Abstract
Extreme oncoplasty is a breast conserving operation, using oncoplastic techniques, in a patient who, in most physicians' opinions, requires a mastectomy. These are generally large, greater than 5 cm multifocal or multicentric tumors. Many will have positive lymph nodes. Most will require radiation therapy, even if treated with mastectomy. Sixty-six consecutive patients with multifocal, multicentric, or locally advanced tumors that spanned more than 50 mm were studied (extreme cases). All patients underwent excision and oncoplastic reconstruction using a standard or split wise pattern reduction and immediate contralateral surgery for symmetry. All received postexcisional standard whole breast radiation therapy with a boost to the tumor bed. The extreme cases were compared with 245 consecutive patients with unifocal or multifocal tumors that spanned 50 mm or less (standard cases). All extreme patients were advised to have a mastectomy; all sought a breast conserving second opinion. Diagnostic evaluation included digital mammography, ultrasound, MRI, and PET-CT (if invasive). Standard cases did extremely well. No ink on tumor was achieved 96% of the time among 245 patients. The median tumor size was 21 mm (mean 23 mm). Margins equal or greater than 1 mm were achieved in 88.6% of patients. Seventeen (6.9%) standard patients underwent re-excision to achieve wider margins and only one patient (0.4%) was converted to mastectomy. With 24 months of median follow-up, three patients (1.2%) experienced local recurrence. For extreme cases, no ink on tumor was achieved 83.3% of the time, which is comparable to published positive margin rates after standard lumpectomy. The median tumor size was 62 mm (mean 77 mm). Margins equal or greater than 1 mm were achieved in 54.5% of patients. Six (9.1%) extreme patients underwent re-excision to achieve wider margins and four patients (6.1%) were converted to mastectomy. With a follow-up of 24 months, one patient (1.5%) experienced a local recurrence. Extreme oncoplasty is a promising new concept. It allows successful breast conservation in selected patients with greater than 5 cm multifocal/multicentric tumors. It may be useful in patients with locally advanced tumors following neo-adjuvant chemotherapy. From a quality of life point of view, it is a better option than the combination of mastectomy, reconstruction, and radiation therapy. Long-term data on recurrence and survival are not available, using this approach. Based on historical data, it is expected the local recurrence will be somewhat higher but that there will be little or no impact on survival.
Keywords: avoiding mastectomy, breast conservation for large tumors, extreme oncoplasty, multifocal/multicentric breast tumors, oncoplastic breast conservation
For 100 years, mastectomy reigned as the only treatment for breast cancer. Then, during the 1970s and 1980s, prospective randomized trials confirmed survival equivalency for breast conservation when compared with mastectomy for patients with tumors 5 cm or smaller 1–6. Although survival was equal, there was a higher local recurrence rate with breast conservation therapy. This was accepted in exchange for a better cosmetic and sensual result, and a happier, more intact patient.
During the last 30 years, there has been significant progress in breast cancer diagnosis and treatment. This includes earlier diagnosis, better hormonal and chemotherapy, improved radiation therapy techniques, and an increased understanding of breast cancer biology and genomics. This progress has led to improved overall and breast cancer specific survival. In addition, it has yielded decreased rates of local recurrence after both mastectomy and breast conservation. Recent prospective randomized trials have reported local recurrence rates less than 1.5% at 5 years for patients randomized to excision plus standard whole breast radiation therapy 7,8. With local recurrence rates this low, breast conservation should be the routine default approach for breast cancer treatment, unless there are compelling reasons to perform a mastectomy.
Prospective randomized data supporting breast conservation exist only for patients with tumors 5 cm or smaller 1–6. Because of this, many patients with larger tumors are denied a chance to pursue breast conservation. When breast conservation is performed for patients with larger tumors, it requires a larger resection which may yield a poor cosmetic result. Neo-adjuvant therapy, to reduce the size of the primary lesion, will convert some tumors to a more appropriate size. For selected patients with larger tumor spans, the surgical answer may be extreme oncoplasty.
Extreme oncoplasty is a breast conserving operation, using oncoplastic techniques, in a patient who, in most physicians' opinions, requires a mastectomy. These are generally large, greater than 5 cm multifocal or multicentric tumors. They may be locally advanced. Many will have positive lymph nodes. Most of these patients will require radiation therapy, even if they are treated with mastectomy. The reason to save a breast like this is that in many cases, breast conservation may be a better alternative.
