Abstract
Although the haptoglobin (Hp) 1-1 genotype is associated with a lower coronary artery disease (CAD) risk in diabetes, we recently reported an increased stroke incidence in type 1 diabetes with Hp 1-1. We, thus, evaluated differences in earlier brain vascular abnormality markers by Hp using neuroimaging. Neuroimaging was completed in 94 participants of the Pittsburgh Epidemiology of Diabetes Complications study with Hp genotyping available (mean age, 49; duration, 41 years). White matter hyperintensities (WMH) volume, lacunar infarcts, and gray matter atrophy were quantified. Sixteen percent were homozygous for Hp 1 and 43% for Hp 2. A significant trend toward increased WMH was observed with greater duration and the number of Hp 1 alleles. Associations were strongest for the interhemispheric connecting fibers of the corpus callosum. Allowing for duration, sex, waist-to-hip ratio, HbA1c, systolic blood pressure, and lipids in models with backward elimination, results were similar. No significant differences by Hp were noted for atrophy or lacunar infarcts. Consistent with its direct association with stroke, the Hp 1-1 genotype is associated with higher WMH in this population. Further, including mechanistic, studies on the role of the Hp genotype in cerebrovascular disease and the implications for worsening cognitive function are needed.
Introduction
White matter hyperintensities (WMH), a cerebrovascular abnormality identifiable via brain MRI, are typical features of the aging brain, are neither benign nor reversible, and predict greater risk of dementia, disability, stroke, and mortality (1). Whether WMH respond to treatment is unknown, although their progression could potentially be delayed (2).
A recent general population study suggested a lower prevalence of the haptoglobin (Hp) 2 allele in stroke (3). Another study, among hypertensive patients without symptomatic vascular disease, noted greater WMH with Hp 1 (4). These findings were surprising given multiple prospective studies in diabetes pointing to increased cardiovascular disease (CVD) risk with Hp 2 allele homozygosity (5–9).
Hp is an acute-phase protein whose major function is binding to free hemoglobin, thus acting as an antioxidant (10). In humans, two classes of alleles exist at the Hp locus (16q22), Hp 1 and Hp 2 (resulting from duplication of exons 3 and 4 [10]), yielding three genotypes (Hp 1-1, 2-1, and 2-2). These allelic protein products differ in structure and function, with Hp 2-2 being a less efficient antioxidant (10,11).
In the Pittsburgh Epidemiology of Diabetes Complications (EDC) study of type 1 diabetes, we observed an increasing coronary artery disease (CAD) incidence with the number of Hp 2 alleles (12) and, interestingly, an inverse association with stroke (13). To further comprehend these discrepant findings, we sought to determine whether the Hp genotype was associated with markers of cerebrovascular abnormalities, including WMH and lacunar infarcts.
Research Design and Methods
The EDC is a representative (14), prospective investigation of a historical, incident childhood-onset type 1 diabetes cohort, diagnosed or seen within 1 year of diagnosis (1950–1980) at Children's Hospital of Pittsburgh (15). After the initial examination (1986–1988), biennial surveys and examinations followed for 24 years. During the 24-year follow-up, participants were invited to a neuroimaging substudy. Of 149 (56.7%) accepting the invitation, 112 were eligible and 95 had usable WMH data; 94 also had Hp genotyping. All participants provided informed consent. The University of Pittsburgh Institutional Review Board approved all study protocols.
Participants underwent brain MRI in a 3T scanner. Two sequences were captured (T1-magnetization prepared rapid acquisition gradient echo and T2-weighted fluid attenuated inversion recovery [FLAIR]). An automated labeling pathway WMH segmentation method was used to quantify volumes and localization of focal WMH (16). Total brain volume constituted the sum of gray and white matter volumes and cerebrospinal fluid obtained using an automated labeling pathway. Tracts examined included the corpus callosum and long connecting tracts vulnerable to cardiovascular risk factors, comprising the superior and inferior longitudinal fasciculus, anterior thalamic radiation, corticospinal tracts, and cingular bundle.
