Abstract
The intrauterine environment during pregnancy is a critical factor in the development of diabetes and obesity in offspring. To determine the effects of maternal exercise during pregnancy on the metabolic health of offspring, 6-week-old C57BL/6 virgin female mice were fed a chow (21%) or high-fat (60%) diet and divided into four subgroups: trained (housed with running wheels for 2 weeks preconception and during gestation), prepregnancy trained (housed with running wheels for 2 weeks preconception), gestation trained (housed with running wheels during gestation), or sedentary (static cages). Male offspring were chow fed, sedentary, and studied at 8, 12, 24, 36, and 52 weeks of age. Offspring from chow-fed dams that trained both before and during gestation had improved glucose tolerance beginning at 8 weeks of age and continuing throughout the 1st year of life, and at 52 weeks of age had significantly lower serum insulin concentrations and percent body fat compared with all other groups. High-fat feeding of sedentary dams resulted in impaired glucose tolerance, increased serum insulin concentrations, and increased percent body fat in offspring. Remarkably, maternal exercise before and during gestation ameliorated the detrimental effect of a maternal high-fat diet on the metabolic profile of offspring. Exercise before and during pregnancy may be a critical component for combating the increasing rates of diabetes and obesity.
Introduction
In recent years, it has become well established that risk patterns for both obesity and type 2 diabetes originate as a consequence of alterations in growth and metabolism during critical windows of prenatal and early postnatal development (1–5). Human studies of maternal undernutrition and low birth weight have shown that adult offspring develop an increased risk for obesity (1), type 2 diabetes (2,3), and cardiovascular disease (4,5). In humans, maternal obesity is also a risk factor for the development of obesity in offspring during childhood (6–8). In animal models, maternal high-fat feeding results in profound changes in offspring health, including increased rates of obesity and percent body fat (9–11), impaired glucose tolerance (12), increased adipocyte lipogenesis and proliferation (13), increased cardiovascular disease (14), decreased β-cell function (12), and increased food intake (15). Thus, both human and animal studies have shown severe metabolic effects of maternal over- and undernutrition, underscoring the need to treat or prevent this problem.
Although exercise in the general population is well established to have numerous health benefits (16,17), the effects of maternal exercise on the metabolic phenotype of offspring are not well understood. Of the limited studies of maternal exercise in animal models, maternal exercise in streptozotocin-treated rats, a model of type 1 diabetes, was shown to result in lower blood glucose concentrations and improved glucose tolerance but elevated insulin concentrations in 4-week-old offspring (18). Maternal exercise in rats (19) and mice (20) has been shown to improve glucose tolerance and insulin sensitivity in offspring; however, whether these effects were specific to the exercising dams could not be determined since the sires were also housed with wheel cages for 10 days of breeding. In the current study, we used a mouse model to determine the effects of maternal exercise, independent of paternal exercise, on the long-term metabolic health of male offspring. We investigated whether the timing of exercise in the dams was critical and determined if maternal exercise could attenuate the detrimental effects of maternal high-fat feeding on offspring health. Our data indicate that maternal exercise, if performed before and during gestation, has profound effects on offspring metabolic health.
Research Design and Methods
Mice and Training Paradigm
Six-week-old C57BL/6 virgin female mice were fed a chow (21% kcal from fat; PharmaServ 9F5020) or high-fat diet (60% kcal from fat; Research Diets Inc.) for 2 weeks preconception and during gestation and were further divided into four subgroups: trained (mice housed with running wheels preconception and during gestation), prepregnancy trained (housed with wheels preconception), gestation trained (housed with wheels during gestation), or sedentary (housed in static cages). This training protocol was used because female mice do not run if they are younger than 6 weeks of age due to inability to adequately pull the wheel. Because 8 weeks of age is the optimal breeding age for mice, mice were trained for 2 weeks and then were bred with the males. The running wheels were 24.5 cm in diameter and 8 cm in width (Nalgene). All male breeders were chow-fed C57BL/6 mice and were sedentary. To control for potential differences in sires, breeding was performed as harems. Offspring were housed in static cages (sedentary) from birth onwards, and male offspring were studied up to 52 weeks of age.
