Abstract
Background
Western lifestyle is associated with high prevalence of allergy, asthma and other chronic inflammatory disorders. To explain this association, we tested the ‘biodiversity hypothesis’, which posits that reduced contact of children with environmental biodiversity, including environmental microbiota in natural habitats, has adverse consequences on the assembly of human commensal microbiota and its contribution to immune tolerance.
Methods
We analysed four study cohorts from Finland and Estonia (n = 1044) comprising children and adolescents aged 0.5–20 years. The prevalence of atopic sensitization was assessed by measuring serum IgE specific to inhalant allergens. We calculated the proportion of five land-use types – forest, agricultural land, built areas, wetlands and water bodies – in the landscape around the homes using the CORINE2006 classification.
Results
The cover of forest and agricultural land within 2–5 km from the home was inversely and significantly associated with atopic sensitization. This relationship was observed for children 6 years of age and older. Land-use pattern explained 20% of the variation in the relative abundance of Proteobacteria on the skin of healthy individuals, supporting the hypothesis of a strong environmental effect on the commensal microbiota.
Conclusions
The amount of green environment (forest and agricultural land) around homes was inversely associated with the risk of atopic sensitization in children. The results indicate that early-life exposure to green environments is especially important. The environmental effect may be mediated via the effect of environmental microbiota on the commensal microbiota influencing immunotolerance.
Keywords: allergen-specific IgE, biodiversity hypothesis, farming environment, Proteobacteria, skin microbiota
Many explanations have been put forward for the rapidly increasing prevalence of allergies, asthma and other chronic inflammatory disorders especially in developed countries 1–3, including the influence of the living environment (rural vs urban, farm vs nonfarm) 4–6, change of diet 7, increased air pollution 8, reduced infections 9 and parasite exposure 10, and increased use of antibiotics 11. Several explanations can be regarded as versions of the ‘hygiene hypothesis’ 10,12–14 or the ‘old friends hypothesis’ 3,15: changes of environment and lifestyle in industrialized countries have reduced the infectious/parasitic burden and exposure to environmental microbes, which may now be insufficient to induce a robust anti-inflammatory regulatory network in the developing immune system 16. The immunomodulatory role of saprophytic bacteria in the soil and vegetation is increasingly recognized 3,10,14,17,18, and the disruption of the ancient connection of humankind with soil may have significant consequences 3,16. Based on this observation and considerations of other possible environmental influences, the hygiene and old friends hypotheses have been extended to the ‘biodiversity hypothesis’, with a focus on childhood exposure to diverse natural environments with diverse macrobiota and microbiota 3,17,19.
Here, we test the biodiversity hypothesis by analysing the relationship between land use around the homes of study subjects, as a measure of environmental biodiversity, and atopic sensitization (atopy) in four cohorts of children aged 0.5–20 years. We observe a strong relationship between the proportion of forest and agricultural land around the home and atopy in children older than 6 years, with prevalence exceeding 30%. The relative abundance of Proteobacteria increased significantly on the skin of healthy individuals along the gradient from built to green environments, suggesting an influence of the environmental microbiota on the human commensal microbiota, which may be related to or reflects the mechanisms by which the habitat gradient influences atopic sensitization.
Materials and Methods
Data sets
We combine data from three studies conducted in Finland and Estonia between 2003 and 2012. The first data set originates from a study testing the hygiene hypothesis in type 1 diabetes and other immune-mediated diseases in childhood (DIABIMMUNE; n = 594). The second data set (LUKAS) consists of children living either on a livestock farms (n = 90) or in nonfarm homes (n = 210) in eastern and central Finland. The third data set (KARA) comprises a random sample of adolescents living in eastern Finland (n = 94). All studies have been approved by ethical boards of University of Helsinki, Helsinki University Central Hospital and Kuopio University Hospital. For further details, see Supporting Information.
Atopy
Atopy (atopic sensitization) was defined based on the sum of IgE antibodies that are specific to inhalant allergens, such that an individual with log10(ΣIgEinhalant) > IgEth was classified as atopic 19, where IgEth is a cut-off level. As the data did not suggest any single cut-off level appropriate for all study cohorts (Fig. S1), we repeated the analyses with several values of IgEth. The minimum value for IgEth considered here is −0.5 on logarithmic scale, corresponding to the commonly used value of 0.35 kUA/L on arithmetic scale. We did not attempt to analyse the prevalence of allergic diseases, such as allergic rhinitis or asthma, for which only self-reported data were available.
