were analysed using the geNorm software (http://www.primerdesign.co.uk/geNorm.asp) (Vandesompele et al. 2002). Table 1 shows the ranking of the six candidate reference genes according to their expression stability in both rat and human endothelial cells treated with the GSK-3β inhibitor, BIO. The stability of the reference genes in human endothelial cells exposed to the pan-Wnt agonist and quercetin was also determined. The three most stable reference genes in human brain endothelial cells exposed to the pan-Wnt agonist and in both rat and human endothelial cells treated with BIO are β-microglobulin, Ubc and Ywhaz. For human brain endothelial cells treated with quercetin, β-microglobulin was the most stably expressed reference gene along with Gapdh and Ubc. It is interesting to note that β-microglobulin and Ubc remain one of the most stable reference genes for use in experiments where activation or down-regulation of β-catenin signalling is undertaken. Although Ywhaz is one of the stable reference genes where there is upregulation, it appears to be the least stable reference gene where there is down-regulation.
Table 1.
Ranking of six candidate reference genes according to their expression stability in RBEC and human (hCMEC/D3) endothelial cells following treatment with pan Wnt agonist, BIO and quercetin
| Wnt agonist |
BIO |
Quercetin |
||
|---|---|---|---|---|
| Reference gene | hCMEC/D3 | RBEC | hCMEC/D3 | hCMEC/D3 |
| 18s rRNA | 6 | 5 | 5 | 4 |
| β-actin | 5 | 6 | 4 | 5 |
| β2-microglobulin | 2 | 1 | 1 | 1 |
| GAPDH | 4 | 4 | 6 | 2 |
| Ywhaz | 3 | 2 | 3 | 6 |
| Ubc | 1 | 3 | 2 | 3 |
The lowest values define the most stable reference genes and thus β-microglobulin and Ubc are seen as the most useful to use as reference in each condition. BIO, 6-bromoindirubin-3-oxime; RBEC, brain endothelial cells.
Preparation of cell lysates, nuclear and cytoplasmic extracts and western blot analysis
To analyse expression at the protein level, western blot analysis was performed on rat or human brain endothelial cells from two wells of a six-well plate for each experimental condition. Cell lysates were prepared by scraping cells directly into sodium dodecyl sulfate–polyacrylamide gel electrophoresis sample buffer. Separate nuclear and cytoplasmic fractions were also prepared using the NE-PER™ nuclear and cytoplasmic extraction kit (Pierce, Rockford, IL, USA) according to the manufacturer’s instructions. Protein concentrations in each fraction were assayed using the BCA™ Protein Assay kit (Pierce), equivalent amounts then applied to each well, separated through 7.5% polyacrylamide and transferred to 0.45 μm nitrocellulose membranes. Following a blocking step with Tris-buffered saline (50 mM Tris and 250 mM NaCl, pH 7.5) supplemented with 0.1% Tween 20 and 5% non-fat milk powder, blots were probed overnight with one of the following antibodies diluted as described in the same buffer: mouse anti-p-gp antibody, C219 (1: 50; ID Labs, Glasgow, UK), rat anti-MRP4 antibody, M41-10 (1: 100; Abcam, Cambridge, UK), mouse anti-BCRP antibody, BXP-21 (1: 100, Chemicon), rabbit anti-β-catenin antibody (1: 2000; Abcam) or rabbit anti-transcription initiation factor IID (TBP) antibody (1: 1000; Santa Cruz Biotechnology, Inc, Santa Cruz, CA, USA). Following three washes in the buffer, the blots were subsequently probed for 30 min with the appropriate peroxidase-conjugated secondary antibody, i.e. anti-mouse, anti-rat and anti-rabbit IgG (all from Abcam) and the proteins then detected using the enhanced chemiluminescence system (GE Healthcare UK Ltd., Little Chalfont, UK). All blots were stained with Ponceau S prior to probing with antibody and also probed with anti-β-actin antibody (1: 10000; Abcam) to verify equal protein loading in each well. Blots were performed at least three times using samples from separate experiments.
Immunocytochemistry
Staining was performed on hCMEC/D3 cells and RBECs grown on glass coverslips. Cells were fixed in ice-cold 100% methanol for 2 min, blocked with 5% goat serum and incubated overnight with one of the primary antibodies in 1% goat serum (v/v), rabbit anti-β-catenin (1: 2000) (Abcam). After washing, a fluorescent-labelled secondary antibody, i.e. Alexa Fluor 488 goat anti-rabbit IgG (1: 500) (Molecular Probes, Eugene, OR, USA), was applied. Cells were mounted in VectaShield® mounting medium containing the nuclear stain 4′,6-diamidino-2-phenylindole (DAPI) (Vector Laboratories, Inc., Burlingame, CA, USA) and viewed with an inverted fluorescence microscope. Quantitation of perinuclear/nuclear β-catenin staining was carried out using at least five frames randomly selected from each slide. Following counting, values were expressed as the ratio of total number of positively stained cells to total number of DAPI stained nuclei. Five separate experiments using this protocol were undertaken.
Assay for p-gp-mediated efflux
Cultured brain endothelial cells were detached from their surfaces by trypsinization, resuspended in F10 Ham’s medium, pre-treated for 30 min without or with 20 μM of the p-gp-inhibitor, verapamil and then exposed to 0.5 μM calcein-acetoxymethyl (AM) for 30 min at 37 °C in the absence or presence of the p-gp-inhibitor, verapamil. The cells were then pelleted and kept on ice until analysis of their fluorescent content via flow cytometry. Any dead cells identified from their scatter characteristics were gated out and the median value of fluorescence for each viable cell population was obtained. Ratios of the median fluorescence in the absence and in the presence of the p-gp inhibitor, verapamil were compared between cell populations exposed to different manipulations to β-catenin. The non-fluorescent ester, calcein-AM enters the cells where it is then cleaved to the fluorescent product, calcein. Calcein-AM is a substrate for p-gp (Holló et al. 1994) that will thus impede its entry and so reduce intracellular accumulation of its fluorescent hydrolysis product, calcein. Hence the higher the intracellular fluorescence, the lower the efflux activity.