Oncoplastic reconstruction generally yields a cosmetic result superior to a mastectomy with immediate reconstruction and radiation therapy. There is less operative and postoperative morbidity with extreme oncoplasty, and finally, radiation therapy is far kinder to breast conservation than to mastectomy with reconstruction 9,10.
This paper reviews a series of extreme oncoplasty patients with large, greater than 5 cm tumors, all of whom were advised to have a mastectomy and compares them with a group of patients with smaller tumors, 5 cm or less, treated with breast conservation using a reduction mammoplasty.
Methods
Sixty-six consecutive patients with multifocal, multicentric, or locally advanced tumors that spanned more than 50 mm were studied. All patients were advised to have a mastectomy and all sought a breast conserving second opinion. Diagnostic evaluation included digital mammography, ultrasound, MRI, and PET-CT (if the lesion was invasive). All patients received multi-disciplinary consultation: including a breast surgical oncologist, plastic surgeon, medical oncologist, and radiation oncologist. These 66 patients are referred to as extreme cases.
All patients underwent excision and oncoplastic reconstruction using a standard wise pattern reduction or split reduction procedure 11, and immediate contralateral surgery for symmetry. All received postexcisional standard whole breast radiation therapy with a boost to the tumor bed. The extreme cases were compared with 245 consecutive patients with unifocal or multifocal tumors that spanned 50 mm or less (standard cases). Groups were compared using 2 × 2 tables and the t-test for independent variables.
Results
Standard cases did extremely well. The current standard for adequate margins, no ink on tumor 12 was achieved 96% of the time among 245 patients. The median tumor size was 21 mm (mean 23 mm). Margins equal or greater than 1 mm were achieved in 88.6% of patients. Seventeen (6.9%) standard patients underwent re-excision to achieve wider margins and only one patient (0.4%) was converted to mastectomy. With an extremely short average follow-up of only 24 months, three patients (1.2%) experienced local recurrence.
For extreme cases, no ink on tumor was achieved 83.3% of the time among 66 patients, which is comparable to published positive margin rates after standard lumpectomy. The median tumor size was 62 mm (mean 77 mm). Margins equal or greater than 1 mm were achieved in 54.5% of patients. Six (9.1%) extreme patients underwent re-excision to achieve wider margins and four patients (6.1%) were converted to mastectomy. With a follow-up of 24 months, one patient (1.5%) experienced a local recurrence. Standard and extreme cases are compared in Table1. Examples of three extreme cases are shown below.
Table 1.
Standard Oncoplasty versus Extreme Oncoplasty
| Standard (≤50 mm) | Extreme (>50 mm) | p-value | |
|---|---|---|---|
| N | 245 | 66 | |
| Mean weight | 142 g | 217 g | <0.01 |
| Mean span | 23 mm | 77 mm | <0.01 |
| No ink on tumor | 236/245 (96%) | 55/66 (83.3%) | <0.01 |
| Margins close but clear (0.1–0.9 mm) | 19/245 (7.8%) | 19/66 (28.8%) | <0.01 |
| Margins (≥1 mm) | 217/245 (88.6%) | 36/66 (54.5%) | <0.01 |
| Re-excision | 17/245 (6.9%) | 6/66 (9.1%) | NS |
| Mastectomy | 1/245 (0.4%) | 4/66 (6.1%) | <0.01 |
| Any local recurrence | 3/245 (1.2%) | 1/66 (1.5%) | NS |
| Mean FU | 24 months | 24 months | NS |
Discussion
In our opinion, the least cosmetically acceptable operation that we do for breast cancer today is the combination of mastectomy, immediate reconstruction, and radiation therapy. While the combination is often necessary and appropriate, it is the last option that we want to offer. Radiation therapy is not kind to the implant reconstruction process. If a tissue expander is used, there are issues regarding timing of expansion versus timing of the radiation therapy 9. At what point does chemotherapy fit into the process and how does chemotherapy affect the sequence of expansion and radiation therapy? Reconstruction generally requires multiple procedures, which then have to be coordinated with other therapies. At what point does the definitive implant go in? How many implant reconstructions fail after radiation therapy?
If an autologous flap is used, it becomes a much longer operation with significant complications and morbidity and a longer hospital stay. If there is delayed healing, it will affect the timing of chemotherapy and radiation therapy. The flap, which may not be of adequate volume, may shrink after radiation therapy. The end result, while often excellent, is generally less optimal than what is routinely achieved with breast conservation.
If radiation therapy is not used after mastectomy, the cosmetic result is better but there is a rim of breast tissue beneath the skin that is left untreated. This includes dermal lymphatics, which may contribute to recurrence.