Glucose and Insulin Tolerance Tests
Glucose tolerance tests (GTTs) were performed as previously described (21). GTTs in dams were performed at day 15 of gestation and mice were fasted for 4 h (0800 h to 1200 h). The shortened duration for the dams was used in order to reduce stress to the pregnant mice (22). For GTTs in offspring, mice were fasted for 11 h (2200 h to 0900 h). Insulin tolerance tests were only performed in offspring as previously described (21).
DEXA and Biochemical Methods
Offspring were anesthetized with ketamine/xylazine (50 mg/mL; injected 0.1 cc per 10 g body weight [bw]), and fat mass and lean mass were measured in offspring by DEXA (Lunar PIXImus2 mouse densitometer). Body weight was measured every 2–3 days and reported every 12 weeks, and food consumption was measured weekly. Retro-orbital sinus bleeds were performed after an overnight fast (2200 h to 900 h); plasma insulin and leptin were measured using mouse ELISA kits (Crystal Chem Inc.); and triglyceride, cholesterol, and free fatty acid were measured by colorimetric assay (Stanbio). Muscle glycogen and triglycerides were determined in tibialis anterior muscles as previously described (23). Tibialis anterior muscle was used for RNA extraction using QIAzol Lysis Reagent (Qiagen). mRNA expression was measured by quantitative RT-PCR (primers in Supplementary Table 1). Tissue processing and immunoblotting were performed as previously described (24). Antibodies used were GLUT4 (AB1346) (Millipore) and HKII (AB37593) (Abcam).
Glucose Clearance In Vivo
Mice were fasted overnight, anesthetized, and injected with either saline (basal) or maximal insulin (16.6 units/kg bw) as previously described (23). Tibialis anterior, soleus, extensor digitorum longus (EDL), and gastrocnemius were dissected and frozen, and accumulation of [3H]2-deoxyglucose-6-P was assessed as previously described (23,25). To determine glucose clearance, we divided the total glucose uptake by the average glucose concentration over the time course of the experiment.
Statistical Analysis
The data are means ± SEM. Statistical significance was defined as P < 0.05 and determined by one- or two-way ANOVA, with Tukey and Bonferroni post hoc analysis.
Results
Maternal Exercise in Dams Fed a Chow Diet Does Not Affect Litter Size or Sex Distribution
Training of dams did not significantly affect maternal body weight (Supplementary Fig. 1A). The amount of wheel running varied based on the timing of the wheel cage housing (Supplementary Fig. 1B and C). There was no difference in pregestation wheel running among the three groups that exercised pregestation. There was also no difference in the wheel running during gestation between the trained and gestation-only trained groups, and these dams exercised at a level of 60% of nonpregnant controls. There was no difference in total distance run between the prepregnancy trained and gestation-only trained dams (Supplementary Fig. 1B and C). There was no difference in glucose tolerance among chow-fed pregnant females at day 15 of gestation (Supplementary Fig. 2A and B). Dams responded with normal training adaptations as indicated by a significant increase in HKII and total GLUT4 in the triceps muscles (Supplementary Fig. 3A–D). Conception rate did not differ among treatments (data not shown), and maternal exercise in chow-fed dams did not affect litter size or sex distribution (Supplementary Table 2).