Land-use description
The coverage of five land-use types, agricultural land, built area, forest, water bodies and wetland, around each home was calculated with the CORINE2006 land-cover data (a publicly available data base containing standardized land-cover classification across Europe), using a buffer with radius of 3 km. For more information on land-use description, see Supporting Information.
Statistical analyses
The prevalence of atopy was analysed using generalized linear models with binomially distributed errors (logistic regression) as implemented in R 20. As the prevalence of atopy was relatively low, the cloglog link function was used in all analyses, as this is better suited for data with uneven representation of zeros and ones than the default probit link function 21. Residual variance was sufficiently homogeneous between data sets such that mixed effect modelling was not needed. For the LUKAS cohort, we also analysed the land-use effect against several potential confounding factors, using the same logistic regression approach as above. Associations between confounding factors and atopy were analysed using generalized linear models with Poisson distributed errors (Poisson regression on counts) as recommended by 21.
Results
Land use and atopy
The four data sets cover a wide range of different environments, which comprise a gradient from forested to built environments (Fig.1A). In the LUKAS and KARA cohorts, the prevalence of atopy decreased systematically by increasing percentage of both agricultural land and forest within 3 km of the home (Fig.1B,C). As these two land-use types had symmetric effects, we describe below land use by the sum of the relative covers of forest and agricultural land, which varies between 0 and 1, and which we call the land-use gradient.
Figure 1.
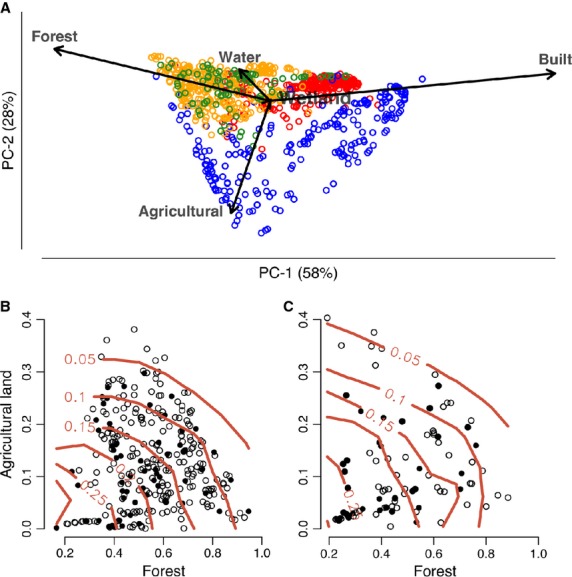
Land-use description. (A) PCA biplot of land-use types in the different data sets: DIABIMMUNE Espoo (red) and Tarto (blue), LUKAS (orange), and KARA (green). Increasing the proportion of both forest and agricultural land in the landscape is associated with a decrease in the average prevalence of atopic sensitization (atopy; red contour lines) in the (B) LUKAS and (C) KARA data sets. Open symbols indicate healthy individuals, and black dots represent atopic individuals (cut-off IgEth = 0.5). Atopy was diagnosed for 6-year-olds in the LUKAS cohort and for 13- to 20-year-olds in the KARA cohort.
For the two youngest age groups combined (0.5–3 years of age), the prevalence of atopy was low and there was no significant association with the land-use gradient (Fig.2A, Table S1). In contrast, in the older subjects with higher prevalence, the association was robustly significant (Fig.2B,C, Table S1). In the oldest age group (13–20 years), the predicted prevalence increased from 0.2 in homes with the land-use gradient approaching 1 to 0.6 in homes with the land-use gradient around 0.2. The results are not sensitive to the exact value of IgEth (Fig. S2), and the results remained largely the same when atopy was defined separately based on indoor and outdoor allergens (Fig. S3). More detailed results for different study cohorts are presented in Table S2.
Figure 2.