A patient with a mastectomy is reminded on a daily basis that she has had breast cancer. Every time she dresses, looks at, or touches her surgically made breast, she remembers she has had breast cancer. The reconstructed breast following mastectomy is functional only in the respect that it appears normal in clothes. It is not normal without clothes and it is generally insensate.
Alternatively, a patient with good cosmetic result following breast conservation does not face the daily reminder of breast cancer. At some point in her future, when chemotherapy and radiation therapy have finished, and additional time has passed, she is simply a normal woman with normal, sensate breasts. Her hair has grown back. Her body looks and feels normal. The conservatively treated, preserved breast can appear completely normal with or without clothes. Hopefully, she thinks about breast cancer only when she sees it discussed on television or when it is time to see her oncologist or breast surgeon.
Breast conservation yields a better quality of life than the combination of mastectomy, reconstruction and radiation therapy 13. A reconstructed breast after mastectomy has minimal or no sensation. Breast conservation generally results in little or no sensory loss.
Breast conservation can generally be done in one operation without drains, as an outpatient surgery. It instantly looks better than a mastectomy. There is less postoperative pain and it is less expensive for the patient as multiple operations and procedures are avoided. There is no foreign body and no donor site. It is more functional when compared with mastectomy and more sensate. The patient often has a better-perceived body image. For these reasons, if it is technically possible and oncologically reasonable, we will always consider breast conservation as the first option for our patients.
Whether for standard or extreme cases, it is important to maintain a multi-disciplinary approach. There are many patients who are relegated to mastectomy as the only option, simply due to an erroneous judgment that a deformity would inevitably result with breast conservation. These patients are referred for plastic surgical consultation with a plan for mastectomy already in place, and therefore are counseled as such. In fact, many of these patients could have breast conservation if sophisticated oncoplastic techniques are employed.
The use of radiation therapy following mastectomy has increased during the last few years following the publication of papers that document not only decreased local recurrence but also increased survival 14,15. Post-mastectomy irradiation is further complicated when immediate reconstruction has been performed. Radiation therapy has long been known to cause skin burning, fibrosis, loss of elasticity, and contraction. When radiation therapy must be given, the results are generally better with autologous tissue, most often a free flap. For most patients, microvascular plastic surgeons are not available or the cost is prohibitive. Accordingly, the majority of reconstructions are device based, performed with a tissue expander followed by a definitive implant. Irradiated implant reconstructions carry a much higher failure rate and lead to multiple additional treatments and surgeries to attempt to salvage them. Regardless of how the reconstruction is performed, the overall functional (esthetic and sensory) outcome is seldom as good as that achieved by oncoplastic reduction excision.
The rationale for breast conserving therapy comes from a group of prospective randomized trials performed in the 1970s 2,4–6. In these trials, the maximum tumor size allowed was 5 cm. When breast preservation is performed in patients with tumors larger than 5 cm, there are no prospective randomized data to support it. Nevertheless, it is commonly done. When breast conservation was first adopted, the recurrence rates were higher for those randomized to BCT compared with mastectomy. Inspite of higher local recurrence rates, survival at 20 years was similar 1,3. Surgeons and patients have long accepted a higher local recurrence rate in exchange for a better esthetic and sensory outcome and a happier patient with no decrease in survival.
Extreme oncoplasty pushes the envelope further. Our patients were all offered mastectomy, the current standard for disease greater than 5 cm. They all declined and sought a modern breast conserving approach. In the past, excising large tumors with an adequate margin generally meant a poor cosmetic result. The use of standard and modified reduction excisions and oncoplastic reconstruction dramatically increases the probability of complete excision with an acceptable esthetic outcome. Moreover, now that the standard for an adequate margin has been relaxed to no ink on tumor 10, the probability of a successful outcome increases. In this series, 83.3% of tumors with a median size of 62 mm (mean 77 mm) were excised with no ink on tumor.
Only four of 66 (6.1%) patients who attempted to save their breast after being advised to have a mastectomy, were converted to mastectomy after final pathology was reviewed. All four had multiple positive or close margins. An additional six patients (9.1%) underwent re-excision and then continued on with BCT. One patient developed a local recurrence.
For selected patients who need a mastectomy based on current standards, such as patients with large multifocal or multicentric tumors, those with small breast size relative to tumor extent, those with locally advanced tumors, or those with a previously irradiated breast that develops local recurrence or a new cancer, the alternative for some of them may be extreme oncoplasty.
