Abstract
Obesity-associated insulin resistance, a common precursor of type 2 diabetes, is characterized by chronic inflammation of tissues, including visceral adipose tissue (VAT). Here we show that B-1a cells, a subpopulation of B lymphocytes, are novel and important regulators of this process. B-1a cells are reduced in frequency in obese high-fat diet (HFD)-fed mice, and EGFP interleukin-10 (IL-10) reporter mice show marked reductions in anti-inflammatory IL-10 production by B cells in vivo during obesity. In VAT, B-1a cells are the dominant producers of B cell–derived IL-10, contributing nearly half of the expressed IL-10 in vivo. Adoptive transfer of B-1a cells into HFD-fed B cell–deficient mice rapidly improves insulin resistance and glucose tolerance through IL-10 and polyclonal IgM-dependent mechanisms, whereas transfer of B-2 cells worsens metabolic disease. Genetic knockdown of B cell–activating factor (BAFF) in HFD-fed mice or treatment with a B-2 cell–depleting, B-1a cell–sparing anti-BAFF antibody attenuates insulin resistance. These findings establish B-1a cells as a new class of immune regulators that maintain metabolic homeostasis and suggest manipulation of these cells as a potential therapy for insulin resistance.
Introduction
Type 2 diabetes currently afflicts 257 million people worldwide, and this number is expected to almost double by 2030 (1). Obesity-associated insulin resistance (IR) is considered to be the primary defect in the natural history of type 2 diabetes (2). Although many factors appear to govern the pathogenesis of IR, chronic low-grade inflammation in insulin-sensitive (IS) tissues, such as the liver and visceral adipose tissue (VAT), appears to play a central role (3). Multiple studies have shown links between increased levels of proinflammatory cytokines, such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), interferon-γ (IFN-γ), and worsened IR (4–6). Conversely, anti-inflammatory cytokine expression (IL-10 and IL-4) is associated with better glucose control (7–9). Similarly, immune cells with anti-inflammatory phenotypes (alternatively activated M2 macrophages, Th2, regulatory T cells [Treg]) are resident in the adipose tissue of lean mice and individuals, whereas proinflammatory cells (classically activated M1 macrophages, Th1) become enriched and expanded in the adipose tissue of obese subjects (3,6,10,11). Lastly, adipose cells are themselves capable of producing immune-related cytokines such as IL-6, IL-18, and B cell–activating factor (BAFF) (12–15). Thus, the complex interactions between innate and adaptive immune cells and adipocytes play a major role in IR.
We have previously shown in diet-induced obese mice that total CD19+ B cells and high-fat diet (HFD)-associated IgG antibodies are pathogenic in IR and that B cell–depleting therapy can alleviate disease (16). B cells also promote systemic and T cell–mediated inflammation in obese mice and humans (9). B cells can be divided into two broad classes, B-1 or B-2 cells; B-1 cells can be further classified as B-1a and B-1b cells (17). B-2 cells are the conventional adaptive B cells that produce antibodies to T cell–dependent antigens and are enriched in secondary lymphoid organs. B-1 cells are enriched in mucosal tissues and in pleural and peritoneal cavities (PerC) and produce natural antibodies, which are a first line of defense against pathogens (17,18). B-1a cells contribute 80% of the natural circulating IgM in the blood of mice (19) and make up the bulk of IL-10–expressing leukocytes in the PerC (20). Recently, human B-1 cells have been identified in umbilical cord and adult peripheral blood based on functional criteria that they share with mouse B-1 cells (21).
Coupled with the fact that B cells are a nonredundant source of IL-10 (20,22) and that B cells from diabetic patients and obese mice demonstrate an impaired anti-inflammatory cytokine profile (9), we hypothesized that B-1a cells might play an important role in glucose metabolism. Here we show that in opposition to B-2 cells, B-1a cells are novel immune regulators that protect against IR. The protective effects of these cells are mediated by IL-10 and polyclonal IgM, and these functions are impaired in obese mice. Depletion of B-2 cells in BAFF knockout mice and BAFF antibody treatment ameliorated IR in these mice. These discoveries suggest that B-2–depleting B-1a–sparing therapies could prove useful in type 2 diabetes.
Research Design and Methods
Mice
C57BL/6J, B cell–deficient μMT (B6.129S2-Ighmtm1Cgn/J), IL-10 EGFP [B6(Cg)-Il10tm1.1Karp/J] and IL-10–deficient (B6.129P2-Il10tm1Cgn/J) mice were purchased from The Jackson Laboratory. Secretory IgM-deficient (sIgMnull) mice (B6;129S4-Ighmtm1Che/J) were a gift from Troy Randall (University of Rochester). BAFF-deficient mice (B6.129S2-Tnfsf13btm1Msc/J) were a gift from Mark Krasnow (Stanford University). The mice were maintained in a pathogen-free, temperature-controlled environment on a 12-h light and dark cycle. The mice were fed a normal chow diet (NCD; 15 kcal% fat; LabDiet) or an HFD (60 kcal% fat; Research Diets) beginning at 6 weeks of age. Mice fed the HFD for at least 6 weeks were considered obese. All mice used in comparative studies were males and were age-matched within individual experiments. The Stanford University Institutional Animal Care and Use Committee approved all protocols.
B-Cell Transfer
To obtain B-2 cells, spleens were mechanically dissociated on 40-µm nylon cell strainers, followed by negative selection with the EasySep Mouse B Cell Enrichment Kit, which depleted CD43+ cells (Stemcell Technologies). B-2 cell purity was >90% as determined by flow cytometry. To obtain B-1a cells, peritoneal cells were harvested by injecting 7 mL RPMI, no phenol (Lonza), plus 3% newborn calf serum into the PerC. CD19+ CD22+ CD5+ B-1a cells were sorted as previously described to ∼95% purity (23). For B-2 versus B-1a experiments, 5 × 106 B-2 and B-1a cells were injected intraperitoneally. For other transfer experiments, 3 × 106 cells were used.