Maternal Exercise Before and During Gestation Improves Glucose Metabolism in Offspring
To determine the effects of maternal exercise on offspring as they age, as well as to determine if the timing of maternal exercise affects offspring health, we compared half-siblings born from sedentary, trained, prepregnancy-trained, or gestation-trained dams. Offspring were sedentary and chow fed, and GTTs were performed in male offspring up to 52 weeks of age. Male offspring of sedentary dams had a worsening of glucose tolerance as they aged (Fig. 1A). These aging effects were fully negated in the offspring throughout the 1st year of life if maternal exercise was performed before and during gestation. Offspring from gestation-only trained dams showed similar improvements in glucose tolerance at 8 and 12 weeks of age, but these effects did not persist over time. Glucose tolerance did not improve in offspring from prepregnancy-trained dams. Fasting insulin, measured at 52 weeks, was only reduced in offspring from dams that trained both before and during gestation (Fig. 1B). The percent body fat and body weights were not lower in the offspring from dams that trained before and during gestation until 52 weeks of age (Fig. 1C and D). Thus, the improvements in glucose tolerance in offspring from dams that trained before and during gestation preceded changes in offspring body weight and body composition (Supplementary Fig. 4A and B), demonstrating that changes in glucose metabolism were not due to changes in body composition (Fig. 1B–D).
Figure 1.
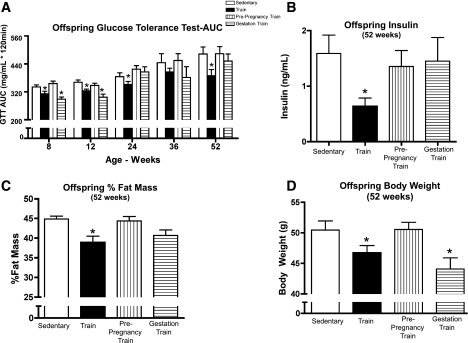
Maternal training during pregnancy improves glucose tolerance, decreases fasting insulin in offspring, and improves percent fat mass and body weight in offspring. A–D: Glucose tolerance was measured in offspring of sedentary, trained, prepregnancy-trained, or gestation-trained dams on a chow diet over a 52-week period, beginning at 8 weeks of age. For GTTs, mice were injected with 2 g glucose/kg bw, i.p. Glucose area under the curve (AUC) of male mice from chow-fed dams (A). Fasting insulin was measured at 52 weeks of age in male offspring (B). Percent fat mass (C) and body weight (D) of male offspring at 52 weeks. Data are expressed as means ± SEM (n = 10–20 litters/group). Asterisks represent differences between trained and all control groups (*P < 0.05).
There was no change in insulin tolerance (Supplementary Fig. 4C) and other serum metabolic markers (Supplementary Table 3), except for cholesterol, which was significantly increased only in offspring from gestation-trained dams. Although the mechanism for this effect is not known, it is important to note that there was no difference in circulating cholesterol concentrations in offspring of dams that exercised both before and during pregnancy, or exercised only during the prepregnancy period. Taken together, these data demonstrate that maternal exercise, when performed before and during gestation, has marked effects on the metabolic health of male offspring. Thus, maternal exercise could cause both epigenetic changes to the ova as well as adaptations in the in utero environment.
Maternal Exercise Before and During Gestation Ameliorates the Detrimental Effect of a Maternal High-Fat Diet
To determine if maternal exercise can ameliorate the deleterious effects of maternal high-fat feeding (7,9,10,26–28), female mice were fed a chow or high-fat diet for 2 weeks prior to breeding and throughout pregnancy and housed in static cages or cages with wheels. Here, we only report data from offspring of sedentary dams and dams that trained both before and during pregnancy. The high-fat maternal diet did not significantly affect body weight of dams or average running distance (Supplementary Fig. 5A and B) but resulted in an impaired glucose tolerance at day 15 of gestation (Supplementary Fig. 5C and D). Exercise training of dams had no effect on litter size, whereas there was a main effect of high-fat feeding to reduce litter size (7.2 ± 0.4 vs. 5.4 ± 0.4 pups/chow-fed vs. high-fat–fed dams, respectively; P = 0.004). There were no significant differences in sex distribution among litters (Supplementary Table 2).