Chronic lacunar-type infarcts were distinguished from WMH and prominent perivascular spaces by careful review. Infarcts were defined as T2 hyperintense/T1 hypointense lesions with irregular borders and central parenchymal loss, which was suppressed on T2/FLAIR sequences. T2 WMH, commonly attributed to chronic small vessel ischemic disease, demonstrated no parenchymal loss and did not suppress centrally on the T2/FLAIR sequences. Prominent perivascular spaces, which can demonstrate central T1 hypointensity and suppression on T2/FLAIR sequences, were distinguished from infarcts by smooth borders, a linear orientation, and the absence of adjacent T2 hyperintensity. Ratings were done by a board-certified neuroradiologist (J.M.M.).
Smoking status was self-reported. Blood pressure was measured with a random zero sphygmomanometer after a 5-minute rest (17), and hypertension was defined as ≥140/90 mmHg or antihypertensive medication use. Glycosylated hemoglobin (HbA1c; DCA 2000 analyzer, Bayer HealthCare, Tarrytown, NY), nonfasting total and HDL cholesterol (HDLc; Cholestech LDX Analyzer, Cholestech Corporation, Hayward, CA), and the urinary albumin-to-creatinine ratio (DCA Vantage Analyzer, Siemens Healthcare Diagnostics) were also measured. Non-HDLc was calculated as total cholesterol minus HDLc. Hp was genotyped by an amplification method (18). When DNA was unavailable, Hp phenotype was assessed (19).
CAD was determined by study-physician diagnosed angina, ischemic electrocardiogram changes (Minnesota codes 1.3, 4.1–4.3, 5.1–5.3, 7.1), revascularization, confirmed myocardial infarction (Minnesota codes 1.1/1.2 or hospital records), or CAD death. Stroke was defined as an acute-onset 24-h neurological deficit, without other evident cause, confirmed by hospital/autopsy records.