Statistical analyses
All values are expressed as the mean ± SEM. All statistical analyses were performed using GraphPad Prism 4 (GraphPad Software, Inc., San Diego, CA, USA). Mean values for expression of genes of interest compared with that of reference genes obtained for each culture condition were compared using one-way anova, followed by Dunnett’s test to compare with controls. Comparisons between two groups were performed using a paired t-test.
Results
Effect of GSK-3β inhibition and of exposure to pan-Wnt agonist on β-catenin in human brain endothelial cells
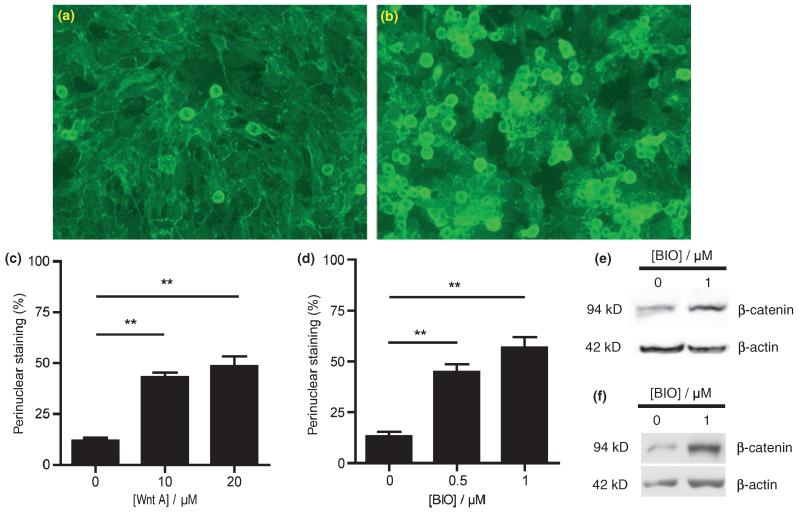
hCMEC/D3 cells were exposed to GSK-3β inhibitor, BIO (Meijer et al. 2004) or to pan-Wnt agonist (Liu et al. 2005) for various times up to 48 h. As shown by immunocytochemical staining (Fig. 1a and b), treatment with BIO for 16 h resulted in nuclear and perinuclear accumulation of β-catenin, indicating that stabilization of cytosolic β-catenin had occurred. Interestingly, a similar nuclear translocation of β-catenin was observed following treatment with pan-Wnt agonist (not shown).
Fig. 1.
Effect of pan Wnt agonist and GSK-3β inhibitor, BIO on stabilization and localization of β-catenin in human brain endothelial cells. Staining for β-catenin is shown in (a) untreated and (b) cells treated with 1 μM BIO for 16 h. The percentage of cells showing nuclear/perinuclear staining for β-catenin 16 h following treatment with (c) pan Wnt agonist or (d) GSK-3β inhibitor, BIO at the concentrations shown was calculated relative to the total number of DAPI-stained nuclei; in each case, values shown as the mean ± SEM are significantly higher than those of untreated control cells (**p < 0.01, n = 5). (e and f) Western blots of (e) whole cell lysates and (f) nuclear fractions from untreated cells and from cells treated with 1 μM BIO for 16 h showing increased intensity of the band corresponding to β-catenin in the treated cells. In each case, the blot is representative of immunoblots resulting from three separate experiments.
To obtain quantitative results, the nuclei were stained simultaneously using DAPI which binds to DNA and labels all nuclei. The proportions of nuclear or perinuclear β-catenin staining relative to DAPI-stained nuclei could thus be calculated. Human brain endothelial hCMEC/D3 cells exposed to 0.5 and 1 μM BIO, a GSK-3β inhibitor, for 16 h showed an increase in the percentage of cells exhibiting nuclear or perinuclear β-catenin staining (45 ± 4%, p < 0.01, n = 5 and 57 ± 5%, p < 0.01, n = 5, respectively) compared with that of control (13 ± 2%, n = 5) (Fig. 1d). Likewise, exposure to the pan-Wnt agonist led to an increase in the percentage of cells exhibiting nuclear or perinuclear β-catenin staining after exposure for 16 h to 10 μM (43 ± 2%, p < 0.01, n = 5) and 20 μM (48 ± 5%, p < 0.01, n = 5) of pan Wnt agonist compared with that of control (12 ± 1%, n = 5) (Fig. 1c).
In addition to the immunocytochemistry results showing changes in subcellular distribution of β-catenin, western blot analysis revealed an increase in β-catenin levels in whole cell lysates taken from the human brain endothelial cells 16 h after treatment with 1 μM BIO (1.55 ± 0.15, p < 0.05, n = 4) (Fig. 1e) indicating β-catenin protein stabilization. These increases following BIO treatment were likewise evident in samples of the nuclear fraction prepared from lysed cells (1.51 ± 0.23, p < 0.01 for treated vs. untreated samples from three separate preparations, each run in triplicate and analysed via two-way anova) (Fig. 1f). Enrichment for nuclear proteins in this fraction was verified using an antibody against the nuclear located transcription initiation factor IID.
p-Glycoprotein expression is enhanced following activation of β-catenin signalling
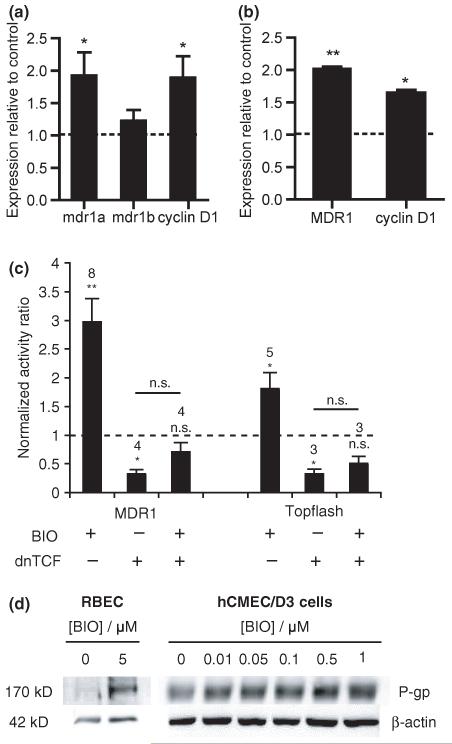
Expression at the mRNA level of the genes encoding the human and rat p-gp, i.e. human MDR1 and rat mdr1a and mdr1b genes, were determined by real time PCR following various treatment conditions in human brain endothelial cells and RBECs. Values of the genes of interest were expressed relative to the geometric mean of the three most stable reference genes using geNorm analysis as described in the Materials and methods section and shown in Table 1. Ratios of the expression compared with that in untreated samples are shown in Fig. 2a and b. Cyclin D1 was used as a positive control as this gene is known to be up-regulated on activation of the Wnt/β-catenin signalling pathway (Shtutman et al. 1999).