Metabolic Studies
Glucose tolerance test (GTT), insulin tolerance test (ITT), and serum insulin were measured as previously described (6). For intraperitoneal GTTs, mice were fasted for 14 h with access to drinking water and then injected intraperitoneally with glucose (2 g/kg body weight). Blood glucose from the tail tip was measured using a blood glucose meter just before the glucose injection and every 15 minutes thereafter. For ITT, mice were fasted for 5 h and then given 1 unit/kg body weight human regular insulin (Eli Lilly). Serum insulin was measured using the Ultra Sensitive Mouse Insulin ELISA Kit (Crystal Chem).
Isolation of VAT-Associated Immune Cells and VAT Lysates
VAT-associated immune cells were isolated from epididymal fat pads as previously described (6). VAT lysates were prepared as previously described (16).
Cell Cultures
Unless otherwise indicated, 300,000 cells were cultured in 200 μL complete RPMI in a 96-well round bottom plate for 24 h at 37°C in 5% CO2. Where noted, cells were stimulated with 1 μg/mL lipopolysaccharide (LPS; Sigma-Aldrich). For macrophage–B-1a cocultures, macrophages (CD19− CD11b+ F4/80+) and B-1a cells (CD19+ F4/80− CD5+) were sorted by FACS and cocultured at a 1:3 ratio for 60 h.
Cytokine and Antibody Measurement
Cytokines and antibodies were measured by ELISA (eBioscience and Bethyl, respectively) and cytometric bead array (BD Biosciences) according to vendors’ instructions. The anti-phosphorylcholine (PC) IgM antibody ELISA was adapted from a previous protocol (24). Antigens were diluted to 5 μg/mL in assay buffer (PBS containing 0.27 mmol/L EDTA and 1% BSA) and applied to a polystyrene enzyme immunosorbent assay plate (Costar) overnight at 4°C. Wells were washed with PBS containing 0.27 mmol/L EDTA and blocked with assay buffer. Detection antibody for the mouse anti-PC IgM ELISA was horseradish peroxidase–conjugated goat anti-mouse IgM and for the human anti-PC ELISA was horseradish peroxidase–conjugated goat anti-human IgM (Bethyl). For the mouse anti-PC IgM ELISA, E06 IgM (Avanti Polar Lipids) was used as the standard. Results for the human anti-PC IgM ELISA were reported as optical density. Antigens probed for the dosage curve were BSA (Sigma-Aldrich), PC-BSA (Biosearch Technologies), human LDL, human high-oxidized LDL (ox-LDL; Kalen Biomedical), and malondialdehyde-modified LDL (Cell Biolabs).
Human Subjects
We obtained sera from 62 age- and BMI-matched overweight to obese IR and IS male and female subjects (mean age IR: 54 ± 9 years, IS: 54 ± 7 years; mean BMI IR: 30.9 ± 2.4 kg/m2; IS: 30.3 ± 2.4 kg/m2). IS was determined by a modified insulin-suppression test, and IR or IS were defined by steady-state plasma glucose (SSPG) levels falling in the top (IR) or bottom (IS) 40th percentile (25). Serum samples were obtained under approval by the Stanford Internal Review Board for Human Subjects.
Flow Cytometry
We used the following gating schemes: total leukocytes (CD45.2+), total B cells (CD19+ CD3−), B-2 (CD19+ CD3− B220hi CD5− CD23+), B-1a (CD19+ CD3− IgM+ IgD− B220lo CD5+ CD23−), B-1b (CD19+ CD3− IgM+ IgD− B220lo CD5− CD23−), regulatory B [Breg] cells (CD19+ B220+ CD22+ CD5− IgM+ IgD+), total T cells (CD19− CD3+), Treg cells (CD19− CD3+ CD4+ CD25+), and macrophages (CD19− CD3− CD11b+ F4/80+). Dead cells were distinguished by Live/Dead Fixable Aqua staining (Life Technologies). Baselines for IL-10 EGFP mice were set using age- and diet-matched C57BL/6J mice. For macrophage intracellular cytokine staining, cells were stimulated with LPS (1 μg/mL; Sigma-Aldrich) and brefeldin A (5 μg/mL; BioLegend) overnight and stained using the Cytofix/Cytoperm Kit (BD Biosciences) according to the vendors’ instructions. Data were acquired on an LSR II flow cytometer (BD Biosciences) and analyzed with FlowJo software (Tree Star).
CD16/32, CD3-PacBlue, CD5-PE-Cy5, CD19-PerCP-Cy5.5, CD22-PE, CD23-PE-Cy7, CD25-PE, CD45.2-APC, B220-APC-Cy7, F4/80-PE, F4/80-PerCP-Cy5.5, IgD-PE, and TNF-α–PE antibodies were from BioLegend. IgM-efluor650, CD4-efluor650, and CD11b-efluor605 antibodies were from eBioscience.
IgM Treatment
We modified a previous protocol for IgM treatment of atherosclerosis (26). We gave 13-week-old HFD-fed B cell–deficient (Bnull) mice 400 μg mouse polyclonal IgM (Rockland Immunochemicals) or 200 μg mouse monoclonal anti-PC IgM (Clone E06, Avanti Polar Lipids) intraperitoneally on days 0, 4, 7, and 11. The polyclonal IgM dose was chosen based on the observation that RAG−/− mice reconstituted with 0.4 mg IgM have serum IgM levels that are similar to wild-type (WT) mice (27) and that the half-life of IgM is 2–3 days (26,28). Two hundred micrograms of monoclonal anti-PC IgM is five times the expected amount of anti–ox-LDL IgM in 400 μg polyclonal IgM (29). Control mice received PBS or 400 μg isotype control (Clone TEPC 183, Sigma-Aldrich). For macrophage-IgM cultures, polyclonal IgM and isotype control were used at 0.5 μg/mL.
BAFF Depletion With BAFF Monoclonal Antibody
Two hundred micrograms per mouse BAFF/B lymphocyte stimulator–specific monoclonal antibody (10F4, GlaxoSmithKline) or isotype control (hamster IgG1) was administered intraperitoneally to mice fed the HFD for 6 weeks on days 0 and 4.
