Abstract
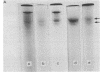
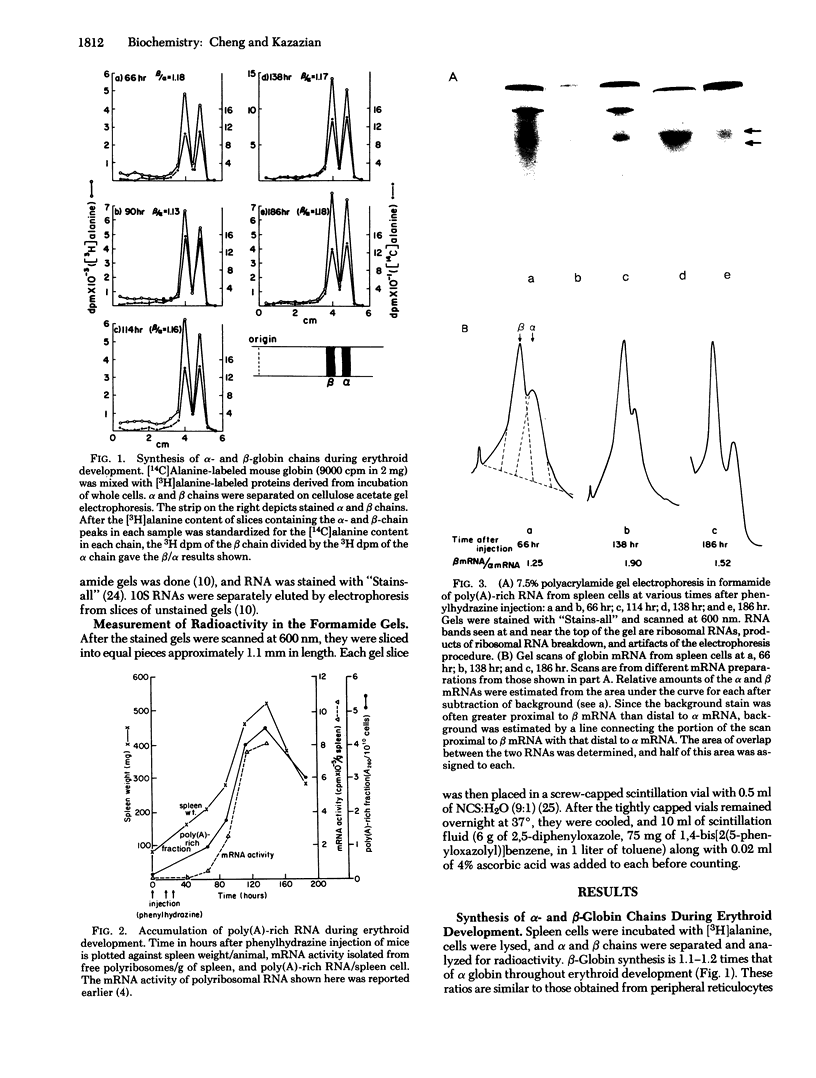
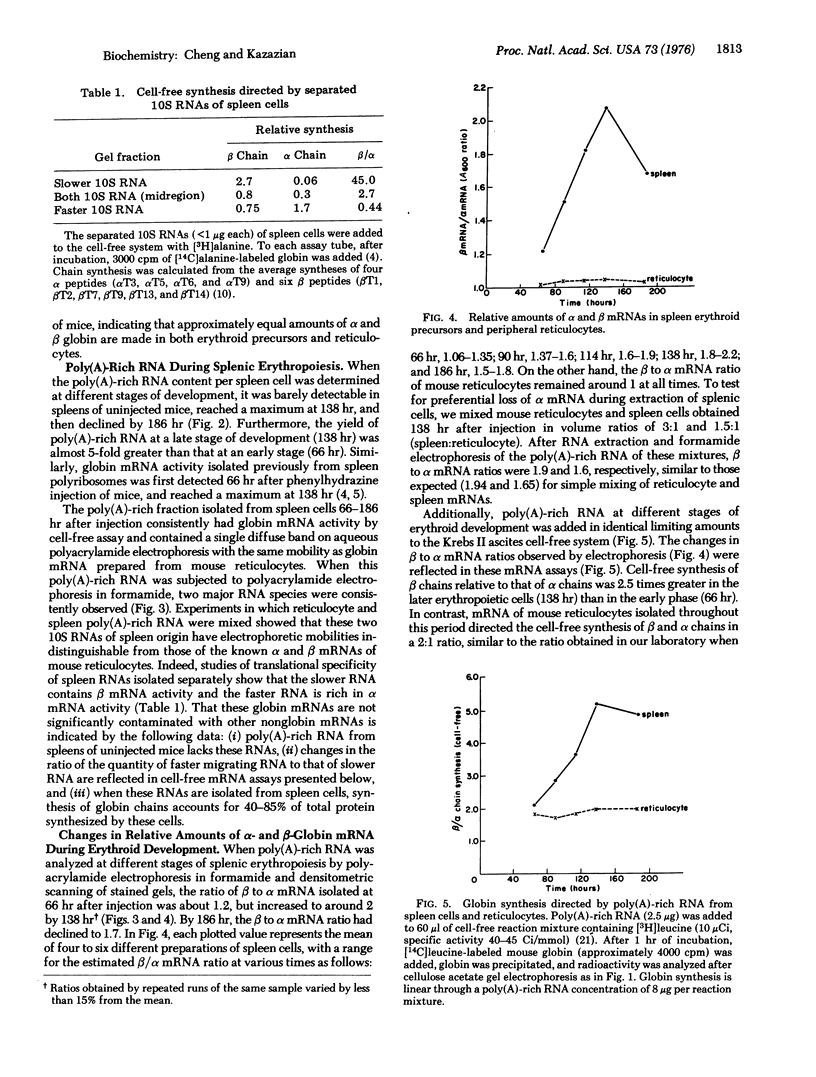
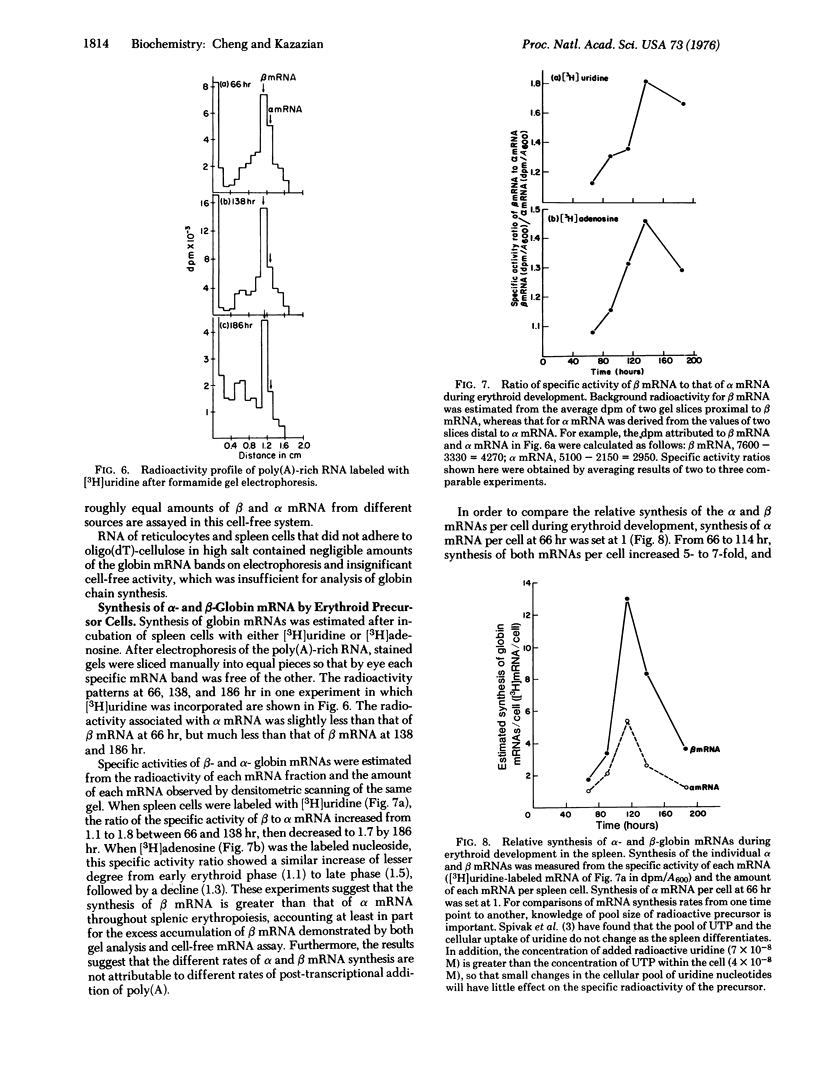
Relative amounts and rates of synthesis of alpha- and beta-globin mRNAs were determined during splenic erythropoiesis in mice. At times after injection of mice with phenylhydrazine, alpha- and beta-globin mRNAs were separated by gel electrophoresis and quantitated by densitometric scanning of stained gels. At 66 hr after injection, the ratio of beta to alpha mRNA is about 1.2. By 138 hr, total globin mRNA is 5-fold greater in spleen cells, and the beta to alpha mRNA ratio approaches 2. This ratio remains around 1 in reticulocytes throughout this period. Analyses of globin products directed by these mRNAs from spleen cells and reticulocytes in the ascites cell-free system reflect the beta to alpha mRNA ratio observed by electrophoresis. Relative rates of synthesis of globin mRNAs were estimated after incubation of spleen cells with either [3H] uridine or [3H] adenosine. Although synthesis of both mRNAs is maximal at 114 hr and then declines sharply, beta mRNA is synthesized at a greater rate than alpha mRNA at every developmental stage. In contrast to the excess accumulation of beta mRNA in spleen cells, synthesis of alpha- and beta-globin chains remains balanced throughout erythroid development. These data suggest that during erythropoiesis in this system, equal synthesis of alpha and beta globin involves regulation at both transcriptional and post-transcriptional levels.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Boime I., Leder P. Protein synthesis directed by encephalomyocarditis virus RNA: properties of a transfer RNA-dependent system. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2303–2307. doi: 10.1073/pnas.68.9.