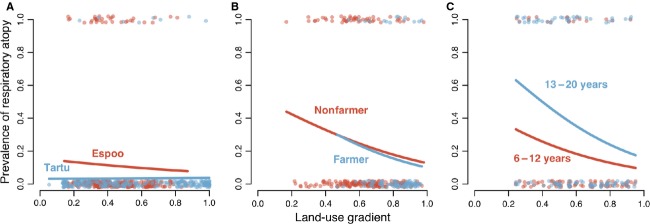
Logistic regression of the prevalence of atopic sensitization against the land-use gradient in three data sets representing different age groups. (A) 3-year-olds from DIABIMMUNE Espoo (red) and Tartu (blue). (B) 6-year-olds from the LUKAS data set, with either farmer or nonfarmer children. (C) Children of 6–12 years and 13–20 years of age from the KARA data set. The lines indicate the regression fit for each cohort. The IgEth for determining atopy was 0.5. Results for other threshold values are given in Supporting Information (Fig. S2).
One of the cohorts (LUKAS) includes children who had moved home since their birth, mostly at the age of 1–2 years (n = 97). In this group, we modelled atopy in relation to land-use types in the surroundings of the home at the time of birth and the new one to which the family had moved. The land-use gradient around the home at birth was a significant predictor of atopy at the age of 6 years, whereas the land-use gradient around the residence at 6 years of age was not (Table1).
Table 1.
Relationship between land-use types around the home at birth and at 6 years of age and prevalence of atopic sensitization at 6 years of age, for children that moved home between birth and 6 years of age in the LUKAS cohort (n = 97). The prevalence of atopy (log10(IgE) > IgEth) at 6 years of age was explained by the land-use gradient associated with the home at birth and the home at 6 years of age in a logistic regression model (the two gradients were positively correlated, r = 0.51, P < 0.001). The regression estimate gives the direction and magnitude of the relationship between the land-use gradient (relative increase in forest and agricultural land) and atopy. OR is odds ratio (exp(Estimate))
| At birth |
At 6 yrsk |
||||||
|---|---|---|---|---|---|---|---|
| IgEth | Atopy prevalence at 6 years | Estimate | OR | P | Estimate | OR | P |
| −0.5 | 0.443 | −0.80 | 0.45 | 0.345 | −0.02 | 0.98 | 0.974 |
| 0 | 0.330 | −1.80 | 0.17 | 0.070 | −0.25 | 0.78 | 0.788 |
| 0.5 | 0.237 | −2.39 | 0.09 | 0.043 | −1.07 | 0.34 | 0.327 |
| 1 | 0.216 | −2.22 | 0.11 | 0.071 | −0.78 | 0.46 | 0.493 |
Farm environment and other confounding factors
In the LUKAS data set, children living on farms had significantly lower prevalence of atopy than children not living on farms (IgEth = 1, Poisson regression on contingency table, testing for the interaction between atopy and farm, estimate = −0.84, P = 0.032). However, this analysis does not take into account the fact that farms are mostly located in environments with a relatively high proportion of forest and/or agricultural land in their surroundings (Fig.2B). When the land-use gradient was included in the model, there was no support for an independent farm effect (Table2).
Table 2.
Effects of the land-use gradient and living on a farm on atopic sensitization (atopy) in the LUKAS data set. Given a sufficiently high IgEth, the land-use gradient (around the home at birth) is a statistically significant predictor of atopy at 6 years of age in the pooled data set, but the farmer status of the family is not, when both predictors are included in the same model. We analysed separately the nonmovers (n = 203; children who had not moved house between birth and 6 years of age) and all individuals (n = 300).