Real-Time PCR Analysis
Tissue was dissociated in TRIzol (Life Technologies), and RNA was extracted and converted to cDNA using the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Primer pairs and probes, including their specificity, orientation (forward [F]; reverse [R]), and sequence were as follows: HPRT1 (F-TGGATACAGGCCAGACTTTGTT, R-CAGATTCAACTTGCGCTCATC), IL-10 (F-TTTGAATTCCCTGGGTGAGA, R-AGACACCTTGGTCTTGGAGC), and IL-6 (F-GATGGATGCTACCAAACTGGA, R-TCTGAAGGACTCTGGCTTTG). Quantitative real-time PCR was performed using SYBR Select (Applied Biosystems) on a QuantStudio 6 Flex machine (Applied Biosystems). Results were normalized to HPRT1 expression.
Statistics
The unpaired Student t test was performed. Values of P < 0.05 were considered statistically significant. Area under the curve (AUC) analysis was performed with correction for the starting glucose level.
Results
B-1a Cells Protect Against Glucose Intolerance
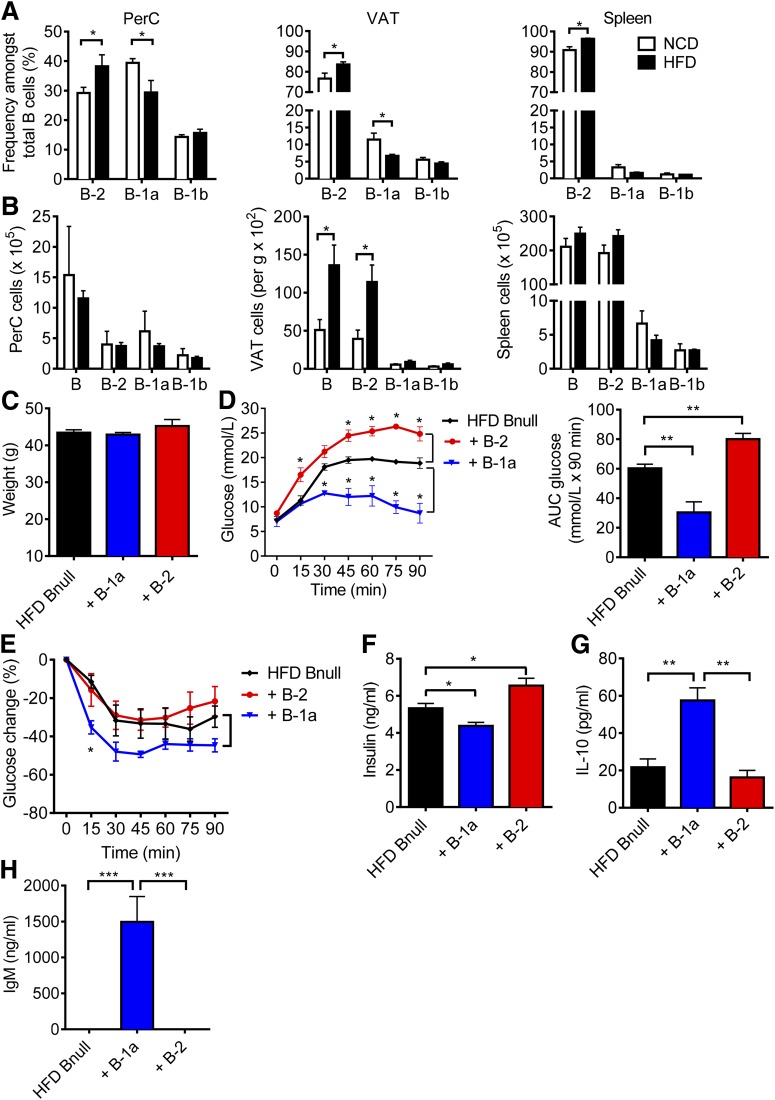
To examine the effects of diet-induced obesity on B cells, we fed C57BL/6J mice the NCD (15 kcal% fat) or the HFD (60 kcal% fat) for 9 weeks, which reliably results in obesity-associated IR and glucose intolerance (16). We subsequently compared the frequencies of CD19+ B-cell subpopulations in the PerC, VAT, and spleen and found that the HFD induced a significant increase in the relative percentage of B-2 cells and a reduction in the percentage of B-1a cells in the PerC and VAT but not in the spleen (Fig. 1A). Absolute cell counts of the B-cell subpopulations indicated that the HFD led to a significant increase of B cells in the VAT, particularly B-2 cells (Fig. 1B).
Figure 1.
B-1a cells play a protective role against glucose intolerance. A: Frequency of CD19+ B-cell subpopulations in the PerC (left panel), VAT (middle panel), and spleen (right panel) from C57BL/6J mice fed the NCD or HFD for 9 weeks (n = 5, representative of three experiments). B: Absolute cell counts of B-cell subpopulations of mice fed the NCD or HFD (n = 5). Body weights (C), GTT with AUC analysis (D), ITT (E), and fasting insulin levels (F) of HFD Bnull recipients, 1 week after receiving PBS, B-1a, or B-2 cells (n = 5). G: IL-10 concentration in 24-h PerC culture supernatant (n = 5). H: Serum IgM concentration (n = 5). Values are given as mean ± SEM. *P < 0.05; **P < 0.005; ***P < 0.0005.
To assess the effects of distinct B-cell subsets on glucose intolerance, we sorted B-1a cells from the PerC and B-2 cells from the spleen of HFD mice (Supplementary Fig. 1) and transferred 5 × 106 of one or the other population into 15-week-old HFD Bnull mice by intraperitoneal injection. Control mice received PBS. When we examined the mice 1 week after transfer, there were no differences in weight among all groups (Fig. 1C). Flow cytometry confirmed the presence of B-1a cells in the PerC and the VAT but not the spleen, whereas B-2 cells were present in all three tissues (Supplementary Fig. 2). Consistent with our previous findings (16), B-2 cells worsened glucose intolerance and increased serum fasting insulin compared with controls (Fig. 1D and F). Remarkably, B-1a cell transfer had the opposite effect: B-1a cells induced marked improvement in glucose tolerance, relative improvement in insulin tolerance, and reduced fasting insulin (Fig. 1D–F). These results suggest that B-1a cells protect against glucose intolerance. Given that the half-life of IgM is 2 days (28), detection of serum IgM and also IL-10 in culture supernatants from PerC cells in B-1a recipient mice confirmed that the transferred B-1a cells remained viable and capable of producing IL-10 and IgM in recipients for at least 1 week (Fig. 1G and H).