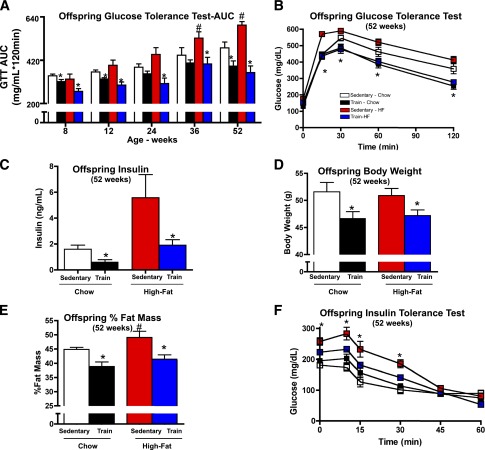
High-fat feeding of sedentary dams resulted in marked glucose intolerance in male offspring as they aged (Fig. 2A). Remarkably, offspring from high-fat–fed, exercise-trained dams (dams that trained before and during gestation) were fully protected from these harmful effects of a maternal high-fat diet. By 24 weeks of age, the detrimental effects of a maternal high-fat diet on glucose tolerance were prevented in offspring from dams that trained before and during gestation (Fig. 2A). In fact, at 52 weeks, offspring from high-fat–fed dams that trained before and during gestation had improved glucose tolerance compared with offspring from high-fat–fed, sedentary dams, and also compared with offspring from chow-fed, sedentary dams (Fig. 2A and B). Offspring from high-fat–fed, exercise-trained dams also had normal plasma insulin concentrations (Fig. 2C) and other serum metabolic markers (Supplementary Table 3), including serum cholesterol.
Figure 2.
Maternal training during pregnancy improves glucose tolerance, decreases fasting insulin, improves peripheral insulin sensitivity, and decreases body weight and percent body fat in offspring from dams fed a high-fat diet. A–F: Glucose tolerance was measured over a 52-week period in offspring of dams that were sedentary or trained and fed a chow or high-fat diet. For GTTs, offspring were injected with 2 g glucose/kg bw, i.p. Glucose area under the curve (AUC) of male offspring from sedentary and trained dams fed a chow or high-fat diet (A). Glucose excursion curve at 52 weeks (B). Fasting serum insulin concentrations at 52 weeks of age (C). Body weight (D) and percent fat mass (E) of male offspring at 52 weeks. F: For insulin tolerance tests, mice were injected with 1 unit insulin/kg i.p. and glucose concentration was measured over 60 min for male offspring. Data are expressed as means ± SEM (n = 10–20/group). Asterisks represent differences between trained and all control groups (*P < 0.05); # represents differences between offspring from sedentary high-fat–fed dams and all control groups (#P < 0.05).
Maternal exercise before and during gestation had clear effects to improve glucose homeostasis in offspring as early as 8 weeks of age. In contrast to this early in life change in glucose tolerance, body weights in offspring from dams that trained before and during gestation were not significantly lower until the offspring were 52 weeks of age (Fig. 2D and Supplementary Fig. 6A). Offspring of high-fat–fed dams had a significantly higher percent fat mass, whereas high-fat feeding in the presence of exercise training in dams resulted in offspring with significantly lower percent fat mass. This beneficial effect on fat mass was also not observed until 52 weeks of age (Fig. 2E and Supplementary Fig. 6B). Insulin tolerance was impaired in offspring from high-fat–fed dams (Fig. 2F), and this effect was attenuated in offspring of high-fat–fed, exercise-trained dams (Fig. 2F). These results demonstrate that maternal high-fat feeding causes pronounced glucose intolerance in male offspring as they age, but exercise training of dams can prevent these detrimental effects of a maternal high-fat diet.