Fig. 2.
Effect of GSK-3β inhibitor, BIO, on p-gp expression in (a) rat brain endothelial cells and (b) human immortalized brain endothelial cells. Cells were exposed to 1 μM BIO for 16 h (hCMEC/D3) or to 5 μM BIO for 5 days (rat) and then harvested for analysis at transcript level (a and b) by qRT-PCR. Expression was firstly estimated relative to the geometric mean of three most stable reference genes using geNorm analysis. Values shown are the mean ± SEM of these amounts expressed relative to the expression in control untreated cells for mdr1a or MDR1, mdr1b and cyclin D1. Significant increases over control are shown (a) for mdr1a (*p < 0.05, n = 6), cyclin D1 (*p < 0.05, n = 6) but not mdr1b in the rat cells and (b) for MDR1 (**p < 0.01, n = 4) and cyclin D1 (*p < 0.05, n = 4) in the human cells. (c) Activation of MDR1 transcription after 24 h or of TCF/LEF-dependent transcription after 16 h following exposure to 1 μM BIO. hCMEC/D3 cells were transfected with pMDR-Luc and pCMV-SEAP or with Topflash DNA and pCMV-SEAP together with empty vector or dnTCF, treated the next day with BIO and later harvested for luciferase and alkaline phosphatase activity. Values shown are the fold increases in luciferase/alkaline phosphatase activity ratio over control untreated cells and are given as mean ± SEM. Number of experiments and statistical significance (paired t-test on the log of the ratios) are shown above each bar. (d) Western blots of brain endothelial cell lysates from rat (RBEC) or human (hCMEC/D3) cells either untreated or exposed to BIO at the concentrations shown for 5 days (rat) or for 24 h (human). The blots were probed with anti-p-gp monoclonal antibody C219. Equal protein loading was verified by probing with anti-β-actin antibody (shown) and Ponceau staining (not shown). Images shown are representative of three independent experiments performed.
Rat brain endothelial cells exposed to 5 μM BIO for 5 days showed significantly increased expression of mdr1a (1.93 ± 0.36, p < 0.05, n = 6) compared with control (Fig. 2a). Increased expression of Cyclin D1 (1.83 ± 0.33, p < 0.05, n = 6) was also demonstrated with BIO treatment. No significant change in the relative expression of mdr1b (1.22 ± 0.17, n = 6) was however evident. Similarly, increases in the expression of MDR1 were noted in hCMEC/D3 cells exposed to 1.0 μM BIO (2.02 ± 0.03, p < 0.01, n = 4) for 24 h (Fig. 2b). Up-regulation of Cyclin D1 (1.64 ± 0.05, p < 0.01, n = 4) was also detected (Fig. 2b). This treatment with BIO resulted in activation of the MDR1 promoter (Fig. 2c) as observed from increases in luciferase/alkaline phosphatase activity in hCMEC/D3 cells transfected with pMDR-Luc and pCMV-SEAP plasmids (fold increases of 2.97 ± 0.41 over untreated controls, n = 8, p < 0.002), an activation that became non-significant after co-transfection with dnTCF. Experiments using hCMEC/D3 cells transfected with the TCF-reporter construct, Topflash and pCMV-SEAP with empty vector or dnTCF (Fig. 2c) showed a similar pattern of responses to treatment for 16 h with 1.0 μM BIO (fold increases of 1.81 ± 0.28 over untreated controls, n = 5, p < 0.05), indicating that BIO at this concentration is capable of activating TCF-dependent transcription in these cells.
Western blot analysis confirmed the increase in p-gp expression in BIO-treated RBECs (2.06 ± 0.77, n = 2) compared with control (Fig. 2d) and the proportionate increase in p-gp protein levels in hCMEC/D3 cells exposed to increasing concentrations of BIO (0.01–1 μM) (Fig. 2d), significant increases being seen at 0.1–1 μM (see Table 2). RBECs required higher BIO concentrations applied for a longer duration, i.e. 5 μM for 5 days to observe an effect whereas exposure of the human brain endothelial cells to ≥ 2 μM BIO resulted in decreased viability of the cells (data not shown).
Table 2.
Analyses of western blots to show the effects of GSK-3β inhibitors and of Wnt agonist on p-gp expression in human brain endothelial cells
| Pharmacological agent | Concentration (μM) | Band density relative to control |
|---|---|---|
| BIO | 0.1 | 1.36 ± 0.14, p < 0.05, n = 3 |
| 0.5 | 1.55 ± 0.05, p < 0.01, n = 3 | |
| 1 | 1.65 ± 0.08, p < 0.01, n = 3 | |
| 1-Azakenpaullone | 0.05 | 1.10 ± 0.08, n = 4 |
| 0.1 | 1.37 ± 0.01, p < 0.05, n = 4 | |
| 0.5 | 1.42 ± 0.14, p < 0.01, n = 4 | |
| LiCl | 5000 | 1.37 ± 0.03, p < 0.01, n = 4 |
| 10 000 | 1.40 ± 0.08, p < 0.01, n = 4 | |
| Wnt agonist | 1.5 | 2.06 ± 0.15, p < 0.05, n = 3 |
| 3 | 1.96 ± 0.13, p < 0.05, n = 3 | |
| 5 | 1.93 ± 0.24, p < 0.05, n = 3 | |
| 10 | 2.29 ± 0.09, p < 0.01, n = 3 | |
| 20 | 3.13 ± 0.37, p < 0.01, n = 3 |
Cell lysates were obtained from cells 24 h following treatment with the stated agents at the concentrations shown and subjected to western blot analyses using the anti-p-gp antibody C219 to visualize the protein. Values shown are the mean ± SEM of band intensities relative to control. BIO, 6-bromoindirubin-3-oxime; Wnt agonist, 2-amino-4-(3,4-(methylenedioxy) benzylamino)-6-(3-methoxyphenyl)pyrimidine; p-gp, p-glycoprotein.