The Protective Effect of B-1a Cells on Glucose Control Is IL-10 Dependent
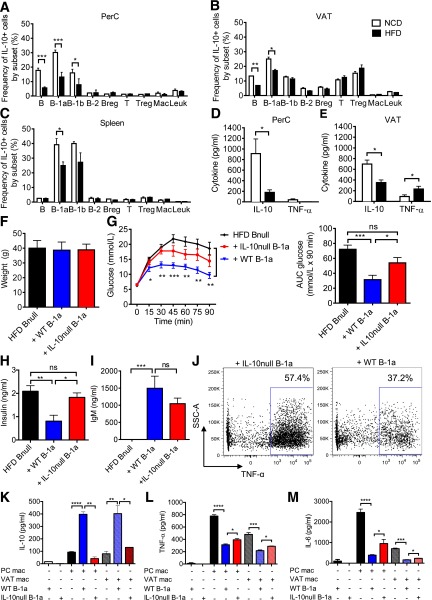
Because B-1a cells are important producers of B cell–derived IL-10, we next examined how diet-induced obesity influences IL-10 production by these cells in vivo. IL-10 EGFP reporter mice on the C57BL/6J background were fed the NCD or HFD for 9 weeks. PerC, VAT, and spleen-resident leukocytes were analyzed for IL-10 expression ex vivo. The results revealed a significant reduction in the frequency of IL-10-GFP+ total B cells and B-1a cells in the PerC. B-1a cells were also reduced in the VAT and spleen of HFD-fed mice (Fig. 2A–C). These cells were distinct from a recently identified population of IL-10–producing CD19+ B220+ CD22+ CD5− IgM+ IgD+ adipose natural Breg cells (22). When we examined the CD19+ B220+ IL-10–GFP+ population, we found that 40–50% of these cells in the PerC and VAT were B220lo CD5+ IgM+ IgD− B-1a cells, whereas 12–20% were of the Breg cell phenotype (Supplementary Fig. 3A).
Figure 2.
IL-10 production is impaired in obese mice. A–C: Frequency of GFP+ cells within each subpopulation in the PerC, VAT, and spleen from IL-10 EGFP mice fed the NCD or HFD for 9 weeks (n = 5–6, representative of three experiments). D and E: IL-10 and TNF-α concentration in 48-h PerC and VAT culture supernatants (n = 6). Body weights (F), GTT with AUC analysis (G), and fasting insulin (H) of HFD Bnull mice 1 week after receiving PBS, WT B-1a, or IL10null B-1a cells (n = 5). I: Serum IgM concentration 1 week after B-1a cell transfer (n = 5). J: TNF-α production in VAT macrophages. FACS plots are representative of two experiments. SSC-A, side scatter-area. Cytokine concentrations of IL-10 (K), TNF-α (L), and IL-6 (M) in 60-h coculture of WT PerC and VAT macrophages with WT or IL-10null B-1a cells (n = 3 per group, two replicates). Values are given as mean ± SEM. *P < 0.05; **P < 0.005; ***P < 0.0005; ****P < 0.0001.
We isolated total PerC and VAT cells from WT C57BL/6J mice fed the NCD or HFD for 9 weeks and cultured them for 48 h. Cells from HFD mice showed decreased IL-10 in PerC (Fig. 2D) and VAT (Fig. 2E) cultures compared with NCD-fed mice. In addition, VAT cell cultures showed increased TNF-α production. Given that B cells comprise a major proportion of CD45+ leukocytes in the PerC (Supplementary Fig. 3B), the loss of IL-10 production in B cells, and particularly in the B-1a cell subset, may explain the reduction in total PerC culture IL-10 production in HFD animals.
We hypothesized that the protective effect of B-1a cells on IR might be mediated by IL-10. To study this, we adoptively transferred 3 × 106 B-1a cells isolated from HFD WT or HFD IL-10null mice intraperitoneally into HFD Bnull recipient mice and monitored the mice for metabolic parameters. One week after transfer, there was no difference in weight in recipient groups (Fig. 2F). IL-10null B-1a recipients exhibited partial restoration of glucose sensitivity, which is consistent with a protective role for IL-10 (Fig. 2G and H). IgM production by IL-10null B-1a cells was not significantly different from IgM production by WT B-1a cells (Fig. 2I). Taken together, these results indicate that the protective effect of B-1a cells partially depends on IL-10.
Next, we explored the interplay between B cells and other known immune mediators of IR. Macrophages have been well established as effectors of IR (30). In lean mice, M2 macrophages, which produce anti-inflammatory cytokines such as IL-10, are enriched in adipose tissue (31). HFD causes accumulation of proinflammatory M1 macrophages in the adipose tissue of obese mice and humans (10,31,32). M1 macrophages are a major source of TNF-α, which alters insulin receptor signaling and induces IR (5). To determine if B-1a–derived IL-10 influences macrophage proinflammatory cytokine production, we stimulated VAT macrophages isolated from recipients of WT or IL-10null B-1a cell transfers. Intracellular cytokine staining showed that TNF-α production by VAT macrophages in mice that received WT B-1a cells was lower than production by VAT macrophages isolated from mice that received IL-10null B-1a cells (Fig. 2J).