Effects of Maternal Exercise on Offspring Skeletal Muscle
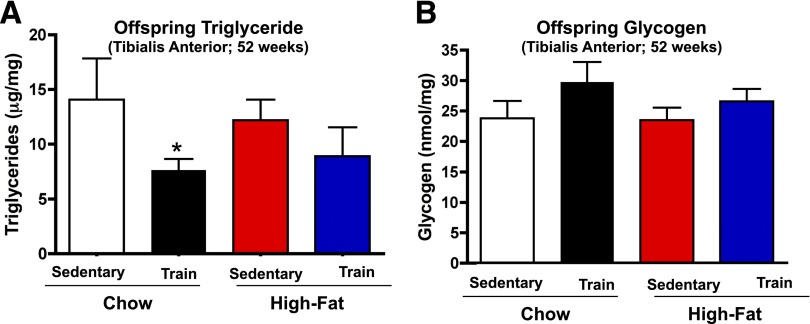
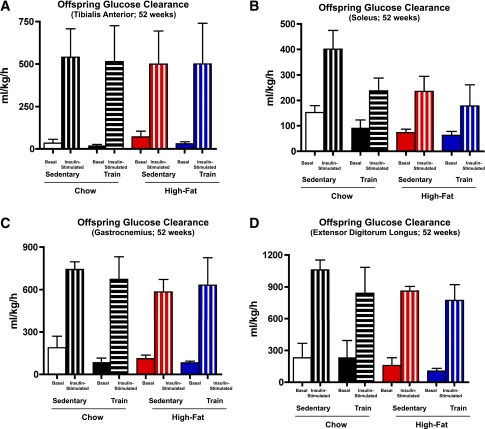
To determine if alterations in the skeletal muscle from offspring of exercise-trained dams was part of the mechanism for improved glucose tolerance, we measured muscle triglyceride and glycogen concentrations, rates of glucose clearance in vivo, and metabolic genes. Glucose clearance was measured in order to account for the mass action of glucose, which is necessary given the difference in glucose tolerance. Consistent with their enhanced metabolic homeostasis, offspring from chow-fed, exercise-trained dams had significantly decreased muscle triglyceride concentrations (Fig. 3A). There was no difference in basal rates of glucose clearance in tibialis anterior, soleus, gastrocnemius, and EDL muscles (Fig. 4A–D). Insulin treatment increased glucose clearance in all muscles studied, but there was no difference in insulin-stimulated glucose clearance among groups (Fig. 4). There were also no differences in muscle glycogen concentrations (Fig. 3B) or expression of a number of muscle metabolic genes, including GLUT4 (Supplementary Fig. 7A), among offspring.
Figure 3.
Muscle triglyceride and glycogen content. Male offspring from sedentary or trained dams fed a chow or high-fat diet were studied at 52 weeks for triglyceride content (A) or glycogen content (B) of tibialis anterior muscle. Data are means ± SEM (n = 5/group).*P < 0.05.
Figure 4.
Maternal exercise reduces insulin-stimulated skeletal muscle glucose clearance in male offspring. A–D: Male offspring from sedentary or trained dams fed a chow or high-fat diet were fasted overnight, anesthetized, and [3H]2-deoxyglucose per gram body weight was administered via retro-orbital injection in the presence of saline (basal) or 1 mg/kg bw glucose (glucose). Glucose clearance was measured in tibialis anterior (A), soleus (B), gastrocnemius (C), and EDL (D). Data are means ± SEM (n = 3–5/group).
Discussion
Maternal exercise in rat and mouse models was previously shown to be associated with an increase in skeletal muscle glucose uptake (19,20). There are differences between our experiments and previous studies that could account for these differences, including species used (mouse vs. rat) (19), mouse strain (C57BL/6 vs. ICR) (20), sex (male vs. female) (19,20), age of offspring at time of experiment (52 vs. 37 weeks) (19), and parental exercise (males also exercised in previous studies) (19,20). In addition, the previous mouse study measured glucose uptake in isolated soleus muscles in vitro, whereas we measured glucose clearance in vivo. Nevertheless, our findings suggest that glucose clearance into skeletal muscle cannot be the only mechanism for the improvement in glucose homeostasis in offspring from exercise-trained dams. In this regard, our finding of lower muscle triglyceride concentrations raises the possibility of enhanced fatty acid oxidation in the muscle of the offspring from trained dams. In addition, the consistently lower circulating insulin concentrations suggest improved β-cell function. Our ongoing work is aimed at determining if multiple metabolic processes (e.g., glucose oxidation or fatty acid metabolism) and multiple tissues (e.g., pancreas, liver, white adipose tissue, brown adipose tissue, or heart) have improved metabolic function in offspring of trained dams.