| Nonmovers |
All |
|||||||
|---|---|---|---|---|---|---|---|---|
| Gradient |
Farm |
Gradient |
Farm |
|||||
| IgEth | Estimate | P | Estimate | P | Estimate | P | Estimate | P |
| −0.5 | −0.53 | 0.230 | −0.16 | 0.340 | −0.42 | 0.140 | −0.24 | 0.480 |
| 0 | −0.86 | 0.150 | −0.29 | 0.170 | −0.47 | 0.032 | −0.41 | 0.278 |
| 0.5 | −1.81 | 0.050 | −0.14 | 0.420 | −1.35 | 0.004 | −0.31 | 0.677 |
| 1 | −1.71 | 0.065 | −0.44 | 0.144 | −1.10 | 0.005 | −0.67 | 0.269 |
Testing of 26 potential confounding factors separately against the land-use gradient did not alter our conclusions about the relationship between land use and atopy (Tables S3 and S4). While none of the interactions between the land-use gradient and the confounding factors was statistically significant, we further examined the land use–atopy relationship separately for the different factor levels of the confounding variables. We found a significant relationship for five confounders: paternal asthma, maternal allergy, exposure to farm milk at young age (1 year), gender and domestic dog exposure at young age (2 months). The association between land use and atopy was stronger among children with a nonasthmatic father (Fig. S4A), a nonallergic mother (Fig. S4B) and those who had not consumed farm milk (Fig. S4C). Also, the association was stronger among boys than girls (Fig. S4D) and among children exposed to a dog at home (Fig. S4E).
While child gender and paternal asthma were evenly distributed along the land-use gradient, maternal allergy (estimate = −1.27, P = 0.0045), consumption of farm milk (estimate = 4.91, P < 0.0001) and domestic exposure to dogs (estimate = 1.89, P = 0.0047) were significantly associated with the land-use gradient. Both farm milk consumption and dog ownership were limited to areas with relatively high cover of green space, >60% and >40%, respectively. When considering these parts of the land-use gradient separately, farm milk consumption and dog ownership were no longer significant predictors of atopy prevalence.
Spatial scale of the land-use description
To evaluate the effect of the size of the spatial buffer, we repeated the analyses with buffers ranging between 0.5 and 10 km for the older age groups (6–20 years) with significant association in the above analyses (Table S1). Increasing the buffer size from 0.5 km initially reduces, but eventually increases, the variance in land-use types around individual homes (Fig.3A). Increasing buffer size is associated with decreasing variance of the land-use gradient, which gradually approaches the regional variance (Fig.3B). The spatial scale of land-use description affects the ability to detect a significant relationship between the land-use gradient and atopy; statistically significant relationships were observed at intermediate scales, from 2 to 5 km (Fig.3C), which encompasses the scale of 3 km used in the other analyses.
Figure 3.
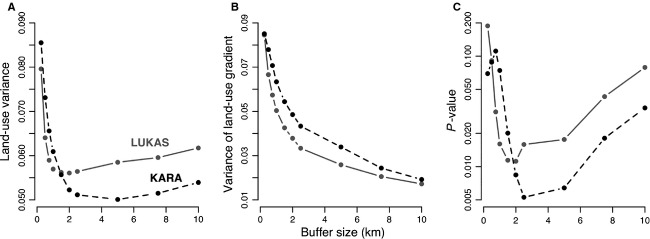
The spatial scale of land-use description. (A) Variance in land-use types (mean across individuals). (B) Variance in the land-use gradient. (C) Statistical significance of the land-use gradient in explaining the prevalence of atopic sensitization (on log-scale). In addition to the land-use gradient, logistic regression models include residence on a farm (LUKAS data set) or age (KARA data set). The IgEth for determining atopy is 0.5 in both cases.
Land-use gradient and human commensal microbiota
We have data available on the skin microbiota for the KARA cohort 19. In this data set, the relative abundance of Proteobacteria was significantly correlated with the land-use gradient in healthy (R2 = 0.19, P = 0.0005; Fig.4) but not in atopic individuals (Fig. S5). The significant relationship stems from the relative abundances of Gammaproteobacteria (R2 = 0.13, P = 0.006) and Betaproteobacteria (R2 = 0.09, P = 0.025), which were significantly associated with the land-use gradient (Fig. S5). The other major bacterial classes showed no significant association (Actinobacteria P = 0.21, Bacilli P = 0.27 and Clostridia P = 0.77).
Figure 4.