Statistical Analysis
Univariate differences by Hp were determined with descriptive statistics. The independence of the Hp-WMH association was evaluated with general linear models, using Bonferroni adjustment for multiple comparisons by Hp and α = 0.008 to account for assessing multiple tracts.
Results
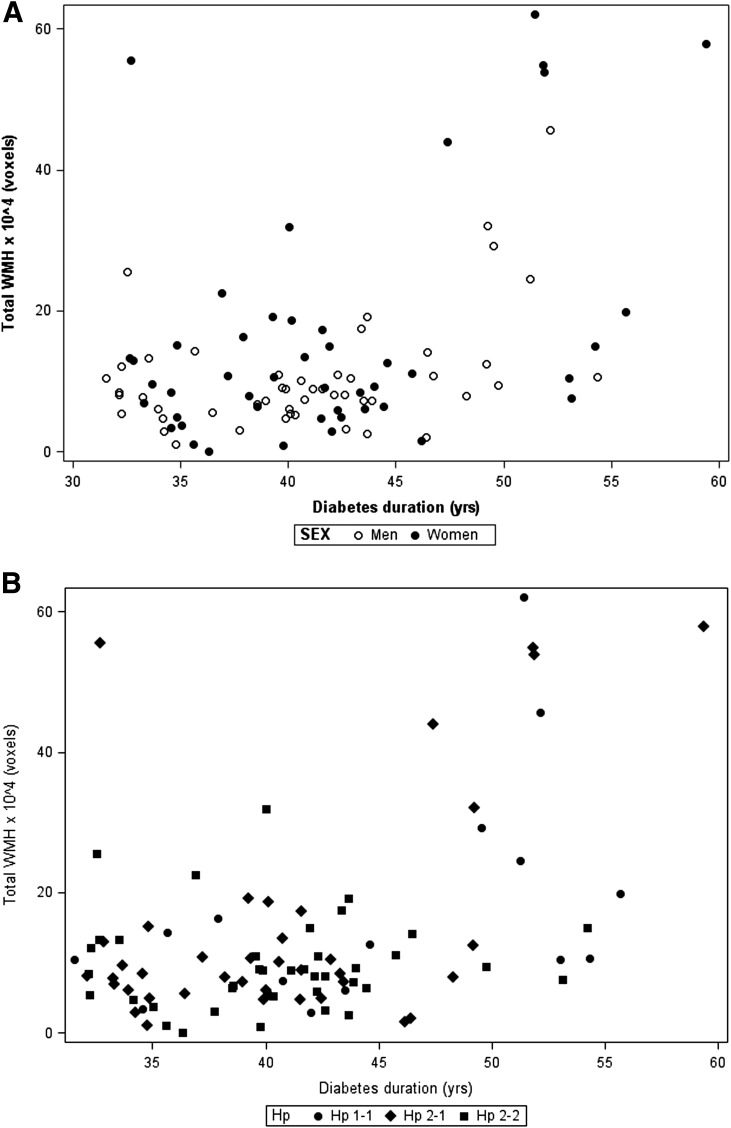
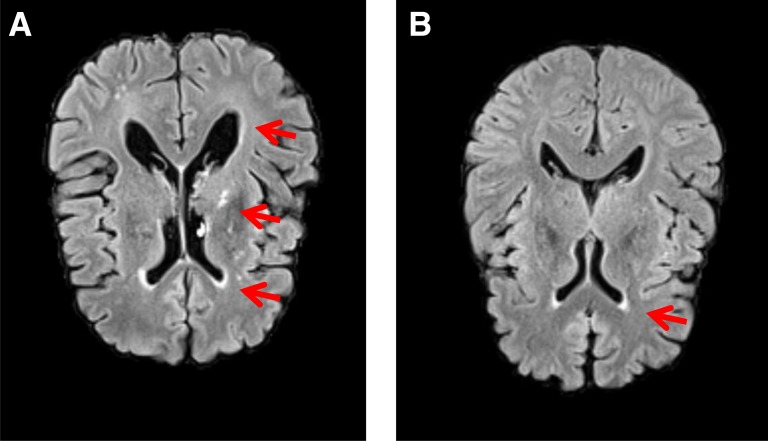
Hp 1 homozygosity was 16% and Hp 2 was 43%. Participant characteristics were similar by Hp, although diabetes duration was longer, non-HDLc was lower, and, among women, HDLc was higher with Hp 1-1 (Table 1). CAD incidence increased from Hp 1-1 to 2-2. Only one participant (Hp 2-1) experienced a clinical stroke; three (one of each Hp type) had a lacunar infarct. All but one (Hp 2-2) had measurable total brain WMH, which increased in extent with duration (Fig. 1A). The extent of WMH increased with diabetes duration to a greater degree in Hp 1-1 (r = 0.49, P = 0.06) than in Hp 2-1 (r = 0.25, P = 0.12) or 2-2 participants (r = 0.15, P = 0.35; Fig. 1B). A direct association between total WMH and Hp 1 allele number was observed (P for trend = 0.02, Table 1), resulting mainly in the interhemispheric connecting fibers of the corpus callosum (P for trend = 0.004). No differences by Hp were seen in other tracks or gray matter atrophy. Figure 2 shows the MRI scan results of an Hp 1-1 and an Hp 2-2 participant of similar age and diabetes duration. Hyperintensities in subcortical and periventricular areas with substantial ventricular enlargement are evident in the Hp 1-1 participant’s scan. Hyperintensities are restricted to the anterior periventricular areas of the Hp 2-2 participant, lacking ventricular enlargement.
Table 1.