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORSOOK H., LINGREL J. B., SCARO J. L., MILLETTE R. L. Synthesis of haemoglobin in relation to the maturation of erythroid cells. Nature. 1962 Oct 27;196:347–350. doi: 10.1038/196347a0. [DOI] [PubMed] [Google Scholar]
- Berns A., Jansen P., Bloemendal H. The separation of alpha- and beta-rabbit globin mRNA by polyacrylamide gel electrophoresis. FEBS Lett. 1974 Oct 15;47(2):343–347. doi: 10.1016/0014-5793(74)81044-6. [DOI] [PubMed] [Google Scholar]
- Bozzini C. E., Barrio Rendo M. E., Devoto F. C., Epper C. E. Studies on medullary and extramedullary erythropoiesis in the adult mouse. Am J Physiol. 1970 Sep;219(3):724–728. doi: 10.1152/ajplegacy.1970.219.3.724. [DOI] [PubMed] [Google Scholar]
- Cheng T., Polmar S. K., Kazazian H. H., Jr Isolation and characterization of modified globin messenger ribonucleic acid from erythropoietic mouse spleen. J Biol Chem. 1974 Mar 25;249(6):1781–1786. [PubMed] [Google Scholar]
- Dahlberg A. E., Dingman C. W., Peacock A. C. Electrophoretic characterization of bacterial polyribosomes in agarose-acrylamide composite gels. J Mol Biol. 1969 Apr 14;41(1):139–147. doi: 10.1016/0022-2836(69)90131-4. [DOI] [PubMed] [Google Scholar]
- Forget B. G., Housman D., Benz E. J., Jr, McCaffrey R. P. Synthesis of DNA complementary to separated human alpha and beta globin messenger RNAs. Proc Natl Acad Sci U S A. 1975 Mar;72(3):984–988. doi: 10.1073/pnas.72.3.984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould H. J., Hamlyn P. H. The molecular weight of rabbit globin messenger RNA's. FEBS Lett. 1973 Mar 15;30(3):301–304. doi: 10.1016/0014-5793(73)80674-x. [DOI] [PubMed] [Google Scholar]