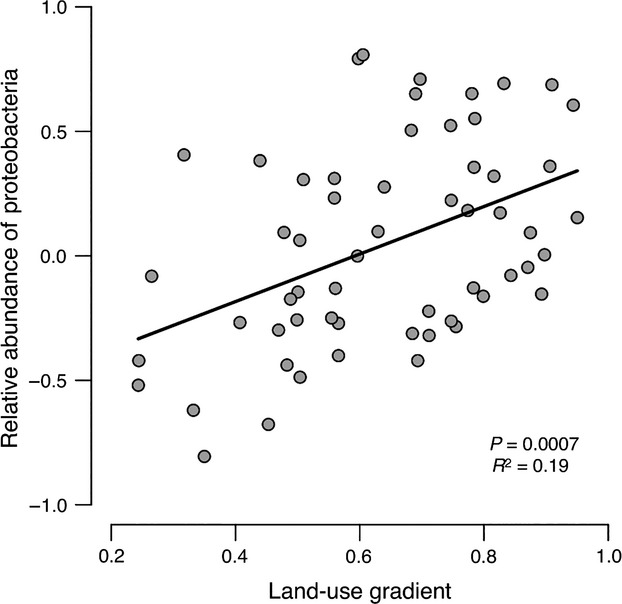
Relative abundance of Proteobacteria on the skin of healthy individuals is associated with the land-use gradient. The log10 relative abundance of all Proteobacteria (Alpha, Beta, Gamma, Delta and Epsilon) plotted against the land-use gradient (proportion of forest and agricultural land around homes, Fig.1). The data are from the KARA cohort.
Discussion
There is substantial evidence for higher prevalence of allergy in cities than in the countryside 5,6,16, but the underlying mechanisms remain unclear. In their review, Schram et al. 5 concluded that it is difficult to differentiate between factors that contribute causally vs a multitude of confounding factors. Here, we have presented a more fine-grained analysis, examining the effect of land use around homes in heterogeneous landscapes but in areas lacking cities, thereby excluding some of the confounders. We found that atopy is associated with low cover of accessible green space within 2 to 5 km from the home. Among children older than 6 years, a dose-dependent relationship emerged, supporting a causal link. In children who had moved in their early childhood, the land use in the surroundings of the home at birth was more strongly associated with atopy at the age of 6 years than the environment surrounding the second home. While this evidence for the importance of early exposure is relatively weak, this result is consistent with studies indicating that early-life exposure to, for example, household pets (e.g. dogs) and farm environment most efficiently reduces the probability of developing asthma/allergy later in life 22–25.
Previous studies conducted in Europe show evidence for growing up on a traditional farm being a significant factor protecting against the development of allergy 4,26. We found the same effect, but when the land-use gradient was included in the model, the farm effect was no longer significant. A difficulty here is that most farms are surrounded mainly by forest and agricultural land. It may also be that living on a farm is confounded by other factors. Riedler et al. 4, studying 3504 children in rural areas in Central Europe, reported that consumption of farm milk in early childhood has a protective effect against atopy. Our analysis also suggests a protective effect for farm milk consumed around 1 year of age, but once again this is confounded by the fact that farm milk consumption was limited to children living in areas with high cover of green space (>60%). Examining the land-use effect in different categories of children, the association between the land-use gradient and atopy was present only in children not exposed to farm milk nor to dogs. This result suggests that the living environment, farm milk and having a dog could offer alternative, possibly complementary routes of protection against allergies.
The biodiversity hypothesis 17 suggests that biodiversity loss due to population growth, rapid urbanization, destruction of natural green areas, deforestation and so forth 16,17,27 leads to reduced contact of humans with beneficial environmental microbes. In support of the biodiversity hypothesis, we found that for healthy individuals about 20% of the variation in the relative abundance of Proteobacteria on the skin of the study subjects was explained by the proportion of forest and agricultural land in the landscape. To our knowledge, no previous study has investigated the relationship between the commensal microbiota and environmental land use, although it has been shown that, for example, microbiota in the gut is affected by diet 28,29. A recent study demonstrated that contact with environmental microbiota via pets can have a considerable impact on the composition of the gastrointestinal microbiota 25. Our results are in line with these previous studies, suggesting that the skin microbiota can change in response to contact with bacteria in the surrounding environment. A puzzling feature in our results, however, is that the relative abundance of Proteobacteria on the skin of atopic individuals was not related to land-use types in the environment, a finding that needs further consideration.