Participant characteristics by Hp genotype
| Hp 1-1 (n = 15) | Hp 2-1 (n = 39) | Hp 2-2 (n = 40) | P value | |
|---|---|---|---|---|
| Age (years) | 52.7 (8.8) | 49.7 (6.8) | 48.0 (6.0) | |
| Age at onset (years) | 7.5 (4.6) | 9.1 (4.1) | 7.6 (4.1) | |
| Duration (years) | 45.2 (7.9) | 40.6 (6.4)* | 40.4 (5.3)* | |
| Females (%, n) | 53.3 (8) | 56.4 (22) | 40.0 (16) | 0.32 |
| BMI (kg/m2) | 25.5 (3.7) | 26.4 (4.7) | 28.4 (4.7) | |
| Waist-to-hip ratio | ||||
| Men | 0.92 (0.05) | 0.92 (0.07) | 0.97 (0.07)† | |
| Women | 0.78 (0.10) | 0.85 (0.07) | 0.85 (0.07) | |
| Ever smoker (%, n) | 40.0 (6) | 21.6 (8) | 21.0 (8) | 0.33‡ |
| HbA1c (%) | 7.4 (1.4) | 8.1 (1.6) | 7.4 (1.2) | |
| Blood pressure (mmHg) | ||||
| Systolic | 122.8 (15.7) | 114.8 (14.5) | 120.7 (14.4) | |
| Diastolic | 63.3 (12.7) | 65.1 (7.5) | 65.8 (9.8) | |
| Pulse (bpm) | 67.5 (14.0) | 68.6 (11.7) | 68.5 (12.7) | |
| Hypertension (%, n) | 20.0 (3) | 18.4 (7) | 21.6 (8) | 0.94‡ |
| HDLc (mg/dL) | ||||
| Men | 41.6 (6.9) | 50.2 (14.3) | 48.0 (14.7) | |
| Women | 88.0 (16.5) | 70.6 (17.2) | 57.3 (19.7)* | |
| Non-HDLc (mg/dL) | 96.1 (26.8) | 96.4 (26.2) | 116.7 (36.9)† | |
| Albumin-to-creatinine ratio (mg/g) | 20.2 (9.6, 61.1) | 12.7 (7.5, 35.6) | 11.2 (5.4, 28.6) | 0.16 |
| CAD (%, n) | 0.0 (0) | 12.8 (5) | 25.0 (10) | 0.06‡ |
| Stroke (%, n) | 0.0 (0) | 2.2 (1) | 0.0 (0) | 1.00‡ |
| Lacunar infarcts (%, n) | 6.7 (1) | 2.2 (1) | 2.4 (1) | 0.53‡ |
| White matter areas (×104) | ||||
| Total brain WMH§ | 12.527 (7.372, 24.582) | 8.345 (6.078, 15.164) | 8.617 (5.427, 12.687) | 0.02** |
| Total corpus callosum WMH§ | 3.916 (2.283, 8.449) | 2.685 (1.219, 6.122) | 2.027 (1.069, 2.977) | 0.004** |
Continuous data are shown as means (SD) or median (interquartile range) and categoric data as indicated.
Significantly different from Hp 1-1 after Bonferroni adjustment for multiple comparisons.
Significantly different from Hp 2-1 after Bonferroni adjustment for multiple comparisons.
Fisher exact test P value.
Logarithmically transformed variables were used.
P value for trend.
Figure 1.
A: Total WMH in men and women with type 1 diabetes. B: Total WMH by Hp genotype and duration of type 1 diabetes.
Figure 2.
Brain MRI scan results of one individual with Hp 1-1 and another with Hp 2-2. A: FLAIR image of a 58.6-year-old individual with type 1 diabetes for 50 years and the Hp 1-1 genotype shows hyperintensities in subcortical areas and periventricular areas, with substantial ventricular enlargement (arrows). B: FLAIR image of a 55.6-year-old individual with type 1 diabetes for 53 years and the Hp 2-2 genotype shows hyperintensities only restricted to the anterior periventricular areas (arrow) but not in the parenchyma and no signs of ventricular enlargement.
In regression models, diabetes duration explained 14% of the total brain WMH variation, whereas Hp contributed a further (nonsignificant) 2.5% (Table 2). For total corpus callosum WMH, duration explained 19.5% of the variance, whereas Hp contributed a further 4% (P = 0.03). Allowing for duration, sex, HbA1c and lipids, results were similar despite the reduction in sample size (n = 88; Ptotal brain = 0.2887, Ptotal corpus callosum = 0.0862).
Table 2.
General linear regression models for total brain and total corpus callosum WMH with the number of Hp 1 alleles (n = 94)
| Outcome | Independent variable | β (SE) | P value | Model R2 |
|---|---|---|---|---|
| Total brain WMH* | Model 1 | 0.1401 | ||
| Diabetes duration | 0.05 (0.01) | 0.0002 | ||
| Model 2 | 0.1648 | |||
| Diabetes duration | 0.04 (0.01) | 0.0008 | ||
| Number of Hp 1 alleles | 0.17 (0.11) | 0.1054 | ||
| Total corpus callosum WMH* | Model 1 | 0.1954 | ||
| Diabetes duration | 0.05 (0.01) | <0.0001 | ||
| Model 2 | 0.2346 | |||
| Diabetes duration | 0.04 (0.01) | <0.0001 | ||
| Number of Hp 1 alleles | 0.20 (0.09) | 0.0281 |
Logarithmically transformed before statistical testing.