- Hori M., Rabinovitz M. Polyribosomal changes during inhibition of rabbit hemoglobin synthesis by an isoleucine antagonist. Proc Natl Acad Sci U S A. 1968 Apr;59(4):1349–1355. doi: 10.1073/pnas.59.4.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Ginder G. D., Snyder P. G., Van Beneden R. J., Woodhead A. P. Further evidence of a quantitative deficiency of chain-specific globin mRNA in the thalassemia syndromes. Proc Natl Acad Sci U S A. 1975 Feb;72(2):567–571. doi: 10.1073/pnas.72.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Itano H. A. Studies on the quantitative control of polypeptide synthesis in human reticulocytes. J Biol Chem. 1968 Apr 25;243(8):2048–2055. [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Snyder P. G., Cheng T. C. Separation of alpha- and beta-globin messenger RNAs by formamide gel electrophoresis. Biochem Biophys Res Commun. 1974 Aug 5;59(3):1053–1061. doi: 10.1016/s0006-291x(74)80086-0. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, Woodhead A. P. Hemoglobin A synthesis in the developing fetus. N Engl J Med. 1973 Jul 12;289(2):58–62. doi: 10.1056/NEJM197307122890202. [DOI] [PubMed] [Google Scholar]
- Kazazian H. H., Jr, cheng T., Polmar S. K., Ginder G. D. Globin mRNA of mouse spleen erythroblasts. Ann N Y Acad Sci. 1974 Nov 29;241(0):170–182. doi: 10.1111/j.1749-6632.1974.tb21876.x. [DOI] [PubMed] [Google Scholar]
- Mathews M., Korner A. Mammalian cell-free protein synthesis directed by viral ribonucleic acid. Eur J Biochem. 1970 Dec;17(2):328–338. doi: 10.1111/j.1432-1033.1970.tb01170.x. [DOI] [PubMed] [Google Scholar]
- Merkel C. G., Kwan S., Lingrel J. B. Size of the polyadenylic acid region of newly synthesized globin messenger ribonucleic acid. J Biol Chem. 1975 May 25;250(10):3725–3728. [PubMed] [Google Scholar]