Conclusions
Extreme Oncoplasty is a new promising concept. It allows successful breast conservation in selected patients with large greater than 5 cm multifocal/multicentric tumors. In addition, it may be useful in patients with locally advanced tumors. From a quality of life point of view, it is a better option than the combination of mastectomy, reconstruction, and radiation therapy. Long-term data on recurrence and survival are not available. Based on historical data, it is expected the local recurrence will be somewhat higher but that there will be little or no impact on survival.
Appendix
Examples of Extreme Oncoplasty
Extreme Case 1: A 65-year-old patient was discovered to have multiple left breast masses spanning approximate 90 mm. Ultrasound guided core biopsy of three of the lesions all revealed low-grade invasive ductal carcinoma. Mastectomy was suggested because of the large span of disease. She sought a second breast conserving option.
Figure 1.
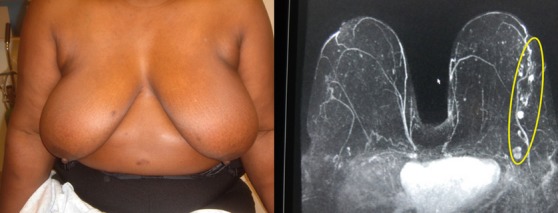
(Left) 65-year old with multiple left breast masses spanning 9 cm discovered on screening mammography, but best seen on MRI (Right).
Figure 2.
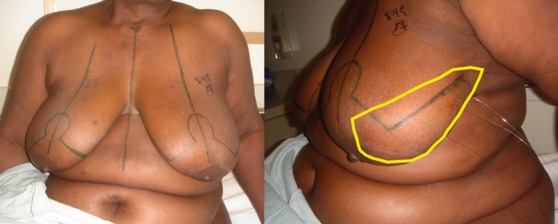
(Left) Marked for a standard left reduction excision with contralateral right reduction for symmetry. (Right) Four bracketing guide wires are in place. A large segment of overlying skin will be removed with underlying breast to include pectoral fascia.
Figure 3.
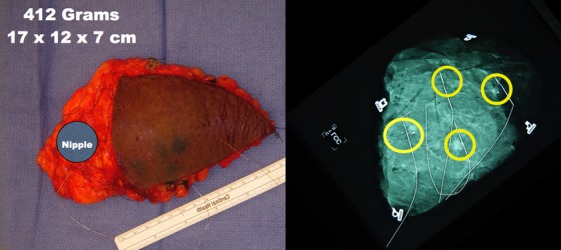
(Left) A 412-gram specimen was removed. The approximate position of the nipple is marked. (Right) Specimen radiograph show four suspicious areas. Final pathology revealed nine foci of invasion. The largest was 12 mm. There was extensive low-grade DCIS spanning 90 mm. All margins greater than 10 mm. Three sentinel lymph nodes were negative.
Figure 4.
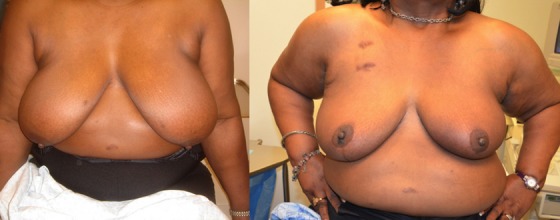
Preop (Left) and 4 years postop (Right). She received chemotherapy and whole breast radiation therapy. There is minimal skin hyperpigmentation on the left and mild breast shrinkage secondary to radiation therapy. There is no evidence of local or distant recurrence.
Extreme Case 2: 48-year old with multiple abnormalities detected on screening mammography. Biopsy of three suspicious areas revealed invasive lobular carcinoma in two and atypical ductal hyperplasia in the third. The disease spanned 81 mm and was in two quadrants. Mastectomy was suggested. She sought an alternative breast conserving approach.
Figure 5.
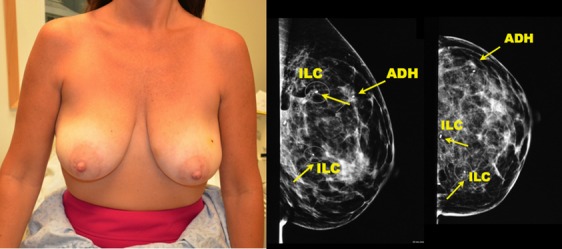
48-year old with multiple abnormalities detected on screening. Mammogram shows multicentric lesions spanning 81 mm. Biopsy revealed two foci of invasive lobular carcinoma and one focus of atypical ductal hyperplasia.