To explore this notion further, we cultured WT macrophages isolated from the VAT or PerC of HFD mice with WT or IL-10null B-1a cells. WT macrophage–WT B-1a cocultures yielded much more IL-10 than the WT macrophage–IL-10null B-1a cocultures (Fig. 2K). The amount of IL-10 in the WT macrophage–WT B-1a cocultures was considerably greater than the sum of IL-10 produced by WT macrophages alone and WT B-1a cells alone, suggesting a synergy between macrophages and B-1a cells that might explain why B-1a–derived IL-10 is effective in controlling glucose intolerance. A similar anti-inflammatory effect, measured by a reduction in TNF-α and IL-6, was seen in cocultures of VAT or PerC macrophages with WT B-1a cells (Fig. 2L and M). This reduction was partially dependent on B-1a–derived IL-10 because IL-10null B-1a cells were significantly less effective at suppressing proinflammatory cytokine production by macrophages. These findings point to an important role for B-1a cells in regulating inflammatory and anti-inflammatory cytokine production by macrophages.
The Protective Effect of B-1a Cells Also Depends on Polyclonal IgM
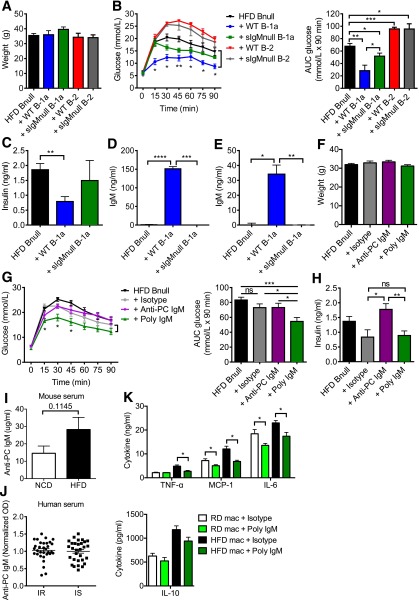
Given that B-1a–derived natural IgM has anti-inflammatory properties in other inflammatory diseases such as atherosclerosis (24), we hypothesized that, in addition to IL-10, secretion of IgM by B-1a cells may play a role in IR. Indeed, B-1 cells from obese db/db mice show an impaired IgM response, which may be due to high glucose levels (33). To explore this, we obtained sIgMnull mice whose B cells do not secrete IgM but still express surface IgM and IgD and undergo class switching to express other Ig isotypes (34), allowing us to study the effect of B cells on IR separately from any effect of IgM. We transferred 3 × 106 B-1a cells or B-2 cells isolated from HFD WT or sIgMnull mice into HFD Bnull recipient mice. One week after transfer, WT B-2 and sIgMnull B-2 cells both worsened glucose tolerance in recipient mice and WT B-1a cells improved glucose tolerance, whereas transfer of sIgMnull B-1a cells resulted in an intermediate phenotype (Fig. 3A–C). IgM was only detectable in the serum and VAT lysate of WT B-1a recipients but not sIgMnull B-1a recipients (Fig. 3D and E). These results suggest that IgM secretion contributes to the promotion of glucose tolerance by B-1a cells.
Figure 3.
Polyclonal IgM, but not monoclonal anti-PC IgM, ameliorates glucose intolerance. Body weights (A) and GTT with AUC (B) 1 week after receiving PBS, WT B-1a, sIgMnull B-1a, WT B-2, or sIgMnull B-2 cells (n = 5–8). Fasting insulin (C), IgM concentration in serum (D), and VAT lysate (E) 1 week after B-1a cell transfer (n = 4). Body weights (F), GTT with AUC (G), and fasting insulin (H) of HFD Bnull mice 1 week after receiving PBS, isotype control, polyclonal IgM, or E06 monoclonal anti-PC IgM (n = 5 for E06 treatment, n = 15 for the rest). I: Anti-PC IgM in serum from NCD and HFD mice (n = 10). J: Anti-PC IgM in serum from IR and IS obese humans (n = 32 and 30). OD, optical density. K: Cytokine concentrations in 24-h supernatants from PerC macrophages cultured with the isotype control or polyclonal IgM (n = 3). MCP-1, monocyte chemoattractant protein-1. Values are given as mean ± SEM. *P < 0.05; **P < 0.005; ***P < 0.0005; ****P < 0.0001.
Anti-atherosclerosis IgM antibodies directed against ox-LDL recognize the PC lipid group (24). Because ∼10% of natural IgM in mice binds to ox-LDL (29), we hypothesized that polyclonal IgM, as well as anti-PC monoclonal IgM antibody (E06) (24), would improve glucose tolerance. After 2 weeks of IgM treatment, there was no statistical difference in weight in all groups (Fig. 3F). Recipients of 400 μg polyclonal IgM showed significantly better glucose tolerance than PBS or isotype-treated controls as well as mice that received 200 μg anti-PC IgM (Fig. 3G). The former also showed improved fasting insulin (Fig. 3H). Hence, polyclonal IgM improves glucose tolerance, but the relevant target antigen does not appear to be a major epitope commonly studied in atherosclerosis. Indeed, PC-BSA–specific IgM ELISA indicated that most of the polyclonal IgM did not bind to PC or ox-LDL (Supplementary Fig. 4). We also investigated the levels of anti-PC IgM in NCD versus HFD mice, as well as in a cohort of 62 overweight to obese men and women who differed from one another only in their IR status, as determined by a modified insulin-suppression test (16). There was no difference in the levels of serum anti-PC IgM in NCD versus HFD mice (Fig. 3I) or between IR versus IS humans (Fig. 3J). To determine if polyclonal IgM affected macrophages, we cultured PerC macrophages with polyclonal IgM or the isotype control. Compared with isotype-treated macrophages, polyclonal IgM–treated macrophages produced significantly less TNF-α, monocyte chemoattractant protein-1, and IL-6 but showed no changes in IL-10 production (Fig. 3K).