Proteobacteria account for at least 40% of all prokaryotic genera and encompass the bulk of gram-negative bacteria 30. Most of the known Proteobacteria are free-living. For example, Lauber et al. 31 studied microbial composition in 53 soil samples, where the relative abundance of Proteobacteria ranged between 0.2 and 0.8, with a mean of 0.54. It is thus reasonable to assume that the relative abundance of Proteobacteria shows substantial variation along the land-use gradient in the present study. This also lends support to the proposition that contact with soil facilitates exposure to beneficial environmental microbiota 16. Many species of Proteobacteria, especially those in the classes of Beta- and Gammaproteobacteria, are pathogenic 30, which may indicate their potential in the early priming of the immune system. Hanski et al. 19 reported that generic diversity of Gammaproteobacteria on the skin was inversely associated with atopy, and an experimental study in a mouse model confirmed a key role for one gammaproteobacterial genus, Acinetobacter, in protecting from atopic sensitization and lung inflammation via the skin 32.
In conclusion, the present results indicate that environmental land use, and hence environmental biodiversity, affects the composition of the human skin microbiota, which in turn may protect against atopy and potentially against other chronic inflammatory disorders. Our results point to a special role for some Proteobacteria in the skin, but other microbes and body parts, not studied here, may be important as well. These results have obvious implications for planning of land use in densely populated regions and highlight the importance of the contact of young children with natural environments. More empirical work is urgently needed on the influence of the environment on the composition of the human commensal microbiota and its stability over time in various parts of the body.
Acknowledgments
We thank Graham Rook and Erica von Mutius, two anonymous reviewers and the Associate Editor for comments on the manuscript. We thank Matti Koski, Katriina Koski and Rauno Tillmann for technical assistance. The DIABIMMUNE study was supported by the European Union Seventh Framework Programme (Grant No. 202063) and The Academy of Finland Centre of Excellence in Molecular Systems Immunology and Physiology (Grant No. 250114). The KARA study was financed by Academy of Finland (Grant No. 138932), European Union Seventh Framework Programme Grant Agreement 261357 (MeDALL) and Helsinki University Hospital (HUS) (Grant No. 8361). The LUKAS study was supported by Academy of Finland (Grant No. 139021) and grants from the Juho Vainio, Päivikki and Sakari Sohlberg, and Finnish Cultural foundations. LR was supported by The Academy of Finland (Grant No. 138695). We thank Jane and Aatos Erkko Foundation for funding.
Author contributions
The study was conceived by IH. LR and IH analysed the data and wrote the manuscript. NF, PA, LvH, JP and TH commented the manuscript. JL wrote scripts to calculate land-cover types around homes. TL, AK, AH and ON contributed to data collection. VT, MK, JP and TH contributed the data and their interpretation.
Conflicts of interest
The authors declare no conflict of interests.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Supplementary information on study methodology and legends for Supplementary Tables and Figures.
Evolution of the specific IgE antibodies to inhalant allergens with age.
Prevalence of atopy in three data sets for different IgE thresholds for defining atopy.
Association between atopy and the habitat gradient separately for specific IgE's to primarily indoor vs outdoor allergens.
The atopy-land-use relationship and the allergy/asthma of parents, the consumption of farm milk at 1 year of age, the gender of the children, and dog ownership at 2mon of age.
Relative abundance of Proteobacteria increases along the land-use gradient for healthy individuals.
Logistic regression model of the prevalence of atopy across the land-use gradient.
Distribution of atopic individuals across the land-use gradient in different data sets (study cohorts), for different ages.
Description of confounding factors used in Table S4.
Environmental land-use remains a significant predictor of allergic sensitization (atopy) in the LUKAS data set after controlling for several confounding factors.