- Morrison M. R., Brinkley S. A., Gorski J., Lingrel J. B. The separation and identification of alpha- and beta-globin messenger ribonucleic acids. J Biol Chem. 1974 Aug 25;249(16):5290–5295. [PubMed] [Google Scholar]
- Nienhuis A. W., Anderson W. F. Hemoglobin switching in sheep and goats: change in functional globin messenger RNA in reticulocytes and bone marrow cells. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2184–2188. doi: 10.1073/pnas.69.8.2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nienhuis A. W., Canfield P. H., Anderson W. F. Hemoglobin messenger RNA from human bone marrow. Isolation and translation in homozygous and heterozygous beta-thalassemia. J Clin Invest. 1973 Jul;52(7):1735–1745. doi: 10.1172/JCI107355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Swan D., Leder P. Differential expression of alpha- and beta-globin genes during differentiation of cultured erythroleukemic cells. J Biol Chem. 1975 Nov 25;250(22):8753–8760. [PubMed] [Google Scholar]
- Shearman J. J., Hamlyn P. H., Gould H. J. Molecular weights of separated rabbit alpha-and beta-globin messenger RNAs. FEBS Lett. 1974 Oct 1;47(1):171–176. doi: 10.1016/0014-5793(74)80452-7. [DOI] [PubMed] [Google Scholar]
- Spivak J. L., Marmor J., Dickerman H. W. Studies on splenic erythropoiesis in the mouse. I. Ribosomal ribonucleic acid metabolism. J Lab Clin Med. 1972 Apr;79(4):526–540. [PubMed] [Google Scholar]
- Spivak J. L., Toretti D., Dickerman H. W. Effect of phenylhydrazine-induced hemolytic anemia on nuclear RNA polymerase activity of the mouse spleen. Blood. 1973 Aug;42(2):257–266. [PubMed] [Google Scholar]
- Staynov D. Z., Pinder J. C., Gratzer W. B. Molecular weight determination of nucleic acids by gel electrophoresis in non-aqueous solution. Nat New Biol. 1972 Jan 26;235(56):108–110. doi: 10.1038/newbio235108a0. [DOI] [PubMed] [Google Scholar]