Figure 6.
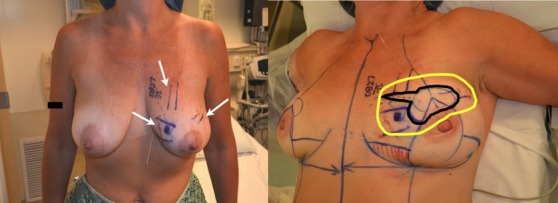
(Left) Two bracketing guide wires have been placed around each lesion. (Right) The patient has been marked for a split reduction excision. The inner black line shows skin that will be removed over the tumors. The outer yellow line shows amount of tissue that will be removed.
Figure 7.
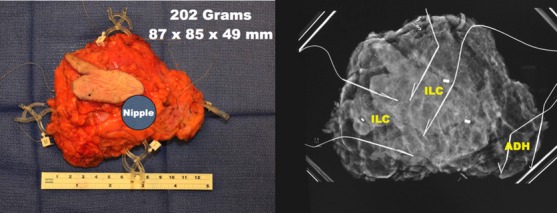
(Left) 202-gram specimen. (Right) Specimen radiograph. Final pathology revealed two foci of invasive lobular carcinoma spanning 42 mm. With ADH, the entire span was 81 mm. Closest margin was 5 mm. There were two negative sentinel lymph nodes.
Figure 8.
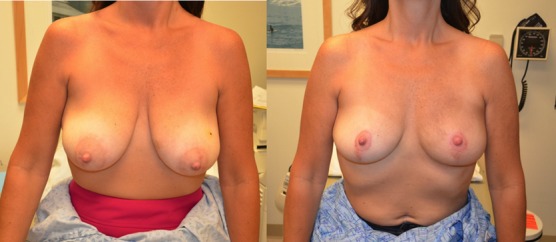
(Left) Preop. (Right) 6 months Postop (3 months post-radiation therapy).
Extreme Case 3: A 42-year old presented with a 6-cm palpable left breast mass, multiple palpable suspicious left axillary lymph nodes, and left breast skin thickening. Core biopsy revealed high-grade invasive ductal carcinoma, ER/PR negative, and HER2 amplified; Ki67 = 80%.
Figure 9.
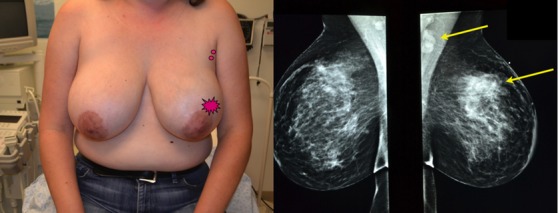
(Left) 42-year old with 6 cm palpable left breast mass and palpable left axillary nodes. Skin thickening present. (Right) Mammogram shows large left breast lesion and suspicious left axillary lymph nodes. Core biopsy reveals high-grade invasive ductal carcinoma (SBR 9/9). ER/PR negative, HER2 amplified. Ki67 = 80%. She was treated with neo-adjuvant Herceptin-based chemotherapy.
Figure 10.
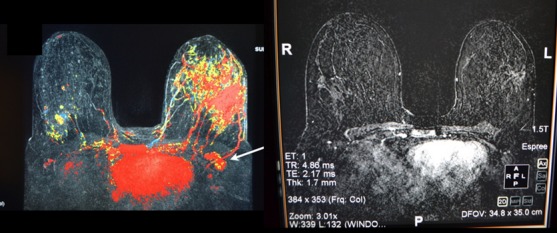
(Left) Preneo-adjuvant chemotherapy MRI confirms extensive left breast lesion. (Right) Postneo-adjuvant chemotherapy MRI shows near complete resolution.
Figure 11.
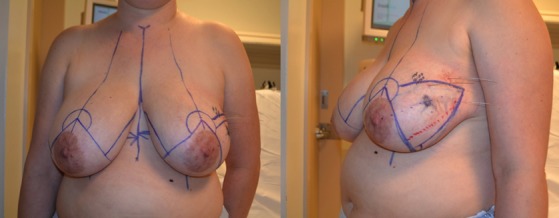
(Left) She has been marked for a split reduction excision. (Right) Lateral view shows a large triangle of skin and underlying breast tissue that will be removed as part of the split reduction.
Figure 12.