Because diabetes duration appeared to drive most of the WMH variation and most of the participants with longer duration carried the Hp 1 allele (Fig. 1B), we sought to evaluate whether duration modified the Hp-WMH relationship. No interaction was observed for total brain WMH (P = 0.35), although a borderline significant effect modification was observed for total corpus callosum WMH (P = 0.07).
Discussion
In this study of middle-aged adults with type 1 diabetes, the Hp 1 allele was associated with greater WMH localized in the interhemispheric connecting fibers of the corpus callosum. This association appeared to be largely driven by diabetes duration, which strongly correlated with WMH and the presence of at least one Hp 1 allele. However, although adjusting for duration rendered the Hp–total brain WMH association nonsignificant, it did not eliminate the Hp–total corpus callosum WMH association.
During the past decade, Hp has been emerging as a major risk factor for the development of diabetic CVD, with most studies having focused on type 2 diabetes. Indeed, five longitudinal studies showed significantly increased cardiovascular risk with Hp 2-2, although a similar association has generally not been observed in nondiabetic populations (5–9). Moreover, despite numerous trial failures to demonstrate cardiovascular benefit with antioxidant supplementation, three trials provided evidence that the protective effect of vitamin E therapy against CVD is restricted to the subgroup with type 2 diabetes and Hp 2-2 (20).
To date, the EDC has been the only study to have evaluated the association between the Hp genotype and CAD incidence in type 1 diabetes, demonstrating increasing risk with the number of Hp 2 alleles (12). Interestingly, although a similar association has generally not been observed in the general population, a greater frequency of the Hp 1 allele in a first symptomatic lacunar stroke was reported in a population with high hypertension prevalence (82%), only 11% of whom had diabetes (3). Furthermore, among hypertensive patients, Hp 1-1 was suggested to be cross-sectionally associated with larger deep white matter lesions (4). Although the nature of these general population study designs allows questions regarding survival bias having affected findings, prospective analyses from our EDC study also suggest a greater incidence of stroke with Hp 1-1 in type 1 diabetes (13), although the number of incident cases was small. Herein, we further report that the extent of WMH in the brain appears linked to the presence of the Hp 1 allele, which correlated with WMH localized in the interhemispheric connecting fibers of the corpus callosum. Thus, although the associations presented here are cross-sectional and we cannot rule out the possibility that residual confounding, competing risk, and/or survival bias have distorted the “true” association between the Hp genotype and extent of WMH in the brain, the prospective relationship between Hp and stroke in EDC demands that we do not readily dismiss these cross-sectional results.
Given the strength and abundance of evidence demonstrating a protective effect of the Hp 1 allele for vascular diabetes complication incidence, however, it is difficult to comprehend the suggested increased risk conferred by the same allele in terms of cerebrovascular disease. If true, these apparently contradictory findings likely relate to existing functional differences between the two Hp allelic protein products as well as hyperglycemia, a distinct feature of diabetes. Most hypotheses and investigations of Hp thus far have focused on associations relating to its major function: the binding of free hemoglobin and its removal from circulation, where the Hp 1 allele has an advantage. Thus, the protection conferred by the Hp 1-1 genotype against CAD development is thought to be mediated by the greater antioxidative and anti-inflammatory capacity of the Hp 1 allele (11). These Hp properties are thought to be of greatest importance in the presence of impaired glucose tolerance for a number of reasons, including the reduced Hp efficiency to prevent oxidation from HbA1c (11).