Other GSK-3β inhibitors such as 1-azakenpaullone (Leost et al. 2000) and LiCl (Stambolic et al. 1996) were also tested on the human brain endothelial cells and western blot analyses undertaken to ascertain the level of p-gp expression following the different treatment conditions. As shown in Table 2, exposure of hCMEC/D3 cells to either of these GSK-3β inhibitors for 24 h resulted in increases in p-gp protein expression. In addition, activation of the MDR1 promoter in the pMDR1-Luc-transfected cells was observed in three out of three experiments 24 h following exposure to LiCl (2.0-, 4.1- and 9.4-fold) though this did not reach significance in a t-test (p = 0.089). Application of the pan-Wnt agonist for 24 h also resulted in an increase in p-gp protein expression in the human brain endothelial cells. At the transcript level, there was also a slight but significant increase in MDR1 levels (1.20 ± 0.02, p < 0.05, n = 3) following exposure to 20 μM pan-Wnt agonist for 8 h.
p-Glycoprotein function is enhanced following activation of β-catenin signalling
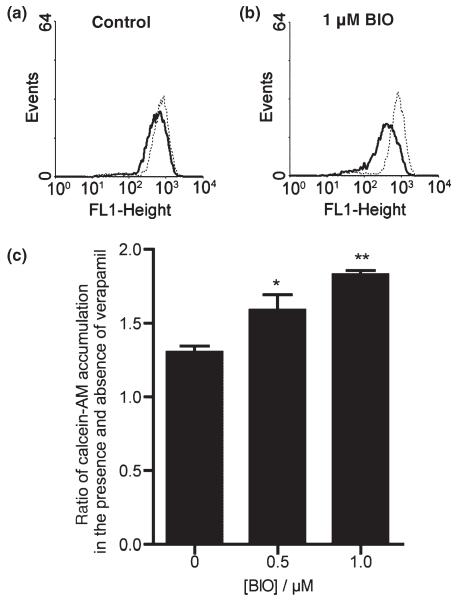
p-Glycoprotein functional activity was assessed in both control cells and in those exposed to BIO by observing the increases in accumulation of fluorescent calcein (Holló et al. 1994) (see Fig. 3) in the presence of p-gp inhibitor, verapamil at 20 μM. In the absence of verapamil i.e. with p-gp activity still present, calcein accumulation was significantly less in the BIO-treated cells (note the lower median fluorescence of the histogram with no verapamil in Fig. 3b compared with that in Fig. 3a). The presence of verapamil clearly increased calcein accumulation in all cases, indicating the presence of p-gp efflux activity (note the rightward shifts of the fluorescence histograms in Fig. 3a and b). These verapamil-mediated increases in calcein accumulation were significantly greater in the cells exposed to 0.5 and 1 μM BIO (1.59 ± 0.11, p < 0.05, n = 4 and 1.83 ± 0.03, p < 0.01, n = 4 respectively) compared with control cells (1.30 ± 0.04, n = 4) indicating the p-gp-mediated efflux activity to be enhanced.
Fig. 3.
Effect of GSK-3β inhibitor, BIO, on p-gp efflux activity in human immortalized brain endothelial cells. (a and b) Show fluorescence histograms of cells (a) untreated or (b) pre-treated with 1 μM BIO for 24 h and exposed either to vehicle for 30 min followed by 0.5 μM calcein-AM for a further 30 min (solid line) or the same procedure but with 20 μM verapamil present throughout (dotted line). Note in the presence of verapamil i.e. with p-gp activity blocked, accumulation of fluorescent calcein, as seen by the median values of the dotted line histograms, is similar in (a) and (b). In the absence of verapamil i.e. with p-gp activity still present, accumulation is significantly less in the BIO-treated cells as seen by comparing the median values of the solid-line histograms in (a) and (b). Median values of background fluorescence (histograms not shown) were very low i.e. 1.4 ± 0.4. (c) Show the increases in accumulation of calcein in the presence of 20 μM verapamil in untreated cells and in cells exposed to 0.5 or 1 μM BIO. Values shown are the mean ± SEM of results from four separate experiments and indicate significant differences from untreated controls for cells treated with 0.5 μM BIO (*p < 0.05) and with 1 μM BIO (**p < 0.01). Median values from fluorescence histograms as shown in (a and b) were used to derive these data.
Expression of other ABC transporters in brain endothelial cells is affected by activation of β-catenin signalling
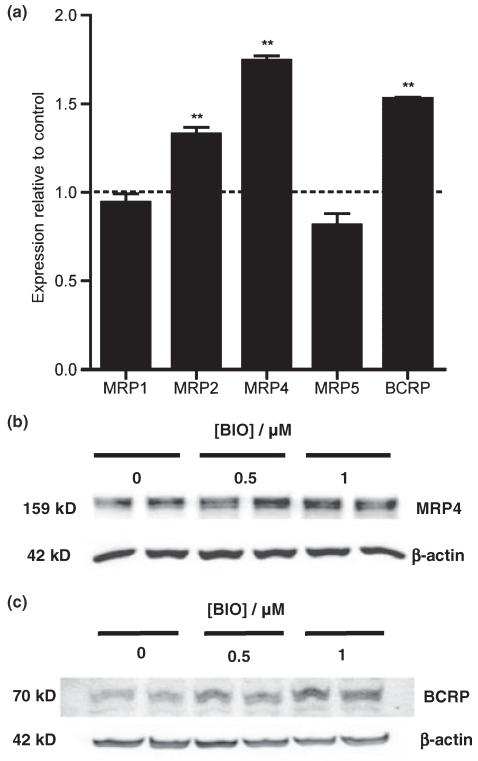
Other ABC transporters known to be present in the blood–brain barrier include MRP4 and BCRP. To investigate the effects of β-catenin signalling on these transporters, hCMEC/D3 cells were exposed to 1.0 μM BIO for 16 h and subsequently analysed by qRT-PCR and by western blotting. Increases in the transcript levels of MRP2 (1.33 ± 0.03, n = 4, p < 0.01), MRP4 (1.74 ± 0.02, n = 4, p < 0.01) and BCRP (1.53 ± 0.01, p < 0.01, n = 4) but not MRP1 (0.94 ± 0.04, n = 4) or MRP5 (0.81 ± 0.06, n = 4) were noted (Fig. 4a).