BAFF Deficiency and BAFF-Depleting Therapy Improve Glucose Tolerance
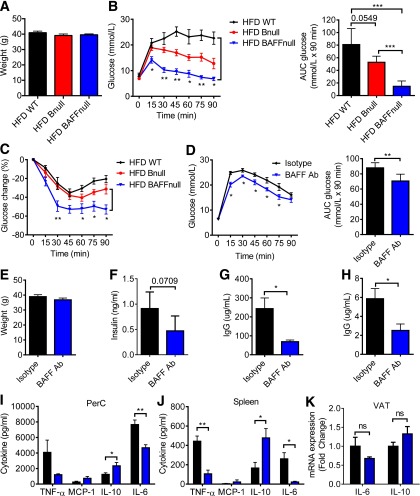
IR can be reversed by depleting mature B cells using an anti-mouse CD20–depleting antibody (16). However, this therapy partly depletes the B-1a population and is also immunosuppressive. Hence, a safer and more efficacious option might be to deplete the B-2 compartment selectively, leaving the B-1a cells intact. BAFF is required for the maintenance of mature B-2 cells in the periphery (35), and BAFF knockout mice have a deficiency of B-2 cells and a relative increase of B-1a and CD23− CD5− B cells (Supplementary Fig. 5A). To study the effect of BAFF on glucose tolerance, we first administered the HFD to WT, BAFF knockout, and Bnull mice for 9 weeks and performed GTT and ITT on these mice (Fig. 4A–C). BAFF knockout mice showed dramatic improvement in glucose tolerance and IR compared with WT and Bnull control mice. This result suggested that B-1a cells could potentially account for the difference.
Figure 4.
BAFF-deficient (BAFFnull) and anti-BAFF antibody (Ab)–treated obese mice exhibit superior glucose metabolic control compared with WT and Bnull mice. Body weights (A), GTT with AUC (B), and ITT (C) of HFD WT, BAFFnull, and Bnull mice (n = 5). GTT with AUC (D), body weights (E), and fasting insulin (F) of HFD WT mice 4 weeks after they received anti-BAFF antibody or isotype control (n = 5). IgG concentration in serum (G) and VAT lysate (H) 5 weeks after anti-BAFF antibody treatment (n = 5). Cytokine concentrations in 24-h cultures of PerC (I) or spleen (J) cells stimulated with LPS from isotype control and BAFF antibody–treated mice (n = 5). K: mRNA expression in VAT from isotype control and BAFF antibody–treated mice (n = 4). Values are given as mean ± SEM. *P < 0.05; **P < 0.005; ***P < 0.0005.
Next, to determine if B-1a cells could be therapeutically manipulated, we treated HFD WT mice with a monoclonal hamster anti-mouse BAFF antibody (clone 10F4) or isotype control hamster IgG while maintaining the mice on the HFD. BAFF antibody caused a relative depletion of B-2 cells in the peripheral blood, spleen, and PerC up to 7 weeks after the first dose and also a reduction in absolute cell counts of total B cells and most B cell subsets (Supplementary Fig. 5B–D). BAFF antibody–treated mice showed improved glucose tolerance compared with the control mice (Fig. 4D–F) and depletion of IgG in serum and VAT lysate (Fig. 4G and H). BAFF antibody–treated mice also displayed a marked anti-inflammatory cytokine profile in PerC and spleen cell cultures (Fig. 4I and J), consistent with a state of reduced systemic and local inflammation, despite the HFD. VAT mRNA expression of IL-6 and IL-10 displayed the same trend, although the differences were not statistically significant (Fig. 4K).
Discussion
This study reveals a novel role for B-1a cells in modulating glucose intolerance in opposition to pathogenic B-2 cells. Consistent with a previous report (33), we observed that the HFD resulted in a decreased frequency of B-1a cells in the PerC and VAT but not in the spleen. At the same time, total B cells and B-2 cells increased in the VAT, in agreement with our previous findings (16). In our previous work, transfer of total CD19+ cells from the spleen of HFD mice worsened IR in recipient mice (16). B-2 cells comprise the dominant B-cell subset in the spleen and were likely responsible for the previously observed effect of total B cells. Indeed, when we transferred B-1a or B-2 cells in the current study, they played opposing roles in IR. These results are similar to those seen in atherosclerosis, a disease that is also characterized by chronic inflammation (36). Hence, obesity appears to simultaneously impair regulatory B-1a cells and cause the expansion of pathogenic B-2 cells.
We also observed that B-1a cells in obese mice produce less IL-10, in keeping with findings that B cells from obese mice and diabetic patients express a proinflammatory cytokine profile (9). B cell–derived IL-10 has been shown to be important in regulating autoimmune and inflammatory diseases, including experimental autoimmune encephalomyelitis and arthritis (37,38). The failure of IL-10–deficient B-1a cells to protect against glucose intolerance suggests that the same holds true for IR. We also found that IL-10–deficient B-1a cells were unable to stimulate macrophages to produce IL-10 or reduce IL-6 and TNF-α secretion. This finding is interesting, because several studies have intimately linked classically activated M1 macrophages with IR (10,31,39). B-1a cells can polarize macrophages toward an alternatively activated phenotype (40) and can downregulate macrophage phagocytic activity (41) and proinflammatory cytokine production (42). In obese mice lacking mature T and B lymphocytes and natural killer cells, macrophages are incapable of causing IR (43). Hence, the defect in B-1a production of IL-10 may exacerbate the proinflammatory state of macrophages in obese individuals.
One intriguing finding is that polyclonal IgM reduces glucose intolerance, but apparently without involvement of ox-LDL. Natural IgM antibodies are circulating IgMs that arise without known immune exposure or vaccination (44). A substantial portion of these antibodies recognize PC and malondialdehyde groups, which are present on oxidation-specific antigens such as ox-LDL (29) and materials shed by apoptotic cells (45). Natural IgM is believed to prevent inflammation by recruiting macrophages and dendritic cells to clear apoptotic debris (24,45). Obesity and IR are associated with hypoxic and apoptotic adipocytes, and IgM may help in the clearance of such debris (46). Moreover, deposition of anti–ox-LDL natural IgM in atherosclerotic lesions helps neutralize ox-LDL and attenuates disease (23,24,47). It was therefore surprising to find that treating HFD Bnull mice with polyclonal IgM, but not monoclonal IgM against PC, improved glucose tolerance. In addition, the level of anti-PC IgM was no different between obese and lean mice or between IS and IR patients. These results do not exclude the possibility that IgM antibodies directed against other potential targets might mediate the beneficial effect of the class.