The importance of Hp to normal physiology, however, expands beyond its role as the scavenger of free hemoglobin. Indeed, Hp represents a potent angiogenic factor, essential for the proliferation and differentiation of endothelial cells in neovascular development (21). Moreover, although Hp is primarily produced by liver hepatocytes (10), Hp is also expressed in the brain, and thus, disruption of the blood-brain barrier is not the only pathway of Hp entry in the brain (22). Interestingly, compared with the other two genotypes, Hp 2-2 has been associated with enhanced angiogenic activity (21). A recent publication (23) shed light on the potential mechanism by which this Hp property may relate to the observed associations with WMH and stroke. It is well established that endothelial dysfunction has a major role in the development and progression of diabetes complications, potentially also including cerebral small vessel disease (24), which underlies white matter lesion and lacunar infarct formation. Investigators suggested that the formation of endothelial cell clusters, which may mitigate cerebral small vessel disease, appeared attenuated in lacunar stroke patients with Hp 1-1 versus Hp 2-2 (23). In addition, cluster numbers were lower when Hp 1-1 was added to endothelial progenitor cell cultures compared with numbers produced by adding other Hp types, suggesting that Hp 1-1 may provide inadequate protection against small vessel disease initiated subsequent to endothelial damage, thus perhaps promoting greater WMH and stroke. Whether this increased angiogenesis after a stroke event continues to be beneficial via formation of functional new blood vessels after the insult is unclear (25), however, given prior evidence that neovascular growth may also lead to pathologic conditions (e.g., diabetic retinopathy) (26).
Undoubtedly, further studies on the role of the Hp genotype in cerebrovascular disease are needed to elucidate the pathophysiology and clinical implications of these associations and possible therapeutic interventions. Moreover, our own findings of diverse effects of Hp on CAD and stroke require validation. It is intriguing, however, to speculate that the failure to demonstrate protection against CVD with vitamin E in the absence of the Hp 2-2 genotype in diabetes may be at least partly explained by an increased stroke risk associated with the Hp 1 allele (20) because vitamin E may increase the risk of bleeding. Lastly, it is becoming evident that the Hp genotype asks us to tell a more intricate story than perhaps originally anticipated, one that will certainly make the search for answers exhilarating.
Article Information
Acknowledgments. The authors thank all study participants for their invaluable contributions as well as the EDC study staff.
Funding. This research was supported by National Institutes of Health grants DK-034818 and DK-089028 and by the Rossi Memorial Fund.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. T.C. researched data, conducted the statistical analysis, and wrote the manuscript. C.R., R.E.F., and T.J.O. researched data and reviewed and edited the manuscript. H.A., J.M.M., and K.N. researched data. T.C. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Debette S, Markus HS. The clinical importance of white matter hyperintensities on brain magnetic resonance imaging: systematic review and meta-analysis. BMJ 2010;341:c3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dufouil C, Chalmers J, Coskun O, et al.; PROGRESS MRI Substudy Investigators . Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation 2005;112:1644–1650 [DOI] [PubMed] [Google Scholar]
- 3.Staals J, Pieters BM, Knottnerus IL, et al. Haptoglobin polymorphism and lacunar stroke. Curr Neurovasc Res 2008;5:153–158 [DOI] [PubMed] [Google Scholar]
- 4.Staals J, Henskens LH, Delanghe JR, et al. Haptoglobin phenotype correlates with the extent of cerebral deep white matter lesions in hypertensive patients. Curr Neurovasc Res 2010;7:1–5 [DOI] [PubMed] [Google Scholar]
- 5.Levy AP, Roguin A, Hochberg I, et al. Haptoglobin phenotype and vascular complications in patients with diabetes. N Engl J Med 2000;343:969–970 [DOI] [PubMed] [Google Scholar]