Fig. 4.
Effect of GSK-3β inhibitor BIO on expression of other ABC transporters in human immortalized brain endothelial cells. Cells were exposed to 1 μM BIO for 16 h and then analysed (a) by qRT-PCR for expression of MRP1, MRP2, MRP4, MRP5 and BCRP. As described in legend to Fig. 2, expression was firstly estimated relative to reference genes. Values shown are the mean ± SEM of these levels of expression relative to that of control untreated cells. Significant increases over control are shown for MRP2 (**p < 0.01, n = 4), MRP4 (**p < 0.01, n = 4) and BCRP (**p < 0.01, n = 4) but not for MRP1 or MRP5. (b and c) Western blots showing expression of (b) MRP4 and (c) BCRP 24 h after exposure to BIO at the concentrations shown. Equal protein loading was verified by probing with anti-β-actin antibody (shown) and Ponceau staining (not shown). Images shown are representative of those obtained from three independent experiments.
Western blot analyses confirmed that there was up-regulation of MRP4 (1.16 ± 0.05, p < 0.01, n = 6 and 1.27 ± 0.01, p < 0.01, n = 4 for 0.5 and 1 μM respectively) and BCRP (1.20 ± 0.04, p < 0.05, n = 4 and 1.27 ± 0.07, p < 0.01, n = 4 for 0.5 and 1 μM respectively) at the protein level in BIO-treated endothelial cells (Fig. 4b and c).
Down-regulation of β-catenin signalling decreases p-glycoprotein expression in brain endothelial cells
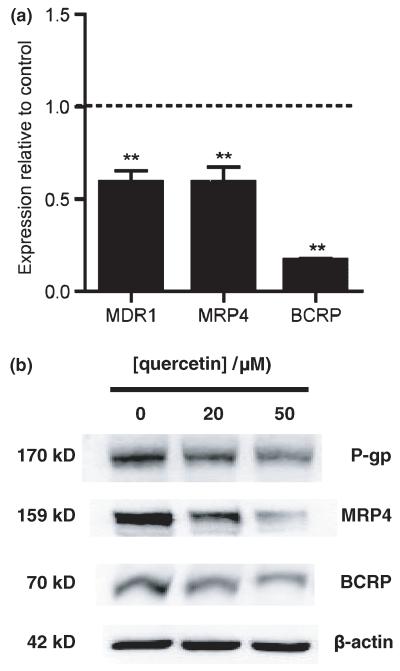
Quercetin was used to mimic down-regulation of β-catenin signalling. This inhibits the transcriptional activity of TCF/β-catenin by suppressing the binding of TCF to its specific DNA-binding sites (Park et al. 2005). Exposing hCMEC/D3 cells to 50 μM quercetin for 8 h resulted in significantly decreased expression at the transcript level of MDR1 (0.59 ± 0.06, p < 0.01, n = 4), MRP4 (0.59 ± 0.09, p < 0.01, n = 4) and BCRP (0.17 ± 0.01, p < 0.01, n = 4) (Fig. 5a). These decreases in efflux pump expression were confirmed at the protein level (Fig. 5b). Western blot analyses showed that expression levels of p-gp (0.80 ± 0.05, p < 0.05, n = 3 and 0.74 ± 0.06, p < 0.05, n = 3 at 20 and 50 μM respectively), MRP4 (0.84 ± 0.08, n = 3 and 0.67 ± 0.07, p < 0.05, n = 3 at 20 and 50 μM, respectively) and BCRP (0.85 ± 0.01, p < 0.01, n = 3 and 0.71 ± 0.04, p < 0.01, n = 3 at 20 and 50 μM respectively) decreased proportionately with quercetin dose compared with that in the untreated control.
Fig. 5.
Effect of quercetin on expression of ABC transporters, p-gp, MRP4 and BCRP in human immortalized brain endothelial cells. Cells were harvested 8 h following exposure to 50 μM quercetin and analysed at transcript level (a) for expression of MDR1, MRP4 and BCRP (**p < 0.01, n = 4 in each case). (b) Western blot analysis of lysates from cells 72 h following exposure to quercetin at the concentrations shown demonstrating decreases at the protein level in expression of p-gp, MRP4 and BCRP. The blots were probed sequentially with anti-p-gp monoclonal antibody C219, anti-MRP4 antibody, M41-10 and anti-BCRP antibody, BXP-21. Equal protein loading was verified by probing with anti-β-actin antibody (shown) and Ponceau staining (not shown). Images shown are representative of three independent experiments performed.
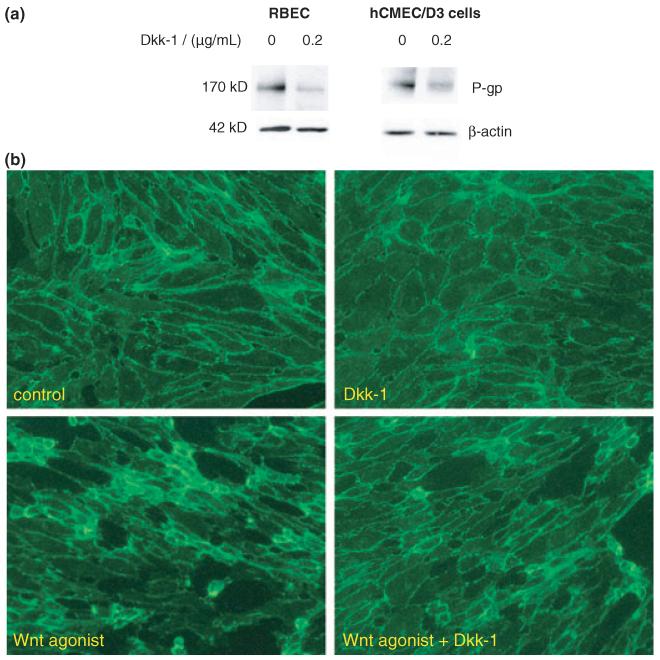
It is known that Wnt signalling pathways can be antagonized by application of the extracellular Wnt inhibitor Dkk-1. This is an endogenously produced inhibitor that binds to and sequesters the co-receptor low density lipoprotein receptor-related proteins 5/6 (LRP5/6) thereby inhibiting Wnt ligand-induced signal transduction and function (Mao et al. 2001). It was found that both RBECs (0.57 ± 0.08, p < 0.01, n = 5) and hCMEC/D3 cells (0.70 ± 0.04, p < 0.01, n = 3) exposed to Dkk-1 (0.2 μg/mL) for 24 h showed decreased levels of p-gp expression compared with that in control (Fig. 6a). As demonstrated by immunocytochemical staining in the hCMEC/D3 cells (Fig. 6b), Dkk-1 also reduced the extent of β-catenin nuclear/perinuclear staining and changes in cell morphology brought about by the pan-Wnt agonist.