In BAFF-deficient mice, which show normal levels and function of B-1a and B-1b cells but markedly reduced B-2 cell frequency (35), we found improved glucose tolerance more than that seen in Bnull mice. We also found that treatment of HFD mice with a monoclonal anti-mouse BAFF antibody improved glucose metabolism, although not to the same extent as genetic knockout of BAFF. This might have been because BAFF antibody was less efficient at depleting splenic B-2 cells, although it is also possible that the remaining CD23+ B cells were transitional T2 and T3 B cells (48). Anti-BAFF therapy is used for the treatment of systemic lupus erythematous, where excess BAFF is associated with a loss of self-tolerance and production of autoantibodies. BAFF has also been shown to induce production of proinflammatory cytokines in adipocytes as well as in monocytes (14,49), resulting in IR. Hence, BAFF-depleting therapy, through its direct anti-inflammatory actions and its effect on the ratio of B-1a to B-2 cells, may be effective in treating glucose intolerance. In a recent study, short-term treatment of five lupus patients with belimumab resulted in a modest reduction in HOMA IR, although the effect was not statistically significant (15). Thus, larger clinical trials in different patient populations will be needed to determine the potential utility of this drug in the treatment of glucose intolerance.
A recent report by Nishimura et al. (22) described a unique subset of Breg cells in the adipose tissue of mainly lean mice that produce IL-10 constitutively and contribute to the maintenance of glucose homeostasis via IL-10. These CD19+ B220+ CD22+ CD5− IgM+ IgD+ cells are distinct from the B-1a cells studied here, not only in their surface phenotype but also in their tissue distribution. In addition, whether they produce IgM or are BAFF-dependent is not known. Nonetheless, it is interesting that two distinct populations of B cells can regulate glucose metabolism through the secretion of IL-10. Because the cells described by Nishimura et al. (22) reside only in adipose tissue and apparently do not circulate, these cells likely function locally, whereas the B-1a cells described in our study serve to regulate glucose metabolism more broadly. Moreover, in our study, IL-10–secreting B-1a cells accounted for most of the B cell–derived IL-10 in VAT in vivo and were at least five times more contributory than the cells described by Nishimura et al. (22). For these reasons, we believe that B-1a cells may be the predominant B-cell population involved in regulating glucose metabolism, at least under the conditions studied here.
Collectively, our data support a model in which B-1a cells oppose B-2 cells and promote IS through production of IL-10 and natural IgM to modulate macrophage and T cell–mediated inflammation. These functions become impaired in obesity, leading to chronic low-grade systemic and local tissue inflammation, which fuels IR. Thus, B-1a cells represent a novel immune subset governing glucose tolerance and provide an important link in the complex interplay of immunity and metabolism.
Supplementary Material
Article Information
Acknowledgments. The authors thank Troy Randall, of the University of Rochester, for providing sIgMnull mice; Mark Krasnow, of Stanford University, for providing BAFF-deficient mice; Tracey McLaughlin, of Stanford University, for providing human serum; and GlaxoSmithKline for providing anti–B lymphocyte stimulator/BAFF antibody 10F4. The authors also thank Joseph C. Gonzalez, of Stanford University, for assistance with experiments.
Funding. This work was partly supported by National Institutes of Health grant 1R01-DK-096038 (E.G.E.), Canadian Institutes of Health Research grant 119414 (D.A.W.), Canadian Diabetes Association grants OG-3-12-3844 (D.A.W.) and CS-5-12-3886 (D.A.W.), National Natural Science Foundation of China grant 81373210 (L.S.), and Shanghai Pujiang Program grant 13PJ1405400 (L.S.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. L.S. and M.H.Y.C. designed and performed research, analyzed data, and wrote the paper. M.N.A. and R.Y. performed research. D.A.W. and E.G.E. designed research, analyzed data, and wrote the paper. E.G.E. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
This article contains Supplementary Data online at http://diabetes.diabetesjournals.org/lookup/suppl/doi:10.2337/db14-0554/-/DC1.
References
- 1.Shaw JE, Sicree RA, Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 2010;87:4–14 [DOI] [PubMed] [Google Scholar]
- 2.Ioannidis I. The road from obesity to type 2 diabetes. Angiology 2008;59(Suppl. 2):39S–43S [DOI] [PubMed] [Google Scholar]
- 3.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 2003;112:1821–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz O, Oberhauser F, Saech J, et al. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS ONE 2010;5:e14328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science 1996;271:665–668 [DOI] [PubMed] [Google Scholar]
- 6.Winer S, Chan Y, Paltser G, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med 2009;15:921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hong EG, Ko HJ, Cho YR, et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes 2009;58:2525–2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ricardo-Gonzalez RR, Red Eagle A, Odegaard JI, et al. IL-4/STAT6 immune axis regulates peripheral nutrient metabolism and insulin sensitivity. Proc Natl Acad Sci U S A 2010;107:22617–22622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DeFuria J, Belkina AC, Jagannathan-Bogdan M, et al. B cells promote inflammation in obesity and type 2 diabetes through regulation of T-cell function and an inflammatory cytokine profile. Proc Natl Acad Sci U S A 2013;110:5133–5138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW Jr. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest 2003;112:1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cipolletta D, Feuerer M, Li A, et al. PPAR-γ is a major driver of the accumulation and phenotype of adipose tissue Treg cells. Nature 2012;486:549–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fried SK, Bunkin DA, Greenberg AS. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6: depot difference and regulation by glucocorticoid. J Clin Endocrinol Metab 1998;83:847–850 [DOI] [PubMed] [Google Scholar]
- 13.Wood IS, Wang B, Jenkins JR, Trayhurn P. The pro-inflammatory cytokine IL-18 is expressed in human adipose tissue and strongly upregulated by TNFalpha in human adipocytes. Biochem Biophys Res Commun 2005;337:422–429 [DOI] [PubMed] [Google Scholar]