(Left) 298-gram specimen. (Right) Specimen radiograph. Microclip marking the lesion is central along with microcalcifications. Final pathology revealed no residual invasive breast cancer. There were a few small scattered foci of residual DCIS. Lymph nodes were negative.
Figure 13.
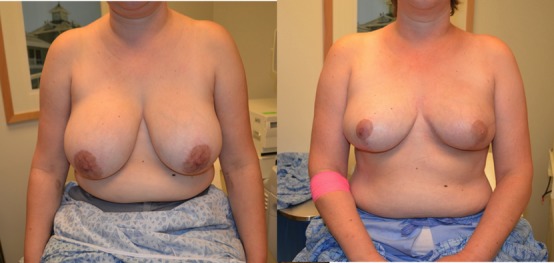
(Left) Preop. (Right) she is 2 years postop and postradiation therapy. MRI and recent PET-CT negative.
References
- 1.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347:1233–41. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 2.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and lumpectomy with or without radiation therapy in the treatment of breast cancer. N Eng J Med. 1985;312:665–73. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- 3.Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347:1227–32. doi: 10.1056/NEJMoa020989. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U, Saccozzi R, Del Vecchio M, et al. Comparing radical mastectomy with quadrantectomy, axillary dissection and radiotherapy in patients with small cancers of the breast. N Engl J Med. 1981;305:6–10. doi: 10.1056/NEJM198107023050102. [DOI] [PubMed] [Google Scholar]
- 5.Lichter A, Lippman M, Danforth D, et al. Mastectomy versus breast conserving therapy in the treatment of stage I and II carcinoma of the breast: a randomized trial at The National Cancer Institute. J Clin Oncol. 1992;10:976–82. doi: 10.1200/JCO.1992.10.6.976. [DOI] [PubMed] [Google Scholar]
- 6.Van Dongen J, Bartelink H, Fentiman I, et al. Randomized clinical trial to assess the value of breast-conserving therapy in stage I and II breast cancer, EORTC 10801 trial. Monogr Natl Cancer Inst. 1992;11:8–15. [PubMed] [Google Scholar]
- 7.Vaidya J, Wenz F, Bulsara M, et al. Risk-adapted targited intraoperative radiotherapy versus whole-breast radiotherapy for breast cancer: 5-year results for local control and overall survival from the TARGIT-A randomised trial. Lancet. 2013;383:603–13. doi: 10.1016/S0140-6736(13)61950-9. [DOI] [PubMed] [Google Scholar]
- 8.Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol. 2013;14:1269–77. doi: 10.1016/S1470-2045(13)70497-2. [DOI] [PubMed] [Google Scholar]
- 9.Behranwala KA, Dua RS, Ross GM, et al. The influence of radiotherapy on capsule formation and aesthetic outcome after immediate breast reconstruction using biodimensional anatomical expander implants. J Plast Reconstr Aesthet Surg. 2006;59:1043–51. doi: 10.1016/j.bjps.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 10.Boughey JC, Hoskin TL, Hartmann LC, et al. Impact of reconstruction and reoperation on long-term patient-reported satisfaction after contralateral prophylactic mastectomy. Ann Surg Oncol. 2014 doi: 10.1245/s10434-014-4053-3. ; DOI: 10.1245/s10434-014-4053-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Silverstein M, Mai T, Savalia N, Vaince F, Guerra L. Oncoplastic breast conservation surgery: the new paradigm. J Surg Oncol. 2014;110:82–9. doi: 10.1002/jso.23641. [DOI] [PubMed] [Google Scholar]
- 12.Moran M, Schnitt S, Giuliano A, et al. Society of Surgical OncologyeAmerican Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. Int J Radiation Oncol Biol Phys. 2014;88:553–64. doi: 10.1016/j.ijrobp.2013.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hau E, Browne L, Capp A, et al. The impact of breast cosmetic and functional outcomes on quality of life: long-term results from the St. George and Wollongong randomized breast boost trial. Breast Cancer Res Treat. 2013;139:115–23. doi: 10.1007/s10549-013-2508-z. . doi:10.1007/s10549-013-2508-z. [DOI] [PubMed] [Google Scholar]
- 14.EBCTCG. Effects of radiotherapy and differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomized trials. Lancet. 2005;366:2087–106. doi: 10.1016/S0140-6736(05)67887-7. [DOI] [PubMed] [Google Scholar]
- 15.EBCTCG. Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast. J Natl Cancer Monogr. 2010;41:162–77. doi: 10.1093/jncimonographs/lgq039. [DOI] [PMC free article] [PubMed] [Google Scholar]