Fig. 6.
Effect of Dkk-1 on expression of p-gp in rat brain endothelial cells (RBEC) and human immortalized brain endothelial cells. (a) Western blots showing decreased expression of p-gp in RBEC and in human immortalized brain endothelial cells (hCMEC/D3) following treatment with 0.2 μg/mL Dkk-1 for 24 h. The blots were probed with anti-p-gp monoclonal antibody C219 and then with anti-β-actin antibody to confirm equal protein loading per well. Images shown are representative of three independent experiments performed. (b) Immunocytochemical staining for β-catenin in human brain endothelial cells to show reduction in the presence of 0.2 μg/mL Dkk-1 of the nuclear/perinuclear localization brought about by 16 h exposure to 20 μM pan Wnt agonist.
Discussion
Previous studies have shown that components of the Wnt/β-catenin signalling pathway are expressed in vascular endothelial cells and influence endothelial cell proliferation and blood vessel formation and maturation (Wright et al. 1999; Masckauchan et al. 2005). This study explores the influence of downstream events in this pathway i.e. β-catenin signalling in a specialized type of vascular endothelial cell, i.e. the endothelial cell lining the brain blood vessels. Our results show that enhancing β-catenin signalling leads to increases in expression of certain multidrug efflux ABC transporters that provide important blood–brain barrier properties to the brain endothelial cells. This is of some interest as components of the Wnt signalling pathway are present in the developing brain blood vessels at the stage at which one of these transporters i.e. p-gp is known to appear (Qin and Sato 1995). This multidrug efflux ABC transporter plays a major role in protection at the blood–brain barrier. Hence its appearance and regulation are important factors in maturation and maintenance of barrier function. Previous studies in other tissues i.e. in intestinal epithelial cells (Yamada et al. 2000) have linked the regulation of MDR1 expression and the β-catenin pathway. The results shown in this study suggest very strongly that β-catenin signalling also contributes to regulation of p-gp expression in brain vasculature.
We show that agents causing activation of β-catenin signalling (inhibitors of GSK-3β, BIO, LiCl and azakenpaullone) can lead to increases in p-gp expression. Interestingly, an agonist at Wnt receptors (pan-Wnt agonist) produced a similar response. By contrast, exposure to quercetin that inhibit β-catenin signalling responses at the transcriptional level resulted in decreases in p-gp expression. The Wnt antagonist (Dkk-1) that blocks the Wnt ligand receptor complex also brought about a decrease in expression. We do not claim any of these agents to have just this single mechanism of action (lithium in particular has multiple modes of action). However, we have demonstrated that stimulators of β-catenin signalling such as BIO up-regulate p-gp expression, stabilize β-catenin levels in the nucleus and activate reporter promoters in a β-catenin/TCF-dependent manner. Hence our collective results would suggest very strongly that it is alterations to β-catenin signalling that are responsible for the changes seen in p-gp expression.
It has yet to be proven that alterations to canonical Wnt/β-catenin signalling would have the same effect. However, the decreases seen in the presence of the Wnt antagonist Dkk-1 is certainly suggestive. Further, the results of the quercetin and Dkk-1 experiments in which down-regulation of p-gp was observed in the absence of applied agonist also suggest strongly that there may be some autocrine activation of the signalling pathways taking place in these cultured endothelial cells. Certainly, there is a report showing that cultured endothelial cells express multiple ligands, receptors and secreted modulators of the canonical Wnt signalling pathway (Goodwin et al. 2006).
It is interesting to note that manipulations to the β-catenin signalling pathway produced similar changes in expression in endothelial cells of both rat and of human origin. There were however differences in sensitivity to the GSK-3β inhibitor, BIO. The human cells responded at low concentrations and brief exposure whilst the RBECs needed higher concentrations and longer duration of exposure to show the same type of response. When similar conditions were applied to the human cells this resulted in toxicity. These apparent species differences may imply that the human GSK-3β enzyme is more sensitive to BIO than that of the rat.
The increases in p-gp expression were shown to be of functional significance as an increase in efflux capability was also evident. This was revealed by exposing the BIO-treated cells to calcein-AM and measuring their capacity to accumulate the fluorescent hydrolysed derivative, calcein. The difference in accumulation in presence and absence of the p-gp inhibitor verapamil was seen to be much greater in the BIO-treated than in the untreated cells, showing a greater capacity for p-gp-mediated efflux of the calcein-AM.
It is interesting to note that only the mdr1a and not the mdr1b isoform of rat p-gp was affected by manipulation of β-catenin signalling. This is the same form that was influenced in rat brain endothelial cells by neural precursor cells (Lim et al. 2007). By contrast, both forms respond by up-regulation following exposure to oxidative challenge (Robertson unpublished).