In summary, although obesity and overnutrition in individuals of reproductive age can propagate risk to subsequent generations via nongenetic or epigenetic and metabolic mechanisms (8,26,29–31,32), our findings suggest that it may be possible to overcome these risks through physical exercise. These findings, if translatable to humans, will have critical implications for the prevention of obesity and type 2 diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank Maura Mulvey, Allen Clermont, and Geetha Sankaranarayan (Joslin Diabetes Center Diabetes Research Center Physiology and Complex Assay cores) for technical assistance; Samantha S. Toombs for editorial contributions; and Dr. Kristy L. Townsend (Joslin Diabetes Center) for critical discussions.
Funding. This work was supported by National Institutes of Health grants R01-DK-101043 and R01-AR-42238 (to L.J.G.) and 5P30-DK-36836 (Joslin Diabetes Center DERC), an American College of Sports Medicine Research Endowment grant (to K.I.S.), and a Mary K. Iacocca Fellowship (to K.I.S.). M.-Y.L. was supported by a mentor-based fellowship awarded to L.J.G. from the American Diabetes Association.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. K.I.S. designed and performed experiments, analyzed the data, and wrote and edited the manuscript. M.-Y.L., K.M.G., and K.S. performed experiments. M.F.H. performed experiments and analyzed the data. L.J.G. designed experiments, analyzed the data, and wrote and edited the manuscript. L.J.G. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db13-1848/-/DC1.
See accompanying article, p. 335.
References
- 1.Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med 1976;295:349–353 [DOI] [PubMed] [Google Scholar]
- 2.Hales CN, Barker DJ, Clark PM, et al. Fetal and infant growth and impaired glucose tolerance at age 64. BMJ 1991;303:1019–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phipps K, Barker DJ, Hales CN, Fall CH, Osmond C, Clark PM. Fetal growth and impaired glucose tolerance in men and women. Diabetologia 1993;36:225–228 [DOI] [PubMed] [Google Scholar]
- 4.Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet 1989;2:577–580 [DOI] [PubMed] [Google Scholar]
- 5.Fall CH, Vijayakumar M, Barker DJ, Osmond C, Duggleby S. Weight in infancy and prevalence of coronary heart disease in adult life. BMJ 1995;310:17–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinhas-Hamiel O, Zeitler P. The global spread of type 2 diabetes mellitus in children and adolescents. J Pediatr 2005;146:693–700 [DOI] [PubMed] [Google Scholar]
- 7.Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH. Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 1997;337:869–873 [DOI] [PubMed] [Google Scholar]
- 8.Hopkins SA, Cutfield WS. Exercise in pregnancy: weighing up the long-term impact on the next generation. Exerc Sport Sci Rev 2011;39:120–127 [DOI] [PubMed] [Google Scholar]
- 9.Gniuli D, Calcagno A, Caristo ME, et al. Effects of high-fat diet exposure during fetal life on type 2 diabetes development in the progeny. J Lipid Res 2008;49:1936–1945 [DOI] [PubMed] [Google Scholar]
- 10.Masuyama H, Hiramatsu Y. Effects of a high-fat diet exposure in utero on the metabolic syndrome-like phenomenon in mouse offspring through epigenetic changes in adipocytokine gene expression. Endocrinology 2012;153:2823–2830 [DOI] [PubMed] [Google Scholar]
- 11.Bayol SA, Simbi BH, Stickland NC. A maternal cafeteria diet during gestation and lactation promotes adiposity and impairs skeletal muscle development and metabolism in rat offspring at weaning. J Physiol 2005;567:951–961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerf ME. High fat programming of beta-cell failure. Adv Exp Med Biol 2010;654:77–89 [DOI] [PubMed] [Google Scholar]