References
- 1.Asher MI, Montefort S, Björkstén B, Lai CK, Strachan DP, Weiland SK, et al. Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross-sectional surveys. Lancet. 2006;368:733–743. doi: 10.1016/S0140-6736(06)69283-0. [DOI] [PubMed] [Google Scholar]
- 2.Eder W, Ege MJ, von Mutius E. The asthma epidemic. N Engl J Med. 2006;355:2226–2235. doi: 10.1056/NEJMra054308. [DOI] [PubMed] [Google Scholar]
- 3.Rook GA. Regulation of the immune system by biodiversity from the natural environment: an ecosystem service essential to health. Proc Natl Acad Sci USA. 2013;110:18360–18367. doi: 10.1073/pnas.1313731110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riedler J, Braun-Fahrländer C, Eder W, Schreuer M, Waser M, Maisch S, et al. Exposure to farming in early life and development of asthma and allergy: a cross-sectional survey. Lancet. 2001;358:1129–1133. doi: 10.1016/S0140-6736(01)06252-3. [DOI] [PubMed] [Google Scholar]
- 5.Schram ME, Tedja AM, Spijker R, Bos JD, Williams HC, Spuls PI. Is there a rural/urban gradient in the prevalence of eczema? A systematic review. Br J Dermatol. 2010;162:964–973. doi: 10.1111/j.1365-2133.2010.09689.x. [DOI] [PubMed] [Google Scholar]
- 6.Nicolaou N, Siddique N, Custovic A. Allergic disease in urban and rural populations: increasing prevalence with increasing urbanization. Allergy. 2005;60:1357–1360. doi: 10.1111/j.1398-9995.2005.00961.x. [DOI] [PubMed] [Google Scholar]
- 7.Devereux G. The increase in the prevalence of asthma and allergy: food for thought. Nat Rev Immunol. 2006;6:869–874. doi: 10.1038/nri1958. [DOI] [PubMed] [Google Scholar]
- 8.Parker JD, Akinbami LJ, Woodruff TJ. Air pollution and childhood respiratory allergies in the United States. Environ Health Perspect. 2009;117:140. doi: 10.1289/ehp.11497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.von Hertzen LC, Laatikainen T, Mäkelä MJ, Jousilahti P, Kosunen TU, Petäys T, et al. Infectious burden as a determinant of atopy—a comparison between adults in Finnish and Russian Karelia. Int Arch Allergy Immunol. 2006;140:89–95. doi: 10.1159/000092251. [DOI] [PubMed] [Google Scholar]
- 10.Yazdanbakhsh M, Kremsner PG, van Ree R. Allergy, parasites, and the hygiene hypothesis. Science. 2002;296:490–494. doi: 10.1126/science.296.5567.490. [DOI] [PubMed] [Google Scholar]
- 11.Tsakok T, McKeever TM, Yeo L, Flohr C. Does early life exposure to antibiotics increase the risk of eczema? A systematic review. Br J Dermatol. 2013;169:983–991. doi: 10.1111/bjd.12476. [DOI] [PubMed] [Google Scholar]
- 12.Strachan DP. Hay fever, hygiene, and household size. Br Med J. 1989;299:1259–1260. doi: 10.1136/bmj.299.6710.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okada H, Kuhn C, Feillet H, Bach J-F. The ‘hygiene hypothesis’ for autoimmune and allergic diseases: an update. Clin Exp Immunol. 2010;160:1–9. doi: 10.1111/j.1365-2249.2010.04139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown EM, Arrieta M-C, Finlay BB. A fresh look at the hygiene hypothesis: how intestinal microbial exposure drives immune effector responses in atopic disease. Semin Immunol. 2013;25:378–387. doi: 10.1016/j.smim.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 15.Rook GAW. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: darwinian medicine and the ‘hygiene’ or ‘old friends’ hypothesis. Clin Exp Immunol. 2010;160:70–79. doi: 10.1111/j.1365-2249.2010.04133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.von Hertzen L, Haahtela T. Disconnection of man and the soil: reason for the asthma and atopy epidemic? J Allergy Clin Immunol. 2006;117:334–344. doi: 10.1016/j.jaci.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 17.von Hertzen L, Hanski I, Haahtela T. Natural Immunity: biodiversity loss and inflammatory diseases are two global megatrends that might be related. EMBO Rep. 2011;12:1089–1093. doi: 10.1038/embor.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maynard CL, Elson CO, Hatton RD, Weaver CT. Reciprocal interactions of the intestinal microbiota and immune system. Nature. 2012;489:231–241. doi: 10.1038/nature11551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanski I, von Hertzen L, Fyhrquist N, Koskinen K, Torppa K, Laatikainen T, et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc Natl Acad Sci USA. 2012;109:8334–8339. doi: 10.1073/pnas.1205624109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.R Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 21.Zuur AF, Ieno EN, Walker NJ, Saveliev AA, Smith GM. Mixed Effects Models and Extensions in Ecology with R. New York: Springer; 2009. [Google Scholar]