- 14.Hamada M, Abe M, Miyake T, et al. B cell-activating factor controls the production of adipokines and induces insulin resistance. Obesity (Silver Spring) 2011;19:1915–1922 [DOI] [PubMed] [Google Scholar]
- 15.Müller N, Schulte DM, Hillebrand S, et al. B lymphocyte stimulator (BLyS) is expressed in human adipocytes in vivo and is related to obesity but not to insulin resistance. PLoS ONE 2014;9:e94282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winer DA, Winer S, Shen L, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med 2011;17:610–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol 2011;11:34–46 [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med 1985;161:1554–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baumgarth N, Herman OC, Jager GC, Brown L, Herzenberg LA, Herzenberg LA. Innate and acquired humoral immunities to influenza virus are mediated by distinct arms of the immune system. Proc Natl Acad Sci U S A 1999;96:2250–2255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Madan R, Demircik F, Surianarayanan S, et al. Nonredundant roles for B cell-derived IL-10 in immune counter-regulation. J Immunol 2009;183:2312–2320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Griffin DO, Rothstein TL. Human “orchestrator” CD11b(+) B1 cells spontaneously secrete IL-10 and regulate T cell activity. Mol Med 2012;18:1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nishimura S, Manabe I, Takaki S, et al. Adipose natural regulatory B cells negatively control adipose tissue inflammation. Cell Metab 2013;18:759–766 [DOI] [PubMed] [Google Scholar]
- 23.Kyaw T, Tay C, Krishnamurthi S, et al. B1a B lymphocytes are atheroprotective by secreting natural IgM that increases IgM deposits and reduces necrotic cores in atherosclerotic lesions. Circ Res 2011;109:830–840 [DOI] [PubMed] [Google Scholar]
- 24.Shaw PX, Hörkkö S, Chang MK, et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance, and protective immunity. J Clin Invest 2000;105:1731–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McLaughlin T, Deng A, Gonzales O, et al. Insulin resistance is associated with a modest increase in inflammation in subcutaneous adipose tissue of moderately obese women. Diabetologia 2008;51:2303–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faria-Neto JR, Chyu KY, Li X, et al. Passive immunization with monoclonal IgM antibodies against phosphorylcholine reduces accelerated vein graft atherosclerosis in apolipoprotein E-null mice. Atherosclerosis 2006;189:83–90 [DOI] [PubMed] [Google Scholar]
- 27.Williams JP, Pechet TT, Weiser MR, et al. Intestinal reperfusion injury is mediated by IgM and complement. J Appl Physiol (1985) 1999;86:938–942 [DOI] [PubMed] [Google Scholar]
- 28.Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol 1988;18:313–316 [DOI] [PubMed] [Google Scholar]
- 29.Chou MY, Fogelstrand L, Hartvigsen K, et al. Oxidation-specific epitopes are dominant targets of innate natural antibodies in mice and humans. J Clin Invest 2009;119:1335–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol 2011;11:738–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007;117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aron-Wisnewsky J, Tordjman J, Poitou C, et al. Human adipose tissue macrophages: m1 and m2 cell surface markers in subcutaneous and omental depots and after weight loss. J Clin Endocrinol Metab 2009;94:4619–4623 [DOI] [PubMed] [Google Scholar]
- 33.Jennbacken K, Ståhlman S, Grahnemo L, Wiklund O, Fogelstrand L. Glucose impairs B-1 cell function in diabetes. Clin Exp Immunol 2013;174:129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boes M, Esau C, Fischer MB, Schmidt T, Carroll M, Chen J. Enhanced B-1 cell development, but impaired IgG antibody responses in mice deficient in secreted IgM. J Immunol 1998;160:4776–4787 [PubMed] [Google Scholar]
- 35.Schiemann B, Gommerman JL, Vora K, et al. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science 2001;293:2111–2114 [DOI] [PubMed] [Google Scholar]
- 36.Kyaw T, Tay C, Hosseini H, et al. Depletion of B2 but not B1a B cells in BAFF receptor-deficient ApoE mice attenuates atherosclerosis by potently ameliorating arterial inflammation. PLoS ONE 2012;7:e29371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol 2002;3:944–950 [DOI] [PubMed] [Google Scholar]
- 38.Mauri C, Gray D, Mushtaq N, Londei M. Prevention of arthritis by interleukin 10-producing B cells. J Exp Med 2003;197:489–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature 2007;447:1116–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong SC, Puaux AL, Chittezhath M, et al. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol 2010;40:2296–2307 [DOI] [PubMed] [Google Scholar]
- 41.Popi AF, Lopes JD, Mariano M. Interleukin-10 secreted by B-1 cells modulates the phagocytic activity of murine macrophages in vitro. Immunology 2004;113:348–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbeiro DF, Barbeiro HV, Faintuch J, et al. B-1 cells temper endotoxemic inflammatory responses. Immunobiology 2011;216:302–308 [DOI] [PubMed] [Google Scholar]
- 43.Behan JW, Ehsanipour EA, Sheng X, et al. . Activation of adipose tissue macrophages in obese mice does not require lymphocytes. Obesity (Silver Spring) 2013;21:1380–1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kaveri SV, Silverman GJ, Bayry J. Natural IgM in immune equilibrium and harnessing their therapeutic potential. J Immunol 2012;188:939–945 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen Y, Park YB, Patel E, Silverman GJ. IgM antibodies to apoptosis-associated determinants recruit C1q and enhance dendritic cell phagocytosis of apoptotic cells. J Immunol 2009;182:6031–6043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cinti S, Mitchell G, Barbatelli G, et al. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res 2005;46:2347–2355 [DOI] [PubMed] [Google Scholar]
- 47.Rosenfeld ME, Palinski W, Ylä-Herttuala S, Butler S, Witztum JL. Distribution of oxidation specific lipid-protein adducts and apolipoprotein B in atherosclerotic lesions of varying severity from WHHL rabbits. Arteriosclerosis 1990;10:336–349 [DOI] [PubMed] [Google Scholar]
- 48.Scholz JL, Crowley JE, Tomayko MM, et al. . BLyS inhibition eliminates primary B cells but leaves natural and acquired humoral immunity intact. Proc Natl Acad Sci U S A 2008;105:15517–15522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kawasaki K, Abe M, Tada F, et al. Blockade of B-cell-activating factor signaling enhances hepatic steatosis induced by a high-fat diet and improves insulin sensitivity. Lab Invest 2013;93:311–321 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.