- 13.Desai M, Ross MG. Fetal programming of adipose tissue: effects of intrauterine growth restriction and maternal obesity/high-fat diet. Semin Reprod Med 2011;29:237–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan I, Dekou V, Hanson M, Poston L, Taylor P. Predictive adaptive responses to maternal high-fat diet prevent endothelial dysfunction but not hypertension in adult rat offspring. Circulation 2004;110:1097–1102 [DOI] [PubMed] [Google Scholar]
- 15.Muhlhausler BS, Ong ZY. The fetal origins of obesity: early origins of altered food intake. Endocr Metab Immune Disord Drug Targets 2011;11:189–197 [DOI] [PubMed] [Google Scholar]
- 16.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Physical Activity and Health, 2011. Atlanta, GA, Centers for Disease Control and Prevention. Available from http://www.cdc.gov/physicalactivity/everyone/health/. Accessed 16 February 2011
- 18.Vanheest JL, Rodgers CD. Effects of exercise in diabetic rats before and during gestation on maternal and neonatal outcomes. Am J Physiol 1997;273:E727–E733 [DOI] [PubMed] [Google Scholar]
- 19.Carter LG, Qi NR, De Cabo R, Pearson KJ. Maternal exercise improves insulin sensitivity in mature rat offspring. Med Sci Sports Exerc 2013;45:832–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carter LG, Lewis KN, Wilkerson DC, et al. Perinatal exercise improves glucose homeostasis in adult offspring. Am J Physiol Endocrinol Metab 2012;303:E1061–E1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stanford KI, Middelbeek RJ, Townsend KL, et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest 2013;123:215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zou M, Arentson EJ, Teegarden D, Koser SL, Onyskow L, Donkin SS. Fructose consumption during pregnancy and lactation induces fatty liver and glucose intolerance in rats. Nutr Res 2012;32:588–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Toyoda T, An D, Witczak CA, et al. Myo1c regulates glucose uptake in mouse skeletal muscle. J Biol Chem 2011;286:4133–4140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lessard SJ, Rivas DA, Alves-Wagner AB, et al. Resistance to aerobic exercise training causes metabolic dysfunction and reveals novel exercise-regulated signaling networks. Diabetes 2013;62:2717–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferré P, Leturque A, Burnol AF, Penicaud L, Girard J. A method to quantify glucose utilization in vivo in skeletal muscle and white adipose tissue of the anaesthetized rat. Biochem J 1985;228:103–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morris MJ, Chen H. Established maternal obesity in the rat reprograms hypothalamic appetite regulators and leptin signaling at birth. Int J Obes (Lond) 2009;33:115–122 [DOI] [PubMed] [Google Scholar]
- 27.Woo M, Isganaitis E, Cerletti M, et al. Early life nutrition modulates muscle stem cell number: implications for muscle mass and repair. Stem Cells Dev 2011;20:1763–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bentham J, Michell AC, Lockstone H, et al. Maternal high-fat diet interacts with embryonic Cited2 genotype to reduce Pitx2c expression and enhance penetrance of left-right patterning defects. Hum Mol Genet 2010;19:3394–3401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci 2008;28:9055–9065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mueller BR, Bale TL. Impact of prenatal stress on long term body weight is dependent on timing and maternal sensitivity. Physiol Behav 2006;88:605–614 [DOI] [PubMed] [Google Scholar]
- 31.Mueller BR, Bale TL. Early prenatal stress impact on coping strategies and learning performance is sex dependent. Physiol Behav 2007;91:55–65 [DOI] [PubMed]
- 32.Barker DJ. In utero programming of chronic disease. Clin Sci (Lond) 1998;95:115–128 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.