- 22.Hesselmar B, Aberg N, Aberg B, Eriksson B, Bjorksten B. Does early exposure to cat or dog protect against later allergy development? Clin Exp Allergy. 1999;29:611–617. doi: 10.1046/j.1365-2222.1999.00534.x. [DOI] [PubMed] [Google Scholar]
- 23.Ownby DR, Johnson C, Peterson EL. Exposure to dogs and cats in the first year of life and risk of allergic sensitization at 6 to 7 years of age. J Am Med Assoc. 2002;288:963–972. doi: 10.1001/jama.288.8.963. [DOI] [PubMed] [Google Scholar]
- 24.von Mutius E, Vercelli D. Farm living: effects on childhood asthma and allergy. Nat Rev Immunol. 2010;10:861–868. doi: 10.1038/nri2871. [DOI] [PubMed] [Google Scholar]
- 25.Fujimura KE, Demoor T, Rauch M, Faruqi AA, Jang S, Johnson CC, et al. House dust exposure mediates gut microbiome Lactobacillus enrichment and airway immune defense against allergens and virus infection. Proc Natl Acad Sci USA. 2013;111:201310750. doi: 10.1073/pnas.1310750111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Mutius E, Braun-Fahrländer C, Schierl R, Riedler J, Ehlermann S, Maisch S, et al. Exposure to endotoxin or other bacterial components might protect against the development of atopy. Clin Exp Allergy. 2000;30:1230–1234. doi: 10.1046/j.1365-2222.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 27.Haahtela T, Holgate S, Pawankar R, Akdis CA, Benjaponpitak S, Caraballo L, et al. The biodiversity hypothesis and allergic disease: world allergy organization position statement. World Allergy Organ J. 2013;6:3. doi: 10.1186/1939-4551-6-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci USA. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turnbaugh PJ, Ridaura VK, Faith JJ, Rey FE, Knight R, Gordon JI. The effect of diet on the human gut microbiome: a metagenomic analysis in humanized gnotobiotic mice. Sci Transl Med. 2009;1:6ra14. doi: 10.1126/scitranslmed.3000322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kersters K, De Vos P, Gillis M, Swings J, Vandamme P, Stackebrandt E. Introduction to the Proteobacteria. The Prokaryotes. 2006;5:3–37. [Google Scholar]
- 31.Lauber CL, Zhou N, Gordon JI, Knight R, Fierer N. Effect of storage conditions on the assessment of bacterial community structure in soil and human-associated samples. FEMS Microbiol Lett. 2010;307:80–86. doi: 10.1111/j.1574-6968.2010.01965.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fyhrquist N, Ruokolainen L, Suomalainen A, Lehtimäki S, Veckman V, Vendelin J, et al. Acinetobacter in the skin microbiota protects from allergic sensitization and inflammation. J Allergy Clin Immunol. 2014;134:1301–1309. doi: 10.1016/j.jaci.2014.07.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information on study methodology and legends for Supplementary Tables and Figures.
Evolution of the specific IgE antibodies to inhalant allergens with age.
Prevalence of atopy in three data sets for different IgE thresholds for defining atopy.
Association between atopy and the habitat gradient separately for specific IgE's to primarily indoor vs outdoor allergens.
The atopy-land-use relationship and the allergy/asthma of parents, the consumption of farm milk at 1 year of age, the gender of the children, and dog ownership at 2mon of age.
Relative abundance of Proteobacteria increases along the land-use gradient for healthy individuals.
Logistic regression model of the prevalence of atopy across the land-use gradient.
Distribution of atopic individuals across the land-use gradient in different data sets (study cohorts), for different ages.
Description of confounding factors used in Table S4.
Environmental land-use remains a significant predictor of allergic sensitization (atopy) in the LUKAS data set after controlling for several confounding factors.