Manipulation of β-catenin signalling also affected expression of other ABC transporters i.e. MRP4 and BCRP known to be present at the blood–brain barrier. This was evident at both transcript and protein levels. Other ABC transporters investigated i.e. MRP1 and MRP5 were however not affected. These two transporters are expressed to only very low levels in brain endothelial cells and are not thought to play a major role in protection at the blood–brain barrier. They are much more highly represented in other brain cell types e.g. astrocytes. It is interesting to note the similar responses of both MRP4 and BCRP to up-regulation of β-catenin with BIO or to blockade of its transcriptional activity with quercetin. Compared with MDR1, there is as yet relatively little known about transcriptional regulation of BCRP and MRP4 (Scotto 2003). There has been some characterization of the BCRP promoter (Bailey-Dell et al. 2001; Zhang et al. 2007) but no reports of the presence of TCF4 binding sequences in the promoter regions of either BCRP or MRP4. Further investigations using reporter assays with the appropriate promoters are thus warranted and are now ongoing.
Up-regulation of p-gp is here observed at mRNA as well as protein level and via activation of its promoter, suggesting that transcriptional events are involved. Many of the numerous studies of p-gp transcriptional activation have focussed on cancer cells and observed that general changes in chromatin structure and histone methylation and/or acetylation lead to changes in the levels of transcriptional activity. Here, we report up-regulation of p-gp by more specific pathways i.e. via β-catenin signalling. Such a pathway may operate in regulating p-gp expression both spatially i.e. at the specific location of the blood–brain barrier and temporally i.e. during brain development. Whether other barrier characteristics, in particular the tight junctions, are similarly influenced by this signalling pathway has yet to be determined though evidence from other studies would suggest that at least some of the factors regulating p-gp and tight junctions do differ (Lim et al. 2007).
In conclusion, the results reported here suggest that β-catenin signalling can significantly influences expression of key components of the blood–brain barrier i.e. the ABC efflux transporters. Such influences have implications for blood–brain barrier integrity following use of drugs that affect such signalling e.g. GSK-3β inhibitors. It remains to be confirmed by more extensive studies whether the upstream activation of this signalling involves Wnt pathways.
Supplementary Material
Acknowledgements
This work was supported by BBSRC grant (S19517). JCL holds scholarships from Gates Cambridge Trust and Overseas Research Students Award Scheme (ORS), HCW from ORS, Cambridge Commonwealth Trust and Corpus Christi College Cambridge and KDK from Polish Ministry of Science and Higher Education. SC is a BBSRC David Phillips Fellow.
Abbreviations used
- ABC
ATP-binding cassette
- AM
acetoxymethyl
- BCRP
breast cancer resistant protein
- BIO
6-bromoindirubin-3′-oxime
- DAPI
4,6-diamidino-2-phenylindole
- dnTCF4
dominant negative mutant TCF4
- GSK-3
glycogen synthase kinase
- MRP
multidrug resistance protein
- p-gp
p-glycoprotein
- RBECs
rat brain endothelial cells
- TCF4
T-cell factor 4
- Wnt agonist
2-amino-4-(3,4-(methylenedioxy) benzylamino)-6-(3-methoxyphenyl)pyrimidine
Footnotes
The following supplementary material is available for this article:
Table S1 Primers used in the polymerase chain reaction.
This material is available as part of the online article from: http://www.blackwell-synergy.com/doi/abs/10.1111/j.1471-4159.2008.05537.x. (This link will take you to the article abstract).
Please note: Blackwell Publishing is not responsible for the content or functionality of any supplementary materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Afonso PV, Ozden S, Prevost M-C, Schmitt C, Seilhean D, Weksler B, Couraud P-O, Gessain A, Romero IA, Ceccaldi PE. Human blood–brain barrier disruption by retroviral-infected lymphocytes: role of myosin light chain kinase in endothelial tight-junction disorganisation. J. Immunol. 2007;179:2576–2583. doi: 10.4049/jimmunol.179.4.2576. [DOI] [PubMed] [Google Scholar]
- Bailey-Dell KJ, Hassel B, Doyle LA, Ross DD. Promoter characterization and genomic organization of the human breast cancer resistance protein (ATP-binding cassette transporter G2) gene. Biochim. Biophys. Acta. 2001;1520:234–241. doi: 10.1016/s0167-4781(01)00270-6. [DOI] [PubMed] [Google Scholar]
- Cucullo L, Couraud P-O, Weklser B, Romero IA, Hossain M, Rapp E, Janigro D. Immortalized human brain endothelial cells and flow-based vascular modeling: a marriage of convenience for rational neurovascular studies. J. Cereb. Blood Flow Metab. 2007;28:312–328. doi: 10.1038/sj.jcbfm.9600525. [DOI] [PubMed] [Google Scholar]
- Goodwin AM, Sullivan KM, D’Amore PA. Cultured endothelial cells display endogenous activation of the canonical Wnt signaling pathway and express multiple ligands, receptors, and secreted modulators of Wnt signaling. Dev. Dyn. 2006;235:3110–3120. doi: 10.1002/dvdy.20939. [DOI] [PubMed] [Google Scholar]
- Hagen T, Sethi JK, Foxwell N, Vidal-Puig A. Signalling activity of β-catenin targeted to different subcellular compartments. Biochem. J. 2004;379:471–477. doi: 10.1042/BJ20031749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holló Z, Homolya L, Davis CW, Sarkadi B. Calcein accumulation as a fluorometric functional assay of the multidrug transporter. Biochim. Biophys. Acta. 1994;1191:384–388. doi: 10.1016/0005-2736(94)90190-2. [DOI] [PubMed] [Google Scholar]
- Leost M, Schultz C, Link A, et al. Paullones are potent inhibitors of glycogen synthase kinase-3beta and cyclin-dependent kinase 5/p25. Eur. J. Biochem. 2000;267:5983–5994. doi: 10.1046/j.1432-1327.2000.01673.x. [DOI] [PubMed] [Google Scholar]