- 6.Levy AP, Hochberg I, Jablonski K, et al.; Strong Heart Study . Haptoglobin phenotype is an independent risk factor for cardiovascular disease in individuals with diabetes: The Strong Heart Study. J Am Coll Cardiol 2002;40:1984–1990 [DOI] [PubMed] [Google Scholar]
- 7.Suleiman M, Aronson D, Asleh R, et al. Haptoglobin polymorphism predicts 30-day mortality and heart failure in patients with diabetes and acute myocardial infarction. Diabetes 2005;54:2802–2806 [DOI] [PubMed] [Google Scholar]
- 8.Roguin A, Koch W, Kastrati A, Aronson D, Schomig A, Levy AP. Haptoglobin genotype is predictive of major adverse cardiac events in the 1-year period after percutaneous transluminal coronary angioplasty in individuals with diabetes. Diabetes Care 2003;26:2628–2631 [DOI] [PubMed] [Google Scholar]
- 9.Cahill LE, Levy AP, Chiuve SE, et al. Haptoglobin genotype is a consistent marker of coronary heart disease risk among individuals with elevated glycosylated hemoglobin. J Am Coll Cardiol 2013;61:728–737 [DOI] [PMC free article] [PubMed]
- 10.Langlois MR, Delanghe JR. Biological and clinical significance of haptoglobin polymorphism in humans. Clin Chem 1996;42:1589–1600 [PubMed] [Google Scholar]
- 11.Costacou T, Levy AP. Haptoglobin genotype and its role in diabetic cardiovascular disease. J Cardiovasc Transl Res 2012;5:423–435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Costacou T, Ferrell RE, Orchard TJ. Haptoglobin genotype: a determinant of cardiovascular complication risk in type 1 diabetes. Diabetes 2008;57:1702–1706 [DOI] [PubMed] [Google Scholar]
- 13.Costacou T, Secrest AM, Ferrell RE, Orchard TJ. Haptoglobin genotype and cerebrovascular disease incidence in type 1 diabetes. Diab Vasc Dis Res 2014;11:335–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wagener DK, Sacks JM, LaPorte RE, Macgregor JM. The Pittsburgh study of insulin-dependent diabetes mellitus. Risk for diabetes among relatives of IDDM. Diabetes 1982;31:136–144 [DOI] [PubMed] [Google Scholar]
- 15.Orchard TJ, Dorman JS, Maser RE, et al. Prevalence of complications in IDDM by sex and duration. Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes 1990;39:1116–1124 [DOI] [PubMed] [Google Scholar]
- 16.Wu M, Rosano C, Butters M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res 2006;148:133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borhani NO, Kass EH, Langford HG, Payne GH, Remington RD, Stamler J. The hypertension detection and follow-up program: Hypertension detection and follow-up program cooperative group. Prev Med 1976;5:207–215 [DOI] [PubMed] [Google Scholar]
- 18.Koch W, Latz W, Eichinger M, et al. Genotyping of the common haptoglobin Hp 1/2 polymorphism based on PCR. Clin Chem 2002;48:1377–1382 [PubMed] [Google Scholar]
- 19.Levy NS, Vardi M, Blum S, et al. An enzyme linked immunosorbent assay (ELISA) for the determination of the human haptoglobin phenotype. Clin Chem Lab Med 2013;51:1615–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vardi M, Blum S, Levy AP. Haptoglobin genotype and cardiovascular outcomes in diabetes mellitus - natural history of the disease and the effect of vitamin E treatment. Meta-analysis of the medical literature. Eur J Intern Med 2012;23:628–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cid MC, Grant DS, Hoffman GS, Auerbach R, Fauci AS, Kleinman HK. Identification of haptoglobin as an angiogenic factor in sera from patients with systemic vasculitis. J Clin Invest 1993;91:977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao X, Song S, Sun G, et al. Neuroprotective role of haptoglobin after intracerebral hemorrhage. J Neurosci 2009;29:15819–15827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rouhl RP, van Oostenbrugge RJ, Damoiseaux JG, et al. Haptoglobin phenotype may alter endothelial progenitor cell cluster formation in cerebral small vessel disease. Curr Neurovasc Res 2009;6:32–41 [DOI] [PubMed] [Google Scholar]
- 24.Farrall AJ, Wardlaw JM. Blood-brain barrier: ageing and microvascular disease—systematic review and meta-analysis. Neurobiol Aging 2009;30:337–352 [DOI] [PubMed] [Google Scholar]
- 25.Ergul A, Alhusban A, Fagan SC. Angiogenesis: a harmonized target for recovery after stroke. Stroke 2012;43:2270–2274 [DOI] [PMC free article] [PubMed]
- 26.Durham JT, Herman IM. Microvascular modifications in diabetic retinopathy. Curr Diab Rep 2011;11:253–264 [DOI] [PubMed] [Google Scholar]