- Lim JC, Wolpaw AJ, Caldwell MA, Hladky SB, Barrand MA. Neural precursor cell influences on blood–brain barrier characteristics in rat brain endothelial cells. Brain Res. 2007;1159:67–76. doi: 10.1016/j.brainres.2007.05.032. [DOI] [PubMed] [Google Scholar]
- Liu J, Wu X, Mitchell B, Kintner C, Ding S, Schultz PG. A small-molecule agonist of the Wnt signaling pathway. Angew. Chem. Int. Ed. Engl. 2005;44:1987–1990. doi: 10.1002/anie.200462552. [DOI] [PubMed] [Google Scholar]
- Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu. Rev. Cell Dev. Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- Longo KA, Kennell JA, Ochocinska MJ, Ross SE, Wright WS, MacDougald OA. Wnt signaling protects 3T3-L1 preadipocytes from apoptosis through induction of insulin-like growth factors. J. Biol. Chem. 2002;277:38239–38244. doi: 10.1074/jbc.M206402200. [DOI] [PubMed] [Google Scholar]
- Mao B, Wu W, Li Y, Hoppe D, Stannek P, Glinka A, Niehrs C. LDL-receptor-related protein 6 is a receptor for Dickkopf proteins. Nature. 2001;411:321–325. doi: 10.1038/35077108. [DOI] [PubMed] [Google Scholar]
- Maretto S, Cordenonsi M, Dupont S, Braghetta P, Broccoli V, Hassan AB, Volpin D, Bressan GM, Piccolo S. Mapping Wnt/beta-catenin signaling during mouse development and in colorectal tumors. Proc. Natl Acad. Sci. USA. 2003;100:3299–3304. doi: 10.1073/pnas.0434590100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masckauchan TN, Kitajewski J. Wnt/Frizzled signaling in the vasculature: new angiogenic factors in sight. Physiology (Bethesda) 2006;21:181–188. doi: 10.1152/physiol.00058.2005. [DOI] [PubMed] [Google Scholar]
- Masckauchan TN, Shawber CJ, Funahashi Y, Li CM, Kitajewski J. Wnt/beta-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis. 2005;8:43–51. doi: 10.1007/s10456-005-5612-9. [DOI] [PubMed] [Google Scholar]
- Mei Q, Richards K, Strong-Basalyga K, Fauty SE, Taylor A, Yamazaki M, Prueksaritanont T, Lin J.h., Hochman J. Using real-time quantitative TaqMan RT-PCR to evaluate the role of dexamethasone in gene regulation of rat P-glycoproteins mdr1a/1b and cytochrome P450 3A1/2. J. Pharmaceut. Sci. 2004;93:2488–2496. doi: 10.1002/jps.20102. [DOI] [PubMed] [Google Scholar]
- Meijer L, Flajolet M, Greengard P. Pharmacological initiators of glycogen synthase kinase 3. Trends Pharmacol. Sci. 2004;25:471–480. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Park CH, Chang JY, Hahm ER, Park S, Kim HK, Yang CH. Quercetin, a potent inhibitor against beta-catenin/Tcf signaling in SW480 colon cancer cells. Biochem. Biophys. Res. Commun. 2005;328:227–234. doi: 10.1016/j.bbrc.2004.12.151. [DOI] [PubMed] [Google Scholar]
- Pattyn F, Robbrecht P, Speleman F, De Paepe A, Vandesompele J. RTPrimerDB: the real-time PCR primer and probe data base, major update 2006. Nucleic Acids Res. 2006;34(Database issue):D684–D688. doi: 10.1093/nar/gkj155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perriere N, Demeuse PH, Garcia E, et al. Puromycin-based purification of rat brain capillary endothelial cell cultures. Effect on the expression of blood–brain barrier-specific properties. J. Neurochem. 2005;93:279–289. doi: 10.1111/j.1471-4159.2004.03020.x. [DOI] [PubMed] [Google Scholar]
- Pulaski L, Kania K, Ratajewski M, Uchiumi T, Kuwano M, Bartosz G. Differential regulation of the human MRP2 and MRP3 gene expression by glucocorticoids. J. Steroid Biochem. Mol. Biol. 2005;96:229–234. doi: 10.1016/j.jsbmb.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Qin Y, Sato TN. Mouse multidrug resistance 1a/3 gene is the earliest known endothelial cell differentiation marker during blood–brain barrier development. Dev. Dyn. 1995;202:172–180. doi: 10.1002/aja.1002020209. [DOI] [PubMed] [Google Scholar]
- Schinkel AH. P-glycoprotein, a gatekeeper in the blood–brain barrier. Adv. Drug Deliv. Rev. 1999;36:179–194. doi: 10.1016/s0169-409x(98)00085-4. [DOI] [PubMed] [Google Scholar]
- Schreibelt G, Kooij G, Reijerkerk A, et al. Reactive oxygen species alter brain endothelial tight junction dynamics via RhoA, PI3 kinase, and PKB signaling. FASEB J. 2007;21:1–11. doi: 10.1096/fj.07-8329com. [DOI] [PubMed] [Google Scholar]
- Scotto KW. Transcriptional regulation of ABC drug transporters. Oncogene. 2003;22:7496–7511. doi: 10.1038/sj.onc.1206950. [DOI] [PubMed] [Google Scholar]
- Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, Ben-Ze’ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl Acad. Sci. USA. 1999;96:5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stambolic V, Ruel L, Woodgett JR. Lithium inhibits glycogen synthase kinase-3 activity and mimics wingless signalling in intact cells. Curr. Biol. 1996;6:1664–1668. doi: 10.1016/s0960-9822(02)70790-2. [DOI] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:1–12. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksler BB, Subileau EA, Perriere N, et al. Blood–brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- Wright M, Aikawa M, Szeto W, Papkoff J. Identification of a Wnt-responsive signal transduction pathway in primary endothelial cells. Biochem. Biophys. Res. Commun. 1999;263:384–388. doi: 10.1006/bbrc.1999.1344. [DOI] [PubMed] [Google Scholar]
- Yamada T, Takaoka AS, Naishiro Y, Hayashi R, Maruyama K, Maesawa C, Ochiai A, Hirohashi S. Transactivation of the multidrug resistance 1 gene by T-cell factor 4/beta-catenin complex in early colorectal carcinogenesis. Cancer Res. 2000;60:4761–4766. [PubMed] [Google Scholar]
- Zhang Y, Zhou G, Wang H, Zhang X, Wei F, Cai Y, Yin D. Transcriptional upregulation of breast cancer resistance protein by 17beta-estradiol in ERalpha-positive cancer cells. Oncology. 2007;71:446–455. doi: 10.1159/